Abstract
Vibrio parahaemolyticus strains carrying the thermostable direct hemolysin (TDH) tdh gene, the TDH-related hemolysin (trh) gene, or both genes are considered virulent strains. We previously demonstrated that the transcription-reverse transcription concerted (TRC) method could be used to quantify the amount of mRNA transcribed from the tdh gene by using an automated detection system. In this study, we devised two TRC-based assays to quantify the mRNAs transcribed from the trh1 and trh2 genes, the two representative trh genes. The TRC-based detection assays for the tdh, trh1, and trh2 transcripts could specifically and quantitatively detect 103 to 107 copies of the corresponding calibrator RNAs. We examined by the three TRC assays the total RNA preparations extracted from 103 strains of Vibrio parahaemolyticus carrying the tdh, trh1, or trh2 gene in various combinations. The tdh, trh1, and trh2 mRNAs in the total RNA preparations were specifically quantified, and the time needed for detection ranged from 9 to 19 min, from 14 to 18 min, and from 9 to 12 min, respectively. The results showed that this automated TRC assays could detect the tdh, trh1, and trh2 mRNAs specifically, quantitatively, and rapidly. The relative levels of TDH determined by the immunological method and that of tdh mRNA determined by the TRC assays for most tdh-positive strains correlated. Interestingly, the levels of TDH produced from the strains carrying both tdh and trh genes were lower than those carrying only the tdh gene, whereas the levels of mRNA did not significantly differ between the two groups.
Vibrio parahaemolyticus can cause seafood-borne gastroenteritis in humans. However, not all strains are considered virulent strains. The Kanagawa phenomenon, β-type hemolysis in Wagatsuma agar was formerly considered the marker of virulent strains because early studies demonstrated that most clinical strains, but few environmental strains, exhibit this phenomenon (16, 29). Thermostable direct hemolysin (TDH) is responsible for the Kanagawa phenomenon. Subsequently, clinical strains lacking the ability to produce TDH but producing a TDH-related hemolysin (TRH) were discovered (5, 6). The strains carrying the tdh gene encoding TDH, the trh gene encoding TRH, or both genes are now considered to be virulent strains (19); the strains carrying these hemolysin genes are strongly associated with clinical cases (11, 30). Therefore, in investigations of the infection by V. parahaemolyticus, the V. parahaemolyticus strains isolated from clinical specimens are usually examined for the tdh and trh genes in most laboratories. The strains carrying the tdh gene, the trh gene, or both genes are then examined for the O:K serotype and, if possible, the DNA fingerprint for epidemiological investigation.
Our study group reported five variants of the tdh genes, tdh1 and tdh2 from a Kanagawa phenomenon-positive strain and tdh3, tdh4, and tdh5 genes from Kanagawa phenomenon-negative strains, and these five variants of the tdh gene share >97% sequence identity (19). The trh gene shares ∼68% sequence identity with the tdh gene (11). Strain-to-strain nucleotide sequence variation in the trh gene is known, and the trh sequence variants can be separated into two groups representing two trh genes: the trh1 and trh2 that share an 84% sequence identity (11). Expression levels of the tdh and trh genes vary. Of various tdh and trh genes, the tdh2 gene is expressed at a very high level and is directly responsible for the Kanagawa phenomenon (19, 26). The high-level tdh2 expression was shown to be due to a strong promoter activity (26). The biological activities of TDH include hemolysis of various species of erythrocytes, cytotoxicity, lethal toxicity for small experimental animals, increased vascular permeability in rabbit skin, and enterotoxicity to rabbits (19). TRH has biological activities similar to TDH (6). The strains carrying the tdh gene are isolated more often than trh-bearing strains from clinical specimens (11, 30). The expression levels of the virulence genes, the tdh or trh, are likely to influence the virulence of the strains carrying these genes. Therefore, it is important to examine whether the tdh and trh genes are expressed in all strains carrying these genes and, if so, to quantitatively determine how much each gene is expressed.
The PCR method is a powerful tool that allows easy and quick detection of the tdh and trh genes with a high sensitivity and specificity (35). However, this detection method can reveal only the presence or absence of these genes. We demonstrated that the transcription-reverse transcription concerted (TRC) method could be applied to quantify the amount of tdh-specific mRNA produced in a Kanagawa phenomenon-positive V. parahaemolyticus strain and its derivatives (9). The TRC method is a method of real-time monitoring of isothermal RNA sequence amplification with a fluorescent probe in which the amplification reaction and its monitoring are carried out by using a simple automated system. The steps in the TRC method include the following: the amplification reaction starts with the binding of the oligonucleotide, named the scissors probe, to the target mRNA at the specific site and is followed by cleavage of the mRNA at the binding site by using the RNase H activity of avian myeloblastosis virus (AMV) reverse transcriptase. The trimmed mRNA is used as the template and participates in the synthesis of the first-strand cDNA with the antisense primer and then the promoter-bearing double-stranded DNA with the promoter primer, with AMV reverse transcriptase. The RNA fragments are synthesized by a T7 RNA polymerase by using the synthesized double-stranded DNA as the template. The synthesized RNAs are subsequently recycled as RNA templates to synthesize double-stranded DNA. The amplified RNA fragments are detected by using the intercalation activating fluorescent (INAF) DNA probe to emit enhanced fluorescence by binding to a complementary sequence. The entire reaction and fluorescence detection are carried out in a specialized and automated instrument called a TRC monitor, which is composed of a round isothermal incubation block and a rotating fluorescence scanning unit (9).
In the present study, we developed assays for the rapid and quantitative detection of the trh1 and trh2 mRNAs by using the TRC method. We then examined 103 V. parahaemolyticus strains carrying the tdh, trh1, or trh2 gene in various combinations that represent the strains isolated from various locations in the world over a 24-year period by using the TRC-based assays for detection of the tdh-, trh1-, and trh2-specific mRNAs to evaluate the features of this method: specificity, rapidity, and quantification.
MATERIALS AND METHODS
Bacterial strains.
V. parahaemolyticus strains used in the present study are from our laboratory stock cultures and are listed in Table 1. Their O:K serovars were determined by the slide agglutination method by using specific rabbit anti-O and anti-K sera as described previously (31).
TABLE 1.
