Abstract
A real-time PCR assay using SYBR Green I for quantification of provirus load in a murine model of AIDS (i.e., LP-BM5 infection) was developed and validated. In this method, data are normalized against the 18S rRNA gene. The method has a dynamic range of 8 logs and a sensitivity of one copy.
The murine model of AIDS consisting of susceptible C57BL/6 mice infected with the retroviral complex LP-BM5 shows many similarities to human AIDS. The etiologic agent in the viral mixture is represented by a replication-defective virus (BM5d) requiring replication-competent helper viruses, such as ecotropic (BM5e) and mink cell focus-forming viruses (14, 15). This murine model of AIDS has been used to test new therapies aimed at protecting both BM5d targets, i.e., lymphocytes and macrophages (9, 11), and new approaches comprising conventional and lympholytic drugs (i.e., fludarabine) aimed at eradicating infection (8, 10). In these studies a real-time PCR assay for provirus quantification would have been very useful.
We developed a PCR method based on detection of the p12 gag gene of the BM5d virus (6) by using the double-stranded DNA binding dye SYBR Green I as a detection system (18). A similar method has been developed to quantify the 18S rRNA gene in the same samples. Thus, this PCR assay can be used, to determine the absolute copy number of BM5d provirus per cell.
For DNA isolation, 10 or 30 mg of tissue or cell pellet was resuspended in 750 μl or at 5,000 cells/μl, respectively, in lysis buffer (TE buffer [10 mM Tris-HCl, 1 mM EDTA; pH 8.0], 0.5% Tween 20, 0.1 mg of proteinase K/ml), followed by incubation at 60°C for 1 to 3 h and then at 95°C for 15 min. After centrifugation to pellet the cell debris, the supernatant was digested with a DNase-free RNase to a final concentration of 20 μg/ml for 30 min at 37°C. Twofold dilutions for each DNA sample (1:5 and 1:10 dilutions for tissues and 1:8 and 1:16 dilutions for cells) were tested to ensure the absence of inhibitor during amplification, and the mean of both results was used for data analysis. The specificity of the primers (DEF-6 and DEF-7 for the 186-bp amplification product of the BM5d gag gene [4] and 18S-F and 18S-R for 151-bp product of the 18S rRNA gene [17]) was confirmed for every PCR run by dissociation curve analysis (ABI Prism 7700 dissociation curve software; Applied Biosystems, Foster City, Calif.). Moreover, the DEF-6 and DEF-7 primers were able to eliminate the interference in virus quantification due to retrovirus-related sequences present in the mouse genome (3, 4, 6). The standard SYBR Green I real-time PCR (real-time PCR) of BM5d and 18S rRNA was performed in a 25-μl final volume. Then, 4 μl of each DNA sample was added to 21 μl containing 1× PCR Gold Buffer (Applied Biosystems), 3 mM MgCl2, 200 μM (each) deoxynucleoside triphosphates, 400 nM concentrations of each primer, a 1:100,000 final dilution (in dimethyl sulfoxide) of SYBR Green I (Molecular Probes, Eugene, Oreg.), 250 nM concentrations of ROX passive reference dye (in dimethyl sulfoxide; Molecular Probes), bovine serum albumin at 200 ng/μl, and 0.625 U of AmpliTaq Gold (Applied Biosystems). Both amplifications were carried out under the same conditions by using an ABI Prism 7700 sequence detection system (Applied Biosystems): after one cycle at 95°C for 10 min, a two-step PCR procedure was used consisting of 15 s at 95°C and 1 min at 60°C for 40 cycles.
A standard curve of 10-fold serial dilutions of pCR-DEF plasmid molecules (4) was obtained for quantification of BM5d copy number in the samples. A second standard curve with 18S rRNA plasmid was generated in order to normalize sample-to-sample variation values of the BM5d (Fig. 1). Moreover, a single-copy detection assay demonstrated that one provirus copy was detected in 100% of the one-cell dilution samples (Table 1). The amplification efficiencies of the curves generated with pCR-DEF and pCR-18S plasmids and target DNA from samples were similar and in the range of 100% (data not shown). This approach can be easily adapted to other applications. In fact, a BLAST search revealed that 18S-F and 18S-R were 100% homologous to mouse, rat, rabbit, and human 18S rRNA.
