Abstract
Pythium insidiosum is a pathogen that causes disease in both animals and humans. Human infection is rare; however, when it does occur, most patients, especially those having underlying hemoglobinopathy syndromes, such as thalassemia, exhibit a severe form. We identified four isolates of P. insidiosum. Two were recovered from tissue biopsy specimens from thalassemic and leukemic patients, one was derived from brain tissue from a thalassemic patient, and another was isolated from a corneal ulcer from a fourth patient. Western blotting and an enzyme-linked immunosorbent assay (ELISA) were performed with a serum sample derived from one thalassemic patient. The methods used to identify the P. insidiosum isolates were based on morphology, nucleic acid sequencing, and a PCR assay. To confirm the identification, portions of the 18S rRNA genes of these four isolates were sequenced. The sequences were shown to be homologous to previously described P. insidiosum DNA sequences. In addition, PCR amplification of the internal transcribed spacer region specific for P. insidiosum was positive for all four isolates. The ELISA with the serum sample from the thalassemic patient gave a positive result from a serum dilution of 1:800. Finally, Western immunoblotting with this serum sample showed positive immunoglobulin G recognition for proteins of 110, 73, 56, 42 to 35, 30 to 28, 26, and 23 kDa. The results of this study show that both molecularly based diagnostic and serodiagnostic techniques are useful for the rapid identification of human pythiosis. The predominant antigens recognized by Western blotting may be useful in the development of a more sensitive and specific diagnostic tool for this disease.
Pythium insidiosum is an aquatic fungus-like pathogen that causes granulomatous disease (or swamp cancer) in animals such as horses, cattle, dogs, and cats (14, 29). Human infection was first diagnosed in two Thai thalassemic patients with subcutaneous granulomatous lesions and was reported in 1986 (28). The causative agent was characterized in detail as P. insidiosum by De Cock in 1987 (6). Since then, several cases of human pythiosis have been reported (3, 12, 24-26, 30, 33). The disease may be manifested as a subcutaneous, systemic, vascular, or ophthalmic type. The systemic type usually occurs in thalassemic or leukemic patients. Most patients have a severe form that resists treatment. Of 34 human cases reported, 17 (50%) were of the vascular type, 14 (41%) were of the ocular type, and 3 (9%) were of the subcutaneous type. The majority of human cases (76%) have been reported in Thailand. Because of the severe progression of the disease, rapidly sensitive and specific tests are necessary for diagnosis and prognosis monitoring.
The organism is classified as an oomycete in the kingdom Straminipila, phylum Oomycota, order Peronosporales, and family Pythiaceae (1, 7, 23). Some scientists have placed Pythium species in the kingdom Chromista, phylum Pseudofungi, class Oomycetes, order Pythiales, and family Pythiaceae (5, 6, 14). P. insidiosum has characteristic nonseptate filaments and motile zoospores. The zoospores can be induced in an induction water medium and trapped by the use of a baiting technique. P. insidiosum is able to attach itself to grass blades, an unidentified water lily, and horse hair, and these are all useful as bait substrates in a baiting procedure (4, 20, 21).
The identification of P. insidiosum isolates in humans has been carried out by culturing with the induction water medium technique. Since identification by use of morphological criteria can be difficult and time-consuming, immunofluorescence and immunodiffusion tests have been developed for the specific and rapid identification of P. insidiosum in cultures (17). Further, human pythiosis can be diagnosed by the detection of immunoglobulin G (IgG) antibodies in the sera of patients by an immunodiffusion test (24) and an enzyme-linked immunosorbent assay (ELISA) (13, 16). Recently, a nested PCR assay was developed for the identification of P. insidiosum in animals, and this technique was used to discover an isolate in a human periorbital lesion (8).