Detection of tdh, trh1, and trh2 genes and tdh1-, and trh2-specific mRNAs in various V. parahaemolyticus strains
| Strain | Isolation
|
Detectiona of:
|
Detectionb of mRNA specific to:
|
Strain designation in Fig. 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sourcec | Locationd | Date (yr) | O:K serovar | tdh | trh1 | trh2 | tdhe | trh1 | trh2 | ||
| AQ3815 | C | IT (Philippines) | 1983 | 4:12 | + | − | − | + (14.1) | − | − | 57 |
| WP1 | C | Japan | Before 1983 | 4:12 | + | − | − | + (15.3) | − | − | 53 |
| VP108 | C | India | 1996 | 3:6 | + | − | − | + (16.4) | − | − | 43 |
| W-3995 | C | Bangladesh | 1981 | 11:46 | + | − | − | + (15.7) | − | − | 48 |
| AQ3810 | C | Singapore | 1983 | 3:6 | + | − | − | + (16.0) | − | − | 44 |
| KX-V138 | C | IT (Indonesia) | 1995 | 3:6 | + | − | − | + (17.4) | − | − | 26 |
| Y-24634 | C | Bangladesh | 1983 | 8:22 | + | − | − | + (17.4) | − | − | 27 |
| AL-37223 | C | Bangladesh | 1996 | 3:6 | + | − | − | + (16.4) | − | − | 36 |
| Q-9604 | C | Bangladesh | 1977 | 4:8 | + | − | − | + (15.7) | − | − | 47 |
| T-26233 | C | Bangladesh | 1979 | 5:15 | + | − | − | + (16.0) | − | − | 45 |
| 97LVP2 | C | Laos | 1997 | 3:6 | + | − | − | + (16.3) | − | − | 37 |
| AN-5034 | C | Bangladesh | 1998 | 4:68 | + | − | − | + (16.5) | − | − | 40 |
| DOH812-14 | C | Taiwan | 1997 | 3:6 | + | − | − | + (16.8) | − | − | 30 |
| BE98-2029 | C | United States | 1998 | 3:6 | + | − | − | + (14.3) | − | − | 25 |
| VP26 | C | Thailand | 1998 | 3:6 | + | − | − | + (16.5) | − | − | 39 |
| VP232 | C | India | 1998 | 4:68 | + | − | − | + (15.7) | − | − | 49 |
| VP1 | C | Korea | 1998 | 3:6 | + | − | − | + (16.6) | − | − | 33 |
| D-1-81 | C | Japan | 1981 | 4:10 | + | − | − | + (16.9) | − | − | 29 |
| D-21-82 | C | Japan | 1982 | 1:38 | + | − | − | + (15.7) | − | − | 50 |
| D-53-83 | C | Japan | 1983 | 1:38 | + | − | − | + (15.8) | − | − | 46 |
| D-7-84 | C | Japan | 1984 | 4:8 | + | − | − | + (14.8) | − | − | 56 |
| D-14-85 | C | Japan | 1985 | 2:3 | + | − | − | + (16.5) | − | − | 42 |
| D-73-86 | C | Japan | 1986 | 3:33 | + | − | − | + (15.2) | − | − | 54 |
| D-19-88 | C | Japan | 1988 | 4:53 | + | − | − | + (13.9) | − | − | 58 |
| D-26-90 | C | Japan | 1990 | 4:63 | + | − | − | + (16.6) | − | − | 35 |
| F192-1 | E (seafood) | Philippine | 1999 | 5:68 | + | − | − | + (16.8) | − | − | 31 |
| D-1-93 | C | Japan | 1993 | 1:56 | + | − | − | + (15.5) | − | − | 51 |
| D-4-94 | C | Japan | 1994 | 4:55 | + | − | − | + (12.4) | − | − | 59 |
| D-3-96 | C | Japan | 1996 | 1:56 | + | − | − | + (15.0) | − | − | 55 |
| TCVP60 | C | Thailand | 1999 | 4:68 | + | − | − | + (16.5) | − | − | 41 |
| TCVP94 | C | Thailand | 1999 | 1:25 | + | − | − | + (17.0) | − | − | 28 |
| TCVP197 | C | Thailand | 1999 | 1:41 | + | − | − | + (16.8) | − | − | 32 |
| 00F390-1 | E (seafood) | Indonesia | 2000 | 12:UT | + | − | − | + (18.4) | − | − | 24 |
| PS02839 | C | Vietnam | 1997 | 3:75 | + | − | − | + (16.6) | − | − | 38 |
| PS06011 | C | Vietnam | 1997 | 4:12 | + | − | − | + (16.6) | − | − | 34 |
| PS14945 | C | Vietnam | 1999 | 11:UT | + | − | − | + (15.4) | − | − | 52 |
| 58 | E | India | 2000 | 7:19 | + | − | − | + (10.5) | − | − | 60 |
| 91A1437 | C | United States | 1991 | 1:UT | + | + | − | + (16.7) | + (14.6) | − | 10 |
| VP104 | C | India | 1996 | 1:UT | + | + | − | + (17.9) | + (16.1) | − | 2 |
| AQ3855 | C | IT (Philippines) | 1983 | 6:18 | + | + | − | + (17.1) | + (15.0) | − | 3 |
| AQ4889 | C | IT (Thailand) | 1993 | 4:12 | + | + | − | + (16.6) | + (16.0) | − | 14 |
| KX-V47 | C | IT (Thailand) | 1995 | 5:UT | + | + | − | + (16.9) | + (14.9) | − | 5 |
| TX-98-792-807-27 | E (oyster) | United States | 1998 | 8:74 | + | + | − | + (19.3) | + (16.2) | − | 1 |
| AQ3776 | C | IT (Thailand) | 1983 | 10:71 | + | + | − | + (12.8) | + (15.0) | − | 22 |
| AQ3860 | C | IT (Philippines) | 1983 | 6:46 | + | + | − | + (16.4) | + (15.1) | − | 13 |
| 94A-7175 | C | United States | 1994 | 4:9 | + | + | − | + (17.0) | + (15.4) | − | 23 |
| 225 | C | Thailand | 1990 | 5:11 | + | + | − | + (15.9) | + (14.6) | − | 18 |
| 91A-3992 | C | United States | 1991 | 1:56 | + | + | − | + (16.9) | + (14.4) | − | 6 |
| 90A-5598 | C | United States | 1990 | 4:63 | + | + | − | + (16.7) | + (16.0) | − | 9 |
| 88A-5320 | C | United States | 1988 | 4:12 | + | + | − | + (16.6) | + (17.4) | − | 11 |
| AQ4781 | C | IT (Hong Kong) | 1992 | 1:UT | + | − | + | + (16.4) | − | + (10.5) | 15 |
| AQ4969 | C | IT (Indonesia) | 1994 | 1:UT | + | − | + | + (16.9) | − | + (12.1) | 7 |
| 275 | C | Thailand | 1990 | 1:1 | + | − | + | + (13.9) | − | + (13.9) | 4 |
| AQ3857 | C | IT (Hong Kong) | 1983 | 1:1 | + | − | + | + (14.7) | − | + (12.0) | 20 |
| AQ4433 | C | IT (Thailand) | 1989 | 1:UT | + | − | + | + (15.4) | − | + (11.7) | 19 |
| AQ4405 | C | IT (Thailand) | 1989 | 3:72 | + | − | + | + (13.5) | − | + (11.8) | 21 |
| AQ4704 | C | IT (Singapore) | 1992 | 1:UT | + | − | + | + (15.8) | − | + (11.7) | 17 |
| AQ4815 | C | IT (Thailand) | 1993 | 1:69 | + | − | + | + (16.9) | − | + (11.0) | 8 |
| AQ4966 | C | IT (Thailand) | 1994 | 1:UT | + | − | + | + (16.3) | − | + (11.9) | 16 |
| KX-V132 | C | IT (Philippines) | 1995 | 3:UT | + | − | + | + (16.6) | − | + (10.9) | 12 |
| 90A-7408 | C | United States | 1990 | 4:12 | − | + | − | − | + (15.8) | − | |
