Abstract
Human T-cell leukemia/lymphoma virus type 1 (HTLV-1) infection can lead to the development of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP), concomitantly with or without other inflammatory disorders such as myositis. These pathologies are considered immune-mediated diseases, and it is assumed that migration within tissues of both HTLV-1-infected CD4+ T cells and anti-HTLV-1 cytotoxic T cells represents a pivotal event. However, although HTLV-1-infected T cells were found in inflamed lesions, the antigenic specificity of coinfiltrated CD8+ T cells remains to be determined. In this study, we performed both ex vivo and in situ analyses using muscle biopsies obtained from an HTLV-1-infected patient with HAM/TSP and sporadic inclusion body myositis. We found that both HTLV-1-infected CD4+ T cells and CD8+ T cells directed to the dominant Tax antigen can be amplified from muscle cell cultures. Moreover, we were able to detect in two successive muscle biopsies both tax mRNA-positive mononuclear cells and T cells recognized by the Tax11-19/HLA-A*02 tetramer and positive for perforin. These findings provide the first direct demonstration that anti-Tax cytotoxic T cells are chronically recruited within inflamed tissues of an HTLV-1 infected patient, which validates the cytotoxic immune reaction model for the pathogenesis of HTLV-1-associated inflammatory disease.
Human T-cell leukemia/lymphoma virus type 1 (HTLV-1) persistent infection is associated with the development of a chronic myelopathy called HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (7). HAM/TSP is characterized pathologically by a perivascular infiltrate of inflammatory cells, mainly in the thoracic spinal cord, associated with demyelination and gliosis (33). Damage to the central nervous system does not appear to be the direct consequence of HTLV-1 infection of neural cells; HAM/TSP is, rather, considered an immune-mediated disease (23). In particular, the cytotoxic T cell (CTL) response to the virus is suspected to play a pivotal pathogenic role. Thus, the frequency of circulating CD8+ T cells directed to the immunodominant viral Tax protein is higher in HAM/TSP patients than in asymptomatic carriers (15, 22); in some HLA-A*201 patients, up to 10% of circulating CD8+ T cells are recognized by the highly specific Tax 11-19/HLA-A*02 tetramer (1). Accounting for the increased immune response, the number of HTLV-1-infected CD4+ T cells, the main target cells of the virus, is higher in HAM/TSP patients than in asymptomatic carriers (24, 34). Because they are activated, infected CD4+ T cells cross the brain blood barrier and access the central nervous system (3). Provirus-containing cells as well as T cells expressing tax mRNA were indeed found within spinal cord lesions (12, 17, 20). CD8+ T cells were found in the cerebrospinal fluid of patients with a frequency of anti-Tax clonotypes significantly higher than in that in blood, attesting to their selective recruitment (16, 21). Whether such selected CD8+ populations are also present within spinal cord lesions is not known to date. However, it is currently assumed that the presence of both HTLV-1-infected CD4+ T cells and anti-HTLV-1 CTLs leads to a local immune response that in turn provokes a chronic inflammation and eventually tissue damage (for a review, see reference 10). Although numerous data plead for this model, the actual presence and the lytic potential of CD8+ T cells directed to HTLV-1 within inflammatory lesions remain to be demonstrated.
Besides or concomitantly with HAM/TSP development, HTLV-1 infection has been associated with inflammatory muscle diseases such as polymyositis (13, 18) and, more rarely, sporadic inclusion body myositis (sIBM) (4, 25). These disorders are believed to be mediated by the immune system (5, 26, 31). The muscle target antigens remain to be identified, although a viral etiology has been suspected in some cases (35). Similar to the lesions observed in the spinal cord of HAM/TSP patients, CD4+ and CD8+ T cells are found in inflammatory lesions; HTLV-1 is detected in infiltrating CD4+ lymphocytes but not in muscle fibers (4, 14, 25). Furthermore, anti-Tax CD8+ clonotypes can be generated from muscle cultures, at least in those from HTLV-1-infected polymyositis patients (28). These findings thus strongly suggest that an immune reaction between HTLV-1-infected CD4+ T cells and anti-HTLV-1 CD8+ T cells also accounts for HTLV-1-associated myositis. It is therefore tempting to speculate that the mechanisms involved in the pathogenesis of HTLV-1-associated myositis closely mirror those of HAM/TSP. As muscle biopsies are more readily available than spinal cord samples, the study of HTLV-1-associated myopathies might be of major help in generally understanding HTLV-1-associated inflammatory disorders.
It was previously reported that HTLV-1 Tax mRNA is produced in mononuclear cells infiltrating the muscle of an HTLV-1-infected patient with sIBM (25). The present study, of a new HTLV-1-infected Caribbean patient suffering from HAM/TSP and sIBM, provides definitive evidence for a chronic immune reaction involving Tax-positive mononuclear cells and cytolytic anti-Tax T lymphocytes within muscle inflammatory lesions.
MATERIALS AND METHODS
Case report.
Patient CB (HLA-A*0201 positive) was a 40-year-old woman suffering from HAM/TSP diagnosed in 1996, who concomitantly developed progressive weakness involving the lower and upper limb muscles. The diagnosis of sIBM was determined from clinical and histological parameters and electromyography showing a myogenic pattern in the deltoid and proximal lower limb muscles. HTLV-1 infection was certified by immunoblotting showing the complete pattern of anti-HTLV-1 antibodies in the plasma and by amplification of HTLV-1 proviral sequences from peripheral blood mononuclear cells (PBMCs).