In this report, we describe the identification of P. insidiosum isolated from four Thai patients who were admitted to Chiang Mai University Hospital from 2001 to 2002. Two isolates were recovered from tissue biopsy specimens from thalassemic and leukemic patients, the third isolate was derived from brain tissue from a thalassemic patient, and the fourth isolate was recovered from a corneal ulcer from a patient. All isolates were identified by colony morphology, zoospore production, nucleic acid sequencing, and PCR assay. Western blotting and an ELISA were performed with a serum sample derived from a thalassemic patient.
MATERIALS AND METHODS
Microorganisms.
Four clinical isolates of P. insidiosum were recovered from four patients with pythiosis and designated MMC44P21-1, MMC44P21-2, MMC45P21-1, and MMC45P21-2 (Table 1). All isolates were maintained on Sabouraud's glucose agar (SGA) at 28°C.
TABLE 1.
P. insidiosum isolates recovered from patients with pythiosis and used in this study
| Isolate | Clinical specimen | Underlying disease of patient |
|---|---|---|
| MMC44P21-1 | Tissue biopsy from left leg | Thalassemia |
| MMC44P21-2 | Tissue biopsy from right ankle | Leukemia with bone marrow transplantation |
| MMC45P21-1 | Craniotomy from brain tissue | Thalassemia |
| MMC45P21-2 | Corneal specimen from left eye | Unknown |
Morphological observations.
Colony morphology and rate of growth were observed by culturing on SGA at pH 5.3, SGA at pH 6.9, malt extract agar, blood agar, and chocolate agar at 37°C and room temperature. Motile zoospores were induced in an aqueous medium (0.5 ml of solution 1 [K2HPO4 · 3H2O, 11.4 g; KH2PO4, 6.8 g; NH4H2PO4, 5.75 g; distilled water, 50 ml], 0.1 ml of solution 2 [MgCl2 · 6H2O, 2.54 g; CaCl2 · 2H2O, 1.84 g; distilled water, 25 ml], and distilled water to 1,000 ml) containing grass blades or rabbit hair by incubation at 37°C. The grass blades and rabbit hair were sterilized by boiling for 20 min before use as described in the original method of Mendoza and Prendas (20).
DNA extraction.
DNA from the filaments of each isolate was extracted by the following rapid method. Filaments of P. insidiosum were suspended in 0.5 ml of lysis buffer (1.5% sodium dodecyl sulfate [SDS], 0.25 M Tris [pH 8.0]), boiled for 30 min, and vortexed for 2 min. DNA was extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) and precipitated with ethanol. The DNA pellet was air dried and resuspended in 50 μl of water. The DNA concentration was measured with a spectrophotometer.
Primers and PCR conditions.
To confirm the identification, portions of the 18S rRNA genes (580-bp fragments) of these four isolates were sequenced. Oligonucleotides used for PCR amplification were designed based on the sequence of the 18S rRNA gene of P. insidiosum from GenBank (accession numbers AF289981 and AF221847): primer Pin1, 5′-TGGCTCTTCGAGTCGGGCAA-3′; and primer Pin2, 5′-GTCGGCATAGTTTATGGTTAAGA-3′. PCR was performed with 100-μl volumes and a thermal cycler (GeneAmp PCR system 2700; Applied Biosystems) programmed as follows: 94°C for 3 min; 35 cycles at 94°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 10 min. The amplified fragments were sequenced, and then BLAST was used to search for homologous sequences in GenBank.
For rapid identification, we designed primers based on the internal transcribed sequence (ITS) regions of P. insidiosum, which were reported by Grooters and Gees (8): primer ITSpy1, 5′-CTGCGGAAGGATCATTACC-3′; and primer ITSpy2, 5′-GTCCTCGGAGTATAGATCAG-3′. PCR was performed with 50-μl volumes containing 10 pmol of each primer, 2.5 U of Taq polymerase (Qiagen GmbH, Hilden, Germany), deoxynucleoside triphosphate mixture (each at 200 μM), 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgCl2, and about 10 ng of DNA template. PCR conditions were as follows: 94°C for 3 min; 35 cycles at 94°C for 45 s, 60°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 10 min. The PCR amplification products were analyzed by agarose gel (1% [wt/vol]) electrophoresis followed by ethidium bromide staining.