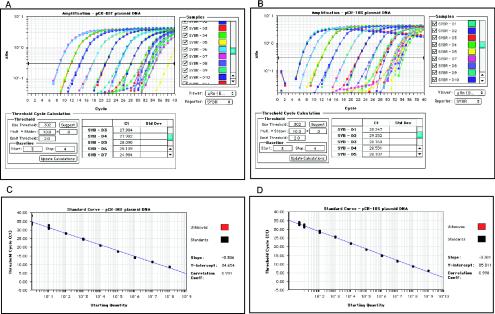
FIG. 1.
Amplification plots (A and B) and standard curves (C and D) for the quantification of BM5d and 18S rRNA by real-time SYBR Green I PCR. pCR-DEF (A) and pCR-18S (B) plasmids were serially 10-fold diluted in water with 108 to 1 copies and 109 to 5 copies per reaction, respectively. Amplification was repeated three or five times (for 109 to 102 dilution range) and 10 times (for 10 to 1 dilution range) for each dilution. The fluorescence intensity (ΔRn) in a logarithmic scale collected in real-time for each sample was plotted against the number of PCR cycles. The horizontal line indicates the fixed fluorescence threshold. (C and D) Calibration curves obtained by linear regression analysis of measured CT values versus log10 input copy number of each dilution for pCR-DEF and pCR-18S plasmids. The slope, the y intercept, and the coefficient of correlation are given in the graph area. Only one representative experiment is shown.
TABLE 1.
Single-copy assay detection
| Sample | CT valuea
|
BM5d copies/cellc | |
|---|---|---|---|
| BM5d | 18S rRNAb | ||
| 1 | 34.55 | 27.38 | 1.08 |
| 2 | 34.53 | 27.46 | 1.09 |
| 3 | 33.68 | 27.60 | 1.98 |
| 4 | 34.32 | 27.44 | 1.27 |
| 5 | 34.66 | 27.40 | 1.00 |
| 6 | 33.90 | 27.34 | 1.70 |
| 7 | 34.40 | 27.51 | 1.20 |
| 8 | 33.16 | 27.58 | 2.84 |
| 9 | 33.20 | 27.34 | 2.76 |
| 10 | 34.62 | 27.28 | 1.03 |
For each sample, the CT value is the average from three replicates of PCR with SYBR Green I and refers to one cell dilution (CT mean ± the standard deviation: 34.10 ± 0.58 for BM5d and 27.43 ± 0.11 for 18S rRNA gene [n = 10]).
The CT values obtained (corresponding to about 246 18S rRNA copies, experimentally calculated [data not shown and Fig. 1]) confirm one cell as the exact starting DNA quantity.
BM5d provirus copies deduced from standard curve analyses.
The high reproducibility of the assay for both plasmids was documented by a coefficient of variation for estimated copy numbers of 19 and 22%, confirming the reliability and correctness of the entire technical procedure over time and over the complete range of quantification (2) (Table 2).
TABLE 2.
Reproducibility of real-time PCR with SYBR Green Ia
| Plasmid and copy no. | Intraassay
|
Interassay
|
||
|---|---|---|---|---|
| %CVCT | %CVCNb | %CVCT | %CVCNc | |
| pCR-DEF | ||||
| 1 | 1.77 | 39.80 | 7.06 | 34.53 |
| 10 | 1.80 | 33.58 | 7.43 | 24.76 |
| 102 | 0.04 | 0.91 | 7.89 | 14.92 |
| 104 | 0.26 | 4.01 | 4.32 | 22.22 |
| 106 | 0.61 | 6.41 | 4.58 | 12.20 |
| 108 | 1.07 | 7.41 | 6.65 | 25.82 |
| pCR-18S | ||||
| 5 | 1.39 | 28.68 | 3.22 | 32.61 |
| 10 | 1.56 | 29.60 | 3.86 | 29.02 |
| 102 | 1.27 | 24.39 | 4.97 | 28.88 |
| 104 | 0.33 | 5.12 | 5.13 | 21.67 |
| 106 | 0.51 | 5.37 | 7.34 | 22.76 |
| 108 | 0.63 | 3.83 | 10.94 | 30.78 |
%CVCN, percent coefficient of variation of copy number; %CVCT, percent coefficient of variation of threshold cycle.
Mean %CVCN = 15.35 (pCR-DEF) and 16.17 (pCR-18S).
Mean %CVCN = 22.41 (pCR-DEF) and 27.62 (pCR-18S). The P value was not significantly different versus intraassay %CVCN.
