Abstract
We evaluated a two-rapid-test serial algorithm using the Determine and Genie II rapid assays, performed on-site in four peripheral laboratories during the French Agence Nationale de Recherches sur le SIDA (ANRS) 1201/1202 Ditrame Plus cohort developed for prevention of mother-to-child transmission of human immunodeficiency virus (HIV) infection in Côte d'Ivoire. A total of 1,039 specimens were retested by two commercial enzyme-linked immunosorbent assays (ELISAs). The following specimens were tested: 315 specimens found on-site to be infected with HIV type 1 (HIV-1), 8 specimens found on-site to be infected with HIV-2, 71 specimens found on-site to be infected with both HIV-1 and HIV-2, 40 specimens found on-site to have indeterminate results for HIV infection, and 605 specimens taken during a quality assurance program. For HIV discrimination, 99 positive serum samples (20 with HIV-1, 8 with HIV-2, and 71 with HIV-1 and HIV-2 on the basis of our rapid test algorithm) were retested by the Peptilav test, Western blot (WB) assays, and homemade monospecific ELISAs. Real-time DNA PCRs for the detection of HIV-1 and HIV-2 were performed with peripheral blood mononuclear cells from 35 women diagnosed on-site with HIV-1 and HIV-2 infections. Compared to the results of the ELISAs, the sensitivities of the Determine and Genie II assays were 100% (95% lower limit [95% LL], 99.1%) and 99.5% (95% confidence interval [95% CI], 98.2 to 99.9%), respectively. The specificities were 98.4% (95% CI, 96.9 to 99.3%) and 100% (95% LL, 99.3%), respectively. All serological assays gave concordant results for infections with single types. By contrast, for samples found to be infected with dual HIV types by the Genie II assay, dual reactivity was detected for only 37 samples (52.1%) by WB assays, 34 samples (47.9%) by the Peptilav assay, and 23 samples (32.4%) by the monospecific ELISAs. For specimens with dual reactivity by the Genie II assay, the rates of concordance between the real-time PCR assays and the serological assays were 25.7% for the Genie II assay, 82.9% for the Peptilav assay, 74.3% for WB assays, and 80% for the homemade ELISAs. Our algorithm provided high degrees of sensitivity and specificity comparable to those of ELISAs. Even if they are rare, women identified by the Genie II assay as being infected with HIV-1 and HIV-2 mostly appeared to be infected only with HIV-2.
Human immunodeficiency virus (HIV) antibody testing is a critical step that allows the implementation of effective prevention and care interventions in HIV-infected individuals. Simple voluntary counseling and testing (VCT) approaches are increasingly required, especially in situations in which the rapid identification of HIV infection is warranted, such as in pregnant women during gestation and in the peripartum period (3, 6, 13). For instance, among pregnant women attending antenatal clinics in Côte d'Ivoire, among whom the prevalence of HIV infection is estimated to be 10%, the increasing implementation of low-cost interventions to reduce mother-to-child transmission (MTCT) with short antiretroviral regimens has created new demands for VCT (22).
For this purpose, the use of standard enzyme-linked immunosorbent assays (ELISAs), designed for batch testing, followed by confirmatory Western blot (WB) tests, if necessary, is now considered time- and money-consuming (5, 23). Sophisticated equipment (such as automatic pipettes, incubators, washers, and readers) must be available, is costly to purchase and maintain, and must be located near clean water and a reliable supply of electricity. The validity of the results obtained by these techniques strongly depends on the skills of the technicians, and their interpretation requires skills training and supervision. These conditions are often lacking in sub-Saharan Africa, at least in district-level hospitals. Finally, given the important delay between HIV antibody testing by standard procedures and the availability of results, a significant number of people do not return for posttest counseling (21).
To face this challenge, about 5 years ago the World Health Organization and the U.S. Centers for Disease Control and Prevention recommended the use of simple and rapid assays in resource-limited settings since their operational characteristics make them more suitable than ELISAs (4, 34). Indeed, most of these assays, which mainly use flowthrough or immunochromatographic membranes and which are presented in kit form, do not require either equipment or refrigeration. The procedures are very easy to perform, and their formats allow persons with minimal instruction and training to perform them correctly (14, 28). A result can be read visually within a few minutes. Even if the cost of these diagnostic procedures remains higher than $1 per test, their cost-effectiveness is better than those of ELISAs in situations in which small numbers of tests are carried out at one time. However, the field performance of these alternative rapid assays is still poorly documented (20, 24, 25, 29, 31). Most of the published evaluations, including those performed in Africa (1, 2, 13, 18, 30), were done retrospectively in reference laboratories with stored plasma and/or with limited numbers of samples taken prospectively. Additionally, it must be pointed out that these evaluations must take into account two supplementary challenges in Africa. First, given rapid assays were initially developed for the detection of B-subtype antibodies, the predominance in Africa of HIV type 1 (HIV-1) non-B subtypes poses another diagnostic problem. In Côte d'Ivoire, CRF02 is the most prevalent strain (M.-L. Chaix, C. Montcho, D. Ekouevi, F. Rouet, L. Bequet, I. Viho, P. Fassinou, C. Welffens-Ekra, V. Leroy, F. Dabis, and C. Rouzioux, Abstr. 11th Conf. Retrovir. Opportunist. Infect., abstr. 657, 2004). Second, both HIV-1 and HIV-2 infections exist in West Africa. Thus, the type-specific diagnosis of HIV-1 and HIV-2 infections and dual HIV-1 and HIV-2 infections is required, particularly because HIV-2 is intrinsically resistant to nonnucleoside reverse transcriptase inhibitors (32), such as nevirapine (NVP), a drug that is largely used for the prevention of MTCT (PMTCT) of HIV-1 (15) but that is inactive against HIV-2.
