Abstract
The serological diagnosis of infection by flaviviruses is complicated by the presence of flavivirus cross-reactive antibodies that produce false-positive results for flavivirus infections, especially in regions where more than one virus is endemic. Current diagnostic reagents for tick-borne flavivirus infection have been found to cross-react with yellow fever- or dengue virus-positive sera. This study utilized recombinant flavivirus E protein domain 3 (rE-D3) as a diagnostic reagent to differentiate between infection by mosquito- and tick-borne flaviviruses. This study found that the use of rE-D3 in an enzyme-linked immunosorbent assay (ELISA)-based format allowed the differentiation between serum specific for either mosquito- or tick-borne flaviviruses, but not among the members of the tick-borne encephalitis (TBE) serocomplex of flaviviruses. Sera derived against several TBE serocomplex rE-D3 were found to cross-react with heterologous rE-D3 within the TBE serocomplex, but not with those from mosquito-borne flaviviruses, in both Western blots and ELISAs. Mouse hyperimmune sera generated against TBE serocomplex viruses were also found to react specifically with TBE serocomplex rE-D3, but not with rE-D3 from mosquito-borne viruses and vice versa. When a similar test using virus-derived antigen was performed, a loss of both specificity and sensitivity was observed. These results indicate that flavivirus rE-D3 would be a useful reagent for the detection of infection by TBE serocomplex flaviviruses, several of which are potential biothreat agents, but would not provide the ability to differentiate among infections by separate members of the serocomplex.
Tick-borne encephalitis (TBE) is a disease endemic to vast areas from western Europe across Asia and into Japan. This disease is characterized by rapid onset of fever with subsequent development of potentially fatal encephalitis (9). TBE found in Europe is typically less severe than that found in central and eastern Asia, and the viruses that cause the different forms of the disease can be distinguished genetically and also by their tick vectors. Three subtypes of TBE have been described based on both serology and genetic data: central European encephalitis (CEE) (or western subtype), Siberian subtype TBE, and Far-eastern subtype TBE (11). The disease caused by the latter two subtypes is often commonly referred to as Russian spring-summer encephalitis (RSSE). The TBE viruses are members of the family Flaviviridae and genus Flavivirus, among which are also several significant mosquito-borne human pathogens, including dengue (DEN), yellow fever (YF), Japanese encephalitis (JE), and West Nile (WN) viruses. The TBE viruses associated with human disease are distinct genetically and antigenically from the mosquito-borne viruses and are hence referred to as the TBE serocomplex. In addition to viruses that cause TBE, there are several other viruses within this serocomplex. Among these are the Langat (LGT) virus, which is not known to infect humans in a natural environment; Powassan virus (POW), which also causes encephalitis; and the Alkhurma (ALK), Kyasanur Forest disease (KFD), and Omsk hemorrhagic fever (OHF) hemorrhagic fever-associated viruses (4). In addition, OHF, KFD, and RSSE viruses are listed as potential biothreat agents by the National Institutes of Health and Centers for Disease Control and Prevention. The possible introduction of these viruses by natural or artificial means into areas where these viruses are nonendemic, as well as the present extensive regions of endemicity, makes the diagnosis of infection by these viruses a major public health objective. The lack of simple and accurate diagnostic assays makes the development of a TBE serocomplex diagnostic kit very important to rapid recognition of the causative agent of disease.
The flaviviruses are small single-stranded RNA viruses with icosohedral symmetry and a host-derived lipid envelope. The viral genome is translated as a single open reading frame that is co- and posttranslationally cleaved to generate three structural and seven nonstructural viral proteins. The major surface protein of the flaviviruses is the envelope (E) protein, which exists on the viral surface in a heterodimer with the viral membrane (M) protein. The E protein is divided into three distinct domains (I to III) that can be distinguished both serologically (5, 14, 18, 19) and within an X-ray crystal structure (15, 17). Domain III (D3) of the E protein is highly antigenic, consists primarily of linear epitopes, and has been proposed as the viral receptor-binding domain based on crystallographic data (17), mapping of neutralizing monoclonal antibodies (1, 2, 5, 20), and direct cell binding studies (M. R. Holbrook, unpublished results).
The viral E protein contains 12 cysteine residues that are completely conserved among all of the flaviviruses. This suggests that the viral E protein framework is essentially conserved while the portions of the E protein exposed to the host immune system may be quite distinct among the different flaviviruses. This supposition is supported by the lack of cross-protection by flavivirus vaccines and among individuals infected with heterologous viruses, despite the ability to generate flavivirus cross-reactive antibodies (6, 12, 16). However, the TBE serocomplex viruses are closely related genetically and there is a high degree of similarity at the amino acid level (7, 8, 10, 13). This characteristic makes serological differentiation among these viruses rather difficult and yet offers the potential for cross-protective vaccines.
To date, commercially available diagnostic tests for flaviviruses are based on enzyme-linked immunosorbent assay (ELISA), antibody capture (PanBio, Brisbane, Australia), or dipstick (Integrated Diagnostics, Baltimore, Md.) methods. There are a number of assays available for testing for mosquito-borne flavivirus infections, most of which are for DEN viruses. Commercially available diagnostic kits for TBE infection include ELISA format kits evaluating infection based on the presence of TBE virus immunoglobulin G (IgG) (16) or IgM (IBL, Hamburg Germany). In a study comparing different diagnostic tests for both sensitivity and specificity, it was found that these kits were reasonably sensitive yet some had serious problems with cross-reactivity with YF- or DEN virus-positive sera (16). Further studies using recombinant subviral particles (SVP) in an ELISA format found high specificity for TBE-positive sera with no cross-reactivity to JE virus-positive sera (22). Commercially available kits were developed for specificity against the Western subtype of TBE virus, while the SVP-based ELISAs used SVP derived from the Far-eastern subtype (strain Oshima 5-10) of TBE as the viral antigen (22). The ability of these assays to detect infection by other subtypes of TBE virus, OHF virus, ALK virus, or KFD virus is unknown.
