Abstract
Cardiac myofibroblasts play an important role in myocardial remodeling. Although α-smooth muscle actin (α-SMA) expression is the hallmark of mature myofibroblasts, its role in regulating fibroblast function remains poorly understood. We explore the effects of the matrix environment in modulating cardiac fibroblast phenotype, and we investigate the role of α-SMA in fibroblast function using loss- and gain-of-function approaches. In murine myocardial infarction, infiltration of the infarct border zone with abundant α-SMA-positive myofibroblasts was associated with scar contraction. Isolated cardiac fibroblasts cultured in plates showed high α-SMA expression localized in stress fibers, exhibited activation of focal adhesion kinase (FAK), and synthesized large amounts of extracellular matrix proteins. In contrast, when these cells were cultured in collagen lattices, they exhibited marked reduction of α-SMA expression, negligible FAK activation, attenuated collagen synthesis, and increased transcription of genes associated with matrix metabolism. Transforming Growth Factor-βl-mediated contraction of fibroblast-populated collagen pads was associated with accentuated α-SMA synthesis. In contrast, serum- and basic Fibroblast Growth Factor-induced collagen pad contraction was associated with reduced α-SMA expression. α-SMA siRNA knockdown attenuated contraction of collagen pads populated with serum-stimulated cells. Surprisingly, α-SMA overexpression also reduced collagen pad contraction, suggesting that α-SMA is not sufficient to promote contraction of the matrix. Reduced contraction by α-SMA-overexpressing cells was associated with attenuated proliferative activity, in the absence of any effects on apoptosis. α-SMA may be implicated in contraction and remodeling of the extracellular matrix, but is not sufficient to induce contraction. α-SMA expression may modulate cellular functions, beyond its effects on contractility.
Keywords: fibroblast, myofibroblast, myocardial infarction, actin, transforming growth factor-β, α-smooth muscle actin, extracellular matrix
INTRODUCTION
In healing tissues, fibroblasts acquire a contractile phenotype, characterized by formation of microfilament bundles, and by de novo expression of α-smooth muscle actin (α-SMA). These activated cells, termed “myofibroblasts” [1],[2],[3],[4],[5],[6],[7] participate in the reparative response, by secreting large amounts of extracellular matrix proteins [8],[9] and may be responsible for contraction of healing wounds [10]. Repair of injured tissues is dependent on timely activation and deactivation of myofibroblasts; prolonged or excessive myofibroblast activity may result in fibrosis and organ dysfunction [11],[12],[13].
The normal mammalian myocardium contains a large number of fibroblast-like cells [14], [15],[16]. In the absence of injury, these interstitial cells remain quiescent; however, a wide range of injurious processes can induce cardiac fibroblast activation. Conversion of cardiac fibroblasts into activated α-SMA-positive myofibroblasts is consistently noted following myocardial infarction, both in human patients [17] and in experimental models [18],[19]. Because the adult mammalian heart has negligible regenerative capacity, activated myofibroblasts play a crucial role in post-infarction cardiac repair, by secreting extracellular matrix proteins, thus protecting the heart from catastrophic rupture. Moreover, the capacity of infarct myofibroblasts to contract the scar may play an important role in protecting the chamber from adverse remodeling [20]. On the other hand, excessive activation of fibroblasts in the infarcted heart may contribute to fibrosis, increase stiffness, and promote both systolic and diastolic dysfunction [21],[12]
Our group and other investigators have used experimental animal models and in vitro approaches to study the role of fibroblasts in the infarcted and remodeling myocardium [22],[23],[24],[25],[26],[27],[28],[29]. In vitro studies investigating myocardial fibrotic responses, have used either cardiac fibroblasts cultured and stimulated in plates, or cells enmeshed in collagen lattices [25],[30],[31],[32]. Assessment of fibroblast-mediated contraction in collagen pads provides a robust and pathophysiologically relevant model of fibroblast function. Although α-SMA expression is a hallmark of the mature myofibroblast, its role in regulation of fibroblast behavior and function remains poorly understood. Studies using fibroblast-populated collagen lattices suggested that α-SMA expression increases fibroblast contractile activity [33], and plays a crucial role in focal adhesion maturation [34]. However, other investigations showed that, at a single cell level, fibroblasts and myofibroblasts were found to exert comparable contractile forces [35]. Because fibroblasts are highly dynamic cells [36], interpretation of the findings derived from different in vitro models requires understanding of the distinct characteristics of fibroblasts in each model. Our study compares cardiac fibroblast phenotype between the two models, and explores the role of α-SMA in fibroblast-mediated matrix contraction.
We report that, in both reperfused and non-reperfused mouse infarcts, scar contraction is associated with marked infiltration of the infarct border zone with myofibroblasts. In vitro, culture of cardiac fibroblasts in collagen pads markedly suppressed α-SMA expression and reduced extracellular matrix synthesis, while promoting synthesis of genes associated with matrix metabolism. Contraction of fibroblast-populated pads in response to serum, or specific growth factors was not consistently associated with upregulation of α-SMA. siRNA knockdown and overexpression experiments demonstrated that α-SMA is involved in collagen pad contraction, but is not sufficient to induce contraction of the matrix. Surprisingly α-SMA expression modulated proliferative activity in cardiac fibroblasts. Our findings highlight the dynamic phenotype of fibroblasts under different conditions and suggest that α-SMA may exert actions independent of its effects on cell contraction.
MATERIALS AND METHODS
Mouse models of reperfused and non-reperfused myocardial infarction
Both male and female, 3–4 month old C57/BL6J mice underwent coronary occlusion/reperfusion protocols as previously described [37]. Mice were anesthetized by isoflurane inhalation (isoflurane 2–3% vol/vol). Non-reperfused myocardial infarction was induced using an open chest model of permanent left coronary artery ligation [38]. Reperfused infarction was induced using a well-characterized closed-chest model of coronary occlusion and reperfusion [39]. Infarcted hearts (after 7 or 28 days of permanent coronary occlusion and after 1h ischemia/7–28 days of reperfusion) were fixed in formalin and embedded in paraffin.
Immunohistochemistry and histology
Infarcted hearts were sectioned systematically from based to apex in 250µm partitions as previously described [39]. One section from each partition was stained for sirius red to identify the healing scar. Scar size was morphometrically assessed using ImagePro software by dividing the total scar area to the total area of the left ventricle (from all partitions). Myofibroblasts in infarcted hearts were identified, as spindle-shaped cells located outside the vascular media with α-SMA immunofluorescence using the anti-α-SMA antibody (1A4, Santa Cruz Biotech) and an Alexa-Fluor 594-labeled secondary antibody (Molecular Probes). Sections were counterstained with DAPI.
Isolation and stimulation of mouse cardiac fibroblasts
Cardiac fibroblasts were isolated from C57/BL6J animals using enzymatic digestion as previously described [40], [41] and were cultured in DMEM/F12 (GIBCO Invitrogen Corporation, Carlsbad, CA) with 10% Fetal Calf Serum (FCS). Cells were serum-starved at passage 2 for 16h and subsequently stimulated with either 10 ng/ml or 50 ng/ml of recombinant TGF-β1 (R&D Systems, Minneapolis MN) for 4–24h. Total RNA was isolated from the stimulated cells using GeneJET RNA Purification Kit (ThermoFisher Scientific) and was reverse transcribed to cDNA using the iScript™ cDNA synthesis kit (Bio-Rad) following the manufacturer’s guidelines. Quantitative PCR was performed using the SsoFast™ EvaGreen® Supermix (Bio-Rad) method on the CFX384™ Real-Time PCR Detection System (Bio-Rad). Primers were synthesized by Integrated DNA Technologies. The following genes were assessed: α-SMA, type I collagen, type III collagen, matrix metalloproteinase (MMP)2, MMP3, MMP8, tissue inhibitor of metalloproteinases (TIMP)1, fibronectin and GAPDH. Each sample was run in triplicate. For experiments with collagen pads, RNA was isolated from the stimulated collagen pads using GeneJET RNA Purification Kit and the RNA obtained was used for qPCR.
Collagen pad contraction assay
Cardiac fibroblasts isolated from adult C57/BL6J mice were cultured to passage 2 and serum-starved overnight (16 hrs). Collagen matrix was prepared on ice by diluting a stock solution of rat 3.0 mg/ml collagen I (GIBCO Invitrogen Corporation, Carlsbad, CA) with 2X MEM and distilled water for a final concentration of 1 mg/ml collagen. Cell suspensions in 2X MEM were mixed with collagen solution to achieve the final 3*105 cells/ml concentration. Subsequently, 500 µl of this suspension was aliquoted to a 24-well culture plate (BD Falcon, San Jose, CA) and allowed to polymerize at 37°C for 30 min. Following polymerization, pads were released from wells, transferred to 6-well culture plate (BD Falcon, San Jose, CA) and cultured in 0% FCS DMEM/F12 for 24 h. After 24h, the pictures of the plates were taken in Bio-Rad ChemiDoc Imager, and the area of each pad was measured using Image Pro software. After incubation, the pads were fixed in formalin and processed in paraffin for subsequent histological analysis. For growth factor stimulation experiments, the collagen pads were suspended in either serum free DMEM/F12 or with 10% fetal bovine serum, or TGF-β1 (1–50ng/ml), or bFGF (50 ng/ml) for 24 hours.