Muscle and blood cultures.
Blood samples were collected at the time of the first biopsy and 2 months later to determine the global virological and immunological parameters of the patient (Table 1) and to purify the PBMCs.
TABLE 1.
Virological and immunological parameters in blood of patient CB
| Date of sampling (mm-day-yr) | Parameter
|
||
|---|---|---|---|
| % proviral loada | Anti-HTLV-1 antibody titersb | Frequency (%) of anti- Tax 11-19 circulating CD8+ T cellsc | |
| 11/28/00 | 19 | 1/40.000 | >0.4 |
| 01/19/01 | 9 | 1/40.000 | >0.4 |
Proviral loads were determined by real-time TaqMan PCR amplification of a fragment of the po1 gene and of the endogenous albumin gene for normalization. Results are expressed as the number of copies for 100 PBMCs.
Antibody titers were measured by indirect immunofluorescence using HTLV-1 chronically infected MT-2 cells and twofold dilutions of plasma.
The frequency of anti-Tax 11-19 effectors was determined by measuring the number of cells producing IFN-γ in response to stimulation with the Tax 11-19 peptide. Results are expressed as the number of positive cells for 100 purified CD8+ T cells.
Polyclonal CTL lines used as positive control for anti-HTLV-1 cytotoxicity were generated from CD8+ T lymphocytes purified from PBMCs by positive selection with anti-CD8-coated magnetic microbeads (MACS reagent; Miltenyi Biotec, Paris, France). Just after being sorted, purified CD8+ T cells were stimulated with 1 μg of phytohemagglutin M (PHA-M; Sigma, Lyon, France)/ml and grown in culture in RPMI medium complemented with 5% fetal calf serum, 5% human serum, and 100 U of interleukin 2 (IL-2) (Roussel Uclaf, Romainville, France)/ml, as previously described (27). The CD8-PBMC population, composed mainly of CD4+ T cells, was used as autologous presenting cells (CB-CD4) after PHA stimulation and short-term culture with IL-2. These cells were also used to generate a chronically HTLV-1-infected CD4+ T-cell line (CB-CD4/HTLV) after stimulation with PHA and long-term culture in the presence of IL-2. Chronic HTLV-1 expression was monitored by indirect immunofluorescence and Western blotting with a monoclonal antibody from the anti-Tax hybridoma 168-A51 (NIH AIDS Research and Reference Reagent Program).
Muscle-cell-infiltrating T lymphocytes were obtained from two independent biopsies of the left deltoid muscle carried out 5 months apart (November 2000 and April 2001). Fresh biopsies were washed in RPMI culture medium to remove contaminating blood and then cut in small pieces; the resulting aggregates were set in culture in a 24-well plate in 2 ml of medium. To permit muscle cell antigen-driven expansion, muscle cell cultures were performed without exogenous stimulation and with a low dose of IL-2 (20 U/ml). Massive expansion of mononuclear cells was apparent after 2 weeks. Then, these cells were sorted into CD4+ and CD8+ T lymphocyte populations by positive selection with magnetic beads (Miltenyi Biotec) and further cultivated in the same medium.
Determination of proviral loads and antibody titers.
HTLV-1 proviral loads were quantified in PBMCs by a real-time TaqMan PCR assay as described previously (6) with the viral pol gene for HTLV-1 quantification and the endogenous albumin gene for normalization. The titers of anti-HTLV-1 antibodies in plasma were quantified by indirect immunofluorescence with the MT-2 cell lines as HTLV-1 antigen-producing cells and twofold dilutions of each plasma sample, as described previously (32).
Chromium release assay and ELISPOT.
Cytotoxic activities of CD8+ T-cell lines were tested against autologous infected T cells (CB-CD4/HTLV) or against short-term culture of autologous PHA-stimulated CD8+ T cells (CB-CD4) pulsed or not pulsed with the HLA-A*02-restricted Tax 11-19 epitope (Neosystem, Strasbourg, France) by the chromium release cytotoxic assay as described previously (27).
The presence of circulating in vivo-primed CD8+ T cells directed to the immunodominant Tax 11-19 epitope was examined by the ELISPOT assay. Briefly, CD8+ T cells freshly sorted from PBMCs were plated at a density of 50,000/well in RPMI medium supplemented with 5% human serum and 5% fetal calf serum, with or without 10 μM purified Tax 11-19 peptide. Specific revelation was performed as described previously (27) after 24 h of stimulation.
TCR repertoire analysis and sequencing.
Total mRNAs were prepared from CD8+ T cells from blood and muscle cell cultures, and immunoscopic analysis of T-cell receptor (TCR) diversity was performed following PCR amplification of each of the 24 Vβ families, as previously described (32). Vβ13.1-specific PCR products were further cloned with the TOPO TA cloning kit (Invitrogen, Cergy Pontoise, France) and sequenced by an automatic procedure.
In situ analyses.
Tissue sections from an HLA-A*0201- and HTLV-1-negative patient also suffering from sIBM were used as controls. For electron microscopy, ultrathin sections of epon-embedded specimens were stained with uranyl acetate-lead citrate. In situ hybridization (ISH) was performed with a tax 33P antisense riboprobe or with a sense tax riboprobe (as a negative control), as described previously (25).
