Abstract
Three strains of cefotaxime (CTX)-resistant Acinetobacter baumannii, FM0209680, FM0300106, and FM0301433, were isolated from transtracheal aspirate cultures of three patients with probable nosocomial infections in a neurosurgery ward in Japan. The CTX MICs for these isolates were greater than 128 μg/ml but were drastically reduced in the presence of 4 μg of clavulanic acid per ml. These strains were also resistant to ceftriaxone, cefpodoxime, and aztreonam but were susceptible to ceftazidime and imipenem. The profile of resistance to various broad-spectrum β-lactams was transferred by conjugation. Strain FM0209680 was not eradicated from case patient 1 by administration of imipenem, ceftazidime, and levofloxacin, even after a 6-month hospitalization period. Strains FM0300106 and FM0301433 were isolated from case patients 2 and 3 during the sixth week following admission, respectively, and then each patient was colonized for 3 weeks. Eradication of FM0300106 was successfully obtained from case patient 2 by imipenem treatment, while administration of imipenem was continued to prevent pneumonia. Prophylactic antimicrobial therapy was discontinued in case patient 3 because of the lack of pneumonic symptoms, and FM0301433 disappeared after the discontinuation of antimicrobial chemotherapy. All three strains carried the blaCTX-M-2 gene, and the appearance of colonies in the growth-inhibitory zones around disks of CTX and aztreonam in double-disk synergy tests suggested inducible β-lactamase production in these A. baumannii strains. The ribotyping investigation suggested that all these strains belong to the same clonal lineage. The plasmids harbored by A. baumannii had the same restriction profile as those harbored by Proteus mirabilis strains previously isolated in a urology ward of the Funabashi Medical Center.
Acinetobacter species, including Acinetobacter baumannii, had been regarded as one of the important groups of opportunistic pathogens implicated in various infections such as pneumonia, urinary tract infection, endocarditis, surgical site infection, meningitis, and septicemia, particularly in immunocompromised patients (4). A. baumannii has recently been rerecognized as an important causative pathogen of nosocomial infections (2). Patients admitted to intensive care units tend to become the main victims of this nosocomial pathogen, which occurs worldwide (12, 22, 29, 54). Increasing therapeutic difficulties due to the acquisition of a profile of multidrug resistance to major groups of antimicrobial agents by various bacterial species have been becoming a serious clinical concern (1, 6, 14, 21, 23, 31, 51). A wide variety of molecular mechanisms for resistance to broad-spectrum β-lactams have been elucidated, i.e., β-lactamase production, mutations of penicillin-binding proteins, and alterations to membrane permeability as well as augmented functioning of the active efflux system (4, 6, 20, 27). Acinetobacter species are renowned for their characteristic nature of readily accepting foreign DNA in order to survive in hazardous environments (15, 36). Various β-lactamases demonstrating broad-spectrum substrate specificities that allow A. baumannii to cope with broad-spectrum β-lactams, such as OXA-type class D β-lactamases (19, 39, 53), metallo-β-lactamases belonging to class B β-lactamases (45, 51, 57), and AmpC-type class C β-lactamases (7), have been found in A. baumannii so far. Moreover, PER-1 and VIB-1 class A β-lactamases demonstrating broad-spectrum substrate specificities have also recently been detected among nosocomially isolated A. baumannii strains in Turkey (52) and were then detected in France (40, 41) and Korea (26).
Since June 2002, the medical microbiology laboratory of the Funabashi Medical Center, Chiba, Japan, isolated three cefotaxime-resistant A. baumannii strains from inpatients in a neurosurgery ward. In the present study, we characterized the molecular mechanism of cefotaxime resistance in A. baumannii strains associated with a nosocomial infection episode.
MATERIALS AND METHODS
Bacterial strains.
Three cefotaxime-resistant A. baumannii strains, FM0209680, FM0300106, and FM0301433, were isolated from cultures of transtracheal aspirates from three different inpatients in the neurosurgery ward of the Funabashi Medical Center since June 2002. Biochemical identification of the isolates was performed with the API 20NE system (bioMérieux, Marcy l'Etoile, France) combined with a complementary test for the ability to grow at 44°C. Alternatively, sequencing of the 16S rRNA gene was performed by the method described by Sasaki et al. (46). β-Lactamase testing was performed on the basis of acidometry by using a commercial product (P/Case test; Nissui Pharmaceutical, Tokyo, Japan). The bacterial strains were stored in Casitone medium (Eiken Chemical, Tokyo, Japan) at room temperature until they were used.
MIC determinations.
MICs were determined by a microdilution broth method with a WalkAway-96 SI system (NEG Combo 5J and NEG MIC 5J panels; Dade Behring, Sacramento, Calif.) with an inoculum of 104 CFU per well. Susceptibility categories were determined according to the criteria of the National Committee for Clinical Laboratory Standards (35).
The ESBL plus panel (Dade Behring) with an inoculum of 104 CFU per well was used as complementary test for MIC measurements. The panel was incubated for 18 h at 35°C, and then the results were assessed visually.
β-Lactamase study.
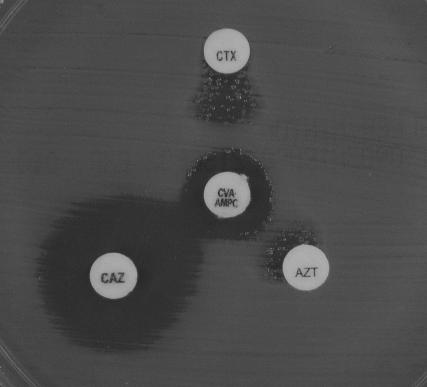
The double-disk synergy test was used to screen for extended-spectrum β-lactamase (ESBL)-producing strains. Kirby-Bauer disks containing cefotaxime (30 μg), ceftazidime (30 μg), aztreonam (30 μg), and amoxicillin-clavulanic acid (20 μg-10 μg) for tests on Mueller-Hinton agar (BBL Microbiology Systems, Cockeysville, Md.) were obtained from Nissui Pharmaceuticals. The distance between the disks was adjusted so that synergy could be detected correctly (48), as shown in Fig. 1.
