Abstract
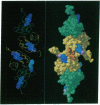
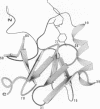
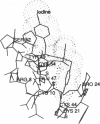
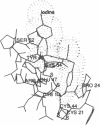
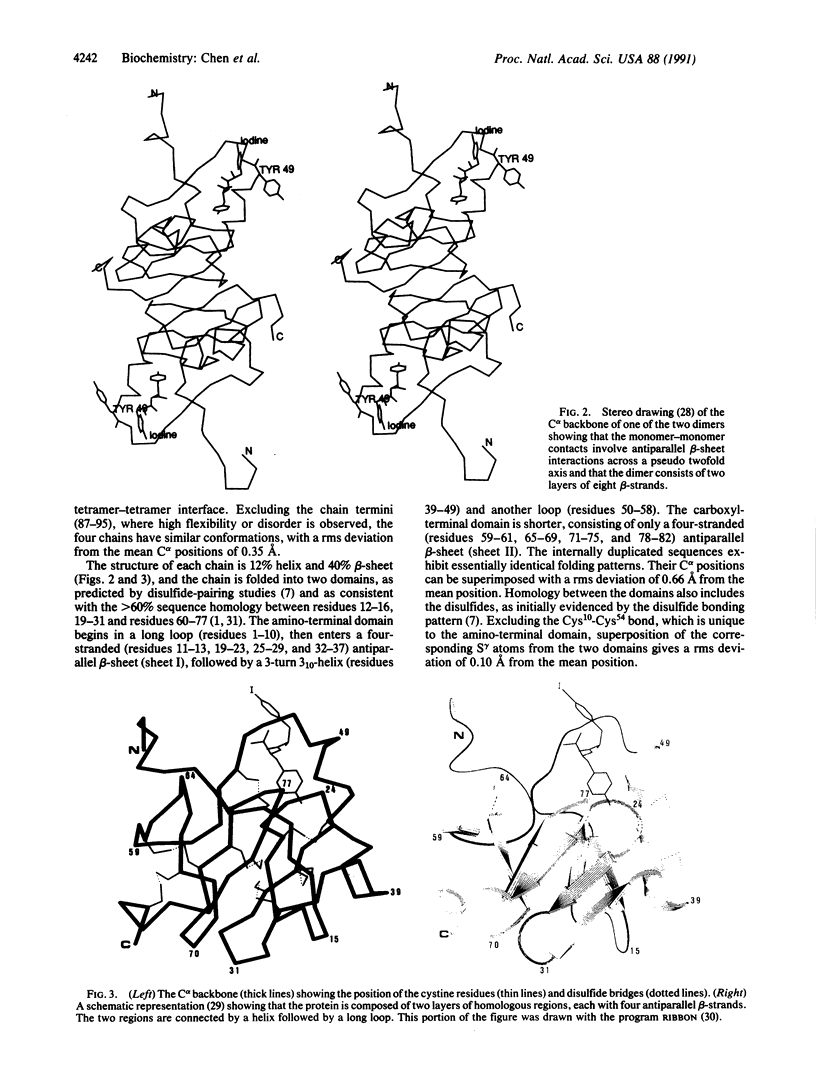
The crystal structure of a dipeptide complex of bovine neurophysin II has been solved at 2.8 A resolution solely by using single-wavelength anomalous scattering data from a single iodinated derivative. The asymmetric unit is an elongated tetramer of dimensions 110 x 40 x 30 A, composed of two dimers related by pseudo twofold symmetry. Each monomer consists of two homologous layers, each with four antiparallel beta-strands. The two regions are connected by a helix followed by a long loop. Monomer-monomer contacts involve antiparallel beta-sheet interactions, which form a dimer with two layers of eight beta-strands. One peptide per monomer occupies the principal hormone-binding pocket formed by part of the amino-terminal region and parts of the connecting helix and loop, with binding to protein consistent with conclusions drawn from solution studies. Dimer-dimer contacts involve the Tyr49 region adjacent to this site. A fifth dipeptide, of unknown biological significance, helps to stabilize one of the monomer-monomer interfaces and the tetramer-tetramer network in the crystal.
