Abstract
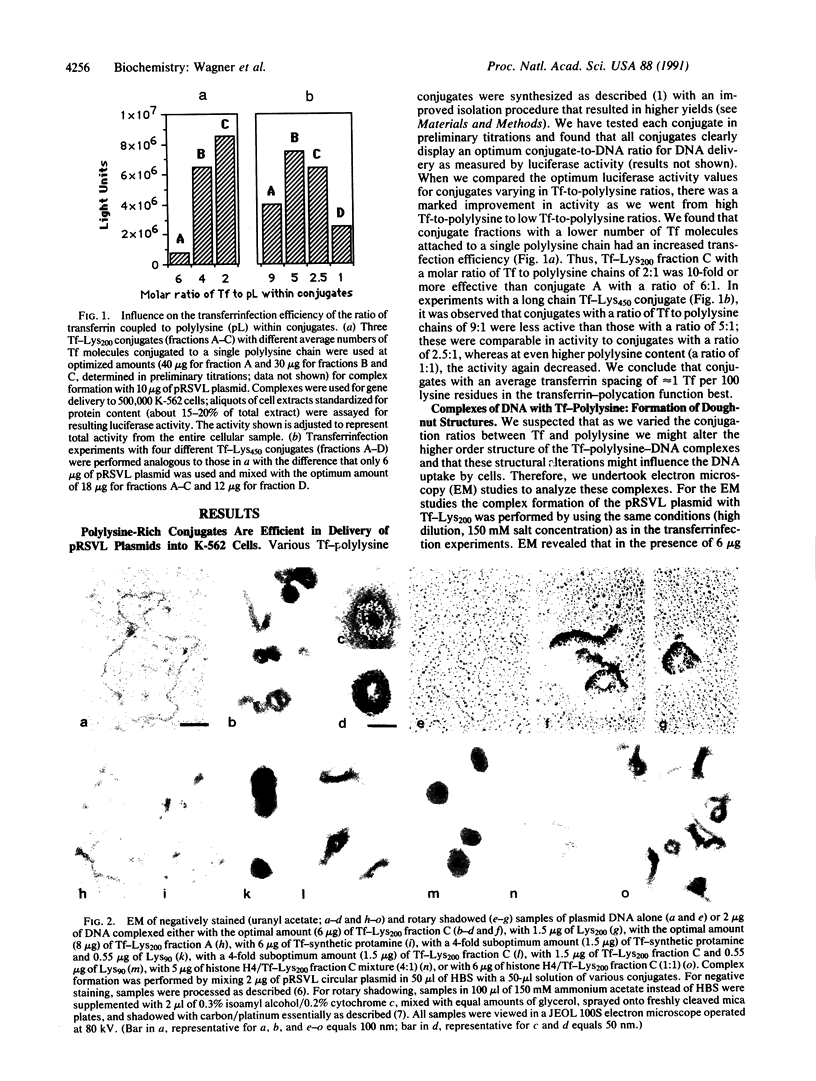
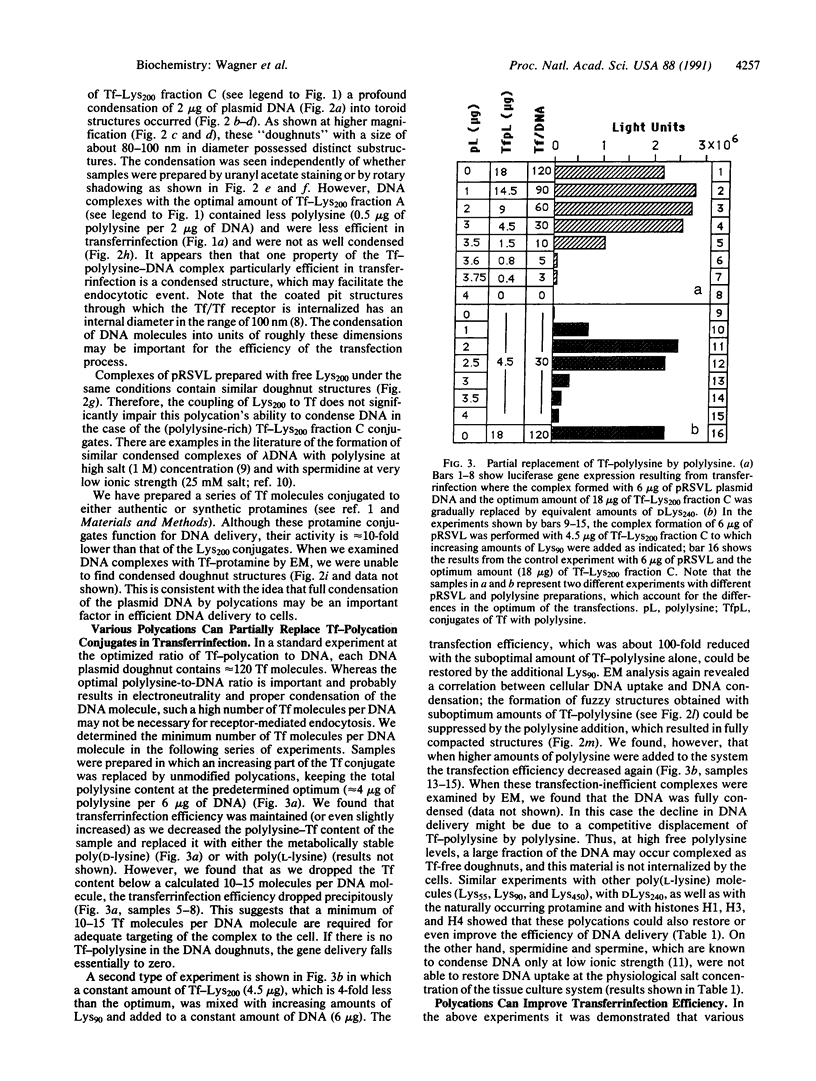
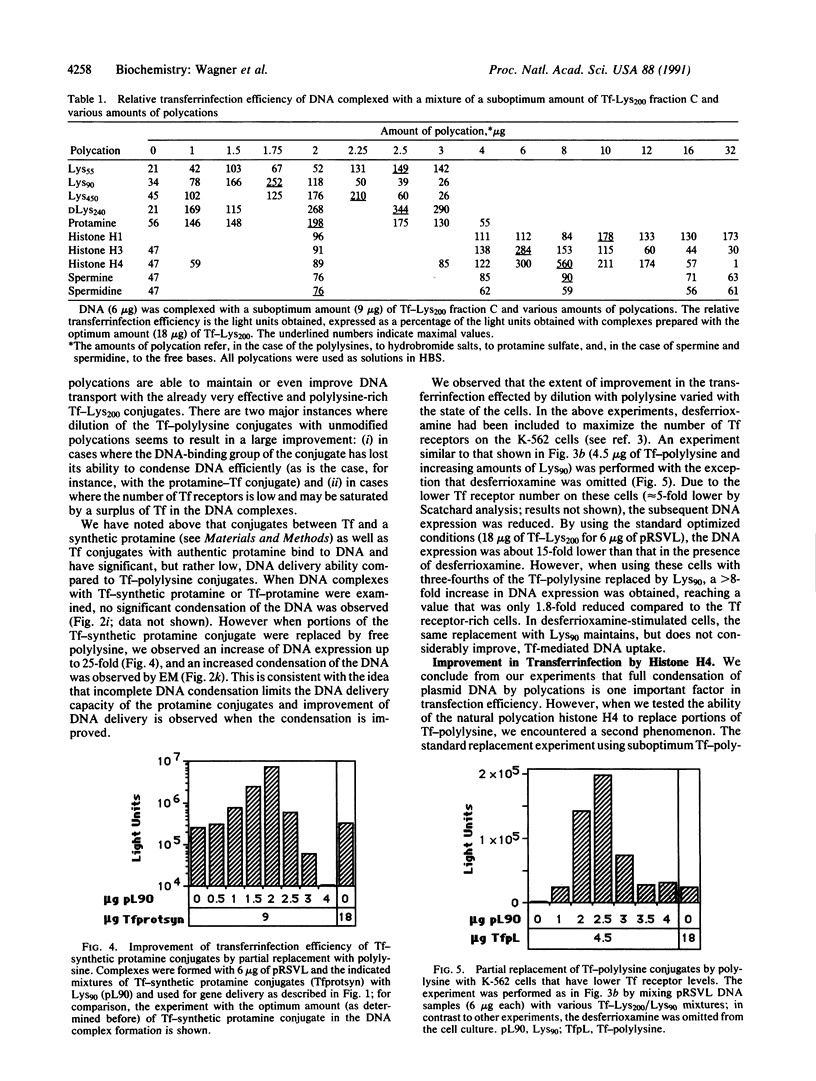
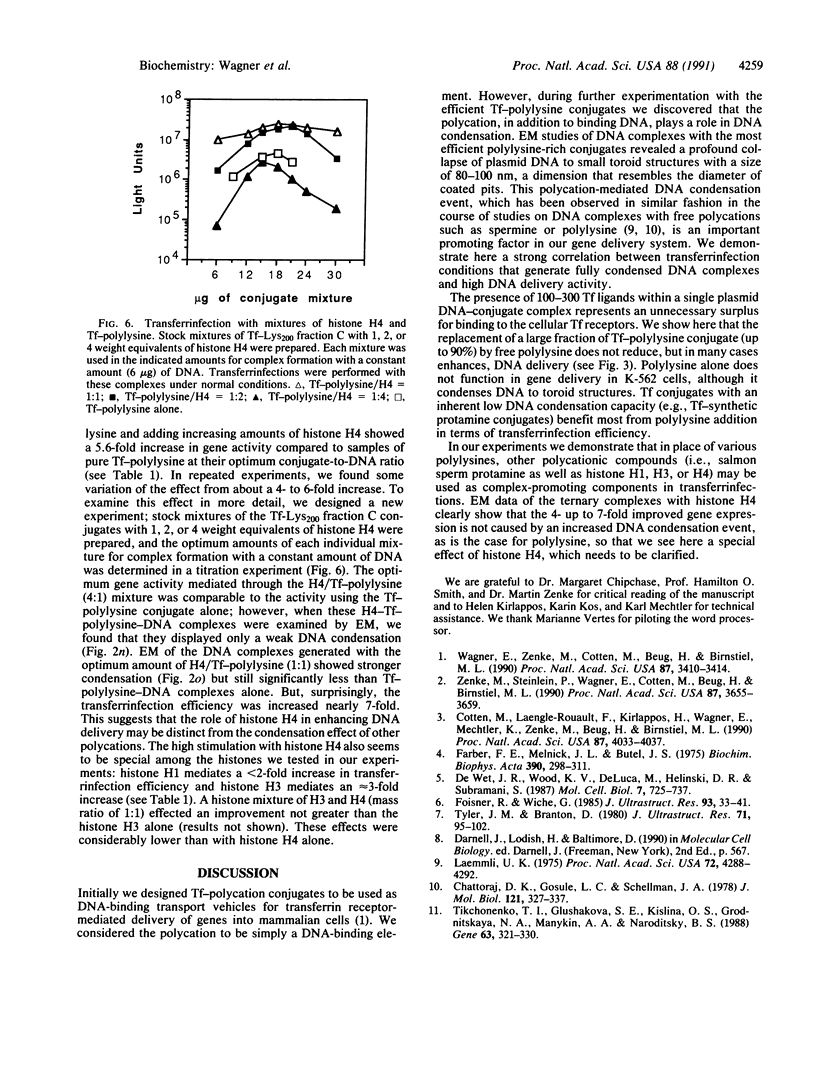
We have previously described a gene delivery system based upon the receptor-mediated endocytosis of DNA complexed with transferrin-polycation conjugates. This delivery system has been found to be very effective for both the internalization and the expression of genetic material in cells that have many transferrin receptors. Upon scrutinization of the parameters involved in this method, which we have termed transferrinfection, we note two important features of the process: the polycation in polycation-transferrin conjugates, as expected, serves to attach the transferrin moiety to the DNA and, in addition, the polycation functions to condense the DNA into a doughnut structure. Electron microscopic analysis of a range of poorly active to highly active transferrinfection samples reveals a strong correlation between DNA condensation and cellular DNA uptake. Furthermore, we