Previous studies from our laboratory used recombinant D3 (rE-D3) derived from WN virus as a reagent to demonstrate the feasibility of using this protein as a very specific reagent to detect the presence of anti-WN antibodies in a group of naturally infected primates (2a). Those studies found that WN virus rE-D3 was sensitive and very specific for WN virus infection and could also differentiate between closely related mosquito-borne flaviviruses. For the diagnosis of flavivirus infection, ideally, one would like a single kit that was able to differentiate infections by different flaviviruses in regions of endemicity. As a next step toward development of such a kit, the current study expands the use of rE-D3 as a potential diagnostic antigen to the TBE serocomplex of flaviviruses and more thoroughly examines the cross-reactivity with rE-D3 derived from other flaviviruses. This study shows that while differentiation between the very similar TBE viruses could not be achieved, this reagent was highly specific for the tick-borne flaviviruses and was much more specific than mouse brain-derived viral antigen in differentiating flavivirus-positive sera in the ELISA format. These results support the feasibility of using rE-D3 as a diagnostic antigen for detection and differentiation of tick-borne flavivirus infection, but find that it is not sufficiently specific in its current format to define individual members of the TBE serocomplex.
MATERIALS AND METHODS
Generation of rE-D3.
rE-D3 protein was expressed in Escherichia coli as a fusion protein with maltose-binding protein as the fusion partner. Expression and purification were essentially done by following the manufacturer's instructions and as previously described (2a). Briefly, the coding sequence for D3 of the viral E protein was cloned into the pMal-c2x expression vector (New England Biolabs). The individual D3 molecules encompassed approximately residues 300 to 395 of the viral E protein. Cloning into the pMal system added an additional serine to the N terminus of the recombinant proteins. The fusion protein was expressed by induction with isopropyl-β-d-thiogalactopyranoside (IPTG). Purification was achieved via lysing the cells by sonication followed by affinity purification over an amylose resin column (New England Biolabs). The fusion protein was cleaved with Factor Xa (Novagen), and the maltose binding protein and rE-D3 were separated by size exclusion chromatography on a Superdex 75 column (Amersham/Pharmacia). D3 was concentrated and stored at 4°C until use. The rE-D3 protein has been found to be extremely stable under very stringent conditions (3, 21) and is stable when stored at 4°C for extended periods.
Antiserum production.
Purified E-D3 was provided to Harlan Bioproducts for Science (Indianapolis, Ind.) for production of rabbit antisera. Antiserum against each rE-D3 protein was produced in two New Zealand White rabbits. Testing of the antisera in ELISA and Western blot assays found little difference between antisera generated in different rabbits against the same antigen (Holbrook, unpublished).
Antigens and MIAF.
Suckling mouse brain-derived viral antigens from DEN virus 2 (DEN2), DEN4, YF vaccine strain 17D, JE strain Nakayama, LGT strain TP21, and POW strain LB were obtained from the World Arbovirus Reference Collection housed at the University of Texas Medical Branch. Mouse hyperimmune ascitic fluid (MIAF) against DEN2, DEN4, JE, YF, WN, LGT, POW, KFD, and RSSE viruses were also obtained from the World Arbovirus Reference Collection.
Western blots.
Ten nanograms of purified rE-D3 was run on sodium dodecyl sulfate-ployacrylamide gel electrophoresis (SDS-PAGE) (12% polyacrylamide) gels and transferred to a nitrocellulose membrane for blotting. The blots were blocked with TBS-Tween (20 mM Tris, pH 7.5, 150 mM NaCl, 0.05% Tween 20) containing 3% dry milk powder (Blotto) for at least 30 min at room temperature. The membranes were probed for 1 h at room temperature with the appropriate antiserum diluted in Blotto at dilutions of 1:800 to 1:1,000, dependent upon the antiserum. Blots were washed three times with Blotto and probed with a horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (Sigma) at a 1:2,000 dilution in Blotto for 1 h at room temperature. The blots were subsequently washed twice with Blotto and three times with TBS-Tween. The presence of rE-D3 was detected by using the ECL enhanced chemiluminescence substrate (Amersham/Pharmacia).
Indirect ELISAs.
Purified rE-D3 or mouse brain-derived viral antigen was used to coat 96-well round-bottom microtiter plates (Falcon) overnight at 4°C in borate saline buffer (120 mM NaCl, 50 mM boric acid, pH 9.0). Preliminary experiments examining the sensitivity of the assay found that wells coated with 10 to 20 ng of rE-D3 provided optimum sensitivity while plates were coated at 1 hemagglutination (HA) unit per well (2a). Wells were blocked with phosphate-buffered saline (PBS)-Tween (PBS with 0.5% Tween 20) containing 3% bovine serum albumin for 30 min at room temperature and then washed once with PBS-Tween prior to incubation with antisera. Twofold serial dilutions of antisera were made in duplicate wells. All dilutions were made in PBS-Tween. Following a 1-h room temperature incubation with primary antibody, the plates were washed with PBS-Tween and then incubated with either HRP-conjugated goat anti-mouse or goat anti-rabbit secondary antibody at a 1:2,000 dilution for 1 h at room temperature. The plates were washed and then incubated with 50 μl of 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma) colorometric detection reagent for 5 min at room temperature. The reaction was stopped with 50 μl of 3 M HCl, and the plates were read at 450 nm with a reference wavelength of 595 nm.