| AQ3749 | C | IT (Singapore) | 1983 | 3:6 | − | + | − | − | + (15.7) | − | |
| AQ4019 | C | IT (Maldives) | 1985 | 3:6 | − | + | − | − | + (16.6) | − | |
| AQ4037 | C | IT (Maldives) | 1985 | 3:6 | − | + | − | − | + (14.2) | − | |
| AQ4235 | C | IT (Thailand) | 1987 | 3:6 | − | + | − | − | + (15.4) | − | |
| AQ4299 | C | IT (Thailand) | 1987 | 3:6 | − | + | − | − | + (15.8) | − | |
| AQ4644 | C | IT (Thailand) | 1991 | 3:6 | − | + | − | − | + (14.8) | − | |
| AQ4733 | C | IT (Singapore) | 1992 | 3:6 | − | + | − | − | + (15.7) | − | |
| AQ4853 | C | IT (Hong Kong) | 1993 | 3:6 | − | + | − | − | + (15.0) | − | |
| AQ4901 | C | IT (Thailand) | 1993 | 3:6 | − | + | − | − | + (15.0) | − | |
| AQ3960 | C | IT (Hong Kong) | 1984 | 4:63 | − | + | − | − | + (15.9) | − | |
| AQ4093 | C | IT (Maldives) | 1986 | 3:6 | − | + | − | − | + (15.6) | − | |
| AQ4095 | C | IT (Maldives) | 1986 | 3:6 | − | + | − | − | + (14.1) | − | |
| AQ4129 | C | IT (Philippines) | 1986 | 3:6 | − | + | − | − | + (15.2) | − | |
| 95A-4675 | C | United States | 1995 | 11:15 | − | − | + | − | − | + (12.4) | |
| AT4 | E (seawater) | Japan | Before 1987 | 4:37 | − | − | + | − | − | + (10.1) | |
| 93A-5463 | C | United States | 1993 | 5:UT | − | − | + | − | − | + (10.8) | |
| 91A-5992 | C | United States | 1991 | 3:59 | − | − | + | − | − | + (9.8) | |
| VP18 | C | India | 1994 | 1:UT | − | − | + | − | − | + (11.2) | |
| VP41 | C | India | 1995 | 1:33 | − | − | + | − | − | + (11.1) | |
| X-13261 | C | Bangladesh | 1982 | 4:UT | − | − | + | − | − | + (11.1) | |
| W-7529 | C | Bangladesh | 1981 | 13:UT | − | − | + | − | − | + (10.8) | |
| W-8274 | C | Bangladesh | 1981 | 1:UT | − | − | + | − | − | + (9.4) | |
| AB-8950 | C | Bangladesh | 1986 | 1:25 | − | − | + | − | − | + (10.3) | |
| Q-6030 | C | Bangladesh | 1977 | 5:UT | − | − | + | − | − | + (10.2) | |
| Q-14337 | C | Bangladesh | 1977 | 3:UT | − | − | + | − | − | + (12.0) | |
| U-5880 | C | Bangladesh | 1980 | 1:56 | − | − | + | − | − | + (10.8) | |
| U-12122 | C | Bangladesh | 1980 | 1:UT | − | − | + | − | − | + (12.4) | |
| U-11868 | C | Bangladesh | 1980 | 4:11 | − | − | + | − | − | + (10.2) | |
| AQ4135 | C | IT (Thailand) | 1986 | 1:UT | − | − | + | − | − | + (10.4) | |
| BAC-98-3574 | C | United States | 1998 | 4:55 | − | − | + | − | − | + (9.8) | |
| VP230 | C | India | 1998 | 1:UT | − | − | + | − | − | + (11.5) | |
| VP276 | C | India | 1998 | 2:28 | − | − | + | − | − | + (10.0) | |
| 68/5 | E (seafood) | Thailand | 1998 | 11:36 | − | − | + | − | − | + (10.0) | |
| AN-20865 | C | Bangladesh | 1998 | 1:UT | − | − | + | − | − | + (15.0) | |
| VP6 | C | India | 1994 | 1:UT | − | − | + | − | − | + (10.7) | |
| DOH272 | C | Taiwan | 1996 | 3:6 | − | − | − | − | − | − | |
| VP56 | E | Japan | 1983 | 2:28 | − | − | − | − | − | − | |
| KV9 | E | Korea | 1998 | 5:17 | − | − | − | − | − | − | |
| 4060 | E | Thailand | 1998 | 3:12 | − | − | − | − | − | − | |
| F25-1 | E (seafood) | Malaysia | 2000 | 5:UT | − | − | − | − | − | − | |
| APCC VP00017 | E | Japan | 2000 | 3:6 | − | − | − | − | − | − | |
| PS01972 | C | Vietnam | 1997 | 8:39 | − | − | − | − | − | − | |
Examined by TRC methods.
C, clinical; E, environmental. More detailed information for the environmental strain is indicated in the parenthesis if available.
IT, international travelers. The origin of travel is indicated in parentheses.
The detection time in minutes (the time to reach the cutoff value after initiation of the reaction) is indicated in parentheses.
Detection of the tdh, trh1, and trh2 genes.
The presence or absence of the tdh, trh1, and trh2 genes in the test strain was determined by the DNA colony hybridization method with the specific DNA probes as described previously (11, 25). Briefly, the DNA probes specific to the tdh, trh1, and trh2 genes were, respectively, 415-, 334-, and 419-bp DNA fragments isolated from recombinant plasmids and were labeled by the random priming method with 32P-labeled dCTP. DNA colony blots were prepared on nitrocellulose membrane, and hybridization was performed under high-stringency conditions (in solution containing 50% formamide). Hybridization signals on the X-ray film were judged visually. The colony blot that gave strong and weak signals with the trh1 and trh2 probes, respectively, were judged to be positive for the trh1 gene. The colony blot that gave weak and strong signals with the trh1 and trh2 probes, respectively, were judged to be positive for the trh2 gene.
Isolation of total RNA from V. parahaemolyticus.
The test strain of V. parahaemolyticus was grown in Luria-Bertani broth containing 1% NaCl (14) with shaking (160 rpm) at 37°C until early log phase (optical density at 600 nm of 1.0). Bacterial cells were collected by centrifugation (15,000 rpm) on a tabletop centrifuge (Centrifuge 5415C; Eppendorf, Hamburg, Germany) from a 1-ml culture, and the total RNA was extracted by using an RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer's specifications. The concentration of the extracted total RNA in RNase-free water was adjusted to 500 ng/μl and stored at −20°C. The stored RNA solution was thawed and diluted 10-fold with RNase-free water immediately before use in the TRC reaction.