demonstrate that the transfection activity of the DNA complex can be increased by addition of free polycation as long as a sufficient quantity of polycation-transferrin conjugates remains in the complex to ensure its binding to the cellular receptor.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Cotten M., Längle-Rouault F., Kirlappos H., Wagner E., Mechtler K., Zenke M., Beug H., Birnstiel M. L. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4033–4037. doi: 10.1073/pnas.87.11.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber F. E., Melnick J. L., Butel J. S. Optimal conditions for uptake of exogenous DNA by Chinese hamster lung cells deficient in hypoxanthine-guanine phosphoribosyltransferase. Biochim Biophys Acta. 1975 May 16;390(3):298–311. doi: 10.1016/0005-2787(75)90350-0. [DOI] [PubMed] [Google Scholar]
- Foisner R., Wiche G. Promotion of MAP/MAP interaction by taxol. J Ultrastruct Res. 1985 Oct-Nov;93(1-2):33–41. doi: 10.1016/0889-1605(85)90083-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Characterization of DNA condensates induced by poly(ethylene oxide) and polylysine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4288–4292. doi: 10.1073/pnas.72.11.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikchonenko T. I., Glushakova S. E., Kislina O. S., Grodnitskaya N. A., Manykin A. A., Naroditsky B. S. Transfer of condensed viral DNA into eukaryotic cells using proteoliposomes. Gene. 1988 Mar 31;63(2):321–330. doi: 10.1016/0378-1119(88)90535-5. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Wagner E., Zenke M., Cotten M., Beug H., Birnstiel M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc Natl Acad Sci U S A. 1990 May;87(9):3410–3414. doi: 10.1073/pnas.87.9.3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Steinlein P., Wagner E., Cotten M., Beug H., Birnstiel M. L. Receptor-mediated endocytosis of transferrin-polycation conjugates: an efficient way to introduce DNA into hematopoietic cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3655–3659. doi: 10.1073/pnas.87.10.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]





