Abstract
The objective of our study was to evaluate parameters influencing the germination of Aspergillus conidia. Inoculum concentration and age significantly influenced germination. Different incubation temperatures revealed significant differences among Aspergillus species. The internal human milieu provides the ideal conditions for the development of invasive disease by Aspergillus fumigatus but restricts invasion by Aspergillus flavus and Aspergillus niger.
Aspergillus spp. are ubiquitous organisms, progressively associated with a growing spectrum of infections in immunocompromised hosts (9, 11). Aspergillus fumigatus is responsible for over 90% of cases of invasive aspergillosis (7).
Germination of conidia is the most crucial step in the development of Aspergillus, as hyphae represent the invasive form (4, 8). Thus, germination rate and hyphal growth represent good indicators of interspecies variability and may be useful in designing potential antifungal compounds (10). Several studies have reported higher MICs for a hyphal inoculum than for a conidial inoculum, supporting the relevance of the developmental state to the outcome of Aspergillus infections (2, 5, 6).
Given the increasing interest in Aspergillus pathogenesis, it is important to standardize germination conditions. Our objective was to establish the ideal inoculum size and to clarify the impact of age of conidia and exposure to Tween 80 (a surface-acting agent that is needed for inoculum preparation) on the germination rate of Aspergillus spp. The temperature and pH of the incubation medium were also evaluated.
Clinical isolates of Aspergillus fumigatus (60 strains), Aspergillus flavus (34 strains), and Aspergillus niger (21 strains from the collection of the Department of Microbiology, Faculty of Medicine, University of Porto, Porto, Portugal) were used. Organisms were grown in Sabouraud agar slants (Difco) at 20°C for 5 and 11 days. Conidia were harvested by flooding the agar surface with phosphate-buffered saline solution (Sigma, St. Louis, MO.) with or without 0.01% Tween 80 (Difco). Conidia were stored at 4°C for up to 5 days.
For the inoculum study, Aspergillus conidia were suspended, in triplicate, in RPMI 1640 medium (Sigma) at a final concentration of 1.4 × 105 to 2 × 106 conidia · ml−1. Evaluation of conidial density was performed with a Neubauer chamber. Incubation was carried out at 37°C.
For the temperature study, Aspergillus conidia (5 × 105 conidia · ml−1) were suspended in RPMI 1640 medium. Incubation was carried out, in triplicate, at 20, 30, 37, and 41°C. All strains were subcultured at the end of the incubation period to assess viability.
For the pH study, Aspergillus conidia (5 × 105 conidia · ml−1) were suspended in RPMI 1640 medium at pH values of 3.5 and 4.5 by using acetic acid (AA), hydrochloric acid (HA), and lactic acid (LA). Controls were prepared in plain RPMI 1640 medium (pH 7.5). After the incubation, each strain was cultivated in Sabouraud agar to assess viability. All tests were performed three times.
The percentage of germination was checked hourly for up to 24 h by visual evaluation of 200 conidia under a phase-contrast microscope (400×; Leitz Laborlux K microscope) (8). The SPSS 11.5 program (SPSS, Inc., Chicago, Ill.) was used for data elaboration. The Wilcoxon signed rank test (3) and Student's t test for paired samples were used for statistical analysis. Data were compared at a significance level of 0.05.
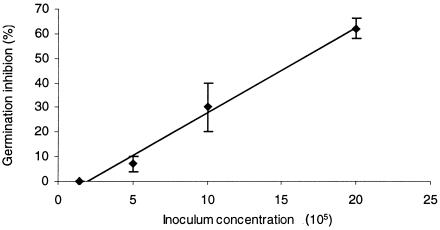
A previous study suggested that high conidial densities are associated with lower germination rates (8). At an inoculum concentration of 1.4 × 105 to 5 × 105 conidia · ml−1, the germination rate of A. fumigatus reached the highest values (Fig. 1). At higher inoculum concentrations, germination efficiency decreased significantly (P < 0.01). With inoculum concentrations of 1 × 106 to 2 × 106 conidia · ml−1, germination decreased by 30 to 60%, respectively (Fig. 1). Results were similar for A. flavus and A. niger strains (data not shown). Thus, 5 × 105 conidia · ml−1 is a density that allows accurate visual counting, being easy to handle. None of the tested strains of Aspergillus showed a significant decrease in germination (P > 0.05) when they were treated with 0.01% Tween 80 (data not shown). Young conidia of A. fumigatus and A. niger showed higher germination rates than old conidia (P < 0.05). In contrast, age did not affect the germination of A. flavus (Table 1).