Full text
PDF

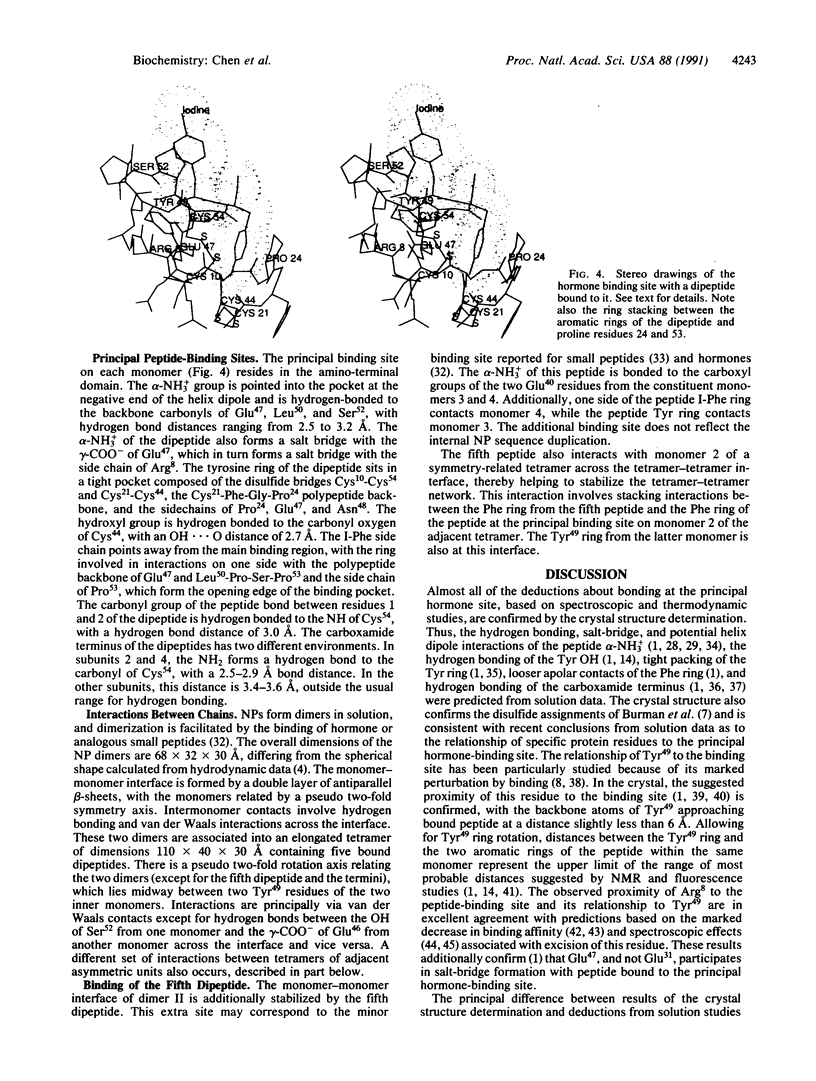

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie D. M., Angal S., Sequeira R. P., Chaiken I. M. Effects of limited tryptic proteolysis of bovine neurophysins on molecular properties of hormone binding, self-association, and antigenicity. Biochemistry. 1982 Dec 7;21(25):6458–6465. doi: 10.1021/bi00268a022. [DOI] [PubMed] [Google Scholar]
- Abercrombie D. M., McCormick W. M., Chaiken I. M. Photoaffinity labeling of the hormone binding site of neurophysin. J Biol Chem. 1982 Mar 10;257(5):2274–2281. [PubMed] [Google Scholar]
- Ando S., McPhie P., Chaiken I. M. Sequence redesign and the assembly mechanism of the oxytocin/bovine neurophysin I biosynthetic precursor. J Biol Chem. 1987 Sep 25;262(27):12962–12969. [PubMed] [Google Scholar]
- Blumenstein M., Hruby V. J., Viswanatha V. The tyrosine ring of oxytocin undergoes hindered rotation when the hormone is bound to neurophysin. Biochem Biophys Res Commun. 1980 May 30;94(2):431–437. doi: 10.1016/0006-291x(80)91249-8. [DOI] [PubMed] [Google Scholar]
- Breslow E., Burman S. Molecular, thermodynamic, and biological aspects of recognition and function in neurophysin-hormone systems: a model system for the analysis of protein-peptide interactions. Adv Enzymol Relat Areas Mol Biol. 1990;63:1–67. doi: 10.1002/9780470123096.ch1. [DOI] [PubMed] [Google Scholar]
- Breslow E., Co R. T., Hanna P., Laborde T. Influence of neurophysin residues 1-8 on the optical activity of neurophysin-peptide complexes. Direct evidence that the 1-8 sequence alters the environment of bound peptide. Int J Pept Protein Res. 1989 Jul;34(1):21–27. doi: 10.1111/j.1399-3011.1989.tb01002.x. [DOI] [PubMed] [Google Scholar]
- Breslow E., Gargiulo P. Effect of low pH on neurophysin-peptide interactions: implications for the stability of the amino-carboxylate salt bridge. Biochemistry. 1977 Jul 26;16(15):3397–3406. doi: 10.1021/bi00634a017. [DOI] [PubMed] [Google Scholar]
- Breslow E., Pagnozzi M., Co R. T. Chemical modification or excision of neurophysin arginine-8 is associated with loss of peptide-binding ability. Biochem Biophys Res Commun. 1982 May 14;106(1):194–201. doi: 10.1016/0006-291x(82)92077-0. [DOI] [PubMed] [Google Scholar]
- Breslow E., Stahl G. L., Walter R. (L-2-hydroxy-3-mercaptopropionic-acid)oxytocin. Circular dichroism studies of conformation and interaction with neurophysin. Int J Pept Protein Res. 1980 Apr;15(4):314–322. [PubMed] [Google Scholar]
