Abstract
Human rotavirus VP4 and VP7 gene sequences were amplified by reverse transcription-PCR from 53% (322 of 607) of fecal specimens collected from children with severe diarrhea who visited hospitals in six urban areas of South Korea in 2000 and 2001. G2 was the most frequently found G type (constituted 50.6%), followed by G1 (30.1%) and G4 (13.0%). Although the P types of high incidence were P[4] (53.1%) and P[8] (21.4%), a significant incidence of P[6] (20.2%) was also noticeable. The commonest G- and P-type combination found in this study was G2P[4], rather than G1P[8], the most prevalent type known worldwide.
Rotaviruses are the major etiological agent of gastroenteritis and cause vomiting, diarrhea, and fever in infants and young children worldwide (8). Rotaviruses are divided into seven groups (groups A to G) on the basis of their antigenic properties. Group A rotaviruses are further divided into the G and the P subtypes according to the antigenic property of the VP7 protein (glycoprotein) and the VP4 protein (protease-susceptible protein), respectively. To date, 10 G types have been identified in humans, but most cases of human infection are associated with four G types (types G1 to G4), of which G1 is the most prevalent worldwide (5, 10). It is well established that the G serotypes coincide with the G genotypes, while P serotypes are classified by a system different from that used to classify P genotypes. Types P[4] and P[8] are most frequently found in humans (according to convention, P genotypes are indicated with brackets). Recent studies, however, indicated the emergence of novel P types in different parts of world (3, 11)
Rotavirus infection is the most common cause of acute diarrhea in infants and young children in South Korea (14). To monitor the diversity of rotavirus strains circulating in the country, we carried out an analysis of 607 fecal specimens collected from infants and young children with acute diarrhea who visited 18 urban hospitals and four clinical laboratories scattered around the six provinces of the country from January 2000 to April 2001. The fecal suspension (10% in phosphate-buffered saline) was centrifuged at 10,000 × g for 10 min, and the double-stranded viral RNA was extracted by treatment of the supernatant with phenol-chloroform-1% sodium dodecyl sulfate, as described previously (6). Reverse transcription (RT)-PCR was performed with the consensus primers Beg9 and End9 to amplify the VP7 gene sequence in full (1,062 bp) and primers Con2 and Con3 to amplify the VP4 gene sequence (877 bp). A second-round, multiplex PCR was performed with primers specific for G types (G1, G2, G3, G4, G8, and G9) and P types (P[4], P[6], P[8], P[9], and P[10]), as described previously (4, 6). The G and P types were determined from the migration rates of the amplicons in a 1.2% agarose gel. Among the 607 stool samples 322 (53.0%) were positive for rotavirus RNA by RT-PCR. The presence of rotaviral antigens was determined by a latex agglutination assay (Biomerieux, Marcy l'Etoile, France) and an enzyme-linked immunosorbent assay (ELISA; Dako Diagnostics, Cambridgeshire, United Kingdom) (discussed below).
G2 was the most prevalent G type found in this study and was detected in 163 (50.6%) of 322 specimens, followed by G1 (97 specimens; 30.1%) and G4 (42 specimens; 13.0%) (Table 1). The most significant difference between the present results and those from previous studies done in South Korea is the strong prevalence of type G2. Type G1 had been predominant in the country from 1987 to 1999 and was detected in more than 75% of specimens in which the rotavirus could be typed, while type G2 constituted less than 18% (14). The emergence of type G2 has recently been reported in other parts of the world (9). We also noticed the emergence of type G4 in the present study. Another study previously conducted in Korea showed an even higher incidence of type G4 (40.9%), although the study was performed with samples from only one city (15). The G types of minor incidence in our study included three strains of type G3 (0.9%) and one strain of type G9 but no strain of type G8, unlike the recent emergence of type G9 in several other countries (2, 7, 13). Mixed infections (constituted 2.8% in total) consisted of types G1 and G4; types G2 and G4; and types G1, G2, and G4.
TABLE 1.