FIG. 1.
Inhibitory effect of concentrations of growing inoculum on the germination rate of 5-day-old A. fumigatus conidia after 8 h of incubation in RPMI 1640 medium at 37°C. Each point represents the mean value ± standard error of the mean for all tested strains of A. fumigatus.
TABLE 1.
Germination rates of 5- and 11-day-old conidia of Aspergillus tested strainsa
| Species (n) | % Germination (mean ± SEM) of conidia
|
% Inhibition of germination | |
|---|---|---|---|
| 5 days old | 11 days old | ||
| A. fumigatus (60) | 58 ± 1.8 | 45 ± 1.9 | 22.4 ± 2.3 |
| A. flavus (34) | 32 ± 3.7 | 31 ± 3.7 | 8.4 ± 3.1 |
| A. niger (21) | 15 ± 2.5 | 12 ± 2 | 19.8 ± 5.4 |
Strains were incubated for 8 h in RPMI 1640 medium at 37°C.
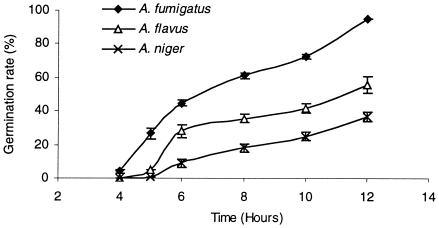
Manavathu et al. (8) have already presented a germination curve for A. fumigatus, but no comparison was made with other Aspergillus species. Our results show that germination rates differ significantly among the most common pathogenic Aspergillus species (P < 0.01). A. fumigatus had the highest germination rate (Fig. 2), followed by A. flavus, both with 5- and 11-day-old spores (data not shown). Following 12 h of incubation, the average germination rate of A. niger strains was about 36.5%. This rate was obtained after 8 h by A. flavus and after 5.5 h by A. fumigatus. Faster germination represents faster development of hyphae, that is, faster onset of tissue invasion.
FIG.2.
Kinetics of germination of 5-day-old conidia of the three tested species of Aspergillus during 12 h of incubation in RPMI 1640 medium at 37°C. Each point represents the mean value ± standard error of the mean for all tested strains of each Aspergillus species.
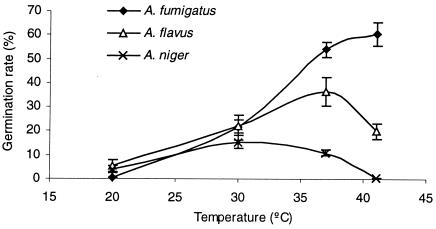
Distinct germination patterns were found at different temperatures (Fig. 3). At 20 and 30°C, germination rates did not differ significantly (P > 0.05) among the Aspergillus species. However, significant differences were found at higher temperatures. At 41°C, germination of A. fumigatus was still enhanced, germination of A. flavus decreased by 45%, and in the case of A. niger no germination was possible for 24 h (conidia remained viable, as ascertained by culture). Accordingly, other Aspergillus species with low pathogenicity demonstrate an optimal temperature for growth of around 30°C, which is lower than the optimal temperature for A. niger (1, 12).
FIG. 3.
Percentage of germination by 5-day-old conidia of the three tested Aspergillus species after incubation for 8 h in RPMI 1640 medium at different temperatures. Each point represents the mean value ± standard error of the mean for all tested strains of each Aspergillus species.