The main goal of the present prospective survey was to validate the use by peripheral laboratories of a two-rapid-test serial algorithm for the screening of HIV infection among pregnant women participating in the French Agence Nationale de Recherches sur le SIDA (ANRS) 1201/1202 Ditrame Plus program, which is evaluating the effectiveness of a short course of zidovudine (ZDV) and intrapartum NVP for the PMTCT of HIV-1 in Abidjan, Côte d'Ivoire (West Africa) (F. Dabis, D. K. Ekouevi, L. Bequet, F. Rouet, A. Horo, P. Fassinou, L. Dequae Merchadou, and V. Leroy, Abstr. 10th Conf. Retrovir. Opportunist. Infect., abstr. 854, 2003).
MATERIALS AND METHODS
Study population and samples.
The study population consisted of pregnant women participating in the ANRS 1201/1202 Ditrame Plus cohort and enrolled in Abidjan from March 2001 to August 2002 (Dabis et al., Abstr. 10th Conf. Retrovir. Opportunist. Infect., abstr. 854, 2003). Eligible women were aged 18 years or older. Consenting women diagnosed with HIV-1 or dual HIV-1 and HIV-2 infections and informed of their serological status were included during prenatal visits, as soon as possible after their first booking (≥36 weeks of gestation). The phylogenetic analysis of HIV-1 strains revealed that women were mostly (∼80%) infected with non-subtype B strains of subtype CRF02 (Chaix et al., Abstr. 11th Conf. Retrovir. Opportunist. Infect., abstr. 657, 2004). This project was approved by the national ethics committees of the AIDS Control Program of Côte d'Ivoire. The diagnosis of HIV infection was based on rapid HIV screening procedures (see “Laboratory methods” section) performed in the laboratories with limited resources of four antenatal clinics located in Abidjan (Avocatier, Anonkouakouté, Sagbé, and Abobo-Sud). One day of training for laboratory personnel was provided at each site by the reference laboratory (Centre de Diagnostic et de Recherches sur le SIDA [CeDReS], CHU Treichville, Abidjan, Côte d'Ivoire). Quality assurance measures included refrigerator and room temperature monitoring, as well as unscheduled visits to review laboratory procedures and records, at least once a year for each laboratory.
The present study was carried out between March 2001 and February 2002 in order to validate the first 10,000 consecutive results obtained in local laboratories by rapid HIV assays. Thus, serum samples were prospectively forwarded to CeDReS for supplementary HIV serology testing for this purpose. The serum specimens selected for further analysis were (i) those drawn at preinclusion from all women diagnosed with HIV-1 infection in local laboratories, (ii) those drawn during VCT from all women diagnosed on-site with HIV-2 infection or dual HIV-1 and HIV-2 infection, (iii) those obtained from women who were initially diagnosed in local laboratories with indeterminate HIV infection and who could be monitored and retested, and (iv) those taken during VCT by way of a quality assurance program (10 to 15 specimens/month/site). Finally, all available peripheral blood mononuclear cells (PBMCs) from women diagnosed on-site with dual HIV-1 and HIV-2 infections and preincluded in the ANRS 1201/1202 Ditrame Plus program were stored at CeDReS at −80°C for further molecular analysis.
Laboratory methods. (i) On-site rapid HIV antibody testing algorithm.
Rapid HIV screening procedures were performed in local laboratories according to a serial testing algorithm with two distinct rapid HIV assays, the Determine HIV-1/2 assay (the Determine assay; Abbott Laboratories, Abbott Park, Ill.) and the Genie II HIV-1/HIV-2 assay (the Genie II assay; Bio-Rad, Marnes-La-Coquette, France). According to World Health Organization recommendations, in settings with limited resources and with an HIV prevalence of about 10% (such as Côte d'Ivoire), the use of serial algorithms, which are about 2.5-fold less costly than parallel algorithms, is advised (34). The Determine assay is an immunochromatographic rapid test for the qualitative detection of HIV-1 and HIV-2 (2) and uses a nitrocellulose strip with HIV-1 and HIV-2 antigens. The results presented on the strips are interpreted visually as red lines. The Genie II test is a dual-recognition rapid enzyme immunoassay and is based on the specific detection of anti-HIV-1 and anti-HIV-2 antibodies by antigens that bind to both antibody binding sites (29). The results are also interpreted visually as gray-blue spots.
Sera that were reactive by the Determine test were then tested by the Genie II assay, which allowed differentiation between HIV-1 and HIV-2. If the assays were concordantly positive (for HIV-1, HIV-2, or HIV-1 and HIV-2), the results were considered true positive and were used for posttest counseling. Sera that reacted negatively by the Determine test were considered true HIV negative and were not further investigated, whereas those that were reactive by the Determine test but negative by the Genie II test were considered indeterminate.
(ii) Supplementary HIV assays. (a) Screening ELISAs.
Screening for HIV infection was performed at CeDReS by the combined use of two third-generation HIV ELISAs: the Vironostika HIV Uni-Form II plus O assay (Organon Teknika, Boxtel, The Netherlands) and the Murex HIV-1.2.O assay (Abbott Laboratories). Each assay was carried out as recommended by the manufacturers. The results of this ELISA-based algorithm were used as the “gold standard” for evaluation of the sensitivities and the specificities of the rapid assays. Sera concordantly reactive or nonreactive by the Organon Teknika and Murex ELISAs were considered true positive or true negative, respectively. Sera with discordant results were considered indeterminate.
(b) Differentiation assays.
Two types of differentiation assays were performed: serological assays and real-time PCR assays. All serological assays for differentiation between HIV-1 and HIV-2 except the home-made HIV-1 and HIV-2 synthetic peptide ELISAs were carried out at CeDReS. The home-made HIV-1 and HIV-2 synthetic peptide ELISAs were performed in a French virology laboratory by F. Simon. HIV-1, HIV-2, and HIV-1-HIV-2 infections were discriminated by five distinct tests: (i) three commercial assays, including the Peptilav 1-2 assay (Bio-Rad) and New LAV blot I and II assays (Bio-Rad), and (ii) and two home-made indirect ELISAs with synthetic peptides that map the gp41/36 region (detection component) and the V3 loop region (differentiation component) of HIV-1 and HIV-2 (26); all samples with an optical density (OD) ≥0.3 (cutoff) were considered reactive.