Detection of TDH production.
Relative levels of TDH produced in the culture broth by V. parahaemolyticus strains were compared by using a commercially available TDH detection kit that was based on the reversed-phase latex agglutination reaction with rabbit anti-TDH immunoglobulin G. The test strain was grown in Luria-Bertani broth containing 1% NaCl with shaking (160 rpm) at 37°C for 18 h. A 50-μl portion of the culture was inoculated into 5-ml of the fresh broth medium and incubated with shaking (160 rpm) at 37°C for 18 h. Twofold dilutions of the culture supernatant were prepared and an agglutination test by using a commercially available TDH detection kit (KAP-RPLA; Denka Seiken Co., Ltd., Tokyo, Japan) was performed according to the manufacturer's instructions. This kit consisted of the latex particles coated with rabbit anti-TDH immunoglobulin G, those coated with immunoglobulin G from a nonimmunized rabbit (a control), purified TDH (a control), and dilution buffer. The agglutination test was carried out in a 96-well V-bottom microtiter plate for 20 h at room temperature. The reciprocal of the highest dilution that gave a positive reaction was defined as the TDH titer.
Preparation of standard RNAs as calibrators.
Standard RNA containing the target region for TRC amplification was prepared by in vitro transcription of the T7 promoter-bearing double-stranded DNA as the template with T7 RNA polymerase. The DNA templates were synthesized from the total DNA extracted from the control strain of V. parahaemolyticus by using PCR (95°C for 30 s, 55°C for 30 s, and 72°C for 3 min; 30 cycles) with a pair of synthetic oligonucleotide primers. The control strains producing the target mRNA were as follows: AQ3815 for the tdh-specific mRNA (18); AQ4037 for the trh1-specific mRNA (20); AT4 for the trh2-specific mRNA (11). The respective genes in the control strains were well characterized (11, 17, 18, 20, 26), and we have been using these strains as the control strains for the DNA colony hybridization tests and PCR assays to detect these genes. The primer pairs for each target mRNA were as follows: 5′-AATTCTAATACGACTCACTATAGGGAGAATTCTGGCAAAGTTATTAATC-3′ and 5′-TTTTATTGTTGATGTTTACATT-3′ for the tdh-specific mRNA; 5′-AATTCTAATACGACTCACTATAGGGAGATCGAGCAATCTTGCTCAAAACCA-3′ and 5′-GTTTAATTTTGTGACATACATTC-3′ for the trh1-specific mRNA; and 5′-AATTCTAATACGACTCACTATAGGGAGATCGAGCAATTTTGCTTAAAATCAT-3′ and 5′-ATTTAAATTTGTGATTTACATTC-3′ for the trh2-specific mRNA. The long primer in each promoter primer pair has the T7 RNA polymerase-binding sequence at its 5′ end (indicated by the underline) to provide the preferred transcription initiation site. The resultant RNA fragments were purified by gel filtration with CHROMA-SPIN 30 columns (Clontech, Palo Alto, Calif.). The concentration of the RNA obtained was estimated by using a high-pressure liquid chromatography system (GPC, G4000SW column; Tosoh Corp., Tokyo, Japan). The calibrator set was prepared by diluting the RNA obtained to the appropriate concentrations with TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]).
TRC reactions.
Synthetic oligonucleotides used for the TRC reaction include a pair of amplification primers (designated as the promoter primer and the antisense primer), a scissors probe to initiate the TRC reaction, and an INAF probe to detect the RNA amplicons. The nucleotide sequence of these oligonucleotides for detection of the tdh-specific mRNA were described previously (9), and those for the trh1- and trh2-specific mRNAs are summarized in Table 2. The sizes of the trh1- and trh2-specific mRNA amplicons were expected to be 154 and 243 bases, respectively. In the INAF probe, oxazole yellow was linked at the nucleotide indicated by the asterisk (Table 2). The sequences indicated by the underlines in the promoter primers are the T7 RNA polymerase-binding sequences (Table 2). The nucleotide sequences of these oligonucleotides were selected by considering the secondary structure of the target transcript as described in Results. The secondary structures of the transcripts from the trh1 and trh2 genes were predicted by using Zucker's computer program obtained through the “mFold” server on the web (http://bioinfo.math.rpi.edu/zuckerm/). The 3′-OH of scissors probes and INAF probes were capped with an amino group and glycolic acid, respectively, to avoid undesired enzymatic elongation by AMV reverse transcriptase.
TABLE 2.
Oligonucleotide primers and probes used in TRC assays for the trh1- and trh2-specific mRNAs
| Target mRNA, primer, and probe | Positiona | Sequence (5′-3′)b |
|---|---|---|
| trh1 | ||
| Promoter primer | 429-453 | AATTCTAATACGACTCACTATAGGGAGATATTCTTCTGTTAGTGATTTCGTTG |
| Antisense primer | 562-582 | ATGATGATTTATTGGAAATAC |
| Scissor probe | 414-433 | GAATAGTTCTGATTTAGGCT |
| INAF probe | 545-561 | ACATAACAAA*CATATGCC |
| trh2 | ||
| Promoter primer | 99-121 | AATTCTAATACGACTCACTATAGGGAGAAAATCATTCGCGATTGATCTGCCA |
| Antisense primer | 322-341 | GTGACCATTGATGTTGACTG |
| Scissor probe | 84-103 | GATTTAGATATTGAAAATAT |
| INAF probe | 259-278 | CGATTGA*CCGTATACATCTT |
The protocol of the TRC assay for detection of the tdh-specific mRNA was described previously (9). The same protocol was used for the detection of trh1- and trh2-specific mRNAs. Briefly, 20 μl of the TRC buffer for each target mRNA was added to 5 μl of RNA extract in a thin-wall PCR tube (Applied Biosystems, Foster City, Calif.), followed by the addition of 5 μl of the enzyme mix. TE buffer in place of the RNA extract was used to serve as the negative control in TRC assays. The TRC buffer contained 90 mM Tris-HCl (pH 8.6), 195 mM KCl, 28 mM MgCl2, 1.5 mM dithiothreitol, 0.38 mM deoxynucleoside triphosphate, 4.5 mM nucleoside triphosphate, 5.4 mM isonine 5′-triphosphate, 0.3 U of RNase inhibitor (TaKaRa, Shiga, Japan)/μl, 1.5 μM promoter primer, 1.5 μM antisense primer, 0.24 μM scissors probe, 23 nM INAF probe, and 16% dimethyl sulfoxide. The enzyme mix consisted of 0.72 mg of bovine serum albumin/ml, 12% sorbitol, 1.3 U of AMV reverse transcriptase (TaKaRa)/μl, 34 U of T7 RNA polymerase (Life Technologies, Rockville, Md.)/μl, and 0.006 U of RNase (TaKaRa)/μl. The TRC reaction tube containing the reaction mix was set in the “TRC monitor” instrument. The temperature of the incubation block was maintained at 41°C, and the RNA amplicons were detected by scanning the fluorescence with the light-emitting diode source (470 nm) and the photomultiplier for fluorescence (520 nm) collection in the instrument at 1-min intervals. Time for fluorescence enhancement to reach a cutoff value of 1.2 was defined as the detection time in the TRC assay.