α-SMA siRNA knockdown and overexpression experiments
For siRNA knockdown, mouse cardiac fibroblasts at passage 1 were seeded at 80% confluence (10 cm dishes) in complete medium and were either transfected with 50 nM ON-TARGET plus siRNA to α-SMA or transfected with a non-silencing control siRNA (Dharmacon) using Lipofectamine® 3000 Reagent (ThermoFisher Scientific). The ONTARGET modification is shown to dramatically decrease the off-target effects of the siRNA. In a pilot experiment we tested the effectiveness of 4 different siRNAs in reducing α-SMA protein levels in unstimulated HT cardiac fibroblasts. We found that although three of the four siRNAs reduced the expression of α-SMA, only one of the duplexes (#4, J-061937-12) reduced α-SMA protein expression by ~70%. We used this siRNA for all following in vitro experiments to achieve targeted knockdown of α-SMA.
The cells were returned to a 5% CO2 incubator and allowed to recover for 24 h. After 24h, the cells were harvested using TrypLE ™ Express reagent, counted and populated on collagen pads (3 *105 cells/ml concentration). The pads were either suspended in serum free DMEM/F12 or stimulated with media containing 10% serum or 10 ng/ml TGF-β for 72 h after which the pads were Imaged using Bio-Rad ChemiDoc Imager and contraction was assessed using Image J software. Cells were plated in parallel dishes to verify knockdown either by Western blots or by fluorescence microscopy.
For α-SMA overexpression experiments, mouse cardiac fibroblasts at passage 1 were seeded at 80% confluence (10 cm dishes) in complete medium and were either transfected with 2.5 ng of αSMA cDNA (Origene ™ Technologies) or transfected with a control entry vector using Lipofectamine®3000 Reagent (ThermoFisher Scientific). The cells were then processed as above.
Immunofluorescence
In order to assess focal adhesion kinase (FAK) activation, cardiac fibroblasts (1*105 cells per well) from wild type mice were seeded on four-well glass culture slides (BD Falcon). Two days later, cells were serum-starved for 16 hours before adding different doses of TGF-β for 65 min. The cells were fixed for 30 min with 4% paraformaldehyde and processed for immunofluorescence with antibodies recognizing phosphorylated FAK (Anti-phospho Y397 FAK, #ab39967, Abcam) and α-SMA (1A4, Santa Cruz Biotech), followed by Alexa Fluor 488– or Alexa Fluor 597–conjugated secondary antibodies respectively. The slides were mounted using VECTASHIELD Antifade Mounting Medium with DAPI (#H-1200). Images were obtained with a Zeiss Axio Imager.M2 microscope and Zeiss AxioVision software.
For the α-SMA knockdown, cardiac fibroblasts from wild type mice were transfected with 50 nM ONTARGETplus siRNA to α-SMA or transfected with a non-silencing control siRNA (Dharmacon) and for overexpression experiments, cells were transfected with 2.5 ng of α-SMA cDNA (Origene TM Technologies) or transfected with a control entry vector using Lipofectamine®3000 Reagent. The cells were returned to a 5% CO2 incubator, allowed to recover for 72 hours, then fixed for 30 min with 4% paraformaldehyde and processed for immunofluorescence with the anti-α-SMA antibody.
Assessment of cell density and size in collagen pads
Fibroblast-populated collagen pads were fixed in formalin and embedded in paraffin for histologic analysis. Histological sections from fibroblast-populated pads were stained with sirius red, and counterstained with hematoxylin, as previously described [25], to identify the fibroblasts and to quantitate their density and cell area. For assessment of cell density 4 random low power (50X) fields were used for each pad; cell density was expressed as the number of fibroblast profiles per unit area. For quantitation of cell area, we traced and measured the area of 30 fibroblasts from each pad using AxioVision software (Zeiss).
Assessment of fibroblast proliferation in collagen pads
Histological sections from fibroblast-populated pads were deparaffinized in xylene, rehydrated through graded alcohols, and permeabilized using Triton-X (0.1%) at room temperature for 8 min, followed by several washes in PBS. Nonspecific antibody binding was blocked by incubation for 1 hour with 10% goat serum in PBS. Immunohistochemical staining was performed with the monoclonal Rat Anti-Mouse Ki-67 Antibody (Clone TEC-3, DAKO) using the Vectastain ABC kit (Vector laboratories). Sections were developed with the Vector Blue Alkaline Phosphatase Substrate (Vector laboratories) and counterstained with the nuclear Fast Red (Sigma, N 8002) to identify nuclei. After two washes in distilled water, the pads were mounted using an aqueous mounting medium (Vecta Mount AQ; Vector laboratories). Quantitative analysis was performed by scanning 30 random fields at 400X magnification. Ki-67 positive cells density was calculated as the number of Ki-67 positive cells per unit area.
Assessment of fibroblast apoptosis
Histological sections from fibroblast-populated pads were deparaffinized, rehydrated, then permeabilized using Triton-X. Apoptotic cells were detected using the fluorescent TUNEL cell death detection kit (in situ cell death Detection Kit-TMR Red; Roche). Sections were mounted with mounting medium that contained DAPI, to counterstain nuclei (VECTASHIELD Mounting Medium with DAPI, Vector Laboratories). 4 random fields at 400X magnification were imaged from each pad, and the number of positive and negative TUNEL+ cells was counted. The density of apoptotic cells was expressed as the number of TUNEL+ cells divided per unit area.
RNA extraction and qPCR
Isolated total RNA from isolated cardiac fibroblasts was reverse transcribed to cDNA using the iScript™ cDNA synthesis kit (Bio-Rad) following the manufacturer’s guidelines. Quantitative PCR was performed using the SsoFast™ EvaGreen® Supermix (Bio-Rad) method on the CFX384™ Real-Time PCR Detection System (Bio-Rad). Primers were synthesized by Integrated DNA Technologies. The following sets of primers were used in the study: TIMP1 forward GCCTGAACACTGTCTACTT reverse TTGCTGCTGTCTGATAGTT; MMP2 forward TCCGCTGCATCCAGACTT, reverse GGTCCTGGCAATCCCTTTGTATA; MMP3 forward ATTTGGGTTTCTCTACTT, reverse GAAGAACTATAAGCATCAG; MMP8 forward TTAGGATGAGCCATAAGT, reverse TTGCTTGGTCTCTTCTAT; collagen I forward GATACTTGAAGAATATGAAC, reverse AATGCTGAATCTAATGAA; collagen III forward TACTCATTCACCAGCATA, reverse GTATAGTCTTCAGGTCTCA; fibronectin forward AGACTTCTCTCCTCAATG, reverse ACCAAACCATAAGAACTTT; GAPDH forward AACGACCCCTTCATTGACCT, reverse CACCAGTAGACTCCACGACA.
Protein extraction and western blotting
Mouse cardiac fibroblasts were transfected with either control non-targeting siRNA or α-SMA siRNA. 96h after transfection, cells were rinsed and lysed on ice in standard radioimmunoprecipitation assay (RIPA) lysis buffer [50mMtris-HCl (pH 8), 150 mM NaCl, 1%Triton X-100, 0.2 mM EDTA, 0.1% SDS, 0.5% sodium deoxycholate, 2 mM phenylmethylsulfonyl fluoride, 10% protease inhibitor cocktail (Roche), 10% phosphatase inhibitor cocktail (Roche)], and protein concentrations were determined with the BCA Assay Reagent (Pierce). Lysates (30 µg-50µg) were subjected to SDS–polyacrylamide gel electrophoresis and transferred onto Immuno-Blot polyvinylidene difluoride membranes (Bio-Rad). The membranes were blocked [5% BSA in tris-buffered saline (TBS)–Tween], incubated with antibodies to α-SMA (1A4, Santa Cruz Biotech), or phosphorylated FAK (Anti-phospho Y397 FAK, #ab39967, Abcam), washed, and then incubated with horseradish peroxidase (HRP)–linked secondary antibody (1% nonfat milk in TBS-Tween for 1 hour). Bound antibodies were detected by enhanced chemiluminescence with SuperSignal West Pico or Femto reagents (Pierce). Signal intensity was measured with ChemiDoc™ MP System (Bio Rad) and analyzed by Image Lab 3.0 software (Bio Rad). Membranes were then stripped and reprobed with an antibody against GAPDH (Santa Cruz Biotechnology) to verify equal loading.
Statistical analysis
Data are expressed as mean ± SEM. For comparisons of two groups unpaired, 2-tailed Student’s t-test using (when appropriate) Welch’s correction for unequal variances was performed. The Mann-Whitney test was used for comparisons between 2 groups that did not show Gaussian distribution. For comparison of multiple groups 1-way ANOVA was performed, followed by t-test corrected for multiple comparisons (Student-Newman-Keuls). The Kruskall-Wallis test, followed by Dunn’s multiple comparison post-test was used when one or more groups did not show Gaussian distribution.