HIV-1 and HIV-2 proviral DNA real-time PCR assays were used at CeDReS as the reference methods for differentiation between HIV-1 and HIV-2, and the results were compared with those of the five discriminatory serological assays mentioned above. These two real-time PCR tests were chosen to amplify a region with a low degree of variability in the long terminal repeat (LTR) gene of the HIV-1 and HIV-2 genomes, respectively. PBMCs were isolated from EDTA-treated blood samples by Ficoll density gradient centrifugation. After cell lysis by proteinase K, DNA was extracted by using the Qiagen procedure (QIAamp DNA blood minikit; Qiagen S.A., Courtaboeuf, France). The concentrations of the DNA extracts were determined by spectrometry at 260 nm. For each specimen, 2 μg of DNA was used. The forward and reverse primers used for amplification of HIV-1 proviral were primer NEC 152 (5′-GCCTCAATAAAGCTTGCCTTGA-3′) and primer NEC 131 (5′-GGCGCCACTGCTAGAGATTTT-3′). The internal HIV-1 TaqMan probe NEC LTR (5′-AAGTAGTGTGTGCCCGTCTGTTRTKTGACT-3′, where K is G+T and R is G+A) carried a 5′ reporter dye, 6-carboxyfluorescein (FAM), and a 3′ quencher dye, 6-carboxytetramethylrhodamine (TAMRA). The sequences of the forward primer (primer Fi2), the reverse primer (primer Da2), and the probe (probe LTR2) for HIV-2 proviral DNA were 5′-AGCAGGTAGAGCCTGGGTGTT-3′, 5′-TCTTTAAGCAAGCAAGCGTGG-3′, and 5′-R-CTTGGCCGGTGCTGGGCAGA-Q-3′ (where R is FAM and Q is TAMRA), respectively. The sensitivities of the two real-time PCRs were validated for each run by using calibrated DNAs from 8E5 cells as HIV-1-positive controls, with a detection level of 10 copies of proviral DNA per 106 cells, and from a homemade HIV-2-infected cell line (called G) as HIV-2-positive controls (mean cycle threshold for the last dilution, 40.8). All runs were also done with negative controls. For both HIV-1 and HIV-2 DNA amplifications, after 1 cycle at 50°C for 2 min and 1 cycle at 95°C for 10 min, a two-step PCR procedure consisting of 15 s at 95°C and 1 min at 60°C for 50 cycles was used. Amplification and data acquisition were carried out with the ABI Prism 7000 sequence detection system (Perkin-Elmer Applied Biosystems, Foster City, Calif.).
Statistical analysis.
The sensitivity and the specificity of the rapid HIV assays were calculated by using the results of the two ELISAs as the gold standard. The sensitivity of each rapid assay was calculated as the number of positive test results obtained on-site divided by the total number of HIV antibody-positive samples obtained by the ELISAs at CeDReS. The specificity was calculated as the number of negative test results obtained on-site divided by the total number of HIV antibody-negative samples obtained by the ELISAs at CeDReS. The corresponding positive and negative predictive values were also determined. The interlaboratory reproducibility of the Determine assay between the on-site laboratories and CeDReS was investigated. Exact binomial 95% confidence intervals (CIs) were calculated for these estimates. Proportions were compared by the χ2 test or Fisher's exact test, if appropriate. The degree of agreement between the serological assays discriminating HIV-1 and HIV-2 was assessed by the use of kappa statistics, with values greater than 0.75 indicating excellent agreement, values between 0.40 and 0.75 indicating fair to good agreement, and values less than 0.40 indicating poor agreement (19). All statistical analyses were performed with Stata software (version 6.0; Stata Corporation, College Station, Tex.).
RESULTS
On-site results of rapid HIV antibody detection assays.
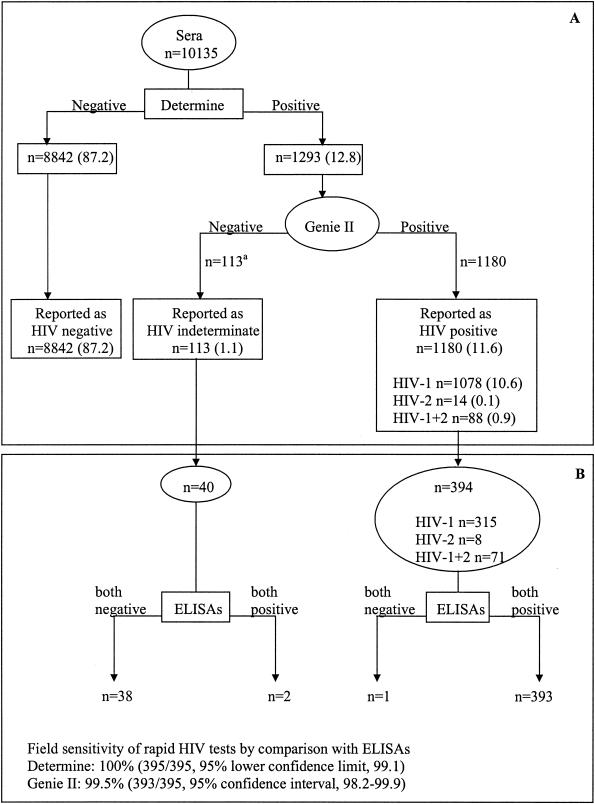
As shown in Fig. 1A, from March 2001 to February 2002, 10,135 serum samples were screened by the Determine rapid assay. A total of 1,293 (12.8%) samples were reactive and further tested by the Genie II assay. Of those specimens initially reactive, 1,180 (11.6%) were also HIV antibody positive by the Genie II assay, which differentiated 1,078 (10.6%) HIV-1-positive samples, 14 (0.1%) HIV-2-positive samples, and 88 (0.9%) samples dually reactive for HIV-1 and HIV-2. Among the remaining 113 (1.1%) samples not confirmed to be positive by the Genie II test, most of them (>90%) exhibited a weak HIV band by the Determine assay. No significant differences in the prevalences of positive and indeterminate HIV results were observed between the four local sites (P = 0.76 and P = 0.48, respectively).
FIG. 1.