G and P types of human rotaviruses found in South Korea (2000 to 2001)a
| P type | No. (%) of rotaviruses
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G4 | G9 | G1/G4 | G2/G4 | G1/G2/G4 | NT | Total | |
| P[4] | 9 | 147 | 2 | 6 | 2 | 5 | 171 (53.1) | |||
| P[6] | 36 | 4 | 18 | 1 | 3 | 2 | 1 | 65 (20.2) | ||
| P[8] | 47 | 4 | 1 | 17 | 69 (21.4) | |||||
| P[9] | 1 | 1 (0.3) | ||||||||
| P[10] | 1 | 1 (0.3) | ||||||||
| P[4]/P[6] | 1 | 4 | 1 | 1 | 7 (2.2) | |||||
| P[4]/P[8] | 3 | 2 | 1 | 6 (1.9) | ||||||
| P[6]/P[8] | 1 | 1 (0.3) | ||||||||
| ND | 1 | 1 (0.3) | ||||||||
| Total (%) | 97 (30.1) | 163 (50.6) | 3 (0.9) | 42 (13.0) | 1 (0.3) | 5 (1.6) | 2 (0.6) | 2 (0.6) | 7 (2.2) | 322 |
Abbreviations: ND, not determined; NT, nontypeable due to insufficient material.
A P type could be assigned to the viruses in all but 1 of the 322 specimens. P[4], P[6], and P[8] types were found in 171 (53.1%), 65 (20.2%), and 69 (21.4%) samples, respectively (Table 1). Types P[9] and P[10] were found in only one sample each. Mixed infections were found in 14 samples (constituting 4.4% of the total): either P[4] and P[6] or P[4] and P[8]. An exception was a mixture of types P[6] and P[8], which was found in one sample. Types P[4] and P[8] have been the predominant P types involved in human rotavirus infections worldwide (5, 16). However, a high incidence of type P[6] strains, as seen in this study, was also reported among infants with diarrhea in Nigeria, India, and Brazil (1, 12, 16). An incidence of type P[6] comparable to that found in this study was also reported in the study previously conducted in South Korea (15). In this study, types P[9] and P[10] were found for the first time in Korea.
Global surveys have indicated that G1P[8], G2P[4], G3P[8], and G4P[8] are the G- and P-type combinations most commonly found in humans (5). The major G- and P-type combinations identified in this study were G2P[4] (147 cases; 45.7%) and G1P[8]) (15.0%). Combinations with a noticeable incidence were G1P[6] (11.2%), G4P[6] (5.6%), and G4P[8] (5.3%). The other combinations of minor frequency were G1P[4](2.9%), G2P[6] (1.3%), G2P[8] (1.3%), and G4P[4] (1.9%). G3P[8], G1P[9], G2P[10], and G9P[6] were found in only one sample each. Genomic reassortment has been postulated to be the main cause of human rotavirus diversity (3). The mixed infections, which probably occur more frequently in populated urban areas, for instance, would facilitate reassortment. Certainly, the emergence of genotypes with atypical combinations, as observed in this study, is consistent with the notion that reassortment occurs in a biased manner.
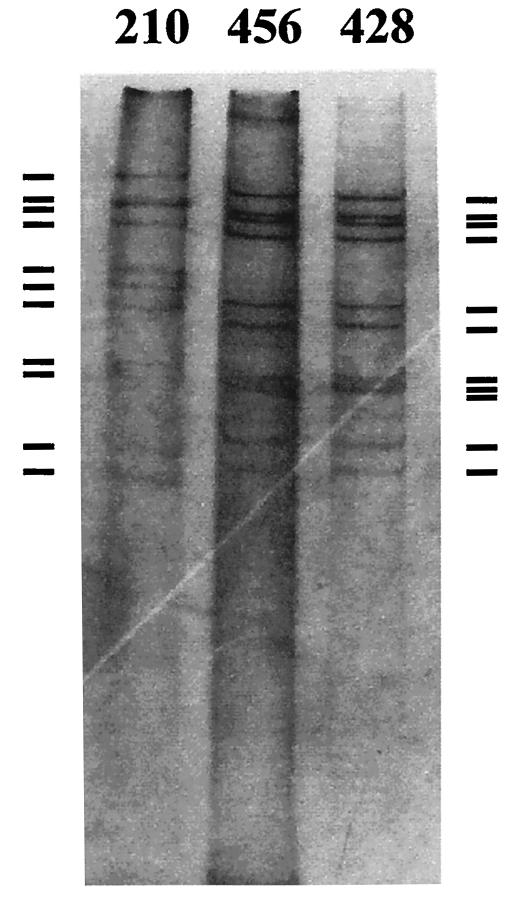
For electropherotype analysis viral RNA was heat denatured and separated in 10% polyacrylamide gels at 20 mA and 75 V for 5 h and stained with silver nitrate (6a). The 4-2-3-2 band pattern characteristic of the group A rotavirus was seen in 239 specimens (among the 322 specimens), comprising 145 (60.7%) samples with short electrophoretic profiles and 94 (39.3%) samples with long electrophoretic profiles, while the electrophoretic profiles could not be determined for 8 samples, as the two smallest RNA segments were not clearly visible, probably due to insufficient material (Table 2). Consistent with the widely accepted notion that type G1 is strongly associated with the long electrophoretic pattern and that type G2 is associated with the short electrophoretic pattern (17), we observed long electrophoretic patterns mostly for type G1 strains (and also for type G4 strains) and short electrophoretic patterns for the majority of type G2 strains. However, a significant portion of G1 strains (12.5%) and some G2 strains (2.3%) showed electrophoretic patterns contrary to this general notion. Interestingly, one specimen in this study displayed an RNA mobility pattern characteristic of that of group C rotaviruses (Fig. 1, specimen 210) and distinctive from that of group A rotaviruses (Fig. 1, specimens 456 and 428, which have the short electrophoretic types). No group C rotavirus has been reported in South Korea, although group C rotaviruses are thought to be endemic among humans throughout the world.