RESULTS
1. Infiltration of the healing infarct with α-SMA-expressing myofibroblasts is associated with scar contraction
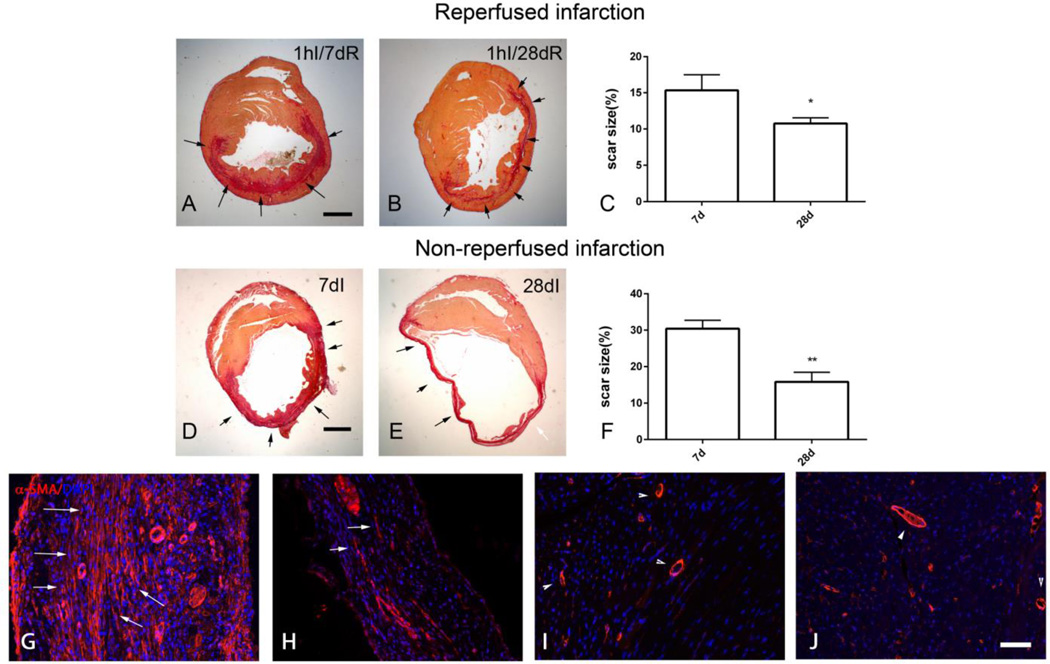
Repair of the adult mammalian heart is associated with replacement of dead cardiomyocytes with a collagen-based scar. We used quantitative morphometric analysis of sirius red-stained sections to study remodeling of the scar in mouse models of reperfused and non-reperfused myocardial infarction. Reperfused myocardial infarction heals through formation of a mid-myocardial scar after 7 days of reperfusion, that spares subendocardial and subepicardial regions (Figure 1A). After 28 days of reperfusion, scar size is significantly decreased (Figure 1B–C), reflecting scar contraction. Permanent coronary occlusion results in transmural infarction of the same territory (Figure 1D). Scar size expressed as a percentage of the left ventricular area is markedly reduced after 28 days of permanent coronary occlusion, reflecting contraction and thinning of the scar and progressive hypertrophy of the non-infarcted segments (Figure 1E–F). Immunofluorescent staining for α-SMA labels abundant myofibroblasts in the infarct border zone after 7 days of coronary occlusion, as spindle-shaped immunoreactive cells, located outside the vascular media (Figure 1G). The number of α-SMA+ myofibroblasts is reduced after 28 days of coronary occlusion (Figure 1H). In contrast, in remote myocardial segments, α-SMA immunoreactivity is predominantly localized in vascular mural cells (Figure 1I–J).
Figure 1.
In healing mouse infarcts, myofibroblast infiltration is associated with scar contraction. A-F: Sirius red staining was performed to label the area of the infarct in mice undergoing reperfused (A–C) and non-reperfused infarction protocols (D- F) (scalebar=1mm). Mouse hearts were sectioned at 250µm partitions and the scar size was assessed by measuring the sirius red-stained area at each level. A. The representative image shows a midmyocardial scar (arrows) after 1h of ischemia and 7 days of reperfusion, sparing the subendocardial and subepicardial region. B. After 1h of ischemia and 28 days of reperfusion, there is marked thinning of the infarcted area (arrows). C. Quantitative analysis shows a marked reduction of scar size after 28 days of reperfusion, reflecting contraction of the scar (*p<0.05, n=15–23/group). D. Permanent coronary occlusion caused a transmural infarct, leading to replacement of dead cardiomyocytes with scar (arrows) in the entire area at risk. E. Marked thinning of the scar was noted after 28 days of permanent occlusion (arrows). F. Quantitative analysis showed a 50% reduction in scar size at the 28-day timepoint, reflecting scar contraction (**p<0.01, n=14/group). G-J. α-SMA staining identifies abundant myofibroblasts in healing myocardial infarcts after 7 days of coronary ischemia (arrows). H. Myofibroblast density is markedly reduced after 28 days of ischemia, as the scar matures (arrows). I-J. In contrast, in the remodeling non-infarcted myocardium, α-SMA immunoreactivity is predominantly localized in the arteriolar media (arrowheads - I, 7 days of ischemia; J, 28 days of ischemia) (scalebar=50µm).
2. Fibroblasts populating free-floating collagen pads induce contraction upon stimulation with serum or TGF-β1
α-SMA+ myofibroblasts infiltrating the infarct may be responsible for contraction of healing myocardial scars. In order to investigate mediators responsible for fibroblast-mediated scar contraction, we used an in vitro model, in which isolated mouse cardiac fibroblasts are enmeshed in free-floating collagen pads. Stimulation with 10% serum induced marked contraction of the fibroblast-populated pads (Supplemental figure 1A, B). TGF-β1 stimulation (1ng/ml) also induced pad contraction; higher concentrations of TGF-β1 (10–50 ng.ml) did not further increase pad contraction (Supplemental figure 1C–D).
3. When enmeshed into free-floating collagen pads, cardiac fibroblasts exhibit markedly reduced baseline synthesis of collagens and α-SMA and decreased FAK activation
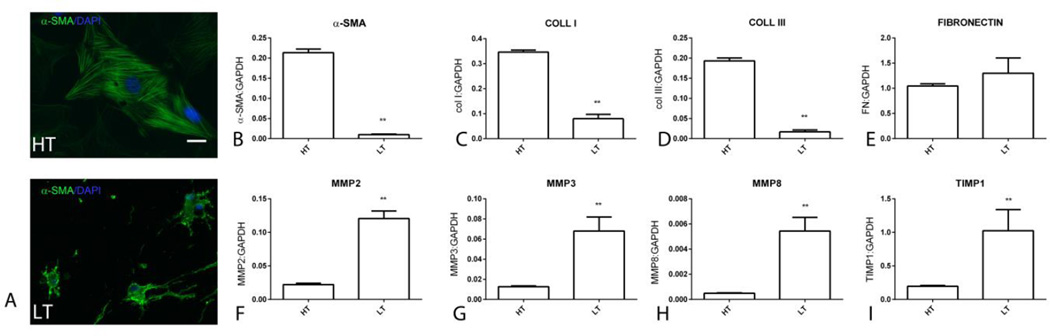
Next, we examined the effects of culture in the collagen pad on cardiac fibroblast morphology and gene expression. Fibroblasts cultured in the high-tension (HT) environment of the plate exhibited a flat, spread out morphology. Immunofluorescent staining showed that fibroblasts cultured in plates exhibited α-SMA incorporation in cytoskeletal filaments (Figure 2A). In contrast, fibroblasts cultured in the low-tension (LT) environment of the collagen pad were elongated, had dendritic projections and had low levels of α-SMA immunofluorescence with punctate cytoplasmic localization (Figure 2B–C). Comparison of gene expression showed that pad fibroblasts had a markedly lower expression of α-SMA, collagen I, and III, when compared with HT fibroblasts. In contrast, fibronectin mRNA expression was comparable between LT and HT fibroblasts. LT fibroblasts exhibited markedly accentuated synthesis of genes associated with matrix metabolism, showing increased MMP2, MMP3, MMP8 and TIMP1 levels (Figure 2).
Figure 2.
Comparison of phenotypic characteristics between high-tension (HT) fibroblasts, cultured in plates, and low-tension (LT) fibroblasts after 24h in collagen pads. A. HT and LT cells were stained for α-SMA. Immunofluorescence shows that HT fibroblasts are large cells with incorporation of α-SMA in the cytoskeleton (scalebar=10µm). In contrast, LT cells in collagen pads exhibit low level punctate α-SMA staining (arrows). qPCR analysis shows that HT fibroblasts exhibit markedly higher α-SMA (B), collagen I (C) and III (D) mRNA synthesis. Fibronectin synthesis was comparable between HT and LT cells (E). LT cells in collagen pads exhibited much higher expression of genes associated with matrix metabolism, such as MMP2 (F), MMP3 (G), MMP8 (H) and TIMP1 (I) (**p<0.01, n=6/group).
Because FAK signaling is implicated in myofibroblast transdifferentiation and activation [42],[43], we compared FAK activity between HT and LT cells. Western blotting for p-FAK and immunofluorescence demonstrated that HT cells had abundant expression of p-FAK in the presence or absence of TGF-b1. In contrast, LT cells showed negligible FAK activity (Supplemental figure 2).