Immunohistochemistry was performed with frozen tissue sections fixed in acetone. The anti-CD8+ and anti-CD4+ (Leu-2a and NU-TH/1) Ki-M7 antibody to the macrophage surface marker CD68, anti-perforin monoclonal antibody (BD Biosciences-Ozyme, Saint Quentin Yvelines, France), and anti-human tumor necrosis factor alpha (anti-TNF-α) antibody (HyCult Biotechnology-TEBU, Le Perray en Yvelines, France) were used. The antigenic specificity of muscle CD8+ T cells was assessed with a phycoerythrin (PE)-conjugated HLA-A*02/Tax 11-19-tetramer (kindly provided by C. Bangham, Imperial College, London, United Kingdom) or a control PE-conjugated HLA-A*02/EBV tetramer (kindly provided by H. Teisserenc, Hôpital Saint Louis, Paris, France), followed by an anti-PE monoclonal antibody (Sigma). All simple labeling was revealed with alkaline phosphatase substrates (Dako, Glostrup, Denmark). Revelation with two secondary antibodies conjugated to alkaline phosphatase or horseradish peroxidase was used for double staining.
RESULTS
We first evaluated the global virological and immunological parameters of the patient with blood samples collected at the time of the first muscle biopsy and 2 months later (Table 1). The mean proviral load determined by real-time PCR was 14 copies/100 PBMCs, and the mean antibody titer to HTLV-1 antigens was 1/40,000. The ELISPOT assay further showed that more than 0.4% of circulating CD8+ T cells corresponded to in vivo-primed cells specific for the dominant HLA-A2-restricted Tax 11-19 epitope. These biological data were fully consistent with the well-known status of HAM/TSP patients, i.e., high proviral load associated with high levels of humoral as well as cellular immune responses (8, 15, 24).
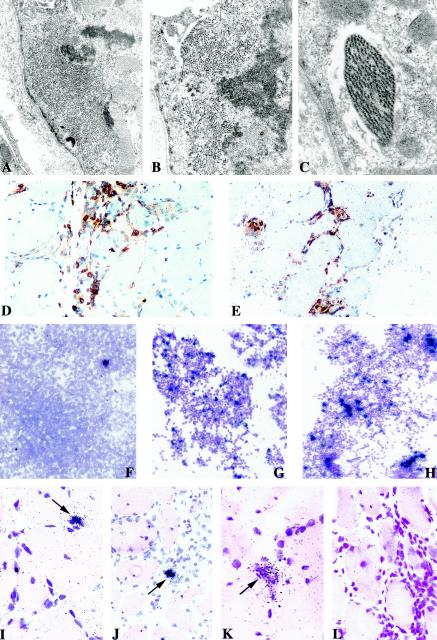
Two biopsies from the left deltoid muscle were then obtained 5 months apart from patient CB. Electromyography and histological examination of the biopsies showed typical features of sIBM, including scattered vacuolated myofibers displaying abnormal subsarcolemmal filaments (Fig. 1A to C) and important interstitial mononuclear cells infiltrates containing macrophages as well as CD4+ (Fig. 1D) and CD8+ (Fig. 1E) T lymphocytes.
FIG.1.
Histological and virological features of muscle tissues and cultures. Electron micrographs of deltoid muscle sections from patient CB showing intracytoplasmic accumulation of abnormal filaments (magnification, ×9,800) (A) and clusters of straight (B) and paired helical (C) filaments seen at higher magnification (×14,000 and ×18,000). Immunochemical detection of CD4+ (D) and CD8+ (E) T cells on muscle sections counterstained with Harris hematoxylin. Detection of tax mRNA-positive cells among fresh PBMCs (F) and CD4+ T lymphocytes sorted from muscle culture (G) compared to PHA-stimulated CD4+ T cells from blood (H) (counterstained with Giemsa; exposure time, 8 days). In situ detection of tax mRNA with an antisense probe on muscle sections from the first (I and J) and the second (K) biopsies; arrows show focal positivity for HTLV-1 in mononuclear infiltrated cells and not in muscle fibers. Negative control analyses were performed with a tissue section from the second biopsy with a sense probe (L). Sections were counterstained with hematoxylin-eosin, and the exposure time was 21 days.
To initiate the exploration of the virological and immunological parameters in tissues, muscle biopsy cultures were performed to recover infiltrating T lymphocytes. Importantly, no mitogen was used in the culture, first, to permit the proliferation of in vivo-infected CD4+ T cells and second, to permit the antigenic stimulation of CD8+ T lymphocytes by any tissue antigen. Such muscle-driven expansions indeed occurred, since massive proliferation of T lymphocytes was apparent in both biopsies after 2 weeks in cultures. Both CD4+ and CD8+ T cells populations were present and were further sorted by magnetic bead purification.
ISH showed that the tax mRNA was produced among CD4+ T cells expanded from the second biopsy (Fig. 1G), consistent with previous observation of HTLV-1 expression in tissues of infected patients with inflammatory disorders (17, 25, 30). Although muscle cell cultures were carried out without mitogen, the number of tax mRNA-positive cells (Fig. 1G) was comparable to that found in a long-term culture of mitogen-stimulated CD4+ T cells from blood (Fig. 1H). This indicates that a large number of HTLV-1-infected cells were already present in the biopsy.