FIG. 1.
Double-disk synergy test with CTX-M-2 ESBL-producing A. baumannii FM0209680. Disks: CTX, cefotaxime at 30 μg; CAZ, ceftazidime at 30 μg; AZT, aztreonam at 30 μg; CVA · AMPC, clavulanic acid at 10 μg and amoxicillin at 20 μg.
The inducibility of AmpC β-lactamase production was tested by disk antagonism tests (30). Disks containing an inducing agent, cefoxitin at 30 μg or ceftazidime at 30 μg, were placed on Mueller-Hinton agar plates (BBL Microbiology Systems). The distance between the disks was adjusted so that the blunting phenomenon of the ceftazidime zone could be detected correctly.
PCR amplification and bla gene sequencing.
The oligonucleotides used as primers for amplification and sequencing are shown in Table 1. A search for the blaTEM (49), blaSHV (41), blaCTX-M-1 (3), blaCTX-M-2 (24), and blaCTX-M-9 (32) genes in the clinical isolates was performed by PCR amplification, as described previously (34). Detection of the ampC gene was performed as described by Bou and Martínez-Beltrán (7).
TABLE 1.
Nucleotide sequences of the oligonucleotides used for PCR amplification and DNA sequencing
| Procedure and gene | Primer | Expected size of PCR amplicon (bp) | Reference |
|---|---|---|---|
| Amplification | |||
| blaTEM-1 | 5′-CCG TGT CGC CCT TAT TCC-3′ | 824 | 50 |
| 5′-AGG CAC CTA TCT CAG CGA-3′ | |||
| blaSHV-1 | 5′-ATT TGT CGC TTC TTT ACT CGC-3′ | 1,051 | 42 |
| 5′-TTT ATG GCG TTA CCT TTG ACC-3′ | |||
| blaCTX-M-1 | 5′-CGG TGC TGA AGA AAA GTG-3′ | 354 | 3 |
| 5′-TAC CCA GCG TCA GAT TAC-3′ | |||
| blaCTX-M-2 | 5′-ACG CTA CCC CTG CTA TTT-3′ | 780 | 24 |
| 5′-GCT TTC CGC CTT CTG CTC-3′ | |||
| blaCTX-M-9 | 5′-GCA GAT AAT ACG CAG GTG-3′ | 393 | 32 |
| 5′-CGG CGT GGT GGT GTC TCT-3′ | |||
| blaAmpC | 5′-ACT TAC TTC AAC TCG CGA CG-3′ | 663 | 7 |
| 5′-TAA ACA CCA CAT ATG TTC CG-3′ | |||
| Sequencing, blaCTX-M-2 | 5′-TTA ATG ATG ACT CAG AGC ATT C-3′ | 902 | 43 |
| 5′-GAT ACC TCG CTC CAT TTA TTG-3′ |
A resistance plasmid carrying the blaCTX-M-2 gene was prepared from the Escherichia coli CSH2 (F− metB, with resistance to both nalidixic acid and rifampin [18]) transconjugant. The 902-bp fragments corresponding to the main frame of the blaCTX-M-2 gene were amplified by PCR with a set of primers (43) and were used for sequencing. The PCR products were labeled with fluorescent materials by using a BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems, Foster City, Calif.). Both strands of the DNA sequences were analyzed with an ABI PRISM 377 XL sequencer analyzer (Applied Biosystems). The nucleotide and deduced amino acid sequences were analyzed and compared with sequences in a database by using the FASTA analysis programs available at the National Institute of Genetics (Mishima, Japan) website (http://www.nig.ac.jp/section/service.html).
Plasmid preparation and restriction endonuclease analysis.
The resident plasmids of three A. baumannii strains and previously isolated Proteus mirabilis strains (34) were prepared by a conventional protocol (38). The plasmids were digested with EcoRI, and the restriction fragment profiles were compared by agarose gel electrophoresis.
Automated ribotyping and analysis.
Because pulsed-field gel electrophoresis analysis failed to give clear results due to the persistent degradation of DNA, we used automated ribotyping for analysis of the genetic relatedness of the three clinical isolates. Ribotyping was performed with the restriction enzyme EcoRI and a RiboPrinter (Qualicon Inc., Wilmington, Del.), as described previously (13, 33). Briefly, a single colony from a 5% sheep blood agar plate was suspended in a sample buffer and heat treated at 80°C for 15 min. After addition of lysis buffer to release the DNA, the sample was loaded into the RiboPrinter system. Further processing including EcoRI digestion, agarose gel separation, transfer to a nylon membrane, and hybridization with a chemiluminescence-labeled DNA probe containing the rRNA operon from E. coli, which was carried out by automated instruments in 8 h. Output ribotype patterns with similarity coefficients of >0.93 were considered a single ribogroup and were automatically given a code number. Computerized ribotypes were finally exported for analysis in text files and imported into BioNumerics software (version 3.0; Applied Maths, Sint-Martens-Latem, Belgium) by using the Qualicon macro. Clustering analysis was performed by the unweighted pair group with arithmetic averages method (48), based on the Dice coefficient (16) for band matching, with a position tolerance setting of 1.0% (default values are 1% position tolerance and 0.5% optimization). Bands for analysis with the Dice coefficient were assigned manually, according to densitometric curves and the accompanying hard-copy photograph.
RESULTS
Identification of A. baumannii isolates and their clinical associations.