4. Distinct effects of TGF-β on collagen and α-SMA synthesis in HT and LT fibroblasts
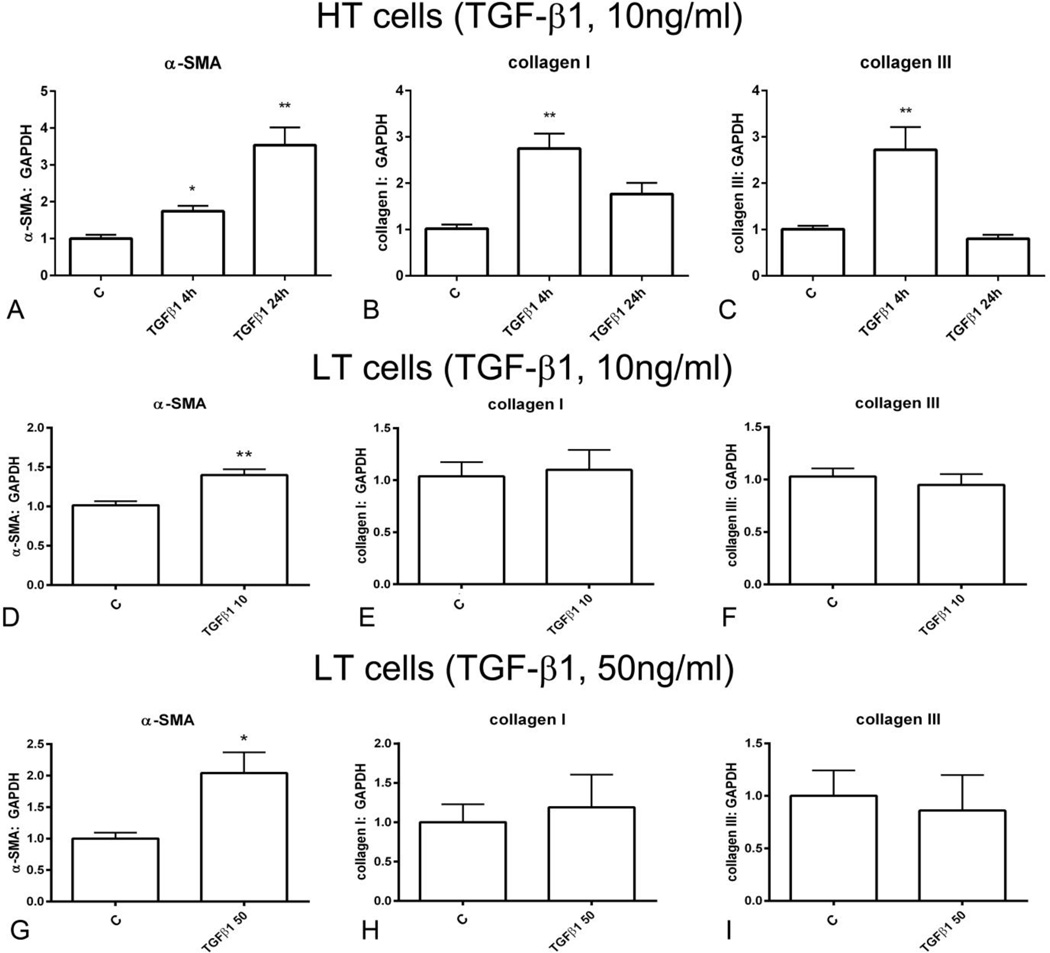
Next, we examined the effects of TGF-β 1 stimulation on α-SMA and collagen mRNA synthesis in HT fibroblasts. TGF-β1 stimulation (10 ng/ml) induced a 1.7-fold upregulation of α-SMA levels after 4 h of stimulation and a 3-fold increase after 24h of stimulation (Figure 3A). Moreover, in HT cells, TGF-β1 induced a transient upregulation of collagen I and III mRNA synthesis after 4h of stimulation (Figure 3B, C). In low-tension cardiac fibroblasts both low (10ng/ml) and high (50ng/ml) concentrations of TGF-β induced a modest (1.5–2.0 fold) upregulation of α-SMA (Figure 3D, G), but did not affect collagen I and collagen III synthesis (Figure 3E–F, H–I).
Figure 3.
Effects of TGF-β1 stimulation on α-SMA and collagen synthesis in high-tension fibroblasts (cultured in plates) and in low-tension cardiac fibroblasts (stimulated in collagen pads). A. In high-tension cells, TGF-β1 (10ng/ml) stimulation for 4–24h markedly upregulated α-SMA mRNA synthesis. B-C. TGF-β1 also induced a transient upregulation of collagen I (B) and III (C). D-F. In low tension cells, stimulation with low concentration TGF-β1 (10 ng/ml) modestly, but significantly increased α-SMA expression (D), without affecting collagen I (E) and III (F) synthesis. G-I. High concentration TGF-β1 (50 ng/ml) also upregulated α-SMA synthesis (G) in low tension cells without affecting collagen I (H) and III (I) levels (*p<0.05, **p<0.01 vs. C, n= 6–12/group).
5. In LT fibroblasts, TGF-β1 stimulation promotes a matrix-preserving fibroblast phenotype
In LT fibroblasts, both low (10ng/ml – Supplemental figure 3A–D) and high (50ng/ml –Supplemental figure 3E–H) TGF-β1 concentrations induced a matrix-preserving program, markedly attenuating MMP2 (Supplemental figure 3A, E), MMP3 (Supplemental figure 3B, F) and MMP8 synthesis (Supplemental figure 3C, G), and upregulating TIMP1 expression (Supplemental figure 3D, H).
6. Serum-induced pad contraction is associated with reduced α-SMA mRNA synthesis
Stimulation with 10% serum markedly increased contraction of fibroblast-populated collagen pads (Supplemental figure 1). Surprisingly, increased pad contraction upon serum stimulation was associated with reduced α-SMA expression (Supplemental figure 4A), suggesting that serum-induced pad contraction was not due to enhanced myofibroblast transdifferentiation. Moreover, serum stimulation did not significantly affect collagen I and III synthesis (Supplemental figure 4B–C), but reduced MMP2 mRNA expression (Supplemental figure 4D) and enhanced TIMP1 expression levels (Supplemental figure 4G). Serum had no significant effects on transcription of the collagenases MMP3 and MMP8 (Supplemental figure 4E, F).
7. Basic fibroblast growth factor (bFGF)-mediated pad contraction is associated with attenuated α-SMA synthesis
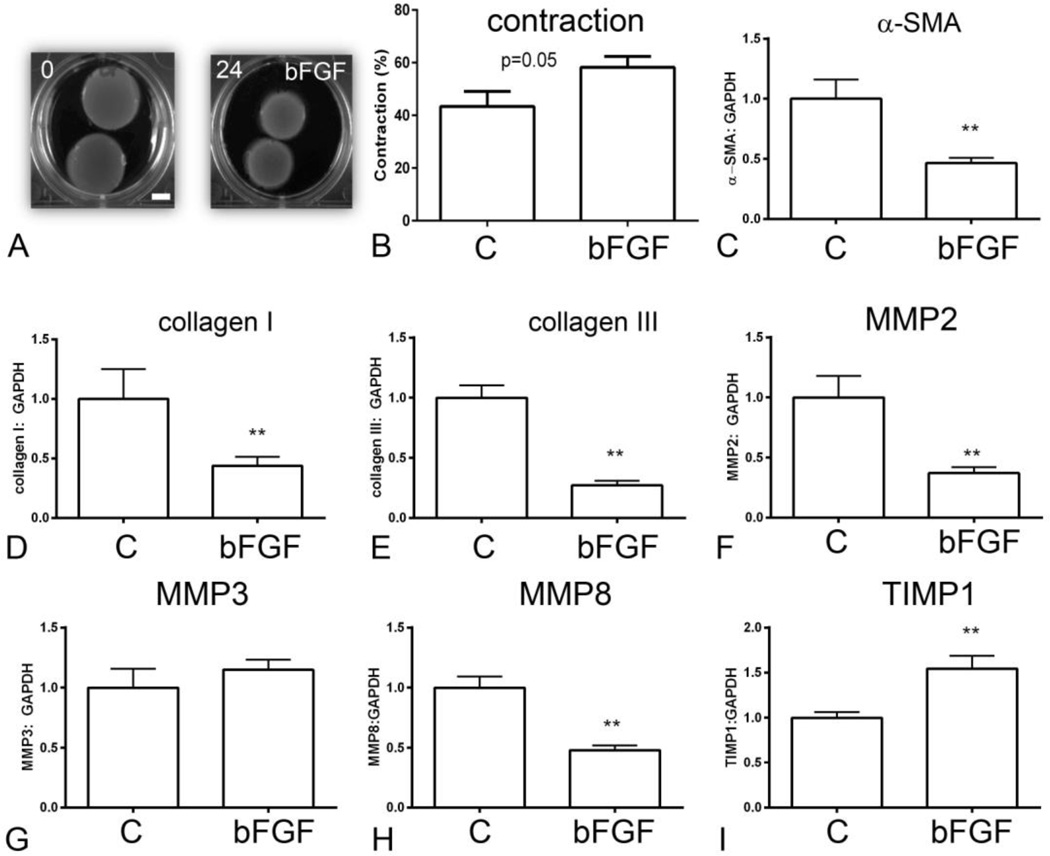
Stimulation with bFGF (50ng/ml) also significantly increased contraction in fibroblast-populated collagen pads (Figure 4A, B). bFGF stimulation markedly reduced α-SMA synthesis in pad fibroblasts (Figure 4C), suggesting that enhanced myofibroblast transdifferentiation is not responsible for bFGF-induced pad contraction. bFGF reduced matrix protein expression in pad fibroblasts, significantly attenuating collagen I and collagen III mRNA synthesis (Figure 4D, E). Moreover, bFGF stimulation reduced MMP2 (Figure 4F) and MMP8 expression (Figure 4H) and increased TIMP1 levels (Figure 4I), changes consistent with activation of a matrix-preserving fibroblast phenotype. bFGF had no effect on MMP3 mRNA expression (Figure 4G).
Figure 4.
Effects of bFGF on collagen pad contraction and fibroblast gene expression. A-B. bFGF enhanced contraction of fibroblast-populated collagen pads (scalebar=5mm). C. bFGF stimulation reduced α-SMA expression by cardiac fibroblasts. bFGF attenuated collagen I (D) and III (E) synthesis. F. bFGF reduced fibroblast MMP2 expression. G. bFGF did not affect MMP3 synthesis (G), but significantly decreased MMP8 expression by cardiac fibroblasts. I. bFGF accentuated TIMP1 expression (**p<0.01, n=8–14/group).