(A) Rapid HIV testing results obtained in four local laboratories by using a serial algorithm for 10,135 serum samples from pregnant women participating in the ANRS 1201/1202 Ditrame Plus program from March 2001 to February 2002. The breakdowns of sample numbers and sources are as follows: n = 4,494 (49.2%) samples from the Anonkouakouté antenatal clinic (site 1), 2,409 (23.8%) samples from Sagbé (site 2); 2,258 (22.3%) samples from Avocatier (site 3), and 474 (4.7%) samples from Abobo-Sud (site 4). a, including 104 samples with a weak HIV band by the Determine assay. (B) HIV ELISA results obtained for 434 serum samples in the reference laboratory (CeDReS) by using a combination of two ELISAs; 315 samples were taken at preinclusion and were found at the peripheral laboratories to be infected with HIV-1; 8 and 71 samples were drawn during VCT from women diagnosed on-site as being infected with HIV-2 and both HIV-1 and HIV-2, respectively; and 40 were taken during the monitoring of women with an indeterminate diagnosis on-site.
Evaluation of the performance of rapid HIV antibody detection assays. (i) Sensitivity.
As shown in Fig. 1B, a total of 434 serum samples could be retested by the ELISAs, including 394 specimens from women in peripheral laboratories with a diagnosis of HIV infection and 40 from women who had an indeterminate diagnosis and who could be retested after a mean duration of 15 days. Overall, HIV antibodies were detected by both ELISAs in 395 samples, including 393 samples that tested HIV positive by rapid assays and 2 samples found to have indeterminate results, i.e., Determine assay positive and Genie II assay negative. Thus, compared to the ELISAs, the field sensitivities of the Determine and the Genie II assays were 100% (395 of 395 samples; 95% lower confidence limit, 99.1%) and 99.5% (393 of 395 samples; 95% CI, 98.2 to 99.9%), respectively. One sample found to be HIV-1 positive on-site was clearly nonreactive by the ELISAs. Finally, the two samples found to have indeterminate results in peripheral laboratories and positive by both ELISAs exhibited indeterminate WB profiles (one isolated weak gp160 band and a gp160 [weak], p55 [weak], and p24 pattern, respectively).
(ii) Quality assurance program.
Overall, 605 serum samples tested on-site were randomly selected and shipped to CeDReS. The field sensitivities and negative predictive values of the rapid assays were 100% compared to the results of the ELISAs (Table 1). HIV antibodies were detected by both the Determine and the Genie II assays performed at the local laboratories in all 49 samples that also tested positive by the HIV ELISAs carried out at CeDReS. The field specificity and positive predictive value of the Determine assay were 98.4% (95% CI, 96.9 to 99.3%) and 84.5% (95% CI, 72.6 to 92.7%), respectively. Both of these values were 100% for the Genie II rapid test (Table 1). No specimen tested positive by the ELISAs and negative by the Determine assay. Among the 556 samples negative by the HIV ELISAs, 9 were positive by the Determine assay (including 5 samples with a weak band). Overall, of these 605 specimens, 345 were retested at CeDReS by the Determine assay. The interlaboratory reproducibility of this test was 99.4% (95% CI, 97.9 to 99.9%) (Table 1). Two samples considered positive by one local laboratory were found to be negative at CeDReS.
TABLE 1.
Quality control results for serological screening for HIV infection with 605 samples from the ANRS 1201/1202 Ditrame Plus program, Abidjan, Côte d'Ivoirea
| Assay | Results at CeDReS
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity
|
Specificity
|
Predictive values
|
Reproducibility
|
|||||||
| Negative
|
Positive
|
|||||||||
| n/Nb | % (95% LCL) | n/Nc | % (95% CI) | n/N | % (95% LCL) | n/N | % (95% CI) | n/Nd | % (95% CI) | |
| Determine | 49/49 | 100 (92.7) | 547/556 | 98.4 (96.9-99.3) | 547/547 | 100 (99.3) | 49/58 | 84.5 (72.6-92.7) | 343/345 | 99.4 (97.9-99.9) |
| Genie II | 49/49 | 100 (92.7) | 556/556 | 100.0 (99.3) | 556/556 | 100 (99.3) | 49/49 | 100.0 (92.7) | ND | ND |
The sensitivities, specificities, and reproducibilities of two rapid assays carried out in four peripheral laboratories were determined by comparison of the results with those obtained in a reference laboratory (CeDReS). Abbreviations: ND, not done; 95% LCL, 95% lower confidence limit; 95% CI, 95% confidence interval.
n, number of samples positive by using rapid assays in local laboratories; N, number of samples positive by the two ELISAs at CeDReS.
n, number of samples negative by using rapid assays in local laboratories; N, number of samples negative by the two ELISAs at CeDReS.
n, number of samples with concordant results by the Determine assay at local laboratories; N, number of samples with concordant results by the Determine assay at CeDReS.
(iii) Differentiation between HIV-1 and HIV-2. (a) Serological assays.
On the basis of the results obtained in the local laboratories by the Genie II assay, 20 serum samples (randomly selected) positive for HIV-1, 8 serum samples positive for HIV-2, and 71 serum samples positive for both HIV-1 and HIV-2 were further analyzed by the Peptilav assay, WB assays for HIV-1 and HIV-2, and synthetic peptide ELISAs for HIV-1 and HIV-2. For samples infected with single HIV types (either HIV-1 or HIV-2), the results of all assays mentioned above were concordant with the results obtained by the Genie II assay. In contrast, for the 71 samples infected with both HIV-1 and HIV-2 by the Genie II assay, dual reactivity by the WB assays, the Peptilav assay, and the synthetic peptide ELISAs was detected in only 37 (52.1%), 34 (47.9%), and 23 (32.4%) specimens, respectively. The 23 samples infected with both HIV-1 and HIV-2 as determined by the ELISAs showed high ODs during screening (median OD, >2.0) and differentiation (median OD, >1.5). The remaining samples were mostly found to be infected with HIV-2 by both WB assays (n = 30), the Peptilav assay (n = 35), and the ELISAs (n = 38). Finally, the synthetic peptide ELISAs detected 10 samples with HIV-1 infections (compared with the detection of HIV-1 infections in 2 and 4 of the 10 samples by the Peptilav and WB assays, respectively). Overall, the level of agreement between the homemade ELISAs and the Peptilav assay was better than that observed between the homemade ELISAs and the WB assays (kappa values = 0.73 and 0.67, respectively).