Strain FM0209680 was derived from the culture of a transtracheal aspirate from a 24-year-old male patient (case patient 1) from whom the first cefotaxime-resistant A. baumannii strain was isolated on 19 June 2002. This patient was admitted to the neurosurgery ward on 14 June with a pneumonia-related condition and had a history as a patient in the same ward in October 1999 due to hemorrhaging of the brain caused by underlying cerebral arteriovenous malformation. Until his hospital discharge, on 6 January 2003, cefotaxime-resistant A. baumannii isolates were recurrently and predominantly detected in cultures of transtracheal aspirates from the patient. These data point out the great difficulty in eradicating the organisms. Strain FM0300106 was then recovered from the culture of a transtracheal aspirate from a 62-year-old female patient (case patient 2) who had been hospitalized in the neurosurgery ward due to brainstem infarction on 24 November 2002. The cefotaxime-resistant A. baumannii strain was first and predominantly detected in the specimen for culture taken on 6 January 2003, although it had not been found in a total of six samples for culture taken up to that time. Strain FM0301433 was recovered from the culture of a transtracheal aspirate from a 65-year-old male patient (case patient 3) who had been hospitalized in the neurosurgery ward due to cerebral hemorrhage on 27 December 2002. As was noted for case patient 2, the cefotaxime-resistant A. baumannii strain was first detected predominantly in the specimen for culture taken on 12 February 2003, although it had not been found in a total of seven specimens for culture obtained up to that time. In both case patients 2 and 3, the eradication of the cefotaxime-resistant A. baumannii strains was achieved within 3 weeks after the strains were first detected. Imipenem therapy was used for case patient 2 because a complication of pneumonia was a serious concern and the organisms disappeared. The cefotaxime-resistant A. baumannii strain was isolated from case patient 3 during prophylactic antimicrobial therapy with gentamicin and cefazolin. Administration of cefazolin may have induced colonization with this organism in case patient 3, in view of the fact that strain FM0301433 was resistant to cefazolin. The prophylactic therapy was then discontinued because no pneumonic clinical findings were noted, and, fortunately, the organism disappeared after 3 weeks.
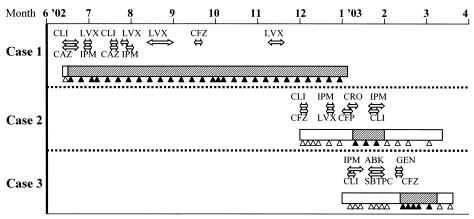
The history of antimicrobial therapy before isolation of cefotaxime-resistant A. baumannii was untraceable for case patient 1 because this patient was transferred from another hospital to the Funabashi Medical Center. Sultamicillin, cefazolin, ceftriaxone, imipenem-cilastatin, cefoperazone-sulbactam, levofloxacin, clindamycin, gentamicin, and arbekacin were used prior to the isolation of A. baumannii in case patients 2 and 3. The sequence of isolation of the three cefotaxime-resistant A. baumannii strains, as well as the antimicrobial agents prescribed for the three case patients, are summarized in Fig. 2.
FIG. 2.
Sequence of isolation of cefotaxime-resistant A. baumannii strains as well as the antimicrobial agents prescribed for three patients. Rectangles, periods of hospitalization; black triangles, times of isolation of cefotaxime-resistant A. baumannii from transtracheal aspirate cultures; open triangles, times that cefotaxime-resistant A. baumannii strains from transtracheal aspirate cultures were negative; hatched rectangles, times of probable colonization or infection with CTX-M-2 producers; double-headed arrows, prescription periods during which each antimicrobial agent was prescribed; CLI, clindamycin; LVX, levofloxacin; CFZ, cefazolin; CAZ, ceftazidime; IPM, imipenem-cilastatin; CRO, ceftriaxone; CFP, cefoperazone-sulbactam; ABK, arbekacin; GEN, gentamicin; SBTPC, sultamicillin.
Three isolates, FM0209680, FM0300106, and FM0301433, which shared the same biochemical profile code, 0041073, by use of the API NE20 system, combined with a positive result for the ability to grow at 44°C as a complementary test, were eventually identified as A. baumannii. The 16S rRNA nucleotide sequences of these microorganisms shared 99% identity with that of A. baumannii type strain ATCC 19606 deposited in the EMBL database (accession number Z93435).
Antibiotic susceptibilities.
The antibiotic susceptibility profiles of all three A. baumannii strains are shown in Table 2. These strains were broadly resistant to penicillins, cephalosporins, cephamycins, and monobactam but were highly susceptible to carbapenems. For all strains, addition of 4 μg of clavulanic acid per ml to the medium for antimicrobial susceptibility testing significantly decreased the MICs of cefotaxime from >128 μg/ml to 2 to 8 μg/ml, while the ceftazidime MICs decreased modestly from 8 μg/ml to 1 to 4 μg/ml.
TABLE 2.
Antibiotic susceptibilities of A. baumannii clinical isolates
| Antibiotica | MIC (μg/ml)b
|
||
|---|---|---|---|
| FM0209680 | FM0300106 | FM0301433 | |
| Ampicillin | >16 | >16 | >16 |
| Amoxicillin-CLA | 8/4 | 8/4 | 16/8 |
| Piperacillin | >64 | >64 | >64 |
| Cefazolin | >16 | >16 | >16 |
| Cefotiam | >16 | >16 | >16 |
| Cefoperazon-SUL | ≤16/8 | ≤16/8 | 32/16 |
| Cefotaxime | >128 | >128 | >128 |
| Cefotaxime-CLA | 2 | 8 | 4 |
| Ceftazidime | 8 | 8 | 8 |
| Ceftazidime-CLA | 1 | 4 | 2 |
| Ceftriaxone | >64 | >64 | >64 |
| Cefpirome | >16 | >16 | >16 |
| Cefepime | >32 | >32 | >32 |
| Cefozopran | >16 | >16 | >16 |
| Cefaclor | >16 | >16 | >16 |
| Cefpodoxime | >64 | >64 | >64 |
| Cefoxitin | >32 | >32 | >32 |
| Cefmetazole | 32 | 32 | >32 |
| Cefotetan | 32 | 32 | 16 |
| Flomoxef | 16 | 16 | 16 |
| Imipenem | ≤0.5 | ≤0.5 | ≤0.5 |
| Meropenem | 1 | 1 | 1 |
| Aztreonam | >64 | >64 | >64 |
| Gentamicin | >8 | >8 | 2 |
| Amikacin | 32 | >32 | 16 |
| Minocycline | 4 | 4 | 4 |
| Levofloxacin | 4 | 4 | 2 |
| Fosfomycin | >16 | >16 | >16 |
CLA, clavulanic acid at a fixed concentration of 4 μg/ml; SUL, sulbactam.