- Breslow E., Weis J., Menendez-Botet C. J. Small peptides as analogs of oxytocin and vasopressin in their interactions with bovine neurophysin-II. Biochemistry. 1973 Nov 6;12(23):4644–4653. doi: 10.1021/bi00747a016. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Burman S., Wellner D., Chait B., Chaudhary T., Breslow E. Complete assignment of neurophysin disulfides indicates pairing in two separate domains. Proc Natl Acad Sci U S A. 1989 Jan;86(2):429–433. doi: 10.1073/pnas.86.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M., Kotelchuck D., Walter R., Breslow E. Evolution of neurophysin proteins: the partial sequence of bovine neurophysin-I (vasopressin-oxytocin-carrier proteins-automated amino-acid-sequence analysis-homology-protein evolution). Proc Natl Acad Sci U S A. 1972 Feb;69(2):431–434. doi: 10.1073/pnas.69.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J., Breslow E. Contribution of the peptide backbone to the binding of peptides and hormones to neurophysin. Biochem Biophys Res Commun. 1981 May 15;100(1):455–462. doi: 10.1016/s0006-291x(81)80118-0. [DOI] [PubMed] [Google Scholar]
- Chauvet M. T., Hurpet D., Chauvet J., Acher R. Identification of human neurophysins: complete amino acid sequences of MSEL- and VLDV-neurophysins. Proc Natl Acad Sci U S A. 1983 May;80(10):2839–2843. doi: 10.1073/pnas.80.10.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth A. J., Hope D. B. Studies on the chemical modification of the tyrosine residue in bovine neurophysin-II. Biochem J. 1970 Feb;116(4):545–553. doi: 10.1042/bj1160545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG M., IRELAND M. BINDING OF VASOPRESSIN AND OXYTOCIN TO PROTEIN IN EXTRACTS OF BOVINE AND RABBIT NEUROHYPOPHYSES. J Endocrinol. 1964 Aug;30:131–145. doi: 10.1677/joe.0.0300131. [DOI] [PubMed] [Google Scholar]
- Hendrickson W. A., Pähler A., Smith J. L., Satow Y., Merritt E. A., Phizackerley R. P. Crystal structure of core streptavidin determined from multiwavelength anomalous diffraction of synchrotron radiation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2190–2194. doi: 10.1073/pnas.86.7.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. A. Stereochemically restrained refinement of macromolecular structures. Methods Enzymol. 1985;115:252–270. doi: 10.1016/0076-6879(85)15021-4. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Thirup S. Using known substructures in protein model building and crystallography. EMBO J. 1986 Apr;5(4):819–822. doi: 10.1002/j.1460-2075.1986.tb04287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Live D. H., Cowburn D., Breslow E. Binding of oxytocin and 8-arginine-vasopressin to neurophysin studied by 15N NMR using magnetization transfer and indirect detection via protons. Biochemistry. 1987 Oct 6;26(20):6415–6422. doi: 10.1021/bi00394a018. [DOI] [PubMed] [Google Scholar]