(b) Real-time PCR assays.
HIV-1 and HIV-2 proviral DNA detection was performed for 35 women preincluded in the ANRS 1201/1202 Ditrame Plus cohort and found on-site to be infected with both HIV-1 and HIV-2 by the Genie II assay (Table 2). Nine of those samples were PCR positive for both HIV-1 and HIV-2 (including two specimens, specimens 1 and 21, negative for HIV-2 only by the homemade ELISAs). In addition, in concordance way with the results of all serological assays except the Genie II assay, 14 samples were PCR positive only for HIV-2 and two samples were PCR positive only for HIV-1. Furthermore, five samples were reactive for HIV-2 (n = 3) or HIV-1 (n = 2) by both real-time PCR assays and the homemade ELISAs. Finally, five women were negative for HIV-2 provirus amplification, whereas the samples from these women showed serological reactivity with HIV-2; two of them (samples 6 and 32) exhibited very weak HIV-2 V3 reactivity (OD, <1) by the homemade ELISAs; one of them (sample 4) exhibited a very low CD4+-cell count (22 cells/mm3). Overall, for these 35 specimens, the rates of concordance between the real-time PCR assays and the serological assays were 25.7% (9 of 35) for the Genie II assay, 82.9% (29 of 35) for the Peptilav assay, 74.3% (26 of 35) for the WB assays, and 80% (28 of 35) for the homemade ELISAs.
TABLE 2.
Serological, virological, and immunological characteristics of the 35 women preincluded in the ANRS 1201/1202 Ditrame Plus program and diagnosed on-site with dual HIV-1 and HIV-2 infections by the Genie II assay
| Patient no. | Serological results by:a
|
HIV-1 and HIV-2 proviral DNA detected by real-time PCRsa | CD4+-T-cells
|
||||
|---|---|---|---|---|---|---|---|
| Genie II assay | Peptilav assay | WB assays for HIV-1 and -2 | Homemade EIAs (gp41/36 and V3) | % | No. of cells/mm3 | ||
| 10 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 32 | 466 |
| 11 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 24.3 | 561 |
| 16 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 7 | 169 |
| 19 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 16.8 | 379 |
| 26 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 36.4 | 530 |
| 33 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 25.6 | 506 |
| 34 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 14.5 | 198 |
| 1 | 1 + 2 | 1 + 2 | 1 + 2 | 1 | 1 + 2 | 15.5 | 165 |
| 21 | 1 + 2 | 1 + 2 | 1 + 2 | 1 | 1 + 2 | 44.4 | 1,038 |
| 7 | 1 + 2 | 2 | 2 | 2 | 2 | 34 | 532 |
| 8 | 1 + 2 | 2 | 2 | 2 | 2 | 42.9 | 645 |
| 12 | 1 + 2 | 2 | 2 | 2 | 2 | 32.9 | 688 |
| 13 | 1 + 2 | 2 | 2 | 2 | 2 | 40.4 | 664 |
| 14 | 1 + 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| 15 | 1 + 2 | 2 | 2 | 2 | 2 | 46.8 | 724 |
| 17 | 1 + 2 | 2 | 2 | 2 | 2 | 46.7 | 1,062 |
| 22 | 1 + 2 | 2 | 2 | 2 | 2 | 42.4 | 1,213 |
| 24 | 1 + 2 | 2 | 2 | 2 | 2 | 28.1 | 451 |
| 25 | 1 + 2 | 2 | 2 | 2 | 2 | 35.5 | 457 |
| 27 | 1 + 2 | 2 | 2 | 2 | 2 | 48.3 | 1,056 |
| 28 | 1 + 2 | 2 | 2 | 2 | 2 | 20.2 | 504 |
| 31 | 1 + 2 | 2 | 2 | 2 | 2 | 39.9 | 555 |
| 35 | 1 + 2 | 2 | 2 | 2 | 2 | 23.6 | 338 |
| 9 | 1 + 2 | 1 | 1 | 1 | 1 | 24.5 | 270 |
| 23 | 1 + 2 | 1 | 1 | 1 | 1 | 22.7 | 392 |
| 18 | 1 + 2 | 2 | 1 + 2 | 2 | 2 | 31.7 | 514 |
| 20 | 1 + 2 | 2 | 1 + 2 | 2 | 2 | 33 | 416 |
| 3 | 1 + 2 | 1 + 2 | 1 + 2 | 2 | 2 | 37.5 | 574 |
| 30 | 1 + 2 | 1 + 2 | 1 | 1 | 1 | 23.3 | 326 |
| 5 | 1 + 2 | 1 + 2 | 1 + 2 | 1 | 1 | 19.8 | 330 |
| 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 | 26.5 | 445 |
| 4 | 1 + 2 | 1 + 2 | 1 + 2 | 1 + 2 | 1 | 3.9 | 22 |
| 6 | 1 + 2 | 2 | 2 | 2 | Both negative | 33.1 | 543 |
| 29 | 1 + 2 | 2 | 2 | 2 | Both negative | 30 | 693 |
| 32 | 1 + 2 | 2 | 2 | 2 | Both negative | 39 | 936 |
1, HIV-1; 2, HIV-2.