NEG Combo 5J and NEG MIC 5J panels and ESBL plus panel were used for MICs determinations.
β-Lactamase production.
The production of β-lactamase was initially judged by the P/Case test (37), which can discriminate between penicillinase (with 1.5 mg of benzylpenicillin as the substrate) and cephalosporinase (with 1.5 mg of cephaloridine and 75 μg of clavulanic acid as the substrate). According to the instructions of the manufacturer, the bacterial colonies are rubbed onto two indicator disks containing benzylpenicillin and cephaloridine with clavulanic acid, respectively. When the strain produces any class A β-lactamase, such as TEM- or SHV-derived β-lactamases or CTX-M-type enzymes, a disk containing benzylpenicillin turns yellow, while the other disk containing cephaloridine with clavulanic acid remains purple, because hydrolysis of cephaloridine by the class A β-lactamases is blocked in the presence of clavulanic acid. If the strain produces class C β-lactamases, the disk containing cephaloridine with clavulanic acid turns yellow, because these enzymes are not blocked by clavulanic acid. The results of the test suggested that all three A. baumannii strains produce both class A and class C β-lactamases.
The isolates suggested to produce class A β-lactamases were investigated further to determine whether they produce ESBLs by the double-disk synergy test. The presence of an ESBL was suggested by the synergy between amoxicillin-clavulanic acid and cefotaxime, ceftazidime, and aztreonam against all three strains. However, growth of scattered colonies was also noted within the expanded growth-inhibitory zones around the cefotaxime and aztreonam disks (Fig. 1). Enhanced production of β-lactamases as well as alterations in membrane protein composition might be implicated in this phenomenon. A significant decrease in the MICs of cefotaxime from >128 μg/ml to 2 to 8 μg/ml in the presence of 4 μg of clavulanic acid per ml (Table 2) suggested the production of some CTX-M-type class A β-lactamases by these isolates.
As described above, the P/Case test suggested that these isolates produce an AmpC cephalosporinase, and the ampC gene (7) was actually detected as a 662-bp PCR amplicon in all three strains, although no obvious inducibility of AmpC β-lactamases was detected in any of them by cefoxitin-ceftazidime disk antagonism tests (data not shown).
No amplicons for the blaTEM, blaSHV, blaCTX-M-1, and blaCTX-M-9 genes were detected in any of the strains except strain FM0301433, which produced an 824-bp amplicon for the blaTEM gene.
Sequencing of blaCTX-M-2 gene and plasmid analysis.
In a preliminary search by PCR, 780-bp amplification products specific for the blaCTX-M-2 genes were observed in all three A. baumannii strains. The nucleotide sequence of the 902-bp amplicon encoding a full-length bla gene, which was amplified with a set of PCR primers (43), was exactly the same as that of the blaCTX-M-2 gene (GenBank accession number X92507), suggesting that all these strains certainly harbor the blaCTX-M-2 gene. The nucleotide sequences of the blaCTX-M-2 gene harbored by the A. baumannii strains were exactly the same as that of a P. mirabilis strain previously isolated in the urology ward of the Funabashi Medical Center (34). Moreover, the blaCTX-M-2-bearing plasmids prepared from three A. baumannii strains were digested into four different EcoRI fragments of approximately 6.6, 6.4, 4.1, and 3.8 kb (data not shown). The restriction fragment profiles of the plasmids were exactly the same as those of plasmids previously isolated from P. mirabilis strains (data not shown).
Ribotyping.
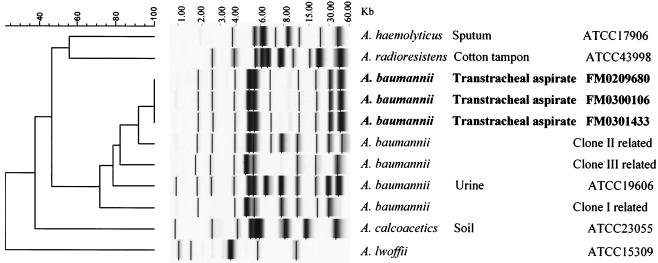
In order to investigate the genetic relationship among FM0209680, FM0300106, and FM0301433, the isolates were analyzed by automated ribotyping. Figure 3 shows the results of a clustering analysis performed with the ribotype patterns of the three isolates and some collection strains of the genus Acinetobacter, including strains with patterns related to those of European outbreak clones I and II (11, 17) and a newly described clone, clone III (S. Brisse, personal communication). The three A. baumannii strains shared the same fingerprint pattern, indicating a clonal lineage. Moreover, the patterns of the three strains showed a high degree of similarity (92%) with those of a clone II strain. Nevertheless, the patterns of all three strains were different from those of the European clones, which, it has been suggested (11), are probably widespread worldwide.
FIG. 3.
EcoRI ribotypes of the three cefotaxime-resistant A. baumannii clinical isolates. A comparative analysis of these isolates with some collection strains of the genus Acinetobacter, including European outbreak clones I, II, and III of A. baumannii, is shown. Clustering was performed by the unweighted pair group method with arithmetic averages, and similarity analysis was based on the use of the Dice coefficient.