RESULTS
Sensitivity and specificity of the TRC assays.
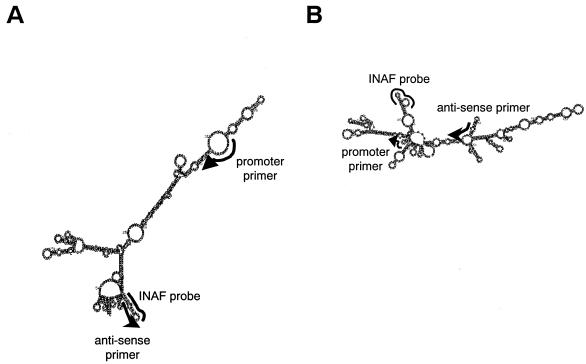
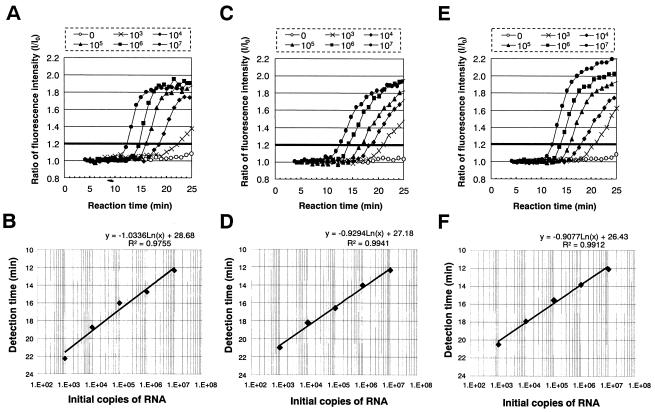
We developed the TRC assays for trh1- and trh2-specific mRNAs in the present study. The secondary structures of mRNA sequences encoded by the trh1 or trh2 gene were predicted by Zucker's computer program (Fig. 1). Primers and probes were designed to bind to the sites relatively free from the secondary structure of the transcripts (Table 2 and Fig. 1). We carried out the TRC assays to detect tdh, trh1, and trh2 mRNAs by using the calibrator RNAs synthesized in vitro (Fig. 2A, C, and E). The profiles shown in Fig. 2A, C, and E indicate the fluorescence enhancement relative to the reaction time. Similar profiles were obtained for all of the TRC assays. The detection time, the time to reach the cutoff value 1.2, yielded a very good linear relationship compared to the number of initial copies ranging from 103 to 107 (Fig. 2B, D, and F). This cutoff value was determined to be ≥3 times the standard deviation of the fluorescence enhancement value of the negative control at 15 min of reaction time. The results indicated that the three TRC assays are quantitative and rapid and that their sensitivity limit is ∼103 copies of mRNA.
FIG. 1.
Secondary structures of the trh1- and trh2-specific mRNA sequences predicted by the Zucker's computer program. (A) trh1-specific mRNA; (B) trh2-specific mRNA. The binding sites of the promoter and antisense primers and INAF probe on the mRNA sequence are indicated by bold lines. Arrowheads indicate the 3′ positions of the primers.
FIG. 2.
Real-time monitoring of the TRC reactions for the RNA calibrators. (A, C, and E) Fluorescent profile of the TRC reaction for the RNA calibrators (A, tdh RNA; C, trh1 RNA; and E, trh2 RNA); the initial copies of the calibrator mRNA (0, 103, 104, 105, 106, and 107) are indicated. (B, D, and F) Correlation between the initial copies of the mRNA calibrators and their detection times (B, tdh RNA; D, trh1 RNA; F, trh2 RNA).
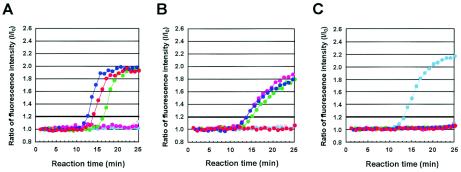
Next, we evaluated the specificity of the three TRC assays with control strains carrying the tdh, trh1, and trh2 genes in various combinations (Fig. 3). The results presented in Fig. 3 show that the three TRC assays could detect the mRNAs transcribed only from the corresponding target genes and suggest that these TRC assays are specific.
FIG. 3.
Real-time monitoring of TRC reactions for RNA preparations of control strains of V. parahaemolyticus by using TRC assays to detect tdh, trh1, and trh2 mRNAs. The profiles of the TRC reactions for detection of the tdh mRNA (A), the trh1 mRNA (B), and the trh2 mRNA (C) are shown. The total RNA extracted from AQ3815 (red dots; tdh1 positive, tdh2 positive, trh1 negative, and trh2 negative), AQ3776 (blue dots; tdh3 positive, tdh4 positive, trh1 positive, and trh2 negative), AQ3860 (green dots; tdh5 positive, trh1 positive, and trh2 negative), AQ4037 (pink dots; tdh negative, trh1 positive, and trh2 negative), AT4 (pale-blue dots; tdh negative, trh1 negative, and trh2 positive), and DOH272 (gray dots; tdh negative, trh1 negative, and trh2 negative) were examined.
Examination of the various V. parahaemolyticus strains by using the TRC assays.
We further evaluated the three TRC assays for their specificity and rapidity of detection with the total RNA extracted from 103 test strains listed in Table 1. These strains were selected from our stock culture collection so as to represent various toxin gene profile groups (combinations of the tdh, trh1, and trh2 genes). These strains were isolated mostly from clinical sources in various parts of the world between 1977 to 2000 and belonged to various O:K serovars. The results of the TRC assays are shown in Table 1. The results of the detection of the tdh-, trh1-, and trh2-specific mRNAs by the TRC assays agreed absolutely with the presence of the tdh, trh1, and trh2 genes detected by the DNA colony hybridization tests. The detection time by the TRC assays, the time to reach the cutoff value after initiation of the reaction, ranged from 9 to 19 min, from 14 to 18 min, and from 9 to 12 min for the tdh-, trh1-, and trh2-specific mRNAs, respectively. These results indicate that the TRC assays can specifically and rapidly detect the tdh-, trh1-, and trh2-specific mRNAs produced in V. parahaemolyticus strains.
Comparison between tdh mRNA transcription level and TDH production level.