- Lord S. T., Breslow E. Synthesis of peptide spin-labels that bind to neurophysin and their application to distance measurements within neurophysin complexes. Biochemistry. 1980 Nov 25;19(24):5593–5602. doi: 10.1021/bi00565a021. [DOI] [PubMed] [Google Scholar]
- Nicolas P., Batelier G., Rholam M., Cohen P. Bovine neurophysin dimerization and neurohypophyseal hormone binding. Biochemistry. 1980 Jul 22;19(15):3565–3573. doi: 10.1021/bi00556a023. [DOI] [PubMed] [Google Scholar]
- Peyton D., Breslow E. Use of perdeuterated peptides in NMR studies of neurophysin-hormone interaction: demonstration of peptide-specific changes in neurophysin resonances. Biochem Biophys Res Commun. 1985 May 16;128(3):1211–1218. doi: 10.1016/0006-291x(85)91069-1. [DOI] [PubMed] [Google Scholar]
- Peyton D., Sardana V., Breslow E. Application of peptide-mediated ring current shifts to the study of neurophysin-peptide interactions: a partial model of the neurophysin-peptide complex. Biochemistry. 1987 Mar 24;26(6):1518–1525. doi: 10.1021/bi00380a004. [DOI] [PubMed] [Google Scholar]
- Peyton D., Sardana V., Breslow E. Dimerization of native and proteolytically modified neurophysins as monitored by proton magnetic resonance spectroscopy: proximity of tyrosine-49 to the subunit interface. Biochemistry. 1986 Oct 21;25(21):6579–6586. doi: 10.1021/bi00369a036. [DOI] [PubMed] [Google Scholar]
- Pitts J. E., Wood S. P., Hearn L., Tickle I. J., Wu C. W., Blundell T. L., Robinson I. C. Crystallisation and preliminary crystallographic data of a porcine neurophysin I-Tyr-Phe-NH2 complex. FEBS Lett. 1980 Nov 17;121(1):41–43. doi: 10.1016/0014-5793(80)81262-2. [DOI] [PubMed] [Google Scholar]
- Rholam M., Nicolas P., Cohen P. Binding of neurohypophyseal peptides to neurophysin dimer promotes formation of compact and spherical complexes. Biochemistry. 1982 Sep 28;21(20):4968–4973. doi: 10.1021/bi00263a021. [DOI] [PubMed] [Google Scholar]
- Rose J. P., Yang D., Yoo C. S., Sax M., Breslow E., Wang B. C. Crystals of modified bovine neurophysin II. Eur J Biochem. 1988 May 16;174(1):145–147. doi: 10.1111/j.1432-1033.1988.tb14074.x. [DOI] [PubMed] [Google Scholar]
- Sur S. S., Rabbani L. D., Libman L., Breslow E. Fluorescence studies of native and modified neurophysins. Effects of peptides and pH. Biochemistry. 1979 Mar 20;18(6):1026–1036. doi: 10.1021/bi00573a015. [DOI] [PubMed] [Google Scholar]
- Sussman J. L. Constrained-restrained least-squares (CORELS) refinement of proteins and nucleic acids. Methods Enzymol. 1985;115:271–303. doi: 10.1016/0076-6879(85)15022-6. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Yoo C. S., Wang B. C., Sax M., Breslow E. Crystals of a bovine neurophysin II-dipeptide amide complex. J Mol Biol. 1979 Jan 15;127(2):241–242. doi: 10.1016/0022-2836(79)90246-8. [DOI] [PubMed] [Google Scholar]