DISCUSSION
This report describes the probable nosocomial transmission of CTX-M-2 β-lactamase-producing A. baumannii strains in a neurosurgery ward, where the majority of inpatients are represented by immunocompromised hosts who have undergone respiratory manipulations, such as a tracheotomy or mechanical ventilation, and where careful and strict monitoring is provided. For case patient 1, a CTX-M-2-producing A. baumannii strain was first isolated on day 5 after admission. During the 6-month hospitalization of this patient, cefotaxime-resistant A. baumannii strains were detected continuously, despite the administration of imipenem, ceftazidime, and levofloxacin, which consequently failed to eradicate the microorganism. For case patients 2 and 3, CTX-M-2-producing A. baumannii strains were detected at the sixth week after admission, and the patients were then colonized for 3 weeks. Imipenem treatment was used to eradicate A. baumannii from case patient 2 because a high risk of pneumonia was a serious concern in this patient. On the other hand, prophylactic therapy was discontinued in case patient 3 because no pneumonic clinical findings appeared and the microorganism consequently disappeared. As shown in Fig. 2, it seems very likely that an A. baumannii strain harboring the blaCTX-M-2 gene was persistently transmitted from case patient 1 to case patient 3 via case patient 2 over a period of 9 months in the same neurosurgery ward.
The three A. baumannii strains, strains FM0209680, FM0300106, and FM0301433, shared the same ribotype pattern, suggesting a clonal lineage. For all three patients, multiple bacterial species other than A. baumannii were frequently coisolated throughout the periods of hospitalization, but fortunately, none was a CTX-M-2 producer. The genes for CTX-M-type β-lactamases are often encoded on plasmids and are easily transmitted among gram-negative bacilli, including members of the family Enterobacteriaceae (5, 38), and glucose nonfermenters, such as Pseudomonas aeruginosa (8). Since no CTX-M-2 ESBL-producing bacterial isolates, including A. baumannii, were previously detected in the neurosurgery ward, it is likely that case patient 1 already carried a CTX-M-2-producing A. baumannii isolate before his rehospitalization. However, in the Funabashi Medical Center, a previous outbreak of nosocomial infections in the urology ward, located on a different floor from the neurosurgery ward, involved 19 inpatients and was related to CTX-M-2-producing P. mirabilis (34). Agarose gel electrophoresis demonstrated that the blaCTX-M-2-bearing plasmids found in both bacterial species had the same EcoRI digestion pattern. Therefore, this finding strongly suggests the possibility of the lateral transfer of the plasmids carrying the blaCTX-M-2 gene between P. mirabilis and A. baumannii in the clinical setting described here.
A. baumannii strains which produce plasmid-mediated CTX-M-type β-lactamases or TEM- and SHV-derived ESBLs are still very rare. The class A β-lactamases PER-1 and VEB-1, which are genetically distant from the predominant TEM- and SHV-derived ESBLs and CTX-M-type enzymes, were first reported among nosocomial A. baumannii isolates in Turkish and French hospitals (41, 52). Similar strains have subsequently been isolated in France (40) and Korea (26). Among the CTX-M-type β-lactamases, the CTX-M-5 gene has been found in A. baumannii (GenBank accession number AF462635); however, no details have been published to date. A. baumannii usually produces a chromosomally encoded AmpC cephalosporinase, but this kind of enzyme generally cannot hydrolyze oxyiminocephalosporins, cephamycins, or carbapenems. Therefore, acquisition of plasmid-mediated enzymes with broad- and extended-spectrum substrate specificities could well allow this bacterial species to survive in present clinical environments. A. baumannii has become one of the major groups of bacteria that causes respiratory infections, especially among patients in intensive care units. Thus, the emergence of CTX-M-2-producing A. baumannii strains could become a serious clinical problem in Japan, because CTX-M-2-producing as well as CTX-M-1-producing microorganisms have already been frequently found among clinical isolates from humans (5, 25, 55) and cattle (47).
Early outbreaks due to β-lactamase-producing strains resulted in epidemics caused by isolates with only a single β-lactamase. However, more complex situations involving multiple-β-lactamase-producing microorganisms have been documented in recent nosocomial outbreaks (7, 9, 10, 56). The three A. baumannii strains reported in the present study produced two β-lactamases, the CTX-M-2 enzyme as well as the AmpC β-lactamase. Moreover, the third strain also produced a TEM-type penicillinase. In the present study, the induction of β-lactamase production in A. baumannii strains was suggested by the growth of colonies within the growth-inhibitory zones around cefotaxime and aztreonam disks. Such inducible class C β-lactamase production by A. baumannii was not detected by previously reported disk antagonism tests with cefoxitin and ceftazidime disks (30), although the presence of the ampC gene was confirmed by PCR. Thus, it is likely that some of the colonies appearing in the growth-inhibitory zone around the cefotaxime and aztreonam disks might be mainly constitutive CTX-M-2 producers. It is also possible that these colonies corresponded to AmpC-hyperproducing mutants in which the regulatory system of AmpC production was disrupted through mutations in the ampC promoter region or in genes implicated in the regulation of ApmC production, such as ampR, ampG, and ampD (28). These mutations might be easily induced in A. baumannii in the presence of cefotaxime, aztreonam, and clavulanic acid. Moreover, all three A. baumannii strains characterized in this study demonstrated resistance to cephamycins, such as cefmetazole, cefoxitin, and cefotetan. This may depend on a continuous and moderate level of production of chromosomal AmpC cephalosporinase as well as alterations in bacterial membrane permeability (44). Functional analysis of the Acinetobacter AmpC cephalosporinase and further characterization of the nature of the bacterial cells appearing around cefotaxime and aztreonam disks will be continued.