8. α-SMA overexpression does not significantly affect contraction of fibroblast-populated pads upon serum stimulation
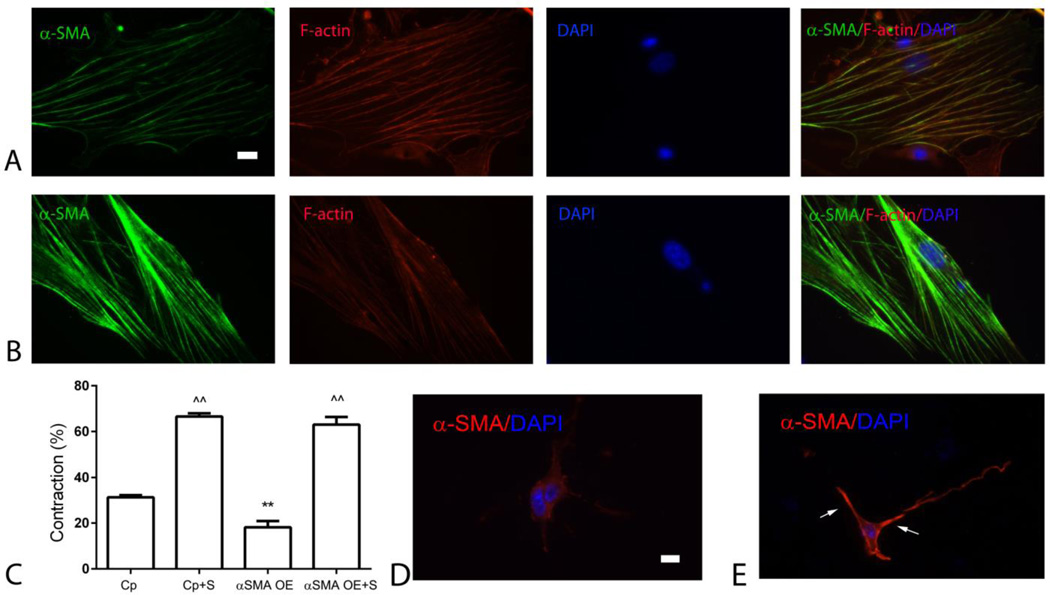
α-SMA expression has been implicated in scar contraction both in vivo, and in fibroblast-populated collagen pads. Our experiments showed that, although contraction of the healing infarct is associated with infiltration of the scar with α-SMA-expressing myofibroblasts (Figure 1), pad fibroblasts express low levels of α-SMA, and accentuated contraction of pads populated with serum or bFGF-stimulated fibroblasts cannot be attributed to increased α-SMA levels (Supplemental figure 4, Figure 4). In order to directly investigate the potential role of α-SMA in mediating collagen pad contraction, we performed α-SMA overexpression experiments. α-SMA-overexpressing cells, cultured in the high-tension environment of the plate exhibited increased α-SMA expression, incorporated into the cytoskeletal stress fibers (Figure 5A–B). Surprisingly, α-SMA overexpression attenuated contraction in pads populated by unstimulated cells, and did not affect contraction by serum-stimulated fibroblasts (Figure 5C). Immunofluorescent staining demonstrated that α-SMA-overexpressing fibroblasts in collagen pads exhibited increased cytoplasmic and sub-membrane α-SMA staining, in the absence of localization in stress fibers (Figure 5D, E).
Figure 5.
α-SMA overexpression in cardiac fibroblasts attenuates baseline pad contraction and does not affect serum-stimulated contraction. A. Isolated high-tension fibroblasts were stained for α-SMA, phalloidin (to identify F-actin filaments) and DAPI. B. α-SMA overexpression markedly increased α-SMA expression and incorporation in cytoskeletal stress fibers. C. α-SMA overexpression (OE) attenuated baseline pad contraction, without affecting serum (S)-induced contraction (**p<0.01 vs. control-plasmid (Cp), n=8–10/group; ^^p<0.01 vs. unstimulated cells). D. In collagen pads, cardiac fibroblasts exhibit low levels of α-SMA immunoreactivity. E. α-SMA-overexpressing cells in collagen pads show high expression of α-SMA with cytoplasmic and sub-membrane localization (arrows) (scalebar=10µm).
9. In fibroblast-populated collagen pads, α-SMA overexpression attenuates the serum-mediated increase in fibroblast density
In order to examine the basis for the surprising attenuation of pad contraction upon α-SMA overexpression, we studied the morphometric characteristics of α-SMA-overexpressing fibroblasts in pads. Serum stimulation induced a marked increase in cell density; α-SMA overexpression attenuated the serum-mediated increase in fibroblast density (Supplemental figure 5A, C). Quantitative morphometric analysis showed that serum stimulation increased mean cell area; α-SMA overexpression attenuated the serum-induced increase in cell size (Supplemental figure 5B, D).
10. α-SMA overexpression reduces fibroblast proliferative activity, but does not affect apoptosis
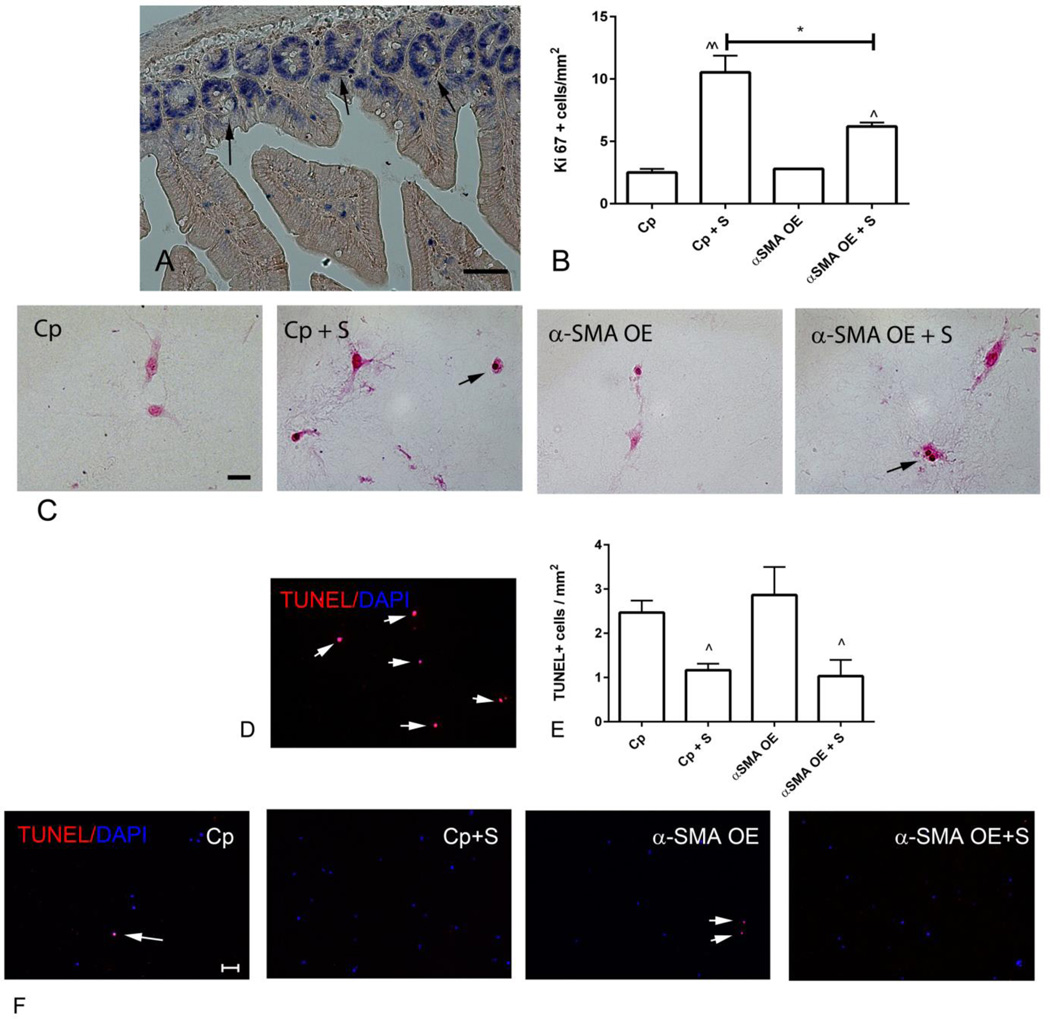
Reduced cell density in collagen pads populated with α-SMA overexpressing fibroblasts may be due to attenuated proliferation or increased cell death. In order to investigate the effects of α-SMA overexpression on fibroblast proliferation, we identified proliferating cells using staining for Ki-67. Sections from mouse bowel were used as a positive control (Figure 6A). Serum stimulation markedly increased the number of proliferating fibroblasts; α-SMA overexpression attenuated serum-mediated fibroblast proliferation (Figure 6B–C). In order to examine whether α-SMA overexpression affects fibroblast apoptosis we performed TUNEL staining (Figure 6D). Serum stimulation reduced the number of apoptotic cells in both α-SMA overexpressing and control cells. α-SMA overexpression did not affect the number of apoptotic cells (Figure 6E–F).
Figure 6.
α-SMA overexpression attenuates fibroblast proliferation, but does not affect apoptosis. A-C. Ki-67 immunohistochemistry was used to identify proliferating cells. A. Sections from the mouse bowel were used as a positive control, identifying proliferating epithelial cells in the base of intestinal crypts (arrows) (scalebar=50µm). B-C. Quantitative analysis of the density of Ki-67+ cells in pads showed that serum (S) increased the density of proliferating cells (C -arrows) (^p<0.05, ^^p<0.01 vs. control, *p<0.05; n=3/group). α-SMA overexpression (OE) did not affect the baseline density of proliferating cells (when compared with control-plasmid (Cp) cells), but attenuated serum-mediated proliferative activity (B- scalebar=20µm). D-F. TUNEL staining was used to identify apoptotic cells (scalebar=50µm). D. DNAse-treated sections served as a positive control (arrows). Small numbers of apoptotic cells were noted in control collagen pads (E, F - arrows). The density of apoptotic cells was reduced in serum-stimulated pads (^p<0.05 vs. corresponding unstimulated cells, n=3/group); however, α-SMA overexpression did not affect fibroblast apoptosis.