RESULTS
Cloning of viral D3.
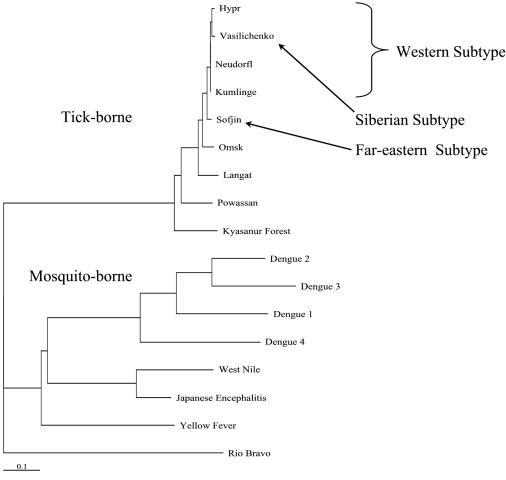
The types of rE-D3 used in these assays were cloned from viruses representing several mosquito-borne flaviviruses and the major clades of the TBE serocomplex, with the exception of the Siberian and Far-eastern subtypes of viruses (Fig. 1). Viral RNA for the Siberian and Far-eastern subtypes was not available as they are biosafety level 4 (BSL-4) agents with restricted availability. Kumlinge (KUM) virus is a strain of CEE, while OHF and KFD viruses are viruses that cause hemorrhagic fever rather than an encephalitic disease that is characteristic of other viruses of the TBE serocomplex and form distinct subgroups within the serocomplex. LGT and POW viruses also represent distinct subgroups of the TBE serocomplex (Fig. 1). LGT is a naturally attenuated virus originally isolated in Malaysia, and POW may represent an older lineage of TBE viruses in North America and Asia (7, 23). In addition to members of the TBE serocomplex, rE-D3 from the mosquito-borne WN virus, YF vaccine strain 17D, and YF wild-type strain Asibi were also produced. The amino acid sequences within D3 of all flaviviruses are similar, but the level of identity within the TBE serocomplex is quite high (Table 1). This high degree of similarity is also seen in Fig. 1, where the representative of the Siberian subtype of TBE (Vasilichenko) groups with the Western subtype viruses, based solely on the amino acid sequence of E-D3. This high degree of similarity makes these viruses difficult to distinguish serologically.
FIG. 1.
Phylogenetic analysis of the flavivirus E protein domain 3 amino acid sequence.
TABLE 1.
Amino acid sequences of E protein domain III of mosquito-and-tick-borne flavinir uses
| Virus | Sequence |
|---|---|
| Positions 300-351 | |
| Mosquito | |
| DEN1 | KGVSYVMCT-GSFKLEKEVAETQHGTVLVQVKYEGTDAPCKIPFSSQDEKGVT |
| DEN2 | KGMSYSMCY-GKFKVVEEIAETQHGTIVIRVQYEGDGSPCKIPLEIMDLDNRH |
| DEN3 | KGMSYAMCL-NTFVLKKEVSETQHGTILIKVEYKGEDAPCKIPFSTEDGQGKA |
| DEN4 | KGMSYTMCS-GKFSIDKEMAETQHGTTVVKVKYEGAGAPCKVPIEIRDVNKEK |
| JE | KGTTYGMCT-EKFSFAKNPADTGHGTVVIELSYSGSDGPCKIPIVSVASLNDM |
| WN | KGTTYGVCS-KAFKFLGTPADTGHGTVVLELQYTGTDGPCKVPISSVASLNDL |
| YF | KGTSYKMCT-DKMSFVKNPTDTGHGTAVMQVKVPKG-APCRIPVMVADDLTAS |
| Tick | |
| RSSE | KGLTYTMCDKTKFTWKRAPTDSGHDTVVMEVTFSGT-KPCRIPVRAVAHGSPD |
| CEE | KGLTYTMCDKTKFTWKRAPTDSGHDTVVMEVTFSGT-KPCRIPVRAVAGHSPD |
| LI | KGLTYTMCDKSKFAWKRTPTDSGHDTVVMEVTFSGS-KPCRIPVRAVAHGSPD |
| LGT | KGLTYTVCDKTKFTWKRAPTDSGHDTVVMEVGFSGT-RPCRIPVRAVAHGVPE |
| POW | KGTTYSMCDKAKFKWKRVPVDSGHDTVVMEVSYTGSDKPCRIPVRAVAHGVPA |
| KFD | KGMTYTVCEGSKFAWKRPPTDSGHDTVVMEVTYTGS-KPCRIPVRAVAHGEPN |
| OHF | KGLTYTMCDKAKFTWKRAPTDSGHDTVVMEVAFSGT-KPCRIPVRAVAHGSPD |
| Positions 352-395 | |
| Mosquito | |
| DEN1 | Q-NGRLITANPIVIDKEK--PVNIEAE-PPFGESYIVVGAGEKALKLSWFKK |
| DEN2 | V-LGRLITVNPIVTEKDS--PVNVEAE-PPLGDSYIIIGVEPGQLKLNWFKK |
| DEN3 | H-NGRLITANPVVTKKEE--PVNIEAE-PPFGESNIVIGIGDKALKINWYRK |
| DEN4 | V-VGRIISSTPLAENTNS--VTNIELE-RPL-DSYIVIGVGNSALTLHWFRK |
| JE | TPVGRLVTVNPFVATSSANSKVLVEME-PPFGDSYIVVGRGDKQINHHWHKA |
| WN | TPVGRLVTVNPFVSVATANAKVLIELE-PPFGDSYIVVGRGEQQINHHWHKS |
| YF | VNKGILVTVNPIASTNED--EVLIEVN-PPFGDSYIIVGTGDSRLTYQWHKE |