DISCUSSION
With the implementation of PMTCT programs with rapid HIV testing in antenatal clinics, women can learn their HIV status quickly and can receive short-course antiretroviral prophylaxis to dramatically reduce the risk of transmitting HIV to their children. Our survey, carried out as part of the ANRS 1201/1202 Ditrame Plus program for PMTCT (Abidjan, Côte d'Ivoire), demonstrated the ability of a serial algorithm to function accurately under actual West African field conditions in the context of the circulation of the predominant subtype, HIV-1 CRF02. Our data confirm and extend the findings of others (1, 2, 17, 18, 29). Despite difficult working conditions in the peripheral laboratories (no air conditioning, frequent power failures, and little equipment), our strategy achieved maximum initial sensitivity with the first-line rapid assay (in our experience, no HIV infection in an HIV-infected woman was missed by using the Determine test) and a high specificity with the second one (the Genie II assay). It seems unlikely that women initially found to have indeterminate results by rapid assays were seroconverters. In fact, they should be reassured early, as they are unlikely to be HIV infected since, in our study, most of them who could be monitored were strictly ELISA negative. As a consequence, we decided to stop the monitoring of these women with indeterminate results (a difficult logistical task) and to assess their HIV antibody status by ELISAs at CeDReS directly with the initial sample with indeterminate results. Finally, during our field evaluation, misclassifications (probably due to technical or labeling errors) were observed very rarely. However, the finding of misclassifications illustrates the need for quality assurance programs in order to perpetuate high performance levels at peripheral sites, in collaboration with a reference laboratory.
Our study revealed the limitation of the use of the Genie II assay in Côte d'Ivoire as a second-line discriminatory rapid test, given its poor performance for the identification of dual HIV-1 and HIV-2 infections. Compared to other discriminatory tests, the Genie II test constituted a reliable tool for the confirmation of infections with a single type but mostly identified women who were infected only with HIV-2 as being infected with both types. Thus, with regard to the homemade ELISA results, the true prevalence of HIV-2 infection reached a rate of 0.5% (instead of the 0.1% rate achieved by the Genie II test), whereas the true prevalence of dual infection declined to 0.2% (instead of the 0.9% prevalence obtained by the Genie II test). This finding has many implications. (i) Given the intrinsic resistance of HIV-2 to NVP, this drug is inactive for PMTCT of HIV-2. In the ANRS 1201/1202 Ditrame Plus cohort, 16 women (of a total of 326, or about 5%) who were initially diagnosed on-site with dual HIV-1 and HIV-2 infections but who were in fact infected only with HIV-2 have been included in this PMTCT program and thus received NVP unnecessarily. For these women, we do not know either the impact of NVP on their own health or that of ZDV to prevent the MTCT of HIV-2, which is known to be quite lower (3 to 4%) than that observed among HIV-1-infected women (10). Since March 2002, we have decided to include in the ANRS 1201/1202 Ditrame Plus program all women with a diagnosis of HIV infection, even those diagnosed with HIV-2 infection only. This group of HIV-2-infected women and neonates does not receive NVP but benefits from ZDV prophylaxis. (ii) A more reliable discriminatory rapid test remains of major concern in our settings (27). At present, we still use the Genie II test at peripheral sites of the ANRS 1201/1202 Ditrame Plus program because patients infected with a single type, HIV-1, represent the vast majority (∼99%) of the cases. However, when an infection with both types is identified by this test, a complementary assay is required. At CeDReS, we use the Peptilav assay, but it remains expensive (about $10 per test). The synthetic peptide strategy based on two homemade ELISAs is much cheaper (less than $1 per test) and appears to be the most cost-effective strategy for HIV type differentiation. Real-time PCR technologies also appeared to be alternative methods for the differentiation of HIV-1 and HIV-2, as has already been demonstrated in France (8, 9, 11) and West African countries, such as Guinea-Bissau (33), Senegal (12), Gambia (16), and Côte d'Ivoire (16). Furthermore, with regard to maternal results (HIV-1, HIV-2, or HIV-1-HIV-2 infection), the appropriate real-time PCR is now selected in our laboratory for the early diagnosis of HIV infection in babies (F. Rouet, N. Coulibaly, D. Ekouevi, M.-L. Chaix, M. Burgard, L. Bequet, F. Dabis, and C. Rouzioux, Abstr. 13th Int. Conf. AIDS STIs Africa, abstr. 879186, 2003).
Our study suffers from several limitations. Some of our results should be considered with caution, due to the small samples sizes. Only one-third of the women with indeterminate results at peripheral laboratories could be monitored and retested, suggesting that HIV seroconversion could not be fully investigated in our survey. The sample size used for the differentiation between HIV-1 and HIV-2 was also very small, limiting the conclusions that can be made from our results. Furthermore, we failed to detect HIV-2 provirus in five maternal samples that were HIV-2 positive with all serological tools. The lack of detectable HIV-2 provirus could be correlated with very low HIV-2 proviral DNA loads or could be due to the lack of detection of particular HIV-2 subtypes. Subtype B strains are frequent in Côte d'Ivoire (7), and some of them could fail to be amplified by our molecular technique.
In conclusion, our study demonstrates that a two-rapid-test serial algorithm routinely performed at peripheral laboratories for a large-scale PMTCT program is highly sensitive and specific for the diagnosis of non-subtype B HIV-1 subtype infections among West African pregnant women. In our setting, when both HIV-1 and HIV-2 are identified by the Genie II rapid test, a supplementary test must be undertaken in a reference laboratory to allow the accurate discrimination between HIV-1 and HIV-2.
Acknowledgments
This work was supported by ANRS (Paris, France) and the French Ministry of Foreign Affairs within Coordinated Action AC12. Didier K. Ekouevi is a fellow of the French Charity Ensemble contre le SIDA (ECS) (Sidaction).
We acknowledge the contributions of the national AIDS authorities in Côte d'Ivoire and women enrolled in the ANRS 1201/1202 Ditrame Plus program.