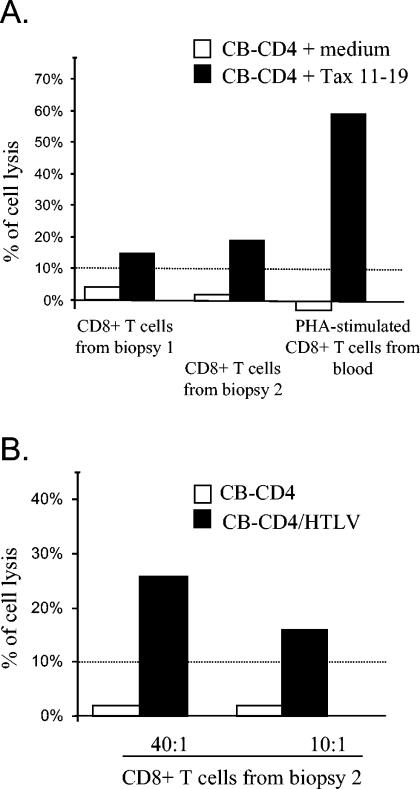
We next investigated whether the CD8+ T-cell populations purified from muscle cultures contain anti-HTLV-1 CTLs. Since no mitogen was added during culture, any specific cytotoxic activity would reflect the presence and efficient presentation of HTLV-1 antigens present in the biopsy. Indeed, significant CTL activity to peptide-pulsed target cells was observed with CD8+ T cells expanded from both biopsies (Fig. 2A). The cytolysis found in CD8+ T cells recovered from muscle cells without mitogenic stimulation represents 20 or 30% of that found for PHA-stimulated CD8+ cells from blood, showing that the muscle-driven stimulation led to the expansion of a relatively high amount of specific and functional cells. Consistently, a cytotoxic activity directed to autologous HTLV-1 chronically infected cells (CB-CD4/HTLV) was found for CD8+ T cells expanded from the second biopsy, even at a low effector:target ratio (Fig. 2B).
FIG. 2.
Cytotoxic activities of CD8+ T cells expanded from muscle cultures. (A) Chromium release assay to measure the cytotoxic activity directed to the immunodominant Tax 11-19 epitope of CD8+ T cells sorted from muscle cultures (performed in absence of mitogen), compared to PHA-stimulated CD8+ T cells from blood. For 4 days, PHA-stimulated CD4+ T lymphocytes from blood (in which HTLV-1 antigen production was still undetectable) incubated with or without the immunodominant Tax 11-19 epitope (10 μM) were used as targets cells (effector/target ratio, 40:1). (B) Chromium release assay to detect the ability of CD8+ T cells sorted after culture of biopsy 2 to kill chronically infected cells (CB-CD4/HTLV) at two effector/target ratios. Nonproducing autologous PHA-stimulated CD4+ cells were also used as a negative control. A percentage of cell lysis up to 10% was considered significant.
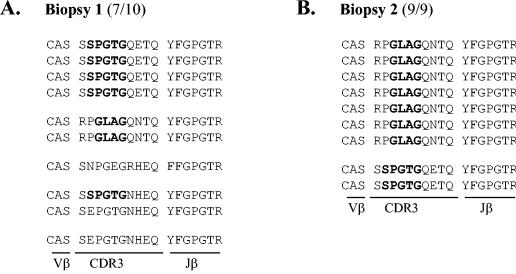
To further characterize the antigenic specificity of muscle-infiltrating CD8+ T lymphocytes, we performed various molecular analyses. Examination of the T-cell receptor diversity of CD8+ T cells expanded from muscle cell culture first revealed a clear limited usage of Vβ chains (data not shown), suggesting that restricted CD8+ T-cell populations were recruited locally. It was recently shown that anti-Tax 11-19 CD8+ T cells preferentially express the TCR Vβ13.1 chain (2) in which conserved motifs within the CDR3 region, including the P/GLA/RG and SPGTG amino acid sequences, were found (2, 29). We then sequenced the Vβ13.1-specific PCR products amplified from the two independent biopsies and from the blood as a control. Ten DNA clones were analyzed for biospy 1, and 9 DNA clones were analyzed for biopsy 2 for 19 clones from blood (Fig. 3). As shown in Fig. 3A, 7 DNA products of 10 from the first biopsy displayed the typical SPGTG or GLAG motifs. Such selectivity of CD8+ T cells was confirmed for biopsy 2, since nine of nine clones exhibited either the GLAG (seven of nine) or the SPGTG (two of nine) sequence (Fig. 3B). The same motifs were also found in Vβ13.1 products amplified in parallel from blood (Fig. 3C) but with a significantly lower frequency (31%), since the SPGTG or the GLAG/PLAG sequence was found for only 6 clones of 19. This formally demonstrates that anti-Tax 11-19 cells were present in the CD8+ T cells amplified from muscle cultures and that such selected clonotypes are highly enriched in muscle tissue compared to blood.
FIG. 3.
Molecular characterization of CD8+ T cells expanded from muscle cultures. Determination of amino acid composition of the TCR β-chain CDR3 sequences of 9 (biopsy 1), 10 (biopsy 2), and 19 (blood) DNA products obtained from Vβ13.1/Cβ-specific PCRs. Amino acid sequences corresponding to the conserved P/GLA/RG and SPGTG motifs previously found in anti-Tax 11-19 CD8+ T clonotypes (2, 29) are in boldface. The number of clones containing the characteristic motifs among the total number of sequenced clones is indicated between brackets.
The above data provided indirect evidence that both anti-HTLV-1 CD8+ lymphocytes and HTLV-1-infected CD4+ T cells were present within muscle cells. To try to directly demonstrate these points, we further performed various in situ detections with frozen biopsies.