| Tick | |
| RSSE | VNVAMLITPNPTIENNGG---GFIEMQLPP-GDNIIYVG----ELSYQWFQK |
| CEE | VNVAMLITPNPTIENNGG---GFIEMQLPP-GDNIIYVG----ELSHQWFQK |
| LI | VNVAMLITPNPTIENDGG---GFIEMQLPP-GDNIIYVG----ELSHQWFQT |
| LGT | VNVAMLITPNPTMENNGG---GFIEMQLPP-GDNIIYVG----DLNHQWFQK |
| POW | VNVAMLITPNPTIETNGG---GFIEMQLPP-GDNIIYVG----DLSQQWFQK |
| KFD | VNVASLITPNPSMENTGG---GFVELQLPP-GDNIIYVG----ELSHQWFQK |
| OHF | VDVAMLITPNPTIENNGG---GFIEMQLPP-GDNIIYVG----ELKHQWFQK |
Western blots.
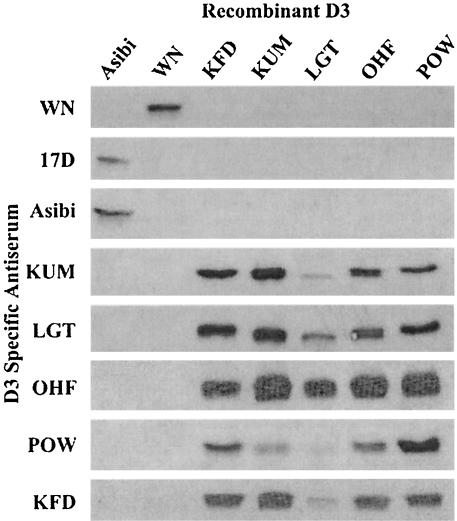
Purified rE-D3 derived from several mosquito- and tick-borne flaviviruses was run on SDS-PAGE gels and transferred to nitrocellulose for blotting with homologous and heterologous rabbit anti-rE-D3-specific antisera. These assays found a significant degree of cross-reactivity between rE-D3 derived from members of the tick-borne flavivirus serocomplex (Fig. 2). All five TBE serocomplex antisera recognized the five TBE serocomplex rE-D3, though the sera tended to cross-react less well with LGT rE-D3, and the rabbit anti-POW rE-D3 antiserum appeared to have less cross-reactivity than other sera. This result is not surprising as LGT and POW viruses are phylogenetically less related than KUM, OHF, and KFD viruses (Fig. 1). None of the rabbit anti-TBE serocomplex antisera recognized rE-D3 derived from the mosquito-borne flaviviruses WN or YF, nor did rabbit anti-YF or anti-WN antisera recognize any of the TBE rE-D3 (Fig. 2). rD3 was not available for the DEN viruses or other members of the JE serocomplex of flaviviruses (e.g., JE, Murray Valley encephalitis, and St. Louis encephalitis viruses).
FIG. 2.
Western blot analysis of rE-D3-specific rabbit antiserum reacting with purified rE-D3 from several flaviviruses.
Viral antigen-based ELISAs.
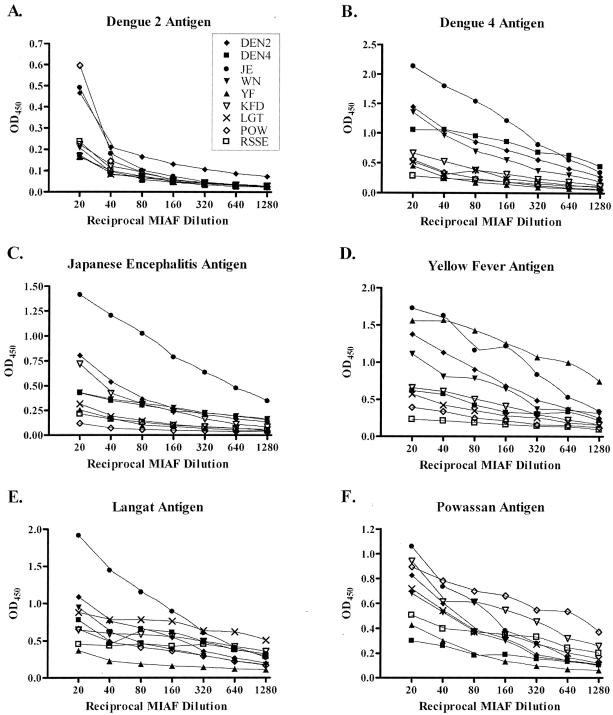
Mouse brain-derived viral antigens were used to coat 96-well plates at 1 HA unit per well. rE-D3-specific sera and MIAF were diluted at twofold serial dilutions, and the sensitivity and specificity of the assay were determined. As seen in Fig. 3, there is a lack of specificity for TBE serogroup viral antigens with MIAF. In all assays, JE MIAF cross-reacted strongly with all of the antigens tested. In fact the only assay that demonstrated clear specificity was that against JE mouse-derived antigen, in which the JE MIAF clearly reacted well with the antigen. In the remaining panels, little specificity was found for MIAF binding to mouse brain-derived viral antigen, clearly demonstrating that this antigen is not suitable for a diagnostic assay. In these experiments, the MIAF were not normalized against homologous rE-D3 or virus-derived antigens prior to performing the experiments. Instead, the MIAF were tested as received from the World Arbovirus Reference Collection. Due to the lack of availability of sera from natural infections, this method was undertaken to mimic the testing of a potentially infected individual in a true diagnostic setting. In some cases, such as is apparent with JE virus MIAF, the reactive antibody titer may be higher than other MIAF and give a higher level of cross-reactivity. Normalization of the MIAF might reduce the cross-reactivity, but it would also bias the experiment.