TABLE 2.
Electropherotypes of human rotaviruses found in South Korea (2000 to 2001)
| G type | P type | No. (%) of isolates with the following electrophoretic pattern:
|
||
|---|---|---|---|---|
| Short | Long | NTa | ||
| G1b | P[4] | 1 | 5 | 1 |
| P[6] | 1 | 23 | 5 | |
| P[8] | 7 | 31 | ||
| P[9] | 1 | |||
| P[4]/P[6] | 1 | |||
| P[4]/P[8] | 2 | |||
| G2c | P[4] | 120 | 3 | 2 |
| P[6] | 3 | |||
| P[10] | 1 | |||
| P[4]/P[6] | 1 | |||
| P[4]/P[8] | 2 | |||
| NT | 1 | |||
| G3 | P[4] | 1 | ||
| P[8] | 1 | |||
| G4 | P[6] | 2 | 7 | |
| P[8] | 1 | 16 | ||
| G9 | P[6] | 1 | ||
| G1-G4 | P[6] | 2 | ||
| P[6]/P[8] | 1 | |||
| G2-G4 | P[4] | 1 | ||
| NT | P[4] | 2 | 1 | |
| Total | 145 (60.7) | 94 (39.3) | 8 | |
NT, nontypeable due to insufficient material.
Among the type G1 isolates, 9 (12.5%) had the short electrophoretic pattern, 63 (87.5%) had the long electrophoretic pattern, and 6 were nontypeable.
Among the type G2 isolates, 128 (97.7%) had the short electrophoretic pattern, 3 (2.3%) had the long electrophoretic pattern, and 2 were nontypeable.
FIG. 1.
Gelelectrophoresis showing the double-stranded RNA patterns of a putative group C rotavirus (lane 210) and two group A rotaviruses (lanes 456 and 428). The viral RNA was separated in a 10% polyacrylamide gel and visualized by silver staining. The bars on the left and right indicate the double-stranded RNA patterns of group C and group A, respectively, of human retrovirus.
The three diagnostic methods that we used in this study are among the assays that are the most frequently used worldwide for rotavirus detection. Although amplification of the VP4 and VP7 gene sequences by RT-PCR was the most sensitive of the three methods (322 of 607 specimens were positive, whereas 307 specimens were positive by ELISA and 306 were positive by the latex agglutination assay), there were certainly overlaps and discordances between the methods. For instance, 26 of the 322 PCR-positive samples were negative by the ELISA, while 52 were negative by the latex agglutination method. Of the 285 PCR-negative specimens, 11 were positive by the ELISA while 36 were negative by the latex agglutination assay.
This is the most recent nationwide surveillance study involving the genotyping of rotaviruses circulating in South Korea. We have not noticed any significant regional or seasonal variations in the prevalent rotavirus types. Further monitoring of the prevalent types of rotaviruses on a global scale as well as a local scale is necessary to understand the dynamic nature of viral transmission.