We first investigated whether HTLV-1 expression occurs within muscle by performing ISH with an anti-sense probe specific for tax mRNA. Foci of tax mRNA-expressing cells were readily detected in the first (Fig. 1I and 1J) and second (Fig. 1K) biopsies, in infiltrated mononuclear cells but not in muscle fiber. In contrast, no signal was detected with a sense tax riboprobe, even in a zone of high inflammation (Fig. 1L). These data demonstrate that HTLV-1 expression occurs chronically in muscle cells. Rare foci of Tax expression were also seen among fresh PBMCs (Fig. 1F). This is consistent with previous findings that HTLV-1 expression in the blood of HAM/TSP patients is rare, approximately 1 positive cell for every 5,000 PBMCs (9).
MHC-1/peptide tetramers are now commonly used to visualize the population of CD8+ T cells that recognized a particular epitope. For example, in HLA-A2-positive individuals suffering from HAM/TSP, Tax 11-19/HLA-A*02 tetramer-reactive cells can represent up to 10% of circulating CD8+ T cells (1). In patient CB, around 5% of PBMCs were recognized by the Tax 11-19/HLA-A*02 tetramer (data not shown).
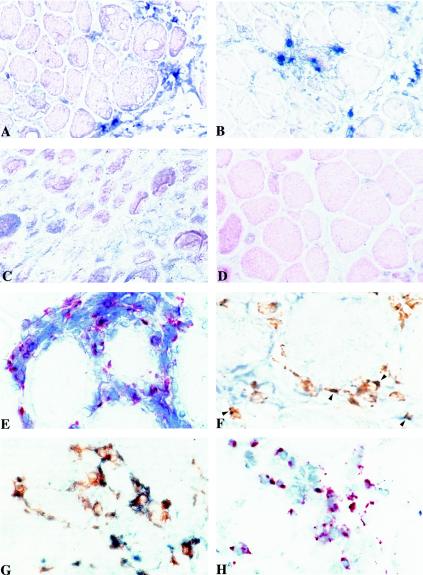
To determine the antigenic specificity of muscle CD8+ T cells, we decided to use the Tax 11-19 tetramer in situ. As shown in Fig. 4, reactivity with the Tax 11-19 tetramer was indeed found among the mononuclear cell infiltrates in both the first (Fig. 4A) and the second (Fig. 4B) biopsies. Only background was found in parallel experiments with muscle sections from an HLA-A*02-uninfected patient with sIBM (Fig. 4C) or a control HLA-A*02/EBV tetramer (Fig. 4D). Perforin-producing cells were also observed (Fig. 4E), and a double-staining experiment using both anti-perforin antibody and the anti-Tax 11-19 tetramer further revealed that such populations include anti-Tax 11-19 T cells (Fig. 4F). Finally, simultaneous detection with anti-CD68 and anti-TNF-α antibodies allowed us to demonstrate that macrophages producing TNF-α were present in inflammatory muscle (Fig. 4G). In addition, we detected the presence of apoptotic cells among mononuclear cell infiltrates (Fig. 4H).
FIG. 4.
Immunochemical analyses of inflammatory lesions within deltoid muscle. Serial 5-μm frozen sections were fixed in acetone, processed for antigen detection, and counterstained with Harris hematoxylin. Staining was performed with the specific PE-conjugated HLA-A*02 Tax 11-19 tetramer on sections from the first (A) and the second (B) biopsies or on a control section from an HLA-A*02 uninfected patient with sIBM (C). Staining with a control HLA-A*02 EBV tetramer was also carried out on the second biospy sample from patient CB (D). Sections from the first biopsy were stained with anti-perforin antibody (E), with both anti-perforin antibody and PE-conjugated HLA-A*02 Tax 11-19 (F) (arrowheads indicate double-stained cells), and with both anti-TNF-α and CD68+ antibodies (G). Detection of apoptotic mononuclear cells by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling method (H) was also performed.
Collectively, these in situ analyses demonstrated that both tax mRNA-producing cells and cytolytic cells directed to an immunodominant Tax CTL epitope were present in the muscle concomitantly with cytokine production and apoptosis among mononuclear cells.
DISCUSSION
Although a pivotal role of the CD8+ T-cell response to HTLV-1 in the development of HTLV-1-associated inflammatory disorders has been suspected for a long time, the presence of cytolytic CD8+ T cells directed to HTLV-1 in inflammatory lesions has never been directly demonstrated. In this study, we provided direct evidence that anti-Tax CD8+ T cells producing perforin are chronically recruited within muscle cell lesions of an HTLV-1-infected patient suffering from HAM/TSP and sIBM.
Our findings first show that HTLV-1 expression exists within muscle lesions, as evidenced by the presence of tax mRNA-positive cells both in muscle cultures and in tissue sections. In tissue sections, only a small proportion of mononuclear cells were positive for tax mRNA, but HTLV-1 expression was maintained over time. This suggests that a low-level but persistent virus expression is sufficient to promote the inflammatory process. Consistent with previous data (14, 25), HTLV-1 expression was confined to mononuclear cell infiltrates, ruling out a direct pathogenic role of the virus on muscle fibers. As was previously shown (9), only rare foci of tax mRNA-positive cells were found among PBMCs. That HTLV-1-expressing cells in muscle were evidenced among foci containing a limited number of cells, whereas such cells were rare among the large population of PBMCs, might indicate that tax mRNA expression is higher in muscle than in blood.