FIG. 3.
ELISAs using MIAF to detect mouse brain-derived viral antigen. MIAF generated against tick-borne flaviviruses are shown by open symbols, while the remaining symbols comprise mosquito-borne flaviviruses.
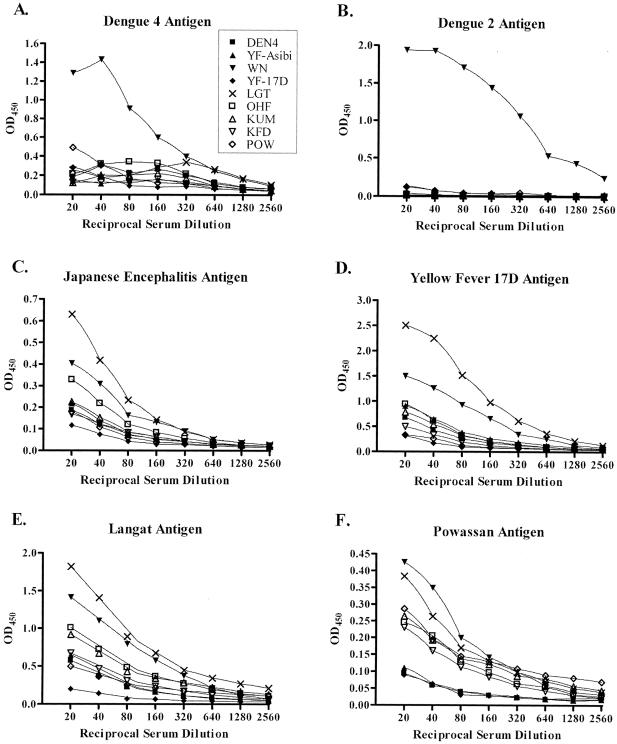
In similar experiments using rabbit anti-rE-D3-specific antiserum to screen against virus-derived antigen, cross-reactivity was also observed. As seen in Fig. 4, both rabbit rE-D3 anti-serum specific for the D3 of LGT and WN viruses reacted with several viral antigens, though the degree of cross-reactivity is not as great as that seen with the MIAF in Fig. 3. Even though specific antiserum was used in the assay, based on results from Western blots (Fig. 2), significant cross-reactivity between mosquito-borne virus antigens and antisera specific for tick-borne viruses was found. Again, the antisera were not normalized prior to use in these experiments to avoid experimental bias. These results, in conjunction with those shown in Fig. 3, demonstrate that the use of mouse brain-derived viral antigen in a diagnostic assay does not provide the specificity required to conclusively identify the agent responsible during flavivirus infection.
FIG. 4.
ELISAs using rE-D3-specific antiserum to detect mouse brain-derived viral antigen.
The majority of the mouse brain-derived viral antigens tested in these experiments were representative of the mosquito-borne flaviviruses. Unfortunately, the assay could not be performed with more TBE serocomplex antigens as some were not available from the World Arbovirus Reference Collection, and others that were available in the collection could not be tested due to concerns about the complete inactivation of the virus during antigen preparation (i.e., live virus might be in the antigen preparations) and inadequate facilities for tested potentially infectious antigens (e.g., BSL-4 for OHF and KFD antigens).
rE-D3-based ELISAs.