11. α-SMA knockdown attenuates contraction in fibroblast-populated collagen pads upon serum stimulation
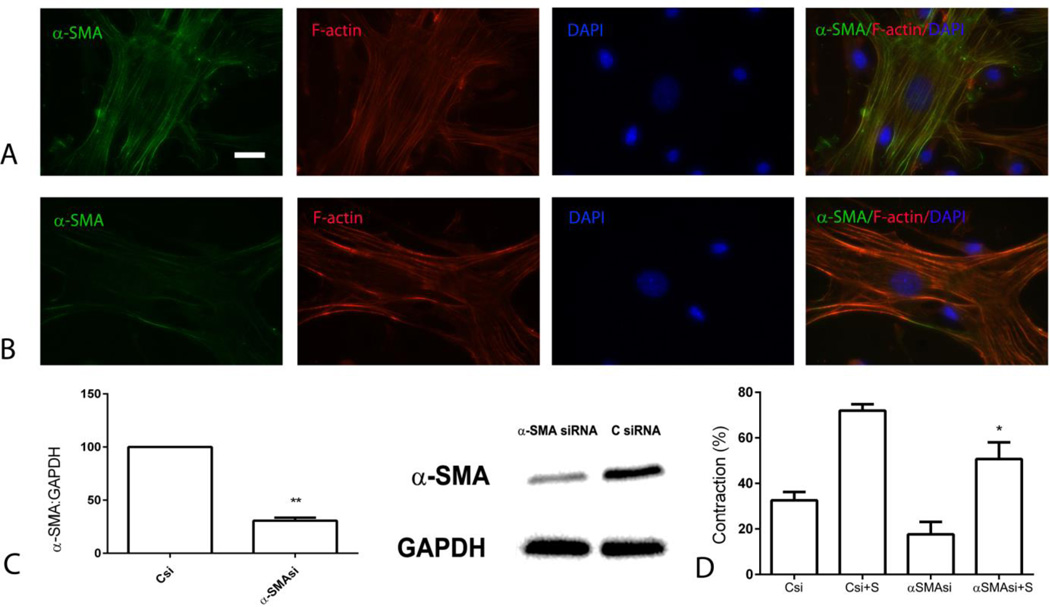
Next, we examined whether α-SMA knockdown affects contraction of fibroblast-populated collagen pads. siRNA knockdown markedly attenuated expression of α-SMA in fibroblasts (Figure 7A–C). α-SMA knockdown markedly reduced contraction of pads populated with serum-stimulated cells, suggesting that α-SMA is implicated in serum-induced pad contraction (Figure 7D).
Figure 7.
α-SMA knockdown attenuates serum-mediated collagen pad contraction. siRNA knockdown experiments were performed to examine the role of α-SMA in contraction of fibroblast-populated collagen pads. A-B. α-SMA staining showed that siRNA knockdown markedly reduced α-SMA content in high-tension cardiac fibroblasts (scalebar=10µm). C. Western blotting confirmed the reduction of α-SMA expression (**p<0.01 vs., control siRNA, n=3/group). D. α-SMA knockdown attenuated serum (S)-induced contraction of collagen pads (*p<0.05 vs. Csi+S, n=3–6/group).
12. α-SMA knockdown increases cell density in serum-stimulated collagen pads
Attenuated serum-mediated contraction in pads populated with α-SMA KD cells occurred despite a significantly increased cell density (Supplemental figure 6A, C). α-SMA KD did not affect cell size in unstimulated and serum-stimulated cells (Supplemental figure 6B, D).
13. α-SMA knockdown accentuates serum-mediated fibroblast proliferation without affecting fibroblast apoptosis
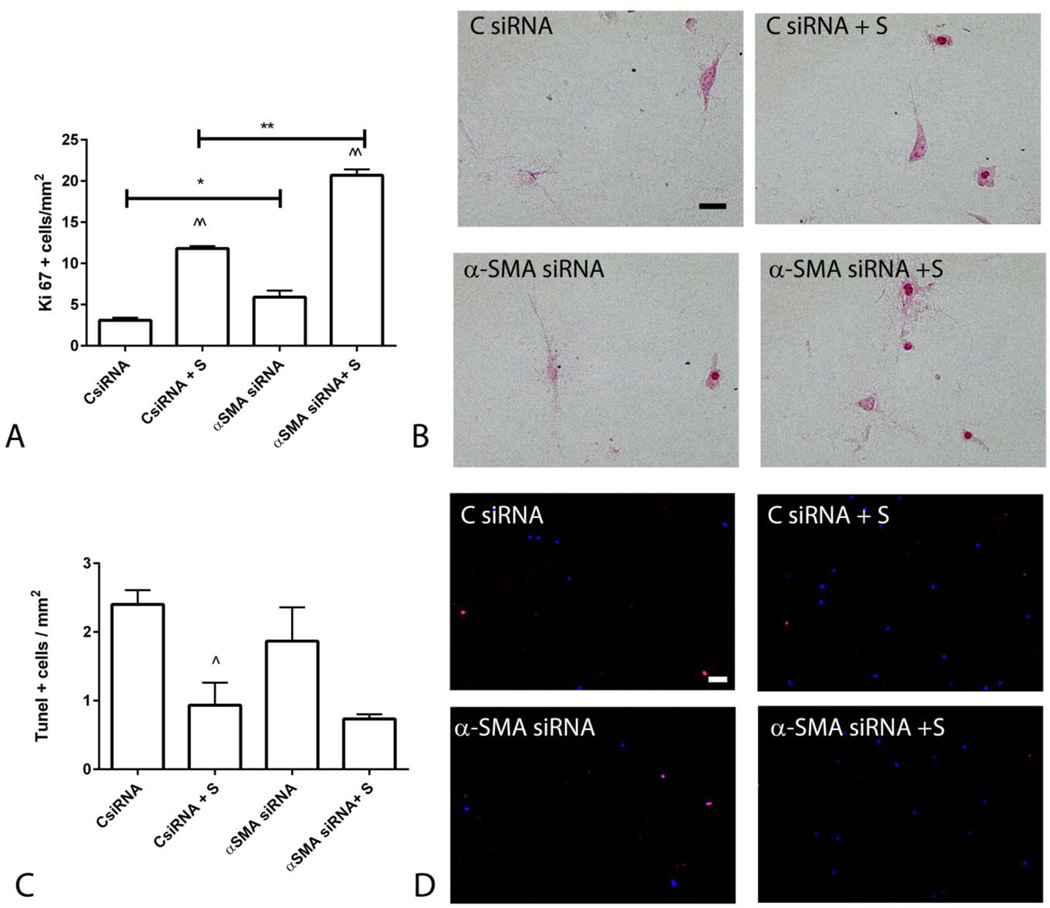
Ki-67 staining showed that α-SMA knockdown increases baseline proliferative activity and accentuates serum-induced proliferation in cardiac fibroblasts (Figure 8A–B). TUNEL staining showed that α-SMA knockdown does not affect fibroblast apoptosis in control and serum-stimulated fibroblasts (Figure 8C–D).
Figure 8.
α-SMA knockdown increased fibroblast proliferation, but had no effects on apoptosis. A-B. Ki-67 staining was used to identify proliferating cells (scalebar=20µm). Serum stimulation (S) markedly increased cell proliferation (^^p<0.01 vs. corresponding controls); α-SMA siRNA knockdown accentuated proliferative activity (*p<0.05, **p<0.01 vs. corresponding controls). C-D. TUNEL staining was used to assess apoptosis (scalebar=50µm). Serum stimulation decreased the density of apoptotic cells (^p<0.05 vs. control); however α-SMA SiRNA knockdown did not affect fibroblast apoptosis (n=3–6/group).
DISCUSSION
Our study demonstrates that cardiac fibroblasts exhibit dynamic phenotypic alterations in response to their microenvironment. Culture of cardiac myofibroblasts in collagen pads causes disassembly of stress fibers and markedly reduces α-SMA and collagen synthesis, while inducing expression of genes associated with matrix metabolism. Although α-SMA expression in infarct myofibroblasts has been implicated in scar contraction, in free-floating collagen pads, serum and bFGF induce contraction, despite a reduction of α-SMA levels. We also report that α-SMA expression in fibroblasts is not sufficient to induce contraction of free-floating collagen pads, but may modulate proliferative activity. Our findings illustrate the remarkable phenotypic plasticity of cardiac fibroblasts and suggest that α-SMA expression in myofibroblasts may have effects beyond scar contraction.
The dynamic changes of the fibroblasts in healing infarcts
Following myocardial infarction, alarmins released by dying cardiomyocytes trigger an intense inflammatory reaction that clears the wound from dead cells, while setting the stage for repair [44]. Secretion of inflammatory mediators and growth factors induces sequential phenotypic alterations in cardiac fibroblasts that contribute to the inflammatory and reparative response. During the early hours after infarction, cardiac fibroblasts may acquire a pro-inflammatory and matrix-degrading phenotype, serving as an important source of cytokines, chemokines and proteases, and mediating leukocyte infiltration [41]. In the inflammatory environment of the infarct, Interleukin (IL)-1 induces MMP expression and reduces fibroblast α-SMA synthesis, delaying myofibroblast transdifferentiation until the wound is cleared from dead cells and matrix debris [41]. Clearance of apoptotic cells and matrix fragments by professional phagocytes is associated with release of anti-inflammatory mediators (such as TGF-β), and with suppression of pro-inflammatory cytokine synthesis. In the infarct border zone, release of bioactive TGF-β co-operates with specialized matrix proteins, triggering conversion of fibroblasts into myofibroblasts [40],[45]. Activated myofibroblasts, the main matrix-producing cells in the healing infarct [46], express large amounts of α-SMA (Figure 1). Because of its abundant expression in infarct myofibroblasts and its known contractile properties, α-SMA has been suggested as a crucial mediator in contraction and remodeling of the healing scar [33].