A massive expansion of anti-HTLV-1 CTLs during muscle culture provided another strong argument for the chronic production of HTLV-1 antigens within tissue. Indeed, since no mitogenic stimulation was performed, any CD8+ T-cell expansion would reflect the production and subsequent presentation of local antigens. Specific amino acid motifs within the CDR3 region of Vβ13.1 anti-Tax 11-19 clonotypes were previously identified (2, 29). Whether a specific CD8+ proliferation driven by HTLV-1 occurred during muscle culture was then examined by sequencing the Vβ13.1 PCR products amplified from the two biopsies and PBMCs as a control. We found that 70 and 100% of DNA clones from the first and second biopsies, respectively, contained a motif found in anti-Tax 11-19 cells for only 31% of DNA clones amplified from PBMCs. Hence, not only cells bearing the molecular signatures of anti-Tax 11-19 clonotypes were present within lymphocytes recovered from muscle but these cells were highly enriched in muscle compared to blood. This is reminiscent of what was recently observed with cerebrospinal fluid samples from HAM/TSP patients, in which a 12-fold enrichment for a particular anti-Tax clone was documented (21). Chromium release assays further showed that CD8+ T cells from muscle were able to kill both Tax 11-19-presenting target cells and autologous infected cells. This demonstrates that CD8+ effectors were generated during muscle culture upon stimulation of CD8+ precursors by HTLV-1-producing cells. Importantly, we obtained direct evidence that such precursor-to effector-differentiation also occurred within tissue. Indeed, we were able to specifically detect anti-Tax 11-19 T cells within lesions by using the highly specific Tax 11-19 tetramer. Furthermore, we also evidenced that such anti-Tax 11-19 cells produce perforin, a hallmark of CD8+ effectors (11). This formally demonstrates that functional CTLs directed to Tax are indeed recruited within inflamed lesions.
The above findings revealed that both Tax-producing T cells and perforin-positive cells directed to a dominant epitope of Tax were present in muscle. Moreover, the recruitment of such populations was maintained over time, since both cell types were found in sections from two successive muscle biopsies. This provides the first direct demonstration that a chronic CTL-mediated immune reaction directed to HTLV-1 occurs locally in the inflammatory tissue of an HTLV-1-infected patient. That such an immune response takes place in the muscle cells of a patient suffering from sIBM strongly supports the notion that chronic HTLV-1 infection and immune recognition are sufficient to trigger the inflammatory process leading to sIBM development. This might also imply that other infectious agents can promote sIBM development, providing that they are chronically present in muscle and constitute potent targets for CD8+ T cells.
Activated CD8+ T cells were previously described in spinal cord lesions (19), but whether this population includes anti-HTLV-1 CTLs was not determined. Since perforin-positive CD8+ T lymphocytes directed to Tax are found within muscle, as we showed here with a patient suffering from both HAM/TSP and sIBM, it seems very likely that such effector cells are also recruited within spinal cord tissue. We propose, therefore, that the specific immune reaction we demonstrated with muscle represents a common pathogenic determinant for the triggering of HTLV-1-associated inflammatory disorders. A remaining issue is how this specific anti-HTLV-1 response is linked to local inflammation and tissue destruction. As documented here, activation of macrophages due to local apoptosis among infiltrated mononuclear cells or other stimulations, leading to secretion of TNF-α, could constitute a major event. Further studies of muscle, a relatively available tissue, could help to elucidate these complex mechanisms and contribute to the design of new approaches for the treatment of HTLV-1-associated inflammatory disorders.
Acknowledgments
We thank P. Goon and C. Bangham for the gift of the HTLV-1 Tax 11-19 tetramer and H. Teisserenc for supplying the control EBV tetramer. We also thank P. Laforet for help in obtaining tissue sections, E. Skrobala for expert technical assistance with EM, and N. Désiré for determining the HTLV-1 proviral loads. We are grateful to D. Ghez for helpful discussion and editing of the manuscript.
This work was supported by the Association Française contre les Myopathies (AFM).