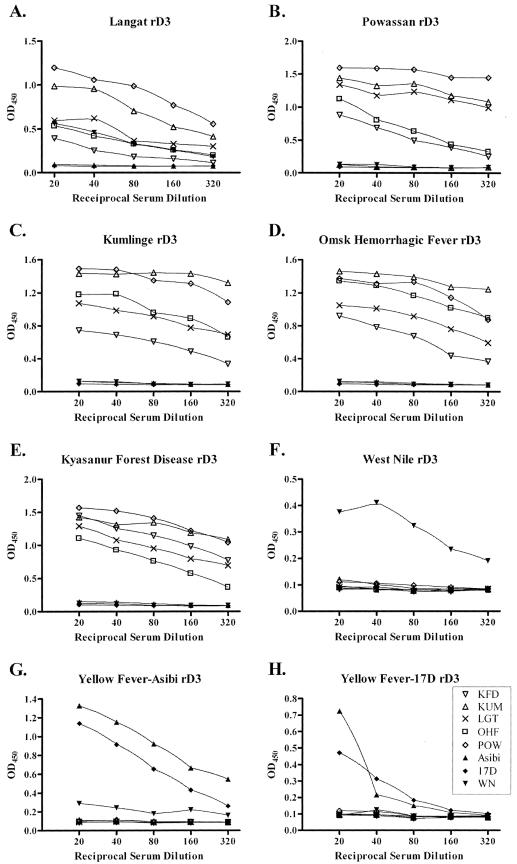
ELISAs using rE-D3 as the antigen, rather than mouse brain-derived viral antigen, demonstrated a much more specific reaction against homologous E-D3-specific antiserum. Both WN and YF rE-D3 reacted only with homologous serum (true for both the YF wild-type Asibi strain and vaccine 17D strain rE-D3) (Fig. 5F to H). The YF Asibi rE-D3 rabbit antiserum cross-reacted with rE-D3 derived from YF vaccine strain 17D, an expected result, as these proteins are nearly identical (Fig. 5G). A similar result was seen in YF 17D rE-D3-coated plates (Fig. 5H). rD3 derived from the TBE serocomplex of viruses, however, were not specific for individual virus rE-D3-specific rabbit antisera, but were cross-reactive with rE-D3 derived from viruses within the TBE serocomplex (Fig. 5A to E). This result supports the Western blot data presented in Fig. 2, where cross-reactivity was seen between the rabbit antisera generated against the recombinant proteins of the TBE serocomplex. These assays found that TBE serocomplex-derived rE-D3 cross-reacted with all of the TBE serocomplex-specific rabbit anti-rE-D3 antisera, but not those derived from the mosquito-borne WN or YF viruses. This assay was also quite sensitive as serum diluted to 1:320 could easily be detected above a 0.2 optical density at 450 nm (OD450) cutoff for a positive test. The cross-reactivity among the TBE serocomplex viruses was somewhat expected as the level of amino acid identity among the E protein D3 from these viruses is very high (Table 1).
FIG. 5.
ELISAs using rabbit anti-rE-D3-specific antiserum to detect rE-D3. Open symbols represent tick-borne flaviviruses.
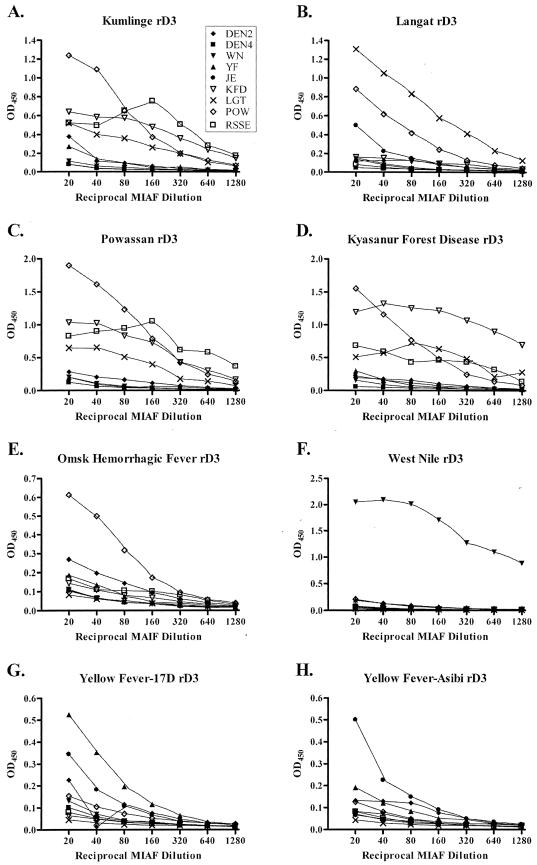
To examine the ability of rE-D3 to detect the presence of IgG in a model for analysis of test serum from a potentially infected individual, MIAF were assayed in plates coated with rE-D3 in experiments similar to those shown above using mouse brain-derived viral antigen. In these experiments, it was found that the rE-D3-coated plates were able to clearly differentiate MIAF derived from TBE serocomplex-infected animals from those of mosquito-borne viruses (Fig. 6). As seen in panels A to E of Fig. 6, TBE serocomplex rE-D3 cross-reacted with the majority of the TBE serocomplex MIAF tested. POW MIAF appeared to cross-react with rE-D3 derived from TBE serocomplex viruses, whereas the RSSE MIAF was somewhat less reactive. POW MIAF was also the only MIAF to react with OHF rE-D3 and with considerably less sensitivity than the other rE-D3-coated plates (Fig. 6E). Unfortunately, OHF-specific MIAF was not available from the World Arbovirus Reference Collection. rE-D3 for mosquito-borne flaviviruses was also highly specific as the WN MIAF reacted only with WN rE-D3, as was previously shown (2a) (Fig. 6F), and the YF-17D rE-D3 reacted with YF MIAF (Fig. 6G), though the sensitivity of this assay was not as high as with the TBE serocomplex rE-D3 or WN rE-D3. Both of the YF rE-D3 cross-reacted with JE MIAF, indicating potentially similar surface amino acid residues.
FIG. 6.
ELISAs using MIAF to detect rE-D3. Open symbols represent TBE serocomplex-specific MIAF.