REFERENCES
- 1.Adah, M. I., A. Rohwedder, O. D. Olaleye, O. A. Durojaiye, and H. Werchau. 1997. Further characterization of field strains of rotavirus from Nigeria VP4 genotype P6 most frequently identified among symptomatically infected children. J. Trop. Pediatr. 43:267-274. [DOI] [PubMed] [Google Scholar]
- 2.Armah, G. E., A. D. Steele, F. N. Binka, M. D. Esona, R. H. Asmah, F. Anto, D. Brown, J. Green, F. Cutts, and A. Hall. 2003. Changing pattern of rotavirus genotypes in Ghana: emergence of human rotavirus G9 as a major cause of diarrhea in children. J. Clin. Microbiol. 41:2317-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunliffe, N. A., J. S. Bresee, J. R. Gentsch, R. I. Glass, and C. A. Hart. 2002. The expanding diversity of rotaviruses. Lancet 359:640-642. [DOI] [PubMed] [Google Scholar]
- 4.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gentsch, J. R., P. A. Woods, M. Ramachandran, B. K. Das, J. P. Leite, A. Alfieri, R. Kumar, M. K. Bhan, and R. I. Glass. 1996. Review of G and P typing results from a global collection of rotavirus strains: implications for vaccine development. J. Infect. Dis. 174(Suppl. 1):S30-S36. [DOI] [PubMed] [Google Scholar]
- 6.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z. Y. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.Herring, A. J., N. F. Inglis, C. K. Ojeh, and D. R. Snodgrass. 1982. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J. Clin. Microbiol. 16:473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iturriza-Gomara, M., B. Isherwood, U. Desselberger, and J. Gray. 2001. Reassortment in vivo: driving force for diversity of human rotavirus strains isolated in the United Kingdom between 1995 and 1999. J. Clin. Microbiol. 75:3696-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapikian, A., Z. Y. Hoshino, and R. M. Chanock. 1996. Rotaviruses, p. 1787-1833. In Field's virology, vol. 2, 4th ed. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 9.Nguyen, V. M., V. T. Nguyen, P. L. Huyuh, D. T. Dang, T. H. T. Nguyen, V. T. Phan, T. L. Nguyen, T. L. Le, B. Ivanoff, J. R. Gentsch, and R. I. Glass. 2001. The epidemiology and disease burden of rotavirus in Vietnam: sentinel surveillance at 6 hospitals. J. Infect. Dis. 183:1707-1712. [DOI] [PubMed] [Google Scholar]
- 10.O'Mahony, J., B. Foley, S. Morgan, J. G. Morgan, and C. Hill. 1999. VP4 and VP7 genotyping of rotavirus samples recovered from infected children in Ireland over a 3-year period. J. Clin. Microbiol. 37:1699-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palombo, E. A. 2002. Genetic analysis of group A rotaviruses: evidence for interspecies transmission of rotavirus genes. Virus Genes 24:11-20. [DOI] [PubMed] [Google Scholar]
- 12.Ramachandran, M., B. K. Das, A. Vij, R. Kumar, S. S. Bhambal, N. Kesari, H. Rawat, L. Bahl, S. Thakur, P. A. Woods, R. I. Glass, M. K. Bhan, and J. R. Gentsch. 1996. Unusual diversity of human rotavirus G and P genotypes in India. J. Clin. Microbiol. 34:436-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santos, N., E. M. Volotao, C. C. Soares, M. C. M. Albuquerque, F. M. da Silva, T. R. B. de Carvalho, C. F. A. Pereira, V. Chizhikov, and Y. Hoshino. 2001. Rotavirus strains bearing genotype G9 or P[9] recovered from Brazilian children with diarrhea from 1997 to 1999. J. Clin. Microbiol. 39:1157-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo, J. K., and J. G. Sim. 2000. Overview of rotavirus infections in Korea. Pediatr. Int. 42:406-410. [DOI] [PubMed] [Google Scholar]
- 15.Song, M. O., K. J. Kim, S. I. Chung, I. Lim, S. Y. Kang, C. N. An, and W. Kim. 2003. Distribution of human group A rotavirus VP7 and VP4 types circulating in Seoul, Korea between 1998 and 2000. J. Med. Virol. 70:324-328. [DOI] [PubMed] [Google Scholar]
- 16.Souza, M. B., M. L. Racz, J. P. Leite, C. M. Soares, R. M. Martins, V. Munford, and D. D. Cardoso. 2003. Molecular and serological characterization of group A rotavirus isolates obtained from hospitalized children in Goiania, Brazil, 1998-2000. Eur. J. Clin. Microbiol. 22:441-443. [DOI] [PubMed] [Google Scholar]
- 17.Trabelsi, A., I. Peenze, C. Pager, M. Jeddi, and D. Steele. 2000. Distribution of rotavirus VP7 serotypes and VP4 genotypes circulating in Sousse, Tunisia, from 1995 to 1999: emergence of natural human reassortants. J. Clin. Microbiol. 38:3415-3419. [DOI] [PMC free article] [PubMed] [Google Scholar]