Western blotting.
Western blotting was performed as follows. Briefly, culture filtrate antigens were prepared from the identified P. insidiosum isolates and concentrated by ammonium sulfate precipitation. Rabbit antiserum was prepared by immunizing white rabbits subcutaneously with a mixture of 1 ml of the concentrated antigens (500 μg) and 1 ml of incomplete Freund adjuvant. After 2 and 4 weeks, the rabbits were immunized subcutaneously again. Two weeks after the final injection, the animals were bled. For immunoblotting, the protein antigens were separated by SDS-polyacrylamide gel electrophoresis and electrotransferred to nitrocellulose membranes. The blots were incubated with skim milk blocking buffer for about 2 h at room temperature and washed in phosphate-buffered saline (PBS) with 0.05% Tween 20 for 20 min (four times for 5 min each time). The blots then were incubated with each serum sample (1:100 dilution) for 45 min at 37°C and washed in PBS-Tween for four 5-min periods. They were subsequently incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5,000 dilution) or anti-human IgG (1:2,000 dilution) for 30 min at 37°C and washed in PBS-Tween four times. The reaction was detected with 4-chloro-1-naphthol as a substrate.
ELISA.
An ELISA was performed to detect the antibody titer in the patient's serum. Ninety-six-well microtiter plates were coated overnight at 4°C with 100 μl of concentrated culture filtrate antigens (500 ng/well). The plates were washed twice with PBS-Tween and blocked with 100 μl of blocking buffer for 45 min at room temperature. Serum samples were diluted twofold from 1:50 to 1:800. Fifty microliters of each dilution was added to each well and incubated for 30 min at 37°C. The plates were washed five times. Fifty microliters of horseradish peroxidase-conjugated goat anti-rabbit IgG or anti-human IgG (1:20,000 dilution) was added and incubated for 30 min at 37°C. After five washes, 100 μl of freshly prepared o-phenylenediamine chromogenic substrate solution was added and incubated in the dark at room temperature for 30 min. The reaction was stopped by the addition of 50 μl of 4 N H2SO4. Optical densities were read at 492 nm with an ELISA plate reader. The cutoff point of this assay was determined to be approximately twice the value of the average optical density of negative controls.
RESULTS
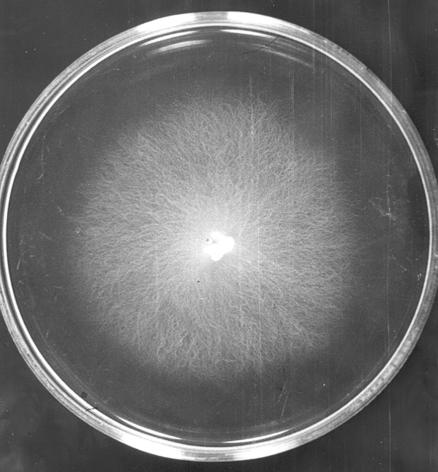
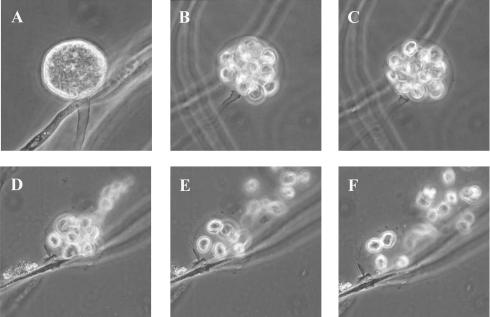
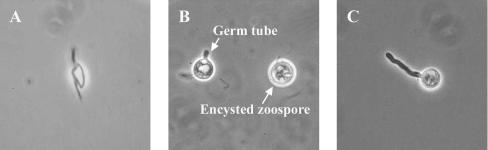
Culture observations revealed the rapid growth of all isolates on several media. Chocolate agar and blood agar appeared to be the best of all the tested media in this experiment. However, to observe colony morphology and pigmentation, brain heart infusion agar and SGA at pH 6.9 were the media of choice. The colonies were creamy white and glabrous (Fig. 1) and grew well at both 37 and 28°C. The organisms grew rather slowly on SGA at pH 5.3 and very slowly on malt extract agar. No growth was observed on Mycosel agar. Microscopically, the organisms produced nonseptate filaments with a diameter of 4 to 10 μm, and no spores were seen. Motile biflagellate zoospores produced from sporangium could be induced in a modified induction medium containing grass blades or rabbit hair (Fig. 2). Approximately 4 to 20 zoospores were found per sporangium. The process of protoplasm flowing to the apical cell to form the sporangium through the cleavage of zoospores until the discharge of all motile zoospores took about 10 to 20 min. Zoospores moved very actively for about 10 to 60 min before encystment and germination (Fig. 3).