DISCUSSION
The development of a specific and sensitive diagnostic assay for detection of flavivirus infection will greatly enhance the ability to treat diseases caused by these viruses. Current diagnostic assays utilize either ELISA or dipstick formats for identification of flavivirus infection (PanBio; Integrated Diagnostics) (6, 16, 22). A number of assays are available for the detection of DEN virus infection. These assays utilize antigen capture and antibody-based ELISAs and dipsticks for detection of virus-specific IgG or IgM. Diagnosis of TBE infection depends on IgG-based ELISAs that are available in Europe (6, 16, 22). However, these tests have limitations, with both sensitivity and cross-reactivity with other flaviviruses (16). The recent utilization of SVP in an ELISA-based diagnostic test for TBE infection shows promise (22). Since this assay uses intact viral M and E proteins, it is likely that the pitfalls that affect the use of complete viral antigen (e.g., cross-reactivity) may impede the employment of this assay in diagnostic settings. The use of reverse transcription-PCR (RT-PCR) is also a potential method for diagnosis of flavivirus infection. However, RT-PCR assays have the significant limitation of requiring advanced techniques, equipment, and reagents that require a cold chain for stability. In addition, RT-PCR detects the presence of virus in patient serum, a condition that is not usually met when patients come to the hospital as the virus is frequently cleared from the bloodstream by the onset of symptoms. Clearly, there is a need to improve the current reagents used for diagnosis of TBE virus infections. The results from the present study indicate that rE-D3 is an excellent tool for differentiating infections caused by TBE serogroup versus mosquito-borne flaviviruses. This reagent would be particularly useful in regions where both tick-borne and mosquito-borne flaviviruses are endemic, such as Asia and North America, as well as economically depressed countries as it is relatively simple and inexpensive to produce.
This study extends previous work from our laboratory examining the use of recombinant flavivirus rE-D3 for the detection of WN virus infection. rD3 derived from the WN virus was found to be very specific and highly sensitive for identifying infection in naturally infected primates (2a). The present study extends the use of rE-D3 as a diagnostic reagent and examines the use of rE-D3 for its potential as a reagent for detecting TBE serocomplex virus infections. Assays using rE-D3-specific homologous and heterologous antisera demonstrated a very high degree of sensitivity and specificity, and tests using mouse hyperimmune serum supported these results. The one major limitation of the rE-D3-based diagnostic assay is the inability to differentiate between the TBE serocomplex viruses. This problem is not surprising given the very high degree of amino acid identity between the rE-D3 of the TBE serocomplex viruses (Table 1). The minimization of potential binding epitopes by using peptide-based diagnostic assays may be required to produce the degree of specificity required to differentiate the TBE serocomplex of viruses immunologically. Consequently, the use of the rE-D3-based ELISAs as a rapid preliminary test for TBE virus infection would need to be supported by further clinical and laboratory tests such as virus isolation or neutralization assays to conclusively identify the virus causing disease. In addition, rE-D3 could be used in a dipstick format by cross-linking the C terminus of the protein to a solid substrate. This format would allow complete exposure of all rE-D3 antibody epitopes to test sera. rE-D3 is an extremely stable protein, as was shown by retention of its structure in up to 4 M urea (3) and at low pH (21). The physical properties of rE-D3 would lend themselves to the use of the rE-D3 reagent under unfavorable environmental conditions such as extreme heat or cold or after extended storage. The use of recombinant protein technology for development of these diagnostic reagents will also minimize the cost of diagnosis, making use of such reagents feasible in economically depressed countries.
Acknowledgments
We would like to thank the UTMB Protein Chemistry Core facility of DNA sequencing and large-scale production of recombinant proteins and David Beasley for cloning of WN E-D3.
Funding was provided by the James W. McLaughlin Fellowship in Infection and Immunity (M.R.H.), the Centers for Disease Control and Prevention (R.E.S.), and the National Institutes for Health (AI-10984) for support of the World Arbovirus Reference Collection.
REFERENCES
- 1.Beasley, D. W., and J. G. Aaskov. 2001. Epitopes on the dengue 1 virus envelope protein recognized by neutralizing IgM monoclonal antibodies. Virology 279:447-458. [DOI] [PubMed] [Google Scholar]