REFERENCES
- 1.Bieganowska, K., P. Hollsberg, G. J. Buckle, D. G. Lim, T. F. Greten, J. Schneck, J. D. Altman, S. Jacobson, S. L. Ledis, B. Hanchard, J. Chin, O. Morgan, P. A. Roth, and D. A. Hafler. 1999. Direct analysis of viral-specific CD8+ T cells with soluble HLA-A2/Tax11-19 tetramer complexes in patients with human T cell lymphotropic virus-associated myelopathy. J. Immunol. 162:1765-1771. [PubMed] [Google Scholar]
- 2.Bourcier, K. D., D. G. Lim, Y. H. Ding, K. J. Smith, K. Wucherpfennig, and D. A. Hafler. 2001. Conserved CDR3 regions in T-cell receptor (TCR) CD8+ T cells that recognize the Tax11-19/HLA-A*0201 complex in a subject infected with human T-cell leukemia virus type 1: relationship of T-cell fine specificity and major histocompatibility complex/peptide/TCR crystal structure. J. Virol. 75:9836-9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavrois, M., A. Gessain, O. Gout, S. Wain-Hobson, and E. Wattel. 2000. Common human T cell leukemia virus type 1 (HTLV-1) integration sites in cerebrospinal fluid and blood lymphocytes of patients with HTLV-1-associated myelopathy/tropical spastic paraparesis indicate that HTLV-1 crosses the blood-brain barrier via clonal HTLV-1-infected cells. J. Infect. Dis. 182:1044-1050. [DOI] [PubMed] [Google Scholar]
- 4.Cupler, E. J., M. Leon-Monzon, J. Miller, C. Semino-Mora, T. L. Anderson, and M. C. Dalakas. 1996. Inclusion body myositis in HIV-1 and HTLV-1 infected patients. Brain 119:1887-1893. [DOI] [PubMed] [Google Scholar]
- 5.Dalakas, M. C., and R. Hohlfeld. 2003. Polymyositis and dermatomyositis muscle biopsy findings in inflammatory myopathies. Understanding the immunopathogenesis of inclusion-body myositis: present and future prospects. Lancet 362:971-982.14511932 [Google Scholar]
- 6.Dehee, A., R. Cesaire, N. Desire, A. Lezin, O. Bourdonne, O. Bera, Y. Plumelle, D. Smadja, and J. C. Nicolas. 2002. Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J. Virol. Methods 102:37-51. [DOI] [PubMed] [Google Scholar]
- 7.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 8.Gessain, A., O. Gout, F. Saal, M. T. Daniel, B. Rio, G. Flandrin, F. Sigaux, O. Lyon-Caen, J. Peries, and G. de-The. 1990. Epidemiology and immunovirology of human T-cell leukemia/lymphoma virus type I-associated adult T-cell leukemia and chronic myelopathies as seen in France. Cancer Res. 50(Suppl.):5692S-5696S. [PubMed] [Google Scholar]
- 9.Gessain, A., A. Louie, O. Gout, R. C. Gallo, and G. Franchini. 1991. Human T-cell leukemia-lymphoma virus type I (HTLV-I) expression in fresh peripheral blood mononuclear cells from patients with tropical spastic paraparesis/HTLV-I-associated myelopathy. J. Virol. 65:1628-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grant, C., K. Barmak, T. Alefantis, J. Yao, S. Jacobson, and B. Wigdahl. 2002. Hum. T cell leukemia virus type I and neurologic disease: events in bone marrow, peripheral blood, and central nervous system during normal immune surveillance and neuroinflammation. J. Cell Physiol. 190:133-159. [DOI] [PubMed] [Google Scholar]
- 11.Hamann, D., P. A. Baars, M. H. Rep, B. Hooibrink, S. R. Kerkhof-Garde, M. R. Klein, and R. A. van Lier. 1997. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 186:1407-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hara, H., M. Morita, T. Iwaki, T. Hatae, Y. Itoyama, T. Kitamoto, S. Akizuki, I. Goto, and T. Watanabe. 1994. Detection of human T lymphotrophic virus type I (HTLV-I) proviral DNA and analysis of T cell receptor V beta CDR3 sequences in spinal cord lesions of HTLV-I-associated myelopathy/tropical spastic paraparesis. J. Exp. Med. 180:831-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higuchi, I., K. Hashimoto, N. Kashio, S. Izumo, M. Inose, K. Izumi, R. Ohkubo, M. Nakagawa, K. Arimura, and M. Osame. 1995. Detection of HTLV-I provirus by in situ polymerase chain reaction in mononuclear inflammatory cells in skeletal muscle of viral carriers with polymyositis. Muscle Nerve 18:854-858. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi, I., K. Hashimoto, E. Matsuoka, R. Rosales, M. Nakagawa, K. Arimura, S. Izumo, and M. Osame. 1996. The main HTLV-I-harboring cells in the muscles of viral carriers with polymyositis are not macrophages but CD4+ lymphocytes. Acta Neuropathol. (Berlin) 92:358-361. [DOI] [PubMed] [Google Scholar]
- 15.Kubota, R., M. Nagai, T. Kawanishi, M. Osame, and S. Jacobson. 2000. Increased HTLV type 1 tax specific CD8+ cells in HTLV type 1-asociated myelopathy/tropical spastic paraparesis: correlation with HTLV type 1 proviral load. AIDS Res. Hum. Retrovir. 16:1705-1709. [DOI] [PubMed] [Google Scholar]
- 16.Kubota, R., S. S. Soldan, R. Martin, and S. Jacobson. 2002. Selected cytotoxic T lymphocytes with high specificity for HTLV-I in cerebrospinal fluid from a HAM/TSP patient. J. Neurovirol. 8:53-57. [DOI] [PubMed] [Google Scholar]
- 17.Lehky, T. J., C. H. Fox, S. Koenig, M. C. Levin, N. Flerlage, S. Izumo, E. Sato, C. S. Raine, M. Osame, and S. Jacobson. 1995. Detection of human T-lymphotropic virus type I (HTLV-I) tax RNA in the central nervous system of HTLV-I-associated myelopathy/tropical spastic paraparesis patients by in situ hybridization. Ann. Neurol. 37:167-175. [DOI] [PubMed] [Google Scholar]