FIG. 1.
Colony of P. insidiosum (4 days old) on SGA (pH 6.9).
FIG. 2.
(A to C) Zoospore production inside the zoosporangium at time intervals of 3 min. (D to F) Rapid release of zoospores.
FIG. 3.
(A) Biflagellate zoospore. (B) Encysted zoospore. (C) Germinated zoospore.
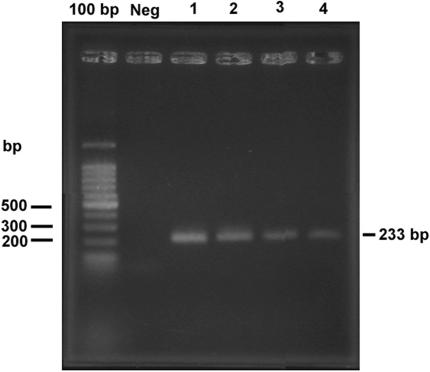
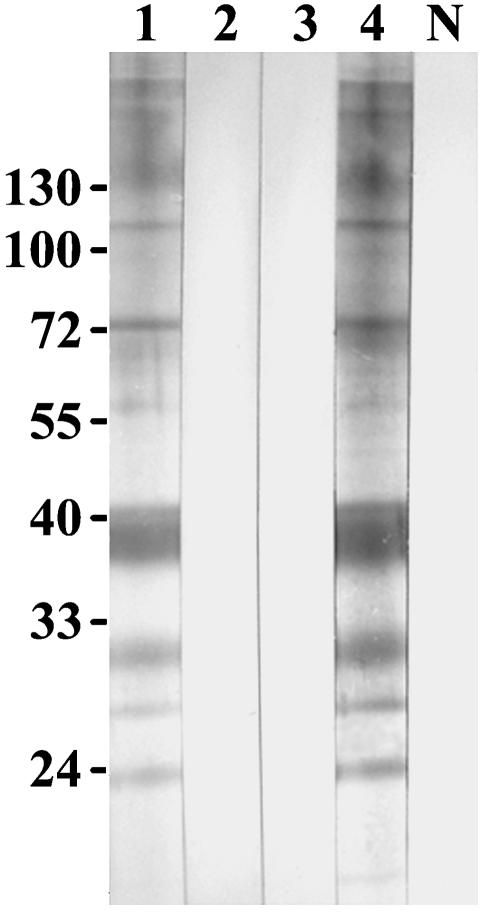
DNA sequencing of a 580-bp fragment of the 18S rRNA gene showed that the sequences of three isolates were 100% homologous to previously described P. insidiosum 18S rRNA sequences. One isolate contained two polymorphic sites (99.65% homology) (data not shown). PCR amplification of the ITS region was positive for all four isolates with primers ITSpy1 and ITSpy2 (Fig. 4). The application of a nested PCR with primers PI-1 and PI-2 produced a negative result for one isolate. This result demonstrated that the previously reported (8) inner primers could not amplify all Thai isolates of P. insidiosum. An ELISA of one serum sample from a thalassemic patient with culture filtrate antigens of P. insidiosum produced a positive result at a serum dilution of 1:800. This isolate was 100% homologous to P. insidiosum in the GenBank database. Finally, a Western immunoblot assay of this serum sample showed positive IgG recognition for proteins of 110, 73, 56, 42 to 35, 30 to 28, 26, and 23 kDa (Fig. 5). The immunoblot pattern for this sample was similar to the reaction with immunized rabbit antiserum. Negative signals were seen in the reactions with preimmunized rabbit antiserum, normal human sera, and a serum sample from a penicilliosis patient.