Culture in a collagen lattice alters the phenotypic characteristics of cardiac fibroblasts
Mechanical tension and changes in the composition of the surrounding extracellular matrix play important roles in modulating the phenotypic and functional characteristics of fibroblasts [47],[48]. Our findings highlight the pronounced effects of the matrix environment on fibroblast phenotype. In culture plates, a large number of cardiac fibroblasts undergo myofibroblast transdifferentiation, forming stress fibers decorated by α-SMA. In contrast, when cardiac fibroblasts are cultured in collagen pads, they acquire a round, or dendritic morphology and express low levels of α-SMA (<5% of the levels in high-tension fibroblasts), exhibiting disassembly of the stress fibers (Figure 2A–B). Activation of FAK signaling may be implicated in acquisition of a myofibroblast phenotype in cells cultured in plates [49] (Supplemental figure 2). In contrast, in the low tension environment of the collagen pad FAK activity is markedly suppressed.
Moreover, pad fibroblasts synthesize much lower amounts of collagen I and III, but exhibit high level synthesis of genes involved in matrix metabolism, such as MMPs and TIMPs (Figure 2C–I)). Thus, high tension fibroblasts resemble activated myofibroblasts in healing infarcts, whereas pad fibroblasts have characteristics similar to the quiescent interstitial populations residing in normal myocardium. The two distinct models of cardiac fibroblast culture provide complementary information regarding the properties of fibroblasts in homeostasis and disease.
Contraction of fibroblast-populated pads is not associated with increased a-SMA expression
When populated with fibroblasts, collagen pads contract recapitulating scar contraction following myocardial infarction. Collagen pad contraction is accentuated when the cells are stimulated with serum, or with the growth factors TGF-β1 and bFGF (Supplemental figure 1, Figure 4). Known associations between myofibroblast phenotype and contractile activity suggest that increased expression of α-SMA, the predominant contractile protein expressed by myofibroblasts, may be involved in pad contraction [33]. Surprisingly, our findings do not support this notion. Increased pad contraction is not consistently associated with higher levels of α-SMA expression by fibroblasts. Although in TGF-β-stimulated cells, increased α-SMA levels were associated with accentuated pad contraction (Supplemental figure 1, Figure 3), serum and bFGF stimulation induced collagen contraction, despite a significant reduction in α-SMA synthesis (Supplemental figure 1, Supplemental figure 4, Figure 4).
Effects of a-SMA knockdown on contraction of fibroblast-populated pads
In order to directly examine whether α-SMA expression mediates contraction of fibroblast-populated collagen pads, we performed siRNA knockdown experiments. α-SMA knockdown attenuated contraction of serum-stimulated collagen pads (Figure 7). The finding may reflect loss of early myofibroblast-induced contraction upon α-SMA downmodulation. Before placement in the pads, cardiac myofibroblasts exhibit high levels of α-SMA expression that may be responsible for early contraction of the pad, until levels are suppressed in the low tension environment of the collagen lattice. Surprisingly, α-SMA knockdown accentuated proliferative activity, without affecting apoptosis (Figure 8). Thus, reduced contraction upon α-SMA knockdown occurred despite an increase in the number of cells populating the pad.
a-SMA overexpression is not sufficient to induce collagen pad contraction, but reduces proliferative activity
We reasoned that, although α-SMA may not be the only factor mediating collagen contraction in fibroblast-populated pads, forced overexpression of α-SMA in fibroblasts may be sufficient to promote contraction. Surprisingly, increased α-SMA expression in cardiac fibroblasts attenuated contraction of collagen pads populated with unstimulated cells, and had no effects on pads containing serum-stimulated fibroblasts (Figure 5). The failure of α-SMA overexpression to induce contraction may be explained by the absence of α-SMA incorporation into stress fibers in cells populating the pads. In the low tension environment of the collagen pad, fibroblasts do not exhibit stress fibers; overexpressing cells exhibit punctate cytoplasmic and sub-membrane α-SMA localization (Figure 5D–E) that would not be expected to accentuate contractile force. Our findings suggest that α-SMA overexpression is not sufficient to increase fibroblast-mediated matrix contraction; this process may require expression of other contractile proteins, or activation of signaling pathways necessary for preservation of stress fibers and for incorporation of α-SMA into the stress fibers [50], [51]. Moreover, the observed inhibitory effects of α-SMA overexpression on pad contraction may reflect changes in proliferative activity of the cells. Reduced serum-induced fibroblast proliferation in α-SMA-overexpressing cells (Figure 6) may be responsible, at least in part, for attenuated pad contraction.
a-SMA as a modulator of fibroblast phenotype
In addition to its known role as a regulator of contractile activity, α-SMA may exert additional actions, modulating pathways critical for cellular responses. Associative studies have suggested that high α-SMA expression may mark a fibroblast subtype with a higher capacity to remodel the extracellular matrix [52]. Whether this is due to direct actions of α-SMA, or represents an epiphenomenon is unclear. Experiments investigating the effects of α-SMA expression on fibroblast migration have produced conflicting results. Lung fibroblasts expressing large amounts of α-SMA exhibited increased migratory activity towards fibronectin [53]. However, other studies demonstrated that fibroblast strains with increased α-SMA expression had reduced migratory capacity and that α-SMA knockdown increased cell motility [54]. α-SMA has been implicated in activation of mechanosensitive signaling pathways. Filamentous α-SMA activates Mitogen Activated Protein Kinase (MAPK) signaling, and cells overexpressing α-SMA exhibit accentuated tensile force-induced activation of p38 [55]. In embryonic stem cells, α-SMA expression may regulate cell fate [56]. In mesenchymal stromal cells, α-SMA overexpression reduced adipogenic potential by inducing Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) nuclear localization [57]. Because overactive YAP/TAZ signaling is associated with a proliferative phenotype [58], activation of this pathway may not explain our observed effects of α-SMA overexpression on cardiac fibroblast proliferation. The potential significance of cytoplasmic α-SMA expression in the absence of stress fiber localization remains unclear.
In vivo actions of a-SMA
Information on the role of α-SMA expression by activated myofibroblasts in vivo remains limited. In a model of cutaneous injury, mice with global loss of α-SMA had delayed contraction and immature organization of cutaneous wounds [59]. Whether these defects result from impaired myofibroblast transdifferentiation, or reflect actions of α-SMA in vascular cells and wound angiogenesis or vascular maturation remains unknown. In vivo approaches specifically targeting α-SMA expression in myofibroblasts are needed to dissect the potential role of α-SMA expression in fibroblast activation and function.
Supplementary Material
HIGHLIGHTS.
In healing infarcts, scar contraction is associated with accumulation of α-SMA-expressing myofibroblasts.
Culture of cardiac myofibroblasts in collagen pads causes disassembly of stress fibers, and reduces α-SMA and collagen synthesis.
Serum and bFGF increase contraction of fibroblast-populated collagen pads, despite attenuating α-SMA synthesis.
α-SMA knockdown attenuates serum-induced contraction of fibroblast-populated collagen pads.
α-SMA overexpression also reduces pad contraction, attenuating fibroblast proliferative activity.