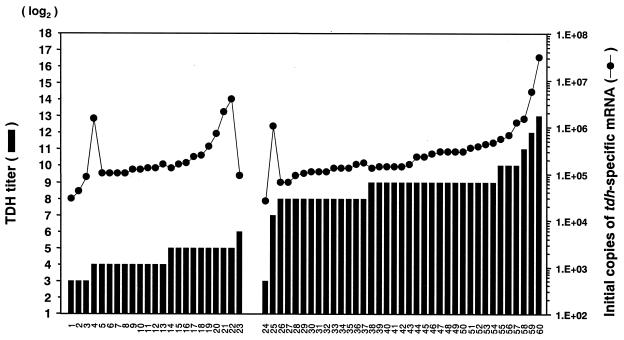
The levels of TDH produced can vary among strains. In the present study we focused on TDH because a commercial kit for detection of TDH, but not TRH, is available. Examination of the twofold dilutions of the culture supernatant by using this detection kit (KAP-RPLA) and determining the end point of the positive reaction allows comparison of the level of TDH produced. We defined the level of TDH determined by this way as the “TDH titer.” Determination of the copy number of the tdh-specific mRNA from the calibration curve in the TRC reaction allows comparison of the levels of the tdh transcription in the different strains. We were interested in comparing the level of the tdh-specific mRNA determined by the TRC assay to the level of TDH produced in test strains to examine whether the level of the tdh transcription is reflected in the level of TDH produced. We could detect the transcription of the tdh gene in all tdh-bearing strains (Fig. 4 and Table 2). The initial copies of mRNA in these strains were greater than 104 copies, and every score was over the threshold, 103 copies. A good correlation between the TDH titer and the quantity of the tdh-specific mRNA was observed among the strains carrying the tdh gene alone with one exception (Fig. 4, strain 25). The strains carrying tdh and trh genes (Fig. 4, strains 1 to 23) produced less TDH than did those carrying the tdh gene alone (Fig. 4, strains 24 to 60) with one exception (Fig. 4, strain 24). The mean and standard error of the TDH titers of the tdh- and trh-positive strains and the tdh-positive strains were 23.0 ± 2.7 and 792.4 ± 234.5, respectively, the difference being ∼35-fold. However, the levels of the tdh-specific mRNA in the tdh and trh gene-positive strains were not comparable to their low TDH titers. The mean and standard error of the initial copies of the tdh-specific mRNA of the tdh and trh gene-positive strains and that of the tdh gene-positive strains were 473,099 ± 188,596 and 1,274,237 ± 833,297, respectively, and the difference was only 2.7-fold.
FIG. 4.
Comparison between levels of TDH and those of tdh-specific mRNA for tdh gene-bearing strains of V. parahaemolyticus. The level of TDH production in each strain is expressed as the TDH titer (explained in the text). The initial copy of the tdh-specific mRNA was determined from the calibration curve in the TRC reaction (Fig. 2B). Designations of the test strains are indicated in Table 1. The strains from 1 to 23 carried the tdh and trh (trh1 or trh2) genes. The strains from 24 to 59 carried the tdh gene alone.
DISCUSSION
The TRC assays for the detection of the tdh, trh1, and trh2 mRNAs are specific, sensitive (detecting 103 mRNA copies), and quantitative (range, 103 to 107 mRNA copies). The TRC reaction is carried out, measured, and recorded by using a simple and automated system, and the results can be obtained rapidly (25 min after onset of the reaction). The easiness and rapidity give these TRC assays advantages over conventional PCR assays in examination of clinical strains for their ability to produce TDH or TRH. In addition, the PCR assays thus far reported (2, 35) could not distinguish the trh1 and trh2 genes that share 84% sequence identity. We have previously distinguished these two trh genes in the test strains by the relative intensities of the hybridization signals with the trh1- and trh2-specific DNA probes in the DNA colony hybridization tests (11). The TRC assays are much easier than the DNA colony hybridization tests, and they can be one of the tools for epidemiological investigation. Furthermore, the TRC assays combined with appropriate RNA preparation methods will be very useful if there is a need for detection of viable virulent strains in clinical and environmental samples.
The TRC assays are more advanced than the PCR or hybridization assays in that the TRC assays detect the tdh and trh genes that are actually expressed but not silent genes. In the present study, we examined 103 strains representing the strains of various toxin gene combinations by the TRC assays and revealed that all tdh and trh genes in the strains were actually transcribed although the levels of transcription among the strains varied. The result suggests that the PCR assays can be used to evaluate the ability of the test strain to produce TDH and TRH in clinical and environmental studies, although the various amounts of the toxins cannot be measured.
Infection by the strain capable of colonizing the host intestine and producing TDH or TRH at a high level is expected to cause damages to the tissues of the susceptible host, and it will result in clinical symptoms. Various clinical symptoms have been reported from V. parahaemolyticus infections. These include diarrhea, abdominal pain, headache, vomiting, fever, weakness, chill, tenesmus, and nausea (15, 27). However, contribution of TDH or TRH to the clinical symptoms of the patients has not been established. The amount of the tdh-specific mRNA determined by the TRC assay generally reflected the level of TDH production (Fig. 4, discussed below). A future study on the association of the expression level of the tdh and trh genes in the clinical strains with the clinical symptoms of the patients would reveal relative importance of TDH and TRH in each of clinical symptoms. Recently, we have investigated 548 cases of V. parahaemolyticus infection in Vietnam between 1997 and 1999, and the symptoms of the patients were recorded (36). The characteristics of the strains isolated from individual patients varied considerably. These characteristics included tdh and trh gene profiles, O:K serotype, and DNA fingerprints (4). These strains and clinical information would be suitable for the study on the association of tdh and trh expression with clinical symptoms.
By using the TRC assays we found that the relative level of TDH production from the tdh gene in test strains can usually be predicted by the level of the tdh transcription, but this correlation cannot be observed in some strains, notably those carrying both tdh and trh genes (Fig. 4). We found in a previous study that the strains carrying both tdh and trh genes produced TDH at lower levels than did the strains carrying a single tdh gene (32). We therefore compared the TDH titer and the level of the tdh-specific mRNA produced from the strains belonging to these two groups. The difference in the amount of mRNA, 2.7-fold, was much lower than the difference in TDH titer at 37-fold, between the two groups. This result suggests that low-level TDH production in the former group is due to the mechanisms not only at a transcriptional level but also at a translational level. We consider that the low-level tdh transcription is due in part to a weak tdh promoter (26). In addition, effect of the trh gene on tdh transcription needs to be evaluated. A small portion of V. parahaemolyticus strains produce urease (1, 3, 7, 10, 12, 21, 23, 24, 37), and almost all urease-positive strains carry the trh (either trh1 or trh2) gene (8, 22, 24, 28, 31, 33, 34). We demonstrated that urease production influenced the transcription of neither the trh nor tdh gene (17). Furthermore, the urease gene cluster did not influence production of TDH from a Kanagawa phenomenon-positive strain (17). Accordingly, a non-urease factor or factors including the trh gene is likely to be responsible for the low-level translation from the tdh mRNA.