FIG. 4.
Single PCR amplification of DNA from four human isolates of P. insidiosum with primers ITSpy1 and ITSpy2 (lanes 1 to 4). The leftmost lane contained a 100-bp DNA ladder marker.
FIG. 5.
Immunoblot of P. insidiosum antigens. Lane 1, reaction with serum from a pythiosis patient. Lanes 2 to 4 and lane N, reactions with serum from a penicilliosis patient, preimmunized rabbit antiserum, immunized rabbit antiserum, and pooled human sera (from healthy persons), respectively. Relative molecular masses are shown on the left in kilodaltons.
These data show that both of our molecularly based diagnostic and serodiagnostic techniques are useful for the rapid identification of human pythiosis. A single PCR assay with primers ITSpy1 and ITSpy2 specifically amplified all four isolates of P. insidiosum. A more efficient nested PCR assay should be developed further. The predominant antigens recognized by the Western blot assay may also be useful in the development of a more sensitive and specific diagnostic tool for this disease.
DISCUSSION
Animal pythiosis, known as swamp cancer, was reported as a subcutaneous disease in horses in New Guinea (2). The disease was later reported in other animals and humans. The causative agent was classified as P. insidiosum on the basis of its morphology of asexual and sexual reproductive units (6). Human pythiosis is severe, and the mortality rate is very high (29) because of the difficulties in early diagnosis and treatment. The histological findings reveal that the organism has broad, nonseptate hyphae like those produced by members of the zygomycetes. The definite morphological identification of this organism from a culture is also problematic. P. insidiosum grows rapidly and produces only sterile hyphae on all media. Its characteristic motile biflagellate zoospores must be induced by use of a special medium. Most mycologists are not familiar with this characteristic of Pythium species. Further, the motile zoospores have a morphology similar to those of other species of oomycetes, such as Pythium species other than P. insidiosum, Phytophthora, and Lagenidium (1, 8, 15). In this study, four isolates of P. insidiosum from patients in northern Thailand were investigated by molecular and immunological methods to confirm their identification as P. insidiosum.
The human isolates of P. insidiosum grew rapidly on several culture media. Despite the fact that the isolates grew well on chocolate agar, it was more practical to observe colony morphology on brain heart infusion agar or SGA at pH 6.9. On these media, colonies of P. insidiosum grew to cover the whole surface of the plates within 1 week. For the induction of asexual zoospores in a water culture, rabbit hair and grass blades were used as bait for colonizaton by this organism. The human isolates of P. insidiosum could colonize both grass blades and rabbit hair. The production of motile biflagellate zoospores was observed after 16 h of incubation at 37°C. When the organism was transferred to new fresh induction medium, zoospores could be seen after 1 h of incubation. Thereafter, identification was confirmed by DNA sequencing of a fragment of the 18S rRNA gene. When the sequences were aligned with the sequence of P. insidiosum (GenBank accession number AF442497), 100% homology was found, with the exception of one isolate from a brain lesion. This isolate had 2 bp that were different from the sequences of the other isolates. A BLAST search showed that the sequence of this part of the 18S rRNA gene was identical to those of other species within the oomycetes, for example, Phytophtora undulata and Lagenidium giganteum. Thus, this section of the 18S rRNA gene was not specific for P. insidiosum. Our results revealed a minor difference within the human isolates of this organism.