A. baumannii tends to accept foreign DNA as a means to adapt to environments hazardous for bacterial growth (15, 36) and has a high survival capacity in any environment with damp conditions and low temperatures. This inherent ability of A. baumannii may facilitate the development of a multidrug resistance profile with the widespread use of antimicrobial agents through the selection of strains with ever accumulating antibiotic resistance profiles in hospital environments. Thus, the emergence of CTX-M-2-producing A. baumannii strains not only may confer the potential for epidemics but also could serve as reservoirs for the plasmid-dependent CTX-M-2 enzyme for dispersal of the blaCTX-M-2 gene among different gram-negative bacterial species. Indeed, although it might be difficult to detect the coproduction of class A β-lactamases like CTX-M-type enzymes in intrinsically cephalosporin-resistant A. baumannii, knowledge of the presence of these burdensome strains would seem to be very important in achieving suitable infection control measures in hospitals on a daily basis.
Acknowledgments
We are grateful to the following for their contributions to infection control at the Funabashi Medical Center: T. Karasawa, Department of Neurosurgery; Y. Yasutoshi, H. Tabeta, H. Kamii, and M. Shoji, Infection Control Team; and Y. Saito, N. Suwa, and M. Toyama, Medical Microbiology Laboratory. We also thank S. Brisse, Unité Biodiversité des Bactéries Pathogèenes Emergentes, Institut Pasteur, for allowing us access to the Acinetobacter ribotypes patterns from the Network for Automated Bacterial Strain Fingerprinting in Europe (GENE project, European Union Fifth Framework Programme).
This work was supported in part by grants H15-Shinkou-9 and H15-Shinkou-10 from the Ministry of Health, Labor and Welfare of Japan.
REFERENCES
- 1.Afzal-Shah, M., and D. M. Livermore. 1998. Worldwide emergence of carbapenem-resistant Acinetobacter spp. J. Antimicrob. Chemother. 41:576-577. [DOI] [PubMed] [Google Scholar]
- 2.Ayan, M., R. Durmaz, E. Aktas, and B. Durmaz. 2003. Bacteriological, clinical and epidemiological characteristics of hospital-acquired Acinetobacter baumannii infection in a teaching hospital. J. Hosp. Infect. 54:39-45. [DOI] [PubMed] [Google Scholar]
- 3.Bauernfeind, A., I. Stemplinger, R. Jungwirth, S. Ernst, and J. M. Casellas. 1996. Sequences of β-lactamase genes encoding CTX-M-1 (MEN-1) and CTX-M-2 and relationship of their amino acid sequences with those of other β-lactamases. Antimicrob. Agents Chemother. 40:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergogne-Berezin, E., and K. J. Towner. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet, R. 2004. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob. Agents Chemother. 48:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bou, G., G. Cerveró, M. A. Domínguez, C. Quereda, and J. Martínez-Beltrán. 2000. Characterization of a nosocomial outbreak caused by a multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: high-level carbapenem resistance in A. baumannii is not due solely to the presence of β-lactamases. J. Clin. Microbiol. 38:3299-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bou, G., and J. Martínez-Beltrán. 2000. Cloning, nucleotide sequencing, and analysis of the gene encoding an AmpC β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 44:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradford, P. A. 2001. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin. Microbiol. Rev. 14:933-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradford, P. A., C. E. Cherubin, V. Idemyor, B. A. Rasmussen, and K. Bush. 1994. Multiply resistant Klebsiella pneumoniae strains from two Chicago hospitals: identification of the extended-spectrum TEM-12 and TEM-10 ceftazidime-hydrolyzing β-lactamases in a single isolate. Antimicrob. Agents Chemother. 38:761-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradford, P. A., C. Urban, A. Jaiswal, N. Mariano, B. A. Rasmussen, S. J. Projan, J. J. Rahal, and K. Bush. 1995. SHV-7, a novel cefotaxime-hydrolyzing β-lactamase, identified in Escherichia coli isolates from hospitalized nursing home patients. Antimicrob. Agents Chemother. 39:899-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brisse, S., D. Milatovic, A. C. Fluit, K. Kusters, A. Toelstra, J. Verhoef, and F. Schmitz. 2000. Molecular surveillance of European quinolone-resistant clinical isolates of Pseudomonas aeruginosa and Acinetobacter spp. using automated ribotyping. J. Clin. Microbiol. 38:3636-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbella, X., A. Montero, M. Pujol, M. A. Domínguez, J. Ayats, M. J. Argerich, F. Garrigosa, J. Ariza, and F. Gudiol. 2000. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J. Clin. Microbiol. 38:4086-4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordevant, C., J. S. Tang, D. Cleland, and M. Lange. 2003. Characterization of members of the Legionellaceae family by automated ribotyping. J. Clin. Microbiol. 41:34-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva, G. J., G. J. Leitao, and L. Peixe. 1999. Emergence of carbapenem-hydrolyzing enzymes in Acinetobacter baumannii clinical isolates. J. Clin. Microbiol. 37:2109-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vries. J., and W. Wackernagel. 2002. Integration of foreign DNA during natural transformation of Acinetobacter sp. by homology-facilitated illegitimate recombination. Proc. Natl. Acad. Sci. USA 99:2094-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dice, L. R. 1945. Measures of the amount of ecological associations between species. J. Ecol. 26:297-302. [Google Scholar]