The ANRS 1201/1202 Ditrame Plus Study Group is organized as follows: principal investigators, INSERM U 593 (ex-330), Université Victor Segalen, Bordeaux, France (F. Dabis, V. Leroy); Centre Hospitalier Universitaire de Yopougon, Abidjan, Côte d'Ivoire (C. Welffens-Ekra, M. Timité-Konan); coordination in Abidjan (Côte d'Ivoire), L. Bequet, D. K. Ekouevi, and I. Viho; clinical team, C. Danel, P. Fassinou, A. Horo, R. Likikouët, H. Touré, C. Amani-Bosse, N. Aka-Akribi, I. Ayekoe, N. Coulibaly, and the Health Centers of Anonkouakouté, Sagbé, Avocatier, Abobo-sud, Niangon-sud, Toit Rouge, and Wassakara; laboratory team, CeDReS, Centre Hospitalier Universitaire de Treichville (A. Inwoley, C. Montcho, F. Rouet); biostatistics and data management, INSERM U 593, Bordeaux (R. Becquet, L. Dequae-Merchadou, C. Sakarovitch, D. Touchard,) and PAC-CI, Abidjan (A. Gerard); psychosocial team, H. Aka-Dogo, A. Desgrées du Lou, and B. Zanou; and scientific committee, S. Blanche, J.-F. Delfraissy, P. Lepage, L. Mandelbrot, C. Rouzioux, and R. Salamon.
REFERENCES
- 1.Andersson, S., Z. da Silva, H. Norrgren, F. Dias, and G. Biberfeld. 1997. Field evaluation of alternative testing strategies for diagnosis and differentiation of HIV-1 and HIV-2 infections in an HIV-1 and HIV-2-prevalent area. AIDS 11:1815-1822. [DOI] [PubMed] [Google Scholar]
- 2.Arai, H., B. Petchclai, K. Khupulsup, T. Kurimura, and K. Takeda. 1999. Evaluation of a rapid immunochromatographic test for detection of antibodies to human immunodeficiency virus. J. Clin. Microbiol. 37:367-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cartoux, M., N. Meda, P. Van de Perre, M. L. Newell, I. de Vicenzi, and F. Dabis. 1998. Acceptability of voluntary HIV testing by pregnant women in developing countries: an international survey. AIDS 12:2489-2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1998. Update: HIV counseling and testing using rapid tests—United States, 1995. Morb. Mortal. Wkly. Rep. 47:211-215. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1990. Update: serologic testing for HIV-1 antibody—United States. Morb. Mortal. Wkly. Rep. 39:69-72. [PubMed] [Google Scholar]
- 6.Dabis, F., and E. R. Ekpini. 2002. HIV-1/AIDS and maternal and child health in Africa. Lancet 359:2097-2104. [DOI] [PubMed] [Google Scholar]
- 7.Damond, F., C. Apetrei, D. L. Robertson, S. Souquiere, A. Lepretre, S. Matheron, J. C. Plantier, F. BrunVezinet, and F. Simon. 2001. Variability of human immunodeficiency virus type 2 (HIV-2) infecting patients living in France. Virology 280:19-30. [DOI] [PubMed] [Google Scholar]
- 8.Damond, F., D. Descamps, I. Farfara, J. N. Telles, S. Puyeo, P. Campa, A. Lepretre, S. Matheron, F. Brun-Vezinet, and F. Simon. 2001. Quantification of proviral load of human immunodeficiency virus type 2 subtypes A and B using real-time PCR. J. Clin. Microbiol. 39:4264-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damond, F., M. Gueudin, S. Pueyo, I. Farfara, D. L. Robertson, D. Descamps, G. Chene, S. Matheron, P. Campa, F. Brun-Vezinet, and F. Simon. 2002. Plasma RNA viral load in human immunodeficiency virus type 2 subtype A and subtype B infections. J. Clin. Microbiol. 40:3654-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Cock, K. M., G. Adjorlolo, E. Ekpini, T. Sibailly, J. Kouadio, M. Maran, K. Brattegaard, K. M. Vetter, R. Doorly, and H. D. Gayle. 1993. Epidemiology and transmission of HIV-2. Why there is no HIV-2 pandemic. JAMA 270:2083-2086. [DOI] [PubMed] [Google Scholar]
- 11.Desire, N., A. Dehee, V. Schneider, C. Jacomet, C. Goujon, P. M. Girard, W. Rozenbaum, and J. C. Nicolas. 2001. Quantification of human immunodeficiency virus type 1 proviral load by a TaqMan real-time PCR assay. J. Clin. Microbiol. 39:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieng-Sarr, A., D. J. Hamel, I. Thior, E. Kokkotou, J. L. Sankale, R. G. Marlink, E. M. Coll-Seck, M. E. Essex, T. Siby, I. Ndoye, S. Mboup, and P. J. Kanki. 1998. HIV-1 and HIV-2 dual infection: lack of HIV-2 provirus with low CD4+ lymphocyte counts. AIDS 12:131-137. [DOI] [PubMed] [Google Scholar]
- 13.Downing, R. G., R. A. Otten, E. Marum, B. Biryahwaho, M. G. Alwano-Edyegu, S. D. K. Sempala, C. A. Fridlund, T. J. Dondero, C. Campbell, and M. A. Rayfield. 1998. Optimizing the delivery of HIV counseling and testing services: the Uganda experience using rapid HIV antibody test algorithms. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18:384-388. [DOI] [PubMed] [Google Scholar]
- 14.Giles, R., K. R. Perry, and J. Parry. 1999. Simple/rapid test devices for anti-HIV screening: do they come up to the mark? J. Med. Virol. 59:104-109. [PubMed] [Google Scholar]
- 15.Guay, L. A., P. Musoke, T. Fleming, D. Bagenda, M. Allen, C. Nakabiito, J. Sherman, P. Bakaki, C. Ducar, M. Deseyve, L. Emel, M. Mirochnick, M. G. Fowler, L. Mofenson, P. Miotti, K. Dransfield, D. Bray, F. Mmiro, and J. B. Jackson. 1999. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet 354:795-802. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa, K., K. Fransen, K. Ariyoshi, J. N. Nkengasong, W. Janssens, L. Heyndrickx, H. Whittle, M. O. Diallo, P. D. Ghys, I. M. Coulibaly, A. E. Greenberg, J. Piedade, W. Canas-Ferreira, and G. van der Groen. 1998. Improved detection of HIV-2 proviral DNA in dually seroreactive individuals by PCR. AIDS 12:1419-1425. [DOI] [PubMed] [Google Scholar]