Grooters and Gee (8) developed nested PCR for the rapid identification of P. insidiosum. The method was specific for several isolates of P. insidiosum from animals, including one isolate from a human periorbital lesion. In addition, the method could differentiate P. insidiosum from other species of the same genus, which produced positive bands of different sizes in the initial PCR. The nested PCR produced positive results only with P. insidiosum. In our experiment, the specific inner primers, PI-1 and PI-2, could amplify three of the four human isolates in both nested and direct PCR assays. Thus, there appears to be some difference in the sequences of the ITS regions of the human isolates. We designed new primers, ITSpy1 and ITSpy2, from the reported sequence of P. insidiosum (8), and we performed a single PCR assay with all four isolates. The results showed a positive PCR amplicon of about 230 bp for all four human isolates. The ITS sequences are good candidates for the identification of P. insidiosum as recommended by Grooters and Gee (8). A more sensitive nested PCR is being developed for future use in the identification of pathogenic P. insidiosum from humans and the environment. This PCR will be performed with more isolates to ensure the specificity of the assay.
Serodiagnosis of human and animal pythiosis by use of an ELISA has been reported (13, 16). We showed that an ELISA can detect IgG antibody at a titer of 1:800 in a serum sample from a patient. The molecular masses of antigens from P. insidiosum which reacted with the IgG antibody in this serum sample were determined by SDS-polyacrylamide gel electrophoresis and immunoblotting. Immunoreactive bands were found at molecular masses of 110, 73, 56, 42 to 35, 30 to 28, 26, and 23 kDa. A previous report showed that in sera from horses with pythiosis, the immunodominant and specific bands were found at molecular masses of 32, 30, and 28 kDa (18). It seems possible that the broad reactive bands of approximately 30 to 28 kDa detected in our study might be the same antigens as those in the study of horse pythiosis. We also found additional reactivities at several molecular masses, especially 42 to 35 kDa, which had strong reactivity. In a study of an emerging oomycosis caused by Lagenidium species in dogs, an immunoblot analysis showed some cross-reactivities with the antigens of P. insidiosum (9). It also suggested that these two genera have some antigenic similarities. Lagenidium species have been reported to cause an emerging disease in animals. This disease should also be considered in the differential diagnosis for any patient with pythiosis (11). Thus, a P. insidiosum-specific antibody that does not react with Lagenidium species would be helpful for a specific diagnosis. Further investigations with more serum samples would be useful for identifying specific antigens of human-pathogenic P. insidiosum.
Several cases of human pythiosis (nearly 80% of the total global cases) have been reported in Thailand. The disease has also been reported in other countries, such as Australia (31), New Zealand (22), the United States (10, 19, 27), Haiti (32), and Malaysia (3). The source of infection and the mode of transmission of the disease are still unknown. A rapid method would assist in the identification of P. insidiosum in nature. In this study, we identified human isolates of P. insidiosum by using molecularly based diagnostic and serodiagnostic techniques. The predominant antigens recognized by Western blotting may be useful in the development of a more sensitive and specific diagnostic tool for pythiosis and may be useful as a clue to understanding the further immunological features of this infection.
Acknowledgments
This work was supported in part by grants from the Royal Golden Jubilee PhD Program, the Thailand Research Fund (for J. Supabandhu and S. Thirach), and the Faculty of Medicine, Chiang Mai University.
REFERENCES
- 1.Alexopoulos, C. J., C. W. Mims, and M. Blackwell. 1996. Introductory mycology, 4th ed., p. 61-85, 683-737. John Wiley & Sons, Inc., New York, N.Y.