- 17.Dijkshoorn, L., H. Aucken, P. Gerner-Smidt, P. Janssen, M. E. Kaufmann, J. Garaizar, J. Ursing, and T. L. Pitt. 1996. Comparison of outbreak and nonoutbreak Acinetobacter baumannii strains by genotypic and phenotypic methods. J. Clin. Microbiol. 34:1519-1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doi, Y., N. Shibata, K. Shibayama, K. Kamachi, H. Kurokawa, K. Yokoyama, T. Yagi, and Y. Arakawa. 2002. Characterization of a novel plasmid-mediated cephalosporinase (CMY-9) and its genetic environment in an Escherichia coli clinical isolate. Antimicrob. Agents Chemother. 46:2427-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donald, H. M., W. Scaife, S. G. B. Amyes, and H. K. Young. 2000. Sequence analysis of ARI-1, a novel OXA β-lactamase responsible for imipenem resistance in Acinetobacter baumannii 6B92. Antimicrob. Agents Chemother. 44:196-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernández-Cuenca, F., L. Martínez-Martínez, M. C. Conejo, J. A. Ayala, E. J. Perea, and A. Pascual. 2003. Relationship between β-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:565-574. [DOI] [PubMed] [Google Scholar]
- 21.Fierobe, L., J. C. Lucet, D. Decre, C. Muller-Serieys, A. Deleuze, M. L. Joly-Guillou, J. Mantz, and J. M. Desmonts. 2001. An outbreak of imipenem-resistant Acinetobacter baumannii in critically ill surgical patients. Infect. Control Hosp. Epidemiol. 22:35-40. [DOI] [PubMed] [Google Scholar]
- 22.Forster, D. H., and F. D. Daschner. 1998. Acinetobacter species as nosocomial pathogens. Eur. J. Clin. Microbiol. Infect. 17:73-77. [DOI] [PubMed] [Google Scholar]
- 23.Go, E. S., C. Urban, J. Burns, B. Kreiswirth, W. Eisner, N. Mariano, K. Mosinka-Snipas, and J. J. Rahal. 1994. Clinical and molecular epidemiology of Acinetobacter infections sensitive only to polymyxin B and sulbactam. Lancet 344:1329-1332. [DOI] [PubMed] [Google Scholar]
- 24.Ishii, Y., A. Ohno, H. Taguchi, S. Imajo, M. Ishiguro, and H. Matsuzawa. 1995. Cloning and sequence of the gene encoding a cefotaxime-hydrolyzing class A β-lactamase isolated from Escherichia coli. Antimicrob. Agents Chemother. 39:2269-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komatsu, M., N. Ikeda, M. Aihara, Y. Nakamachi, S. Kinoshita, K. Yamasaki, and K. Shimakawa. 2001. Hospital outbreak of MEN-1-derived extended spectrum β-lactamase-producing Klebsiella pneumoniae. J. Infect. Chemother. 7:94-101. [DOI] [PubMed] [Google Scholar]
- 26.Kwon, N. Y., J. D. Kim, and H. J. Pai. 2002. The resistance mechanism of β-lactam antimicrobials in clinical isolates of Acinetobacter baumannii. Korean J. Intern. Med. 17:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limansky, A. S., M. A. Mussi, and A. M. Viale. 2002. Loss of a 29-kilodalton outer membrane protein in Acinetobacter baumannii is associated with imipenem resistance. J. Clin. Microbiol. 40:4776-4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindquist, S., M. Galleni, F. Lindberg, and S. Normark. 1989. Signalling proteins in enterobacterial AmpC β-lactamase regulation. Mol. Microbiol. 3:1091-1102. [DOI] [PubMed] [Google Scholar]
- 29.Ling, M. L., A. Ang, M. Wee, and G. C. Wang. 2001. A nosocomial outbreak of multiresistant Acinetobacter baumannii originating from an intensive care unit. Infect. Control Hosp. Epidemiol. 22:48-49. [DOI] [PubMed] [Google Scholar]
- 30.Livermore, D. M., and D. F. J. Brown. 2001. Detection of β-lactamase-mediated resistance. J. Antimicrob. Chemother. 48(Suppl. S1):59-64. [DOI] [PubMed] [Google Scholar]
- 31.Lyytikäinen, O., S. Köljalg, M. Härmä, and J. Vuopio-Varvika. 1995. Outbreak caused by two multiresistant Acinetobacter baumannii clones in a burns unit: emergence of resistance to imipenem. J. Hosp. Infect. 31:41-54. [DOI] [PubMed] [Google Scholar]
- 32.Ma, L., Y. Ishii, M. Ishiguro, H. Matsuzawa, and K. Yamaguchi. 1998. Cloning and sequencing of the gene encoding Toho-2, a class A β-lactamase preferentially inhibited by tazobactam. Antimicrob. Agents Chemother. 42:1181-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagano, N., J. Sato, C. Cordevant, Y. Nagano, F. Taguchi, and M. Inoue. 2003. Presumed endocarditis caused by BRO β-lactamase-producing Moraxella lacunata in an infant with Fallot's tetrad. J. Clin. Microbiol. 41:5310-5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagano, N., N. Shibata, Y. Saitou, Y. Nagano, and Y. Arakawa. 2003. Nosocomial outbreak of infections by Proteus mirabilis that produces extended-spectrum CTX-M-2 type β-lactamase. J. Clin. Microbiol. 41:5530-5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Committee for Clinical Laboratory Standards. 2002. Performance standards for antimicrobial susceptibility testing. Twelfth informational supplement. Approved standard M100-S12. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 36.Nielsen, K. M., M. D. van Weerelt, T. N. Berg, A. M. Bones, A. N. Hagler, and J. D. van Elsas. 1997. Natural transformation and availability of transforming DNA to Acinetobacter calcoaceticus in soil microcosms. Appl. Environ. Microbiol. 63:1945-1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamoto, R., and M. Inoue. 1995. P/Case test for β-lactamase detection. Media Circle 40:173-180. (In Japanese.) [Google Scholar]