00F390-1 (Fig. 4, strain 24) was exceptional among the strains carrying the tdh gene alone in that it produced TDH and tdh-specific mRNA at the lowest levels. This may be due to a mutation in the tdh promoter. Analysis of the tdh promoter sequence would confirm this hypothesis (26).
The mechanism responsible for the exceptionally high tdh transcription in some strains also needs to be explained. The high level of the tdh-specific mRNA in strain AQ3776 belonging to the tdh- and trh-positive group (Fig. 4, strain 22) was much higher than those of the other strains in this group. It was previously reported that this strain is exceptional in that it carries the two tdh genes (tdh3 and tdh4); the tdh3 gene is on the chromosome; however, the tdh4 gene exists on a plasmid (18). The high-level tdh transcription in AQ3776 may be due to the copy number effect of the tdh4 gene. TDH production was not very high compared to the high-level tdh transcription in this strain. The translation from the tdh transcript may be repressed by some mechanism, but this aspect has not been studied thus far. The tdh transcriptions were very high in the two strains among the strains carrying the tdh gene alone (Fig. 4, strains 59 and 60). The exact mechanism involved in the very high tdh transcriptions in these strains is not known. The tdh promoters in these strains may have up mutation. Alternatively, tdh transcription may be stimulated by some factor more strongly in these strains than in other strains. The transmembrane regulator encoded by the toxRS genes stimulates tdh gene variants to different degrees (13). A further study is needed to examine these possibilities.
Acknowledgments
We are grateful to Yohko Takeda for technical assistance.
This research was supported, in part, by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Abbott, S. L., C. Powers, C. A. Kaysner, Y. Takeda, M. Ishibashi, S. W. Joseph, and J. M. Janda. 1989. Emergence of a restricted bioserovar of Vibrio parahaemolyticus as the predominant cause of Vibrio-associated gastroenteritis on the West Coast of the United States and Mexico. J. Clin. Microbiol. 27:2891-2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bej, A. K., D. P. Patterson, C. W. Brasher, M. C. Vickery, D. D. Jones, and C. A. Kaysner. 1999. Detection of total and hemolysin-producing Vibrio parahaemolyticus in shellfish using multiplex PCR amplification of tlh, tdh, and trh. J. Microbiol. Methods 36:215-225. [DOI] [PubMed] [Google Scholar]
- 3.Cai, Y. L., and Y. X. Ni. 1996. Purification, characterization, and pathogenicity of urease produced by Vibrio parahaemolyticus. J. Clin. Lab. Anal. 10:70-73. [DOI] [PubMed] [Google Scholar]
- 4.Chowdhury, A., M. Ishibashi, V. D. Thiem, D. T. N. Tuyet, T. V. Tung, B. T. Chien, L. von Seidlein, D. G. Canh, J. Clemens, D. D. Trach, and M. Nishibuchi. 2004. Emergence and serovar transition of Vibrio parahaemolyticus pandemic strains isolated during a diarrhea outbreak in Vietnam between 1997 and 1999. Microbiol. Immunol. 48:319-327. [DOI] [PubMed] [Google Scholar]
- 5.Honda, S., I Goto, I Minematsu, N. Ikeda, N. Asano, M. Ishibashi, Y. Kinoshita, M. Nishibuchi, T. Honda, and T. Miwatani. 1987. Gastroenteritis due to Kanagawa negative Vibrio parahaemolyticus. Lancet i:331-332. [DOI] [PubMed] [Google Scholar]
- 6.Honda, T., Y. Ni, and T. Miwatani. 1988. Purification and characterization of a hemolysin produced by a clinical isolate of Kanagawa phenomenon-negative Vibrio parahaemolyticus and related to the thermostable direct hemolysin. Infect. Immun. 56:961-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huq, M. I., D. Huber, and G. Kibryia. 1979. Isolation of urease producing Vibrio parahaemolyticus strains from cases of gastroenteritis. Indian J. Med. Res. 70:549-553. [PubMed] [Google Scholar]
- 8.Iida, T., O. Suthienkul, K.-S. Park, G.-Q. Tang, R. K. Yamamoto, M. Ishibashi, K. Yamamoto, and T. Honda. 1997. Evidence for genetic linkage between the ure and trh genes in Vibrio parahaemolyticus. J. Med. Microbiol. 8:639-645. [DOI] [PubMed] [Google Scholar]
- 9.Ishiguro, T., J. Saitoh, R. Horie, T., Hayashi, T. Ishizuka, S. Tsuchiya, K. Yasukawa, T. Kido, Y. Nakaguchi, M. Nishibuchi, and K. Ueda. 2003. Intercalation activating fluorescence DNA probe and its application to homogeneous quantification of a target sequence by isothermal sequence amplification in a closed vessel. Anal. Biochem. 314:77-86. [DOI] [PubMed] [Google Scholar]
- 10.Kelly, M. T., and E. M. D. Stroh. 1989. Urease-positive, Kanagawa-negative Vibrio parahaemolyticus from patients and the environment in the Pacific Northwest. J. Clin. Microbiol. 27:2820-2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kishishita, M., N., Matsuoka, K., Kumagai, S., Yamasaki, Y. Takeda, and M., Nishibuchi. 1992. Sequence variation in the thermostable direct hemolysin-related (trh) gene of Vibrio parahaemolyticus. Appl. Environ. Microbiol. 58:2449-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lam, S., and M. Yeo. 1980. Urease-positive Vibrio parahaemolyticus strain. J. Clin. Microbiol. 12:57-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin, Z., K. Kumagai, K. Baba, J. J. Mekalanos, and M. Nishibuchi. 1993. Vibrio parahaemolyticus has a homolog of the Vibrio cholerae toxRS operon that mediates environmentally induced regulation of the thermostable direct hemolysin gene. J. Bacteriol. 175:3844-3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Miwatani, T., and Y. Takeda. 1976. Vibrio parahaemolyticus: a causative bacterium of food poisoning, p. 104-109. Saikon Publishing, Tokyo, Japan.