- 2.Auswick, P. K. C., and J. W. Copeland. 1974. Swamp cancer. Nature 250:84. [DOI] [PubMed] [Google Scholar]
- 3.Badenoch, P. R., D. J. Coster, B. L. Wetherall, H. T. Brettig, M. A. Rozenbilds, A. Drenth, and G. Wagels. 2001. Pythium insidiosum keratitis confirmed by DNA sequence analysis. Br. J. Ophthalmol. 85:502-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaiprasert, A., K. Samerpitak, W. Wanachiwanawin, and P. Thasnakorn. 1990. Induction of zoospore formation in Thai isolates of Pythium insidiosum. Mycoses 33:317-323. [DOI] [PubMed] [Google Scholar]
- 5.Corliss, J. O. 1994. An interim utilitarian (‘user friendly') hierarchical classification and characterization of the protists. Acta Protozool. 33:1-51. [Google Scholar]
- 6.De Cock, A. W. A. M., L. Mendoza, A. A. Padhye, L. Ajello, and L. Kaufman. 1987. Pythium insidiosum sp. nov., the etiologic agent of pythiosis. J. Clin. Microbiol. 25:344-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dick, M. W. 2001. Straminipilous fungi: systematics of the peronosporomycetes including accounts of the marine straminipilous protist, the plasmodiophorids and similar organisms, p. 670. Kluwer Academic Publishers, London, England.
- 8.Grooters, A. M., and M. K. Gee. 2002. Development of a nested polymerase chain reaction assay for the detection and identification of Pythium insidiosum. J. Vet. Intern. Med. 16:147-152. [DOI] [PubMed] [Google Scholar]
- 9.Grooters, A. M., E. C. Hodgin, R. W. Bauer, C. J. Detrisac, N. R. Znajda, and R. C. Thomas. 2003. Clinicopathologic findings associated with Lagenidium sp. infection in 6 dogs: initial description of an emerging oomycosis. J. Vet. Intern. Med. 17:637-646. [DOI] [PubMed] [Google Scholar]
- 10.Heath, J. A., T. E. Kiehn, A. E. Brown, M. P. LaQuaglia, L. J. Steinherz, G. Bearman, M. Wong, and P. G. Steinherz. 2002. Pythium insidiosum pleuropericarditis complicating pneumonia in a child with leukemia. Clin. Infect. Dis. 35:E60-E64. [DOI] [PubMed] [Google Scholar]
- 11.Holloway, S. A. 2003. The oomycetes—fungi with teeth and flippers? J. Vet. Intern. Med. 17:607-608. [PubMed] [Google Scholar]
- 12.Imwidthaya, P. 1994. Human pythiosis in Thailand. Postgrad. Med. J. 70:558-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krajaejun, T., M. Kunakorn, S. Niemhom, P. Chongtrakool, and R. Pracharktam. 2002. Development and evaluation of an in-house enzyme-linked immunosorbent assay for early diagnosis and monitoring of human pythiosis. Clin. Diagn. Lab. Immunol. 9:378-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendoza, L. 1998. Pythium insidiosum, p. 617-630. In L. Collier, A. Balows, and M. Sussman (ed.), Topley & Wilson's microbiology and microbial infections, 9th ed. Arnold, London, England.
- 15.Mendoza, L., F. Hernandez, and L. Ajello. 1993. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. J. Clin. Microbiol. 31:2967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendoza, L., L. Kaufman, W. Mandy, and R. Glass. 1997. Serodiagnosis of human and animal pythiosis using an enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 4:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendoza, L., L. Kaufman, and P. Standard. 1987. Antigenic relationship between the animal and human pathogen Pythium insidiosum and nonpathogenic Pythium species. J. Clin. Microbiol. 25:2159-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendoza, L., V. Nicholson, and J. F. Prescott. 1992. Immunoblot analysis of the humoral immune response to Pythium insidiosum in horses with pythiosis. J. Clin. Microbiol. 30:2980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendoza, L., S. H. Prasla, and L. Ajello. 2004. Orbital pythiosis: a non-fungal disease mimicking orbital mycotic infections, with a retrospective review of the literature. Mycoses 47:14-23. [DOI] [PubMed] [Google Scholar]
- 20.Mendoza, L., and J. Prendas. 1988. A method to obtain rapid zoosporogenesis of Pythium insidiosum. Mycopathologia 104:59-62. [DOI] [PubMed] [Google Scholar]
- 21.Miller, R. 1983. Investigation into the biology of the three phycomycotic agents pathogenic for horses in Australia. Mycopathologia 81:23-28. [DOI] [PubMed] [Google Scholar]
- 22.Murdoch, D., and D. Parr. 1997. Pythium insidiosum keratitis. Aust. N. Z. J. Ophthalmol. 25:177-179. [DOI] [PubMed] [Google Scholar]
- 23.Patterson, D. J. 1989. Stramenopila: chromophytes from a protistan perspective, p. 357-379. In J. C. Green, B. S. C. Leadbeater, and W. L. Diver (ed.), The chromophyte algae: problems and perspectives. Clarendon, Oxford, England.