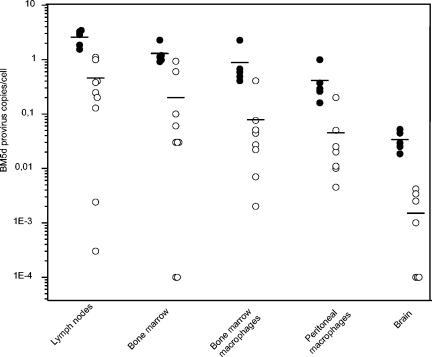
LP-BM5-infected C57BL/6 mice treated with a potent antiretroviral therapy were used to validate the method developed. The two drugs selected were two reverse transcription inhibitors, the nucleoside analogues zidovudine (AZT) and didanosine (DDI), which are known to be very effective at inhibiting the disease progression in murine AIDS (1, 13). The real-time PCR was able to detect small changes in proviral copies in a broad dynamic range with accurate discrimination between the copy number of infected and infected/treated tissues, in which, after the therapeutic regimen, a decline of at least 1 log unit was shown (Fig. 2). In particular, the range varied from ca. 3 to 0.0015 provirus copies/cell in all samples tested. Especially in brain tissue, characterized by limited BM5d infection, the great sensitivity of the real-time PCR is emphasized by the detection of as few as one or two provirus copies per 1,000 cells in treated samples. A competitive PCR (cPCR) previously described (5) was found to be 100 times less sensitive and to have a smaller linear quantitative range (from 100 to 106 copies) compared to real-time PCR. The real-time PCR results correlate with the results obtained with the cPCR on lymph nodes (percent inhibitions of 76% ± 6% and 80% ± 8% were obtained with real-time PCR and cPCR, respectively [mean ± the SD of five mice] in AZT-treated mice versus infected mice; P > 0.1), and bone marrow (percent inhibitions of 64% ± 3% and 71% ± 5% with real-time PCR and cPCR, respectively; P > 0.1) because the quantifications were in the dynamic range of detection of both assays: in fact, the BM5d copies detected in lymph nodes and bone marrow of infected and treated animals were ca. 104 to 103 and 103 to 102, respectively, for 40 ng of DNA (data not shown). In brain tissue, the lack of correlation (percent inhibitions of 90% ± 6% and 48% ± 23% were obtained, respectively, with real-time PCR and cPCR; P = 2.7 × 10−3) between results obtained by the two methods was due to the low level of infection in this tissue (about 3 copies per 100 cells in infected mice), where a further decline of proviral DNA after antiretroviral therapy brings all samples to the lower limit of detection of cPCR.
FIG. 2.
BM5d provirus load in infected (•) and AZT-DDI-treated (○) mice, as measured by real-time SYBR Green I PCR, in tissues and macrophages as described in the text. The BM5d copies were deduced from linear regression analyses of the standard curve and divided by the actual number of cells, calculated after 18S rRNA gene normalization, to obtain the BM5d provirus copies/cell. Bars show the mean for each group. The real-time PCR performed on mock-infected (control) mice gave negative results.
The present study demonstrates that the real time-PCR is a sensitive, specific, and reproducible assay and offers a significant improvement for the quantitation of provirus load compared to cPCR. We believe that this quantitative protocol is readily adaptable to already-existing pathogen primer sets used for quantification. We plan to apply this flexible, inexpensive, and easy method based on normalization by quantitation of 18S rRNA to human immunodeficiency virus type 1 (HIV-1) provirus DNA load determination in peripheral blood mononuclear cells and tissue reservoirs. The existence of a long-lived reservoir of HIV makes the monitoring of proviral load an important tool for research in the dynamics of HIV-1 replication and viral clearance in patients undergoing antiretroviral therapy and in the rational design of agents that, in conjunction with highly active antiretroviral therapy, can be used to decrease or eliminate infected latent reservoirs (7, 12, 16).
Acknowledgments
This study was supported by Ministero della Sanità, Istituto Superiore di Sanità Progetto AIDS (no. 31D.65).