Acknowledgments
FUNDING SOURCES
Supported by NIH grants R01 HL76246 and R01 HL85440, grants from the Department of Defense Congressionally-directed medical research programs (CDMRP) (Dr Frangogiannis), and by an American Heart Association (AHA) Founders’ affiliate post-doctoral award (Dr Shinde).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: None
REFERENCES
- 1.Gabbiani G, Majno G. Dupuytren’s contracture: fibroblast contraction? An ultrastructural study. Am J Pathol. 1972;66:131–146. [PMC free article] [PubMed] [Google Scholar]
- 2.Gabbiani G, Le Lous M, Bailey AJ, Bazin S, Delaunay A. Collagen and myofibroblasts of granulation tissue. A chemical, ultrastructural and immunologic study. Virchows Arch B Cell Pathol. 1976;21:133–145. doi: 10.1007/BF02899150. [DOI] [PubMed] [Google Scholar]
- 3.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 4.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 5.Davis J, Molkentin JD. Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol. 2014;70:9–18. doi: 10.1016/j.yjmcc.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 7.Hinz B. Myofibroblasts. Exp Eye Res. 2016;142:56–70. doi: 10.1016/j.exer.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Darby IA, Zakuan N, Billet F, Desmouliere A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci. 2016;73:1145–1157. doi: 10.1007/s00018-015-2110-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deb A, Ubil E. Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol. 2014;70:47–55. doi: 10.1016/j.yjmcc.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–404. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travers JG, Kamal FA, Robbins J, Yutzey KE, Blaxall BC. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res. 2016;118:1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair. 2013;6:5. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35(suppl II):17–26. [PubMed] [Google Scholar]
- 16.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD. Revisiting Cardiac Cellular Composition. Circ Res. 2016;118:400–409. doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 18.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000;48:89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 19.Vracko R, Thorning D. Contractile cells in rat myocardial scar tissue. Lab Invest. 1991;65:214–227. [PubMed] [Google Scholar]
- 20.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. doi: 10.1038/nrcardio.2014.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cunnington RH, Northcott JM, Ghavami S, Filomeno KL, Jahan F, Kavosh MS, Davies JJ, Wigle JT, Dixon IM. The Ski-Zeb2-Meox2 pathway provides a novel mechanism for regulation of the cardiac myofibroblast phenotype. J Cell Sci. 2014;127:40–49. doi: 10.1242/jcs.126722. [DOI] [PubMed] [Google Scholar]
- 23.Flack EC, Lindsey ML, Squires CE, Kaplan BS, Stroud RE, Clark LL, Escobar PG, Yarbrough WM, Spinale FG. Alterations in cultured myocardial fibroblast function following the development of left ventricular failure. J Mol Cell Cardiol. 2006;40:474–483. doi: 10.1016/j.yjmcc.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Squires CE, Escobar GP, Payne JF, Leonardi RA, Goshorn DK, Sheats NJ, Mains IM, Mingoia JT, Flack EC, Lindsey ML. Altered fibroblast function following myocardial infarction. J Mol Cell Cardiol. 2005;39:699–707. doi: 10.1016/j.yjmcc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lal H, Ahmad F, Zhou J, Yu JE, Vagnozzi RJ, Guo Y, Yu D, Tsai EJ, Woodgett J, Gao E, Force T. Cardiac Fibroblast GSK-3beta Regulates Ventricular Remodeling and Dysfunction in Ischemic Heart. Circulation. 2014 doi: 10.1161/CIRCULATIONAHA.113.008364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeglinski MR, Davies JJ, Ghavami S, Rattan SG, Halayko AJ, Dixon IM. Chronic expression of Ski induces apoptosis and represses autophagy in cardiac myofibroblasts. Biochim Biophys Acta. 2016;1863:1261–1268. doi: 10.1016/j.bbamcr.2016.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Vivar R, Humeres C, Munoz C, Boza P, Bolivar S, Tapia F, Lavandero S, Chiong M, Diaz-Araya G. FoxO1 mediates TGF-beta1-dependent cardiac myofibroblast differentiation. Biochim Biophys Acta. 2016;1863:128–138. doi: 10.1016/j.bbamcr.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 29.Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, Lu Y, Stanbouly S, Huang J, Rojas M, Vondriska TM, Stefani E, Deb A. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–590. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lijnen P, Petrov V, Fagard R. Transforming growth factor-beta 1-mediated collagen gel contraction by cardiac fibroblasts. J Renin Angiotensin Aldosterone Syst. 2003;4:113–118. doi: 10.3317/jraas.2003.011. [DOI] [PubMed] [Google Scholar]
- 31.Carthy JM, Garmaroudi FS, Luo Z, McManus BM. Wnt3a induces myofibroblast differentiation by upregulating TGF-beta signaling through SMAD2 in a beta-catenin-dependent manner. PLoS One. 2011;6:e19809. doi: 10.1371/journal.pone.0019809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frunza O, Russo I, Saxena A, Shinde AV, Humeres C, Hanif W, Rai V, Su Y, Frangogiannis NG. Myocardial Galectin-3 Expression Is Associated with Remodeling of the Pressure-Overloaded Heart and May Delay the Hypertrophic Response without Affecting Survival, Dysfunction, and Cardiac Fibrosis. Am J Pathol. 2016;186:1114–1127. doi: 10.1016/j.ajpath.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinz B, Celetta G, Tomasek JJ, Gabbiani G, Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity. Mol Biol Cell. 2001;12:2730–2741. doi: 10.1091/mbc.12.9.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell. 2003;14:2508–2519. doi: 10.1091/mbc.E02-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wrobel LK, Fray TR, Molloy JE, Adams JJ, Armitage MP, Sparrow JC. Contractility of single human dermal myofibroblasts and fibroblasts. Cell Motil Cytoskeleton. 2002;52:82–90. doi: 10.1002/cm.10034. [DOI] [PubMed] [Google Scholar]
- 36.Chen W, Frangogiannis NG. Fibroblasts in post-infarction inflammation and cardiac repair. Biochim Biophys Acta. 2013;1833:945–953. doi: 10.1016/j.bbamcr.2012.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W, Saxena A, Li N, Sun J, Gupta A, Lee DW, Tian Q, Dobaczewski M, Frangogiannis NG. Endogenous IRAK-M attenuates postinfarction remodeling through effects on macrophages and fibroblasts. Arterioscler Thromb Vasc Biol. 2012;32:2598–2608. doi: 10.1161/ATVBAHA.112.300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saxena A, Shinde AV, Haque Z, Wu YJ, Chen W, Su Y, Frangogiannis NG. The role of Interleukin Receptor Associated Kinase (IRAK)-M in regulation of myofibroblast phenotype in vitro, and in an experimental model of non-reperfused myocardial infarction. J Mol Cell Cardiol. 2015;89:223–231. doi: 10.1016/j.yjmcc.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem. 2013;61:555–570. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saxena A, Chen W, Su Y, Rai V, Uche OU, Li N, Frangogiannis NG. IL-1 Induces Proinflammatory Leukocyte Infiltration and Regulates Fibroblast Phenotype in the Infarcted Myocardium. J Immunol. 2013;191:4838–4848. doi: 10.4049/jimmunol.1300725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leask A. Focal Adhesion Kinase: A Key Mediator of Transforming Growth Factor Beta Signaling in Fibroblasts. Adv Wound Care (New Rochelle) 2013;2:247–249. doi: 10.1089/wound.2012.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lagares D, Busnadiego O, Garcia-Fernandez RA, Kapoor M, Liu S, Carter DE, Abraham D, Shi-Wen X, Carreira P, Fontaine BA, Shea BS, Tager AM, Leask A, Lamas S, Rodriguez-Pascual F. Inhibition of focal adhesion kinase prevents experimental lung fibrosis and myofibroblast formation. Arthritis Rheum. 2012;64:1653–1664. doi: 10.1002/art.33482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lugrin J, Parapanov R, Rosenblatt-Velin N, Rignault-Clerc S, Feihl F, Waeber B, Muller O, Vergely C, Zeller M, Tardivel A, Schneider P, Pacher P, Liaudet L. Cutting Edge: IL-1alpha Is a Crucial Danger Signal Triggering Acute Myocardial Inflammation during Myocardial Infarction. J Immunol. 2015;194:499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arslan F, Smeets MB, Riem Vis PW, Karper JC, Quax PH, Bongartz LG, Peters JH, Hoefer IE, Doevendans PA, Pasterkamp G, de Kleijn DP. Lack of fibronectin-EDA promotes survival and prevents adverse remodeling and heart function deterioration after myocardial infarction. Circ Res. 2011;108:582–592. doi: 10.1161/CIRCRESAHA.110.224428. [DOI] [PubMed] [Google Scholar]
- 46.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 47.Dobaczewski M, de Haan JJ, Frangogiannis NG. The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. J Cardiovasc Transl Res. 2012;5:837–847. doi: 10.1007/s12265-012-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–911. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thannickal VJ, Lee DY, White ES, Cui Z, Larios JM, Chacon R, Horowitz JC, Day RM, Thomas PE. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 50.Varney SD, Betts CB, Zheng R, Wu L, Hinz B, Zhou J, Van De Water L. Hic-5 is required for myofibroblast differentiation by regulating mechanically dependent MRTF-A nuclear accumulation. J Cell Sci. 2016;129:774–787. doi: 10.1242/jcs.170589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson S, DiCesare L, Tan I, Leung T, SundarRaj N. Rho-mediated assembly of stress fibers is differentially regulated in corneal fibroblasts and myofibroblasts. Exp Cell Res. 2004;298:574–583. doi: 10.1016/j.yexcr.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Arora PD, McCulloch CA. Dependence of collagen remodelling on alpha-smooth muscle actin expression by fibroblasts. J Cell Physiol. 1994;159:161–175. doi: 10.1002/jcp.1041590120. [DOI] [PubMed] [Google Scholar]
- 53.Kawamoto M, Matsunami T, Ertl RF, Fukuda Y, Ogawa M, Spurzem JR, Yamanaka N, Rennard SI. Selective migration of alpha-smooth muscle actin-positive myofibroblasts toward fibronectin in the Boyden’s blindwell chamber. Clin Sci (Lond) 1997;93:355–362. doi: 10.1042/cs0930355. [DOI] [PubMed] [Google Scholar]
- 54.Ronnov-Jessen L, Petersen OW. A function for filamentous alpha-smooth muscle actin: retardation of motility in fibroblasts. J Cell Biol. 1996;134:67–80. doi: 10.1083/jcb.134.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, Fan J, Laschinger C, Arora PD, Kapus A, Seth A, McCulloch CA. Smooth muscle actin determines mechanical force-induced p38 activation. J Biol Chem. 2005;280:7273–7284. doi: 10.1074/jbc.M410819200. [DOI] [PubMed] [Google Scholar]
- 56.Clement S, Stouffs M, Bettiol E, Kampf S, Krause KH, Chaponnier C, Jaconi M. Expression and function of alpha-smooth muscle actin during embryonic-stem-cell-derived cardiomyocyte differentiation. J Cell Sci. 2007;120:229–238. doi: 10.1242/jcs.03340. [DOI] [PubMed] [Google Scholar]
- 57.Talele NP, Fradette J, Davies JE, Kapus A, Hinz B. Expression of alpha-Smooth Muscle Actin Determines the Fate of Mesenchymal Stromal Cells. Stem Cell Reports. 2015;4:1016–1030. doi: 10.1016/j.stemcr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu F, Lagares D, Choi KM, Stopfer L, Marinkovic A, Vrbanac V, Probst CK, Hiemer SE, Sisson TH, Horowitz JC, Rosas IO, Fredenburgh LE, Feghali-Bostwick C, Varelas X, Tager AM, Tschumperlin DJ. Mechanosignaling through YAP and TAZ drives fibroblast activation and fibrosis. Am J Physiol Lung Cell Mol Physiol. 2015;308:L344–L357. doi: 10.1152/ajplung.00300.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibrahim MM, Chen L, Bond JE, Medina MA, Ren L, Kokosis G, Selim AM, Levinson H. Myofibroblasts contribute to but are not necessary for wound contraction. Lab Invest. 2015;95:1429–1438. doi: 10.1038/labinvest.2015.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.