- 24.Pracharktam, R., P. Chongtrakool, B. Sathapatayavongs, P. Jayanetra, and L. Ajello. 1991. Immunodiffusion test for diagnosis and monitoring of human pythiosis insidiosi. J. Clin. Microbiol. 29:2661-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasertwitayakij, N., W. Louthrenoo, N. Kasitanon, K. Thamprasert, and N. Vanittanakom. 2003. Human pythiosis, a rare cause of arteritis: case report and literature review. Semin. Arthritis Rheum. 33:204-214. [DOI] [PubMed] [Google Scholar]
- 26.Sathapatayavongs, B., P. Leelachaikul, R. Pracharktam, V. Atichartakarn, S. Sriphojanart, P. Trairatvorakul, S. Jirasiritham, S. Nontasut, C. Eurvilaichit, and T. Flegel. 1989. Human pythiosis associated with thalassemia hemoglobinopathy syndrome. J. Infect. Dis. 159:274-280. [DOI] [PubMed] [Google Scholar]
- 27.Shenep, J. L., B. K. English, L. Kaufman, T. A. Pearson., J. W. Thompson, R. A Kaufman, G. Frisch, and M. G. Rinaldi. 1998. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin. Infect. Dis. 27:1388-1393. [DOI] [PubMed] [Google Scholar]
- 28.Thianprasit, M. 1986. Fungal infection in Thailand. Jpn. J. Dermatol. 96:1343-1345. [Google Scholar]
- 29.Thianprasit, M., A. Chaiprasert, and P. Imwidthaya. 1996. Human pythiosis. Curr. Trop. Med. Mycol. 7:43-54. [PubMed] [Google Scholar]
- 30.Thitithayanont, A., L. Mendoza, A. Chuansumrit, R. Pracharktam, J. Laothamatas, B. Sathapatayavongs, S. Lolekha, and L. Ajello. 1998. Use of an immunotherapeutic vaccine to treat a life-threatening arteritic infection caused by Pythium insidiosum. Clin. Infect. Dis. 27:1394-1400. [DOI] [PubMed] [Google Scholar]
- 31.Triscott, J. A., D. Weedon, and E. Cabana. 1993. Human subcutaneous pythiosis. J. Cutan. Pathol. 20:267-271. [DOI] [PubMed] [Google Scholar]
- 32.Virgile, R., H. D. Perry, B. Pardanani, K. Szabo, E. K. Rahn, J. Stone, I. Salkin, and D. M. Dixon. 1993. Human infectious corneal ulcer caused by Pythium insidiosum. Cornea 12:81-83. [DOI] [PubMed] [Google Scholar]
- 33.Wanachiwanawin, W., M. Thianprasit, S. Fucharoen, A. Chaiprasert, N. Sudasna, N. Ayudhya, N. Sirithanaratkul, and A. Piankijagum. 1993. Fatal arteritis due to Pythium insidiosum infection in patients with thalassaemia. Trans. R. Soc. Trop. Med. Hyg. 87:296-298. [DOI] [PubMed] [Google Scholar]