- 16.Miyamoto, Y., T. Kato, Y. Obara, S. Akiyama, K. Takizawa, and S. Yamai. 1969. In vitro hemolytic characteristics of Vibrio parahaemolyticus: its close correlation with human pathogenicity. J. Bacteriol. 100:1147-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakaguchi, Y., J. Okuda, T. Iida, and M. Nishibuchi. 2003. The urease gene cluster of Vibrio parahaemolyticus does not influence the expression of the thermostable direct hemolysin (TDH) gene or the TDH-related hemolysin gene. Microbiol. Immunol. 47:233-239. [DOI] [PubMed] [Google Scholar]
- 18.Nishibuchi, M., and J. B. Kaper. 1990. Duplication and variation of the thermostable direct hemolysin (tdh) gene in Vibrio parahaemolyticus. Mol. Microbiol. 4:87-99. [DOI] [PubMed] [Google Scholar]
- 19.Nishibuchi, M., and J. B. Kaper. 1995. Thermostable direct hemolysin gene of Vibrio parahaemolyticus: a virulence gene acquired by a marine bacterium. Infect. Immun. 63:2093-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishibuchi, M., T. Taniguchi, T. Misawa, V. Khaeomanee-iam, T. Honda, and T. Miwatani. 1989. Cloning and nucleotide sequence of the gene (trh) encoding the hemolysin related to the thermostable direct hemolysin of Vibrio parahaemolyticus. Infect. Immun. 57:2691-2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolan, C. M., J. Ballard, C. A. Kaysner, J. L. Lilja, L. P. Williams, Jr., and F. C. Tenover. 1984. Vibrio parahaemolyticus gastroenteritis: an outbreak associated with raw oysters in the Pacific Northwest. Diagn. Microbiol. Infect. Dis. 2:119-128. [DOI] [PubMed] [Google Scholar]
- 22.Obata, H., A. Kai, K. Sekiguchi, S. Matsushita, S. Yamada, T. Ito, K. Ohta, and K. Kudoh. 1996. Detection of the trh gene in Vibrio parahaemolyticus isolated from overseas traveler's diarrhea and their biochemical characteristics. J. Jpn. Assoc. Infect. Dis. 40:815-820. (In Japanese with English summary.) [DOI] [PubMed] [Google Scholar]
- 23.Oberhofer, T. R., and J. K. Podgore. 1982. Urea-hydrolyzing Vibrio parahaemolyticus associated with acute gastroenteritis. J. Clin. Microbiol. 16:581-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okuda, J., M. Ishibashi, S. Abbott, J. M. Janda, and M. Nishibuchi. 1997. Analysis of the thermostable direct hemolysin (tdh) gene and the tdh-related hemolysin (trh) genes in urease-positive strains of Vibrio parahaemolyticus isolated on the west coast of the United States. J. Clin. Microbiol. 35:1965-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okuda, J., M. Ishibashi, E. Hayakawa, T. Nishino, Y. Takeda, A. K. Mukhopadhyay, S. Garg, S. K. Bhattacharya, G. B. Nair, and M. Nishibuchi. 1997. Emergence of a unique O3:K6 clone of Vibrio parahaemolyticus in Calcutta, India, and isolation of strains from the same clonal group from Southeast Asian travelers arriving in Japan. J. Clin. Microbiol. 35:3150-3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda, J., and M. Nishibuchi. 1998. Manifestation of the Kanagawa phenomenon, the virulence-associated phenotype, of Vibrio parahaemolyticus depends on a particular single base change in the promoter of the thermostable direct hemolysin gene. Mol. Microbiol. 30:499-511. [DOI] [PubMed] [Google Scholar]
- 27.Oliver, J. D., and J. B. Kaper. 1997. Vibrio species, p. 228-264. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers. ASM Press, Washington, D.C.
- 28.Osawa, R., T. Okitsu, H. Morozumi, and S. Yamai. 1996. Occurrence of urease-positive Vibrio parahaemolyticus in Kanagawa, Japan, with specific reference to presence of thermostable direct hemolysin (TDH) and the TDH-related-hemolysin genes. Appl. Environ. Microbiol. 62:725-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakazaki, R., K. Tamura, T. Kato, Y. Obara, S. Yamai, and K. Hobo. 1968. Studies on the enteropathogenic, facultatively halophilic bacteria, Vibrio parahaemolyticus. III. Enteropathogenicity. Jpn. J. Med. Sci. Biol. 21:325-331. [DOI] [PubMed] [Google Scholar]
- 30.Shirai, H., H. Ito, T. Hirayama, Y. Nakamoto, N. Nakabayashi, K. Kumagai, Y. Takeda, and M. Nishibuchi. 1990. Molecular epidemiologic evidence for association of thermostable direct hemolysin (TDH) and TDH-related hemolysin of Vibrio parahaemolyticus with gastroenteritis. Infect. Immun. 58:3568-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suthienkul, O., M. Ishibashi, T. Iida, N. Nettip, S. Supavej, B. Eampokalap, M. Makino, and T. Honda. 1995. Urease production correlates with possession of the trh gene in Vibrio parahaemolyticus strains isolated in Thailand. J. Infect. Dis. 172:1405-1408. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki, N., S. Hashimoto, M. Ishibashi, Y. B. Kim, J. Okuda, and M. Nishibuchi. 1997. Levels of thermostable direct hemolysin production by Vibrio parahaemolyticus strains carrying both tdh and trh genes. J. Jpn. Assoc. Infect. Dis. 71:1221-1225. (In Japanese with English summary.) [DOI] [PubMed] [Google Scholar]
- 33.Suzuki, N., Y. Ueda, H. Mori, K. Miyagi, K. Noda, H. Hirose, Y. Oosumi, M. Ishibashi, M. Yoh, K. Yamamoto, and T. Honda. 1994. Serotypes of urease producing Vibrio parahaemolyticus and their relation to possession of tdh and trh genes. J. Jpn. Assoc. Infect. Dis. 69:757-758. (In Japanese with English summary.) [DOI] [PubMed] [Google Scholar]
- 34.Suzuki, N., Y. Ueda, H. Mori, Y. Takegaki, K. Miyagi, K. Noda, H. Hirose, H. Hashimoto, Y. Oosumi, M. Ishibashi, and T. Honda. 1995. Correlation between trh possession and urease production of clinical isolates of Vibrio parahaemolyticus. J. Jpn. Assoc. Infect. Dis. 68:1068-1074. (In Japanese with English summary.) [DOI] [PubMed] [Google Scholar]
- 35.Tada, J., T. Ohashi, N. Nishimura, Y. Shirasaki, H. Ozaki, S. Fukushima, J. Takano, M. Nishibuchi, and Y. Takeda. 1992. Detection of thermostable direct hemolysin gene (tdh) and the thermostable direct hemolysin-related hemolysin gene (trh) of Vibrio parahaemolyticus by polymerase chain reaction. Mol. Cell. Probes 6:477-487. [DOI] [PubMed] [Google Scholar]
- 36.Tuyet, D. T., T. D. Thiem, L. von Seidlei, A. Chowdhury, E. Park, D. G. Canh, B. T. Chien, T. V. Tung, A. Naficy, M. R. Rao, M. Ali, H. Lee, T. H. Sy, M. Nichibuchi, J. Clemens, and D. D. Trach. 2002. Clinical, epidemiologic, and socioeconomic analysis of an outbreak of Vibrio parahaemolyticus in Khanh Hoa province, Vietnam. J. Infect. Dis. 186:1615-1620. [DOI] [PubMed] [Google Scholar]
- 37.Zen-Yoji, H., R. A. Le Clair, K. Ota, and T. S. Montague. 1973. Comparison of Vibrio parahaemolyticus cultures isolated in the United States with those isolated in Japan. J. Infect. Dis. 127:237-241. [DOI] [PubMed] [Google Scholar]