- 38.Pagani, L., E. Dell'Amico, R. Migliavacca, M. M. D'Andrea, E. Giacobone, G. Amicosante, E. Romero, and G. M. Rossolini. 2003. Multiple CTX-M-type extended-spectrum β-lactamases in nosocomial isolates of Enterobacteriaceae from a hospital in northern Italy. J. Clin. Microbiol. 41:4264-4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paton, R., R. S. Miles, J. Hood, and S. G. B. Amyes. 1993. ARI-1: β-lactamase mediated imipenem resistance in Acinetobacter baumannii. Int. J. Antimicrob. Agents 2:81-88. [DOI] [PubMed] [Google Scholar]
- 40.Poirel, L., A. Karim, A. Mercat, I. Le Thomas, H. Vahaboglu, C. Richard, and P. Nordmann. 1999. Extended-spectrum β-lactamase-producing strain of Acinetobacter baumannii isolated from a patient in France. J. Antimicrob. Chemother. 43:157-165. [PubMed] [Google Scholar]
- 41.Poirel, L., O. Menuteau, N. Agoli, C. Cattoen, and P. Nordmann. 2003. Outbreak of extended-spectrum β-lactamase VEB-1-producing isolates of Acinetobacter baumannii in a French hospital. J. Clin. Microbiol. 41:3542-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prinarakis, E. E., E. Tzelepi, M. Gazouli, A. F. Mentis, and L. S. Tzouvelekis. 1996. Characterization of a novel SHV β-lactamase variant that resembles the SHV-5 enzyme. FEMS Microbiol. Lett. 139:229-234. [DOI] [PubMed] [Google Scholar]
- 43.Radice, M., C. Gonzealez, P. Power, M. C. Vidal, and G. Gutkind. 2001. Third-generation cephalosporin resistance in Shigella sonnei, Argentina. Emerg. Infect. Dis. 7:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raimondi, A., F. Sisto, and H. Nikaido. 2001. Mutation in Serratia marcescens AmpC β-lactamase producing high-level resistance to ceftazidime and cefpirome. Antimicrob. Agents Chemother. 45:2331-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riccio, M. L., N. Franceschini, L. Boschi, B. Caravelli, G. Cornaglia, R. Fontana, G. Amicosante, and G. M. Rossolini. 2000. Characterization of the metallo-β-lactamase determinant of Acinetobacter baumannii AC-54/97 reveals the existence of blaIMP allelic variants carried by gene cassettes of different phylogeny. Antimicrob. Agents Chemother. 44:1229-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sasaki, T., T. Nishiyama, M. Shintani, and T. Kenri. 1997. Evaluation of a new method for identification of bacteria based on sequence homology of 16S rRNA gene. PDA J. Pharm. Sci. Technol. 51:242-247. [PubMed] [Google Scholar]
- 47.Shiraki, Y., N. Shibata, Y. Doi, and Y Arakawa. 2004. Escherichia coli producing CTX-M-2 β-lactamase in cattle, Japan. Emerg. Infect. Dis. 10:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sirot, J. 1996. Detection of extended-spectrum plasmid-mediated β-lactamases by disk diffusion. Clin. Microbiol. Infect. 2:S35-S39. [DOI] [PubMed] [Google Scholar]
- 49.Sneath, P. H. A., and R. R. Sokal. 1973. Numerical taxonomy. W. H. Freeman & Company, San Francisco, Calif.
- 50.Sutcliffe, J. G. 1978. Nucleotide sequence of the ampicillin resistance gene of Escherichia coli plasmid pBR322. Proc. Natl. Acad. Sci. USA 75:3737-3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi, A., S. Yomoda, I. Kobayashi, T. Okubo, M. Tsunoda, and S. Iyobe. 2000. Detection of carbapenemase-producing Acinetobacter baumannii in a hospital. J. Clin. Microbiol. 38:526-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vahaboglu, H., R. Öztürk, G. Aygün, F. Coskunkan, A. Yaman, A. Kaygusuz, H. Leblebicioglu, I. Balik, K. Aydin, and M. Otkun. 1997. Widespread detection of PER-1-type extended-spectrum β-lactamases among nosocomial Acinetobacter and Pseudomonas aeruginosa isolates in Turkey: a nationwide multicenter study. Antimicrob. Agents Chemother. 41:2265-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vila, J., M. Navia, J. Ruiz, and C. Casals. 1997. Cloning and nucleotide sequence analysis of a gene encoding an OXA-derived β-lactamase in Acinetobacter baumannii. Antimicrob. Agents Chemother. 41:2757-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang, S. H., W. H. Sheng, Y. Y. Chang, L. H. Wang, H. C. Lin, M. L. Chen, H. J. Pan, W. J. Ko, S. C. Chang, and F. Y. Lin. 2003. Healthcare-associated outbreak due to pan-drug resistant Acinetobacter baumannii in a surgical intensive care unit. J. Hosp. Infect. 53:97-102. [DOI] [PubMed] [Google Scholar]
- 55.Yagi, T., H. Kurokawa, N. Shibata, K. Shibayama, K. Shimokata, and Y. Arakawa. 2000. A preliminary survey of extended-spectrum β-lactamases (ESBLs) in clinical isolates of Klebsiella pneumoniae and Escherichia coli in Japan. FEMS Microbiol. Lett. 184:53-56. [DOI] [PubMed] [Google Scholar]
- 56.Yang, Y., N. Bhachech, P. A. Bradford, B. D. Jett, D. F. Sahm, and K. Bush. 1998. Ceftazidime-resistant Klebsiella pneumoniae and Escherichia coli isolates producing TEM-10 and TEM-43 from St. Louis. Antimicrob. Agents Chemother. 42:1671-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yum, J. H., K. Yi, H. Lee, D. Yong, K. Lee, J. M. Kim, G. M. Rossolini, and Y. Chong. 2002. Molecular characterization of metallo-β-lactamase-producing Acinetobacter baumannii and Acinetobacter genomospecies 3 from Korea: identification of two new integrons carrying the blaVIM-2 gene cassettes. J. Antimicrob. Chemother. 49:837-840. [DOI] [PubMed] [Google Scholar]