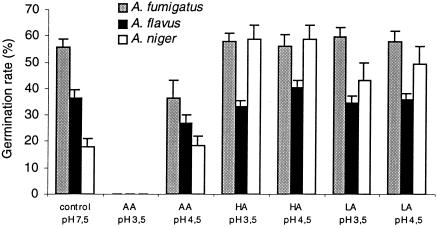
HA and LA did not influence the germination of A. fumigatus or A. flavus (Fig. 4) but promoted the germination rate of A. niger to values similar to those of A. fumigatus; the mechanism responsible for such an effect is still unknown. AA at pH 4.5 reduced the germination of A. fumigatus and A. flavus, which was completely inhibited at pH 3.5, although conidia remained viable following incubation for 120 h.
FIG. 4.
Effect of different acidic pH values produced by the addition of AA, HA, and LA on the germination rate of 5-day-old conidia of different Aspergillus species. Each bar represents the mean value + standard error of the mean for all tested strains of each Aspergillus species after 8 h of incubation in RPMI 1640 medium at 37°C.
Our results strongly suggest that environmental conditions like pH and temperature may play a crucial role in selecting and promoting pathogenic species of Aspergillus. A. fumigatus is the species most able to adapt to extreme changes in environmental conditions, as is probably the case during development of deep-seated infections. The internal milieu of the human body seems to provide excellent conditions for the growth of and invasion by A. fumigatus, while such activities are restricted in the cases of A. flavus and A. niger.
REFERENCES
- 1.Gock, M. A., A. D. Hocking, J. I. Pitt, and P. G. Poulos. 2003. Influence of temperature, water activity and pH on growth of some xerophilic fungi. Int. J. Food Microbiol. 81:11-19. [DOI] [PubMed] [Google Scholar]
- 2.Guarro, J., C. Llop, C. Aguilar, and I. Pujol. 1997. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of filamentous fungi. Antimicrob. Agents Chemother. 41:2760-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill, B. 1991. The Wilcoxon signed rank test, p. 126-127. In B. Hill (ed.), Principles of medical statistics, 12th ed. Edward Arnold, London, England.
- 4.Hogan, L. H., B. S. Klein, and S. M. Levitz. 1996. Virulence factors of medically important fungi. Clin. Microbiol. Rev. 9:469-488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lass-Flörl, C., M. Nagl, C. Speth, H. Ulmer, M. P. Dierich, and R. Würzner. 2001. Studies of in vitro activities of voriconazole and itraconazole against Aspergillus hyphae using viability staining. Antimicrob. Agents Chemother. 45:124-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lass-Flörl, C., M. Nagl, E. Gunsilius, C. Speth, H. Ulmer, and R. Würzner. 2002. In vitro studies on the activity of amphotericin B and lipid-based amphotericin B formulations against Aspergillus conidia and hyphae. Mycoses 45:166-169. [DOI] [PubMed] [Google Scholar]
- 7.Latgé, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manavathu, E. K., J. Cutright, and P. H. Chandrasekar. 1999. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J. Clin. Microbiol. 37:858-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reichenberg, F., J. M. Habicht, A. Gratwohl, and M. Tamm. 2002. Diagnosis and treatment of invasive pulmonary aspergillosis in neutropenic patients. Eur. Respir. J. 19:734-755. [DOI] [PubMed] [Google Scholar]
- 10.Sheehan, D. J., C. A. Hitchcock, and C. M. Sibley. 1999. Current and emerging azole antifungal agents. Clin. Microbiol. Rev. 12:40-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soubani, A. O., and P. H. Chandrasekar. 2002. The clinical spectrum of pulmonary aspergillosis. Chest 121:1988-1999. [DOI] [PubMed] [Google Scholar]
- 12.Torres, M. R., A. J. Ramos, J. Soler, V. Sanchis, and S. Marín. 2003. SEM study of water activity and temperature effects on the initiál growth of Aspergillus ochraceus, Alternaria alternata and Fusarium verticillioides on maize grain. Int. J. Food Microbiol. 81:185-193. [DOI] [PubMed] [Google Scholar]