REFERENCES
- 1.Basham, T., C. D. Rios, T. Holdener, and T. C. Merigan. 1990. Zidovudine (AZT) reduces virus titer, retards immune dysfunction, and prolongs survival in the LP-BM5 murine induced immunodeficiency model. J. Infect. Dis. 161:1006-1009. [DOI] [PubMed] [Google Scholar]
- 2.Broccolo, F., G. Locatelli, L. Sarmati, S. Piergiovanni, F. Veglia, M. Andreoni, S. Butto, B. Ensoli, P. Lusso, and M. S. Malnati. 2002. Calibrated real-time PCR assay for quantitation of human herpesvirus 8 DNA in biological fluids. J. Clin. Microbiol. 40:4652-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casabianca, A., and M. Magnani. 1994. A p12 gag gene homologue is present in the mouse genome. Biochem. Mol. Biol. Int. 32:691-696. [PubMed] [Google Scholar]
- 4.Casabianca, A., C. Orlandi, A. Fraternale, and M. Magnani. 2003. A new one-step RT-PCR method for virus quantitation in murine AIDS. J. Virol. Methods 110:81-90. [DOI] [PubMed] [Google Scholar]
- 5.Casabianca, A., G. Vallanti, and M. Magnani. 1998. Competitive PCR for quantification of BM5d proviral DNA in mice with AIDS. J. Clin. Microbiol. 36:2371-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chattopadhyay, S. K., D. N. Sengupta, T. N. Fredrickson, H. C. Morse III, and J. W. Hartley. 1991. Characteristics and contributions of defective, ecotropic, and mink cell focus-inducing viruses involved in a retrovirus-induced immunodeficiency syndrome of mice. J. Virol. 65:4232-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun, T. W., R. T. Davey, Jr., M. Ostrowski, J. Shawn Justement, D. Engel, J. I. Mullins, and A. S. Fauci. 2000. Relationship between preexisting viral reservoirs and the re-emergence of plasma viremia after discontinuation of highly active anti-retroviral therapy. Nat. Med. 6:757-761. [DOI] [PubMed] [Google Scholar]
- 8.Fraternale, A., A. Casabianca, A. Tonelli, L. Chiarantini, G. Brandi, and M. Magnani. 2001. New drug combinations for the treatment of murine AIDS and macrophage protection. Eur. J. Clin. Investig. 31:248-252. [DOI] [PubMed] [Google Scholar]
- 9.Fraternale, A., A. Casabianca, C. Orlandi, A. Cerasi, L. Chiarantini, G. Brandi, and M. Magnani. 2002. Macrophage protection by addition of glutathione (GSH)-loaded erythrocytes to AZT and DDI in a murine AIDS model. Antivir. Res. 56:263-272. [DOI] [PubMed] [Google Scholar]
- 10.Fraternale, A., A. Casabianca, C. Orlandi, L. Chiarantini, G. Brandi, G. Silvestri, and M. Magnani. 2002. Repeated cycles of alternate administration of fludarabine and zidovudine plus didanosine inhibits murine AIDS and reduces proviral DNA content in lymph nodes to undetectable levels. Virology 302:354-362. [DOI] [PubMed] [Google Scholar]
- 11.Fraternale, A., A. Tonelli, A. Casabianca, G. Vallanti, L. Chiarantini, G. F. Schiavano, U. Benatti, A. De Flora, and M. Magnani. 1999. Role of macrophage protection in the development of murine AIDS. J. Acquir. Immune Defic. Syndr. 21:81-89. [PubMed] [Google Scholar]
- 12.Hamer, D. H., S. Bocklandt, L. McHugh, T. W. Chun, P. M. Blumberg, D. M. Sigano, and V. E. Marquez. 2003. Rational design of drugs that induce human immunodeficiency virus replication. J. Virol. 77:10227-10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvie, P., R. F. Omar, N. Dusserre, N. Lansac, A. Desormeaux, P. Gourde, M. Simard, M. Tremblay, D. Beauchamp, and M. G. Bergeron. 1996. Ribavirin potentiates the efficacy and toxicity of 2′,3′-dideoxyinosine in the murine acquired immunodeficiency syndrome model. J. Pharmacol. Exp. Ther. 279:1009-1017. [PubMed] [Google Scholar]
- 14.Jolicoeur, P. 1991. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. FASEB J. 5:2398-2405. [DOI] [PubMed] [Google Scholar]
- 15.Morse, H. C., III, S. K. Chattopadhyay, M. Makino, T. N. Fredrickson, A. W. Hugin, and J. W. Hartley. 1992. Retrovirus-induced immunodeficiency in the mouse: MAIDS as a model for AIDS. AIDS 6:607-621. [DOI] [PubMed] [Google Scholar]
- 16.Pierson, T., J. McArthur, and R. F. Siliciano. 2000. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu. Rev. Immunol. 18:665-708. [DOI] [PubMed] [Google Scholar]
- 17.Schmittgen, T. D., and B. A. Zakrajsek. 2000. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J. Biochem. Biophys. Methods 46:69-81. [DOI] [PubMed] [Google Scholar]
- 18.Wittwer, C. T., M. G. Herrmann, A. A. Moss, and R. P. Rasmussen. 1997. Continuous fluorescence monitoring of rapid cycle DNA amplification. BioTechniques 22:130-138. [DOI] [PubMed] [Google Scholar]