- 17.Kassler, W. J., M. G. Alwano-Edyegu, E. Marum, B. Biryahwaho, P. Kataaha, and B. Dillon. 1998. Rapid HIV testing with same-day results: a field trial in Uganda. Int. J. STD AIDS 9:134-138. [DOI] [PubMed] [Google Scholar]
- 18.Koblavi-Deme, S., C. Maurice, D. Yavo, T. S. Sibailly, K. N′guessan, Y. Kamelan-Tano, S. Z. Wiktor, T. H. Roels, T. Chorba, and J. N. Nkengasong. 2001. Sensitivity and specificity of human immunodeficiency virus rapid serologic assays and testing algorithms in an antenatal clinic in Abidjan, Ivory Coast. J. Clin. Microbiol. 39:1808-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis, J., and G. Koch. 1977. The measurement of observer agreement for categorical data. Biometrics 33:159-174. [PubMed] [Google Scholar]
- 20.Lien, T. X., N. T. K. Tien, G. F. Chanpong, C. T. Cuc, V. T. Yen, R. Soderquist, K. Laras, and A. Corwin. 2000. Evaluation of rapid diagnostic tests for the detection of human immunodeficiency virus types 1 and 2, hepatitis B surface antigen, and syphilis in Ho Chi Minh City, Vietman. Am. J. Trop. Med. Hyg. 62:301-309. [DOI] [PubMed] [Google Scholar]
- 21.Malonza, I. A., B. A. Richardson, J. K. Kreiss, J. J. Bwayo, and G. C. J. Stewart. 2003. The effect of rapid HIV-1 testing on uptake of perinatal HIV-1 interventions: a randomized clinical trial. AIDS 17:113-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meda, N., V. Leroy, I. Viho, P. Msellati, S. Yaro, L. Mandelbrot, C. Montcho, O. Manigart, and F. Dabis. 2002. Field acceptability and effectiveness of the routine utilization of zidovudine to reduce mother-to-child transmission of HIV-1 in West Africa. AIDS 16:2323-2328. [DOI] [PubMed] [Google Scholar]
- 23.Mylonakis, E., M. Paliou, M. Lally, T. P. Flanigan, and J. D. Rich. 2000. Laboratory testing for infection with the human immunodeficiency virus: established and novel approaches. Am. J. Med. 109:568-576. [DOI] [PubMed] [Google Scholar]
- 24.Nogueira, S. A., J. S. Lambert, A. L. Albuquerque, R. Rodrigues, S. Reis, R. Bornia, M. Dias, R. Barbosa, D. Sztanjbock, A. L. Santos, W. Blattner, and N. T. Constantine. 2001. Assessment of a rapid HIV test strategy during labor—a pilot study from Rio de Janeiro, Brazil. J. Hum. Virol. 4:278-282. [PubMed] [Google Scholar]
- 25.Palmer, C. J., J. M. Dubon, E. Koenig, E. Perez, A. Ager, D. Jayaweera, R. R. Cuadrado, A. Rivera, A. Rubido, and D. A. Palmer. 1999. Field evaluation of the Determine rapid human immunodeficiency virus diagnostic test in Honduras and the Dominican Republic. J. Clin. Microbiol. 37:3698-3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon, F., S. Souquiere, F. Damond, A. Kfutwah, M. Makuwa, E. Leroy, P. Rouquet, J. Berthier, J. Rigoulet, A. Lecu, P. T. Telfer, I. Pandrea, J. C. Plantier, F. Barre-Sinoussi, P. Roques, M. C. Muller-Trutwin, and C. Apetrei. 2001. Synthetic peptide strategy for the detection of and discrimination among highly divergent primate lentiviruses. AIDS Res. Hum. Retrovir. 17:937-952. [DOI] [PubMed] [Google Scholar]
- 27.Sommerfelt, M. A., I. Ohlsson, I. Flolid, R. Thorstensson, and B. Sorensen. 2004. A simple semi-rapid HIV-1&2 confirmatory immunoassay using magnetic particles J. Virol. Methods 115:191-198. [DOI] [PubMed] [Google Scholar]
- 28.Spielberg, F., and W. Kassler. 1996. Rapid test for HIV antibody: a technology whose time has come. Ann. Intern. Med. 125:509-511. [DOI] [PubMed] [Google Scholar]
- 29.Stetler, H. C., T. C. Granade, C. A. Nunez, R. Meza, S. Terrell, L. Amador, and J. R. George. 1997. Field evaluation of rapid HIV serologic tests for screening and confirming HIV-1 infection in Honduras. AIDS 11:369-375. [DOI] [PubMed] [Google Scholar]
- 30.Urassa, W., S. Nozohoor, S. Jaffer, K. Karama, F. Mhalu, and G. Biberfeld. 2002. Evaluation of an alternative confirmatory strategy for the diagnosis of HIV infection in Dar Es Salaam, Tanzania, based on simple rapid assays. J. Virol. Methods 100:115-120. [DOI] [PubMed] [Google Scholar]
- 31.van den Berk, G. E. L., P. H. J. Frissen, R. M. Regez, and P. J. G. M. Rietra. 2003. Evaluation of the rapid immunoassay determine HIV 1/2 for detection of antibodies to human immunodeficiency virus types 1 and 2. J. Clin. Microbiol. 41:3868-3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Ende, M. E., A. M. Prins, K. Brinkman, M. Keuter, A. Veenstra, S. A. Danner, H. G. M. Niesters, A. D. M. E. Osterhaus, and M. Schutten. 2003. Clinical, immunological and virological response to different antiretroviral regimens in a cohort of HIV-2-infected patients. AIDS 17:S55-S61. [DOI] [PubMed] [Google Scholar]
- 33.Walther-Jallow, L., S. Andersson, Z. Da Silva, and G. Biberfeld. 1999. High concordance between polymerase chain reaction and antibody testing of specimens from individuals dually infected with HIV types 1 and 2 in Guinea-Bissau, West Africa. AIDS Res. Hum. Retrovir. 15:957-962. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. 1998. The importance of simple/rapid assays in HIV testing. Wkly. Epidemiol. Rec. 73:321-328. [PubMed] [Google Scholar]