- 2.Beasley, D. W. C., and A. D. Barrett. 2002. Identification of neutralizing epitopes within structural domain III of the West Nile virus envelope protein. J. Virol. 76:13097-13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a.Beasley, D. W. C., M. R. Holbrook, A. P. A. Travassos da Rosa, L. Coffey, A.-S. Carrara, K. Phillippi-Falkenstein, R. P. Bohn, Jr., M. S. Ratterree, K. M. Lillibridge, G. V. Ludwig, J. Estrada-Franco, S. C. Weaver, R. B. Tesh, R. E. Shope, and A. D. T. Barrett. 2004. Use of a recombinant envelope protein subunit antigen for specific serological diagnosis of West Nile virus infection. J. Clin. Microbiol. 42:2759-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhardwaj, S., M. R. Holbrook, R. E. Shope, A. D. T. Barrett, and S. J. Watowich. 2001. Biophysical characterization and vector-specific antagonist activity of domain III of the tick-borne flavivirus envelope protein. J. Virol. 75:4002-4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burke, D. S., and T. P. Monath. 2001. Flaviviruses, p. 1043-1125. In D. M. Knipe and P. M. Howley (ed.), Field's virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 5.Crill, W. D., and J. T. Roehrig. 2001. Monoclonal antibodies that bind to domain III of the dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobler, G., J. Treib, S. T. Kiessig, W. V. Blohn, G. Frosner, and A. Haass. 1996. Diagnosis of tick-borne encephalitis: evaluation of sera with borderline titers with the TBE-ELISA. Infection 24:405-406. [DOI] [PubMed] [Google Scholar]
- 7.Gould, E. A., X. de Lamballerie, P. M. Zanotto, and E. C. Holmes. 2001. Evolution, epidemiology, and dispersal of flaviviruses revealed by molecular phylogenies. Adv. Virus Res. 57:71-103. [DOI] [PubMed] [Google Scholar]
- 8.Gritsun, T. S., T. V. Frolova, V. V. Pogodina, V. A. Lashkevich, K. Venugopal, and E. A. Gould. 1993. Nucleotide and deduced amino acid sequence of the envelope gene of the Vasilchenko strain of TBE virus: comparison with other flaviviruses. Virus Res. 27:201-209. [DOI] [PubMed] [Google Scholar]
- 9.Gritsun, T. S., V. A. Lashkevich, and E. A. Gould. 2003. Tick-borne encephalitis. Antivir. Res. 57:129-146. [DOI] [PubMed] [Google Scholar]
- 10.Gritsun, T. S., K. Venugopal, P. M. Zanotto, M. V. Mikhailov, A. A. Sall, E. C. Holmes, I. Polkinghorne, T. V. Frolova, V. V. Pogodina, V. A. Lashkevich, and E. A. Gould. 1997. Complete sequence of two tick-borne flaviviruses isolated from Siberia and the UK: analysis and significance of the 5′ and 3′-UTRs. Virus Res. 49:27-39. [DOI] [PubMed] [Google Scholar]
- 11.Heinz, F. X., M. S. Collett, R. H. Purcell, E. A. Gould, C. R. Howard, M. Houghton, R. J. M. Moorman, C. M. Rice, and H. J. Thiel. 2000. Family Flaviviridae, p. 859-878. In M. H. V. Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. Carstens, M. K. Estes, S. Lemon, J. Maniloff, M. A. Mayo, D. McGeogch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. 7th International Committee for the Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 12.Holzmann, H., M. Kundi, K. Stiasny, J. Clement, P. McKenna, C. Kunz, and F. X. Heinz. 1996. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J. Med. Virol. 48:102-107. [DOI] [PubMed] [Google Scholar]
- 13.Lin, D., L. Li, D. Dick, R. E. Shope, H. Feldmann, A. D. T. Barrett, and M. R. Holbrook. Analysis of the complete genome of the tick-borne flavivirus Omsk hemorrhagic fever virus. Virology, in press. [DOI] [PubMed]
- 14.Mandl, C. W., F. Guirakhoo, H. Holzmann, F. X. Heinz, and C. Kunz. 1989. Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J. Virol. 63:564-571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modis, Y., S. Ogata, D. Clements, and S. C. Harrison. 2003. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc. Natl. Acad. Sci. USA 100:6986-6991. (First published 20 May 2003; 10.1073/pnas.0832193100.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niedrig, M., D. Vaisviliene, A. Teichmann, U. Klockmann, and S. S. Biel. 2001. Comparison of six different commercial IgG-ELISA kits for the detection of TBEV-antibodies. J. Clin. Virol. 20:179-182. [DOI] [PubMed] [Google Scholar]
- 17.Rey, F. A., F. X. Heinz, C. W. Mandl, C. Kunz, and S. C. Harrison. 1995. The envelope glycoprotein from tick-borne encephalitis at 2 Å resolution. Nature 375:291-298. [DOI] [PubMed] [Google Scholar]
- 18.Starapoli, I., M. P. Frenkiel, F. Megret, and V. Deubel. 1997. Affinity-purified dengue-2 virus envelope glycoprotein induces neutralizing antibodies and protective immunity in mice. Vaccine 15:1946-1954. [DOI] [PubMed] [Google Scholar]
- 19.Takegami, T., H. Miyamoto, H. Nakamura, and K. Yasui. 1982. Biological activities of the structural proteins of Japanese encephalitis virus. Acta Virol. 26:312-320. [PubMed] [Google Scholar]
- 20.Thullier, P., C. Demangel, H. Bedouelle, F. Megret, A. Jouan, V. Deubel, J. Mazie, and P. Lafaye. 2001. Mapping of a dengue virus neutralizing epitope critical for the infectivity of all serotypes: insight into the neutralization mechanism. J. Gen. Virol. 82:1885-1892. [DOI] [PubMed] [Google Scholar]
- 21.White, M. A., D. Liu, M. R. Holbrook, R. E. Shope, A. D. Barrett, and R. O. Fox. 2003. Crystallization and preliminary X-ray diffraction analysis of Langat virus envelope protein domain III. Acta Crystallogr. D Biol. Crystallogr. 59:1049-1051. [DOI] [PubMed] [Google Scholar]
- 22.Yoshii, K., D. Hayasaka, A. Goto, M. Obara, K. Araki, K. Yoshimatsu, J. Arikawa, L. Ivanov, T. Mizutani, H. Kariwa, and I. Takashima. 2003. Enzyme-linked immunosorbent assay using recombinant antigens expressed in mammalian cells for serodiagnosis of tick-borne encephalitis. J. Virol. Methods 108:171-179. [DOI] [PubMed] [Google Scholar]
- 23.Zanotto, P. M., G. F. Gao, T. Gritsun, M. S. Marin, W. R. Jiang, K. Venugopal, H. W. Reid, and E. A. Gould. 1995. An arbovirus cline across the northern hemisphere. Virology 210:152-159. [DOI] [PubMed] [Google Scholar]