- 18.Leon-Monzon, M., I. Illa, and M. C. Dalakas. 1994. Polymyositis in patients infected with human T-cell leukemia virus type I: the role of the virus in the cause of the disease. Ann. Neurol. 36:643-649. [DOI] [PubMed] [Google Scholar]
- 19.Levin, M. C., T. J. Lehky, A. N. Flerlage, D. Katz, D. W. Kingma, E. S. Jaffe, J. D. Heiss, N. Patronas, H. F. McFarland, and S. Jacobson. 1997. Immunologic analysis of a spinal cord-biopsy specimen from a patient with human T-cell lymphotropic virus type I-associated neurologic disease. N. Engl. J. Med. 336:839-845. [DOI] [PubMed] [Google Scholar]
- 20.Moritoyo, T., T. A. Reinhart, H. Moritoyo, E. Sato, S. Izumo, M. Osame, and A. T. Haase. 1996. Human T-lymphotropic virus type I-associated myelopathy and tax gene expression in CD4+ T lymphocytes. Ann. Neurol. 40:84-90. [DOI] [PubMed] [Google Scholar]
- 21.Muraro, P. A., K. P. Wandinger, B. Bielekova, B. Gran, A. Marques, U. Utz, H. F. McFarland, S. Jacobson, and R. Martin. 2003. Molecular tracking of antigen-specific T cell clones in neurological immune-mediated disorders. Brain 126:20-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagai, M., R. Kubota, T. F. Greten, J. P. Schneck, T. P. Leist, and S. Jacobson. 2001. Increased activated human T cell lymphotropic virus type I (HTLV-I) Tax11-19-specific memory and effector CD8+ cells in patients with HTLV-I-associated myelopathy/tropical spastic paraparesis: correlation with HTLV-I provirus load. J. Infect. Dis. 183:197-205. [DOI] [PubMed] [Google Scholar]
- 23.Nagai, M., and M. Osame. 2003. Human T-cell lymphotropic virus type I and neurological diseases. J. Neurovirol. 9:228-235. [DOI] [PubMed] [Google Scholar]
- 24.Nagai, M., K. Usuku, W. Matsumoto, D. Kodama, N. Takenouchi, T. Moritoyo, S. Hashiguchi, M. Ichinose, C. R. Bangham, S. Izumo, and M. Osame. 1998. Analysis of HTLV-I proviral load in 202 HAM/TSP patients and 243 asymptomatic HTLV-I carriers: high proviral load strongly predisposes to HAM/TSP. J. Neurovirol. 4:586-593. [DOI] [PubMed] [Google Scholar]
- 25.Ozden, S., A. Gessain, O. Gout, and J. Mikol. 2001. Sporadic inclusion body myositis in a patient with human T cell leukemia virus type 1-associated myelopathy. Clin. Infect. Dis. 32:510-514. [DOI] [PubMed] [Google Scholar]
- 26.Pignone, A., G. Fiori, A. Del Rosso, S. Generini, and M. Matucci-Cerinic. 2002. The pathogenesis of inflammatory muscle diseases: on the cutting edge among the environment, the genetic background, the immune response and the dysregulation of apoptosis. Autoimmun. Rev. 1:226-232. [DOI] [PubMed] [Google Scholar]
- 27.Pique, C., A. Ureta-Vidal, A. Gessain, B. Chancerel, O. Gout, R. Tamouza, F. Agis, and M. C. Dokhelar. 2000. Evidence for the chronic in vivo production of human T cell leukemia virus type I Rof and Tof proteins from cytotoxic T lymphocytes directed against viral peptides. J. Exp. Med. 191:567-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito, M., I. Higuchi, A. Saito, S. Izumo, K. Usuku, C. R. Bangham, and M. Osame. 2002. Molecular analysis of T cell clonotypes in muscle-infiltrating lymphocytes from patients with human T lymphotropic virus type 1 polymyositis. J. Infect. Dis. 186:1231-1241. [DOI] [PubMed] [Google Scholar]
- 29.Saito, M., G. P. Taylor, A. Saito, Y. Furukawa, K. Usuku, J. N. Weber, M. Osame, and C. R. Bangham. 2001. In vivo selection of T-cell receptor junctional region sequences by HLA-A2 human T-cell lymphotropic virus type 1 Tax11-19 peptide complexes. J. Virol. 75:1065-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tangy, F., M. Ossondo, J. C. Vernant, D. Smadja, O. Bletry, A. C. Baglin, and S. Ozden. 1999. Hum. T cell leukemia virus type I expression in salivary glands of infected patients. J. Infect. Dis. 179:497-502. [DOI] [PubMed] [Google Scholar]
- 31.Tawil, R., and R. C. Griggs. 2002. Inclusion body myositis. Curr. Opin. Rheumatol. 14:653-657. [DOI] [PubMed] [Google Scholar]
- 32.Ureta-Vidal, A., C. Pique, Z. Garcia, A. Dehee, P. Tortevoye, N. Desire, A. Gessain, B. Chancerel, O. Gout, F. A. Lemonnier, and M. Cochet. 2001. Human T cell leukemia virus type I (HTLV-I) infection induces greater expansions of CD8 T lymphocytes in persons with HTLV-I-associated myelopathy/tropical spastic paraparesis than in asymptomatic carriers. J. Infect. Dis. 183:857-864. [DOI] [PubMed] [Google Scholar]
- 33.Wu, E., D. W. Dickson, S. Jacobson, and C. S. Raine. 1993. Neuroaxonal dystrophy in HTLV-1-associated myelopathy/tropical spastic paraparesis: neuropathologic and neuroimmunologic correlations. Acta Neuropathol. (Berlin) 86:224-235. [DOI] [PubMed] [Google Scholar]
- 34.Yamano, Y., M. Nagai, M. Brennan, C. A. Mora, S. S. Soldan, U. Tomaru, N. Takenouchi, S. Izumo, M. Osame, and S. Jacobson. 2002. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8(+) T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood 99:88-94. [DOI] [PubMed] [Google Scholar]
- 35.Ytterberg, S. R. 1996. Infectious agents associated with myopathies. Curr. Opin. Rheumatol. 8:507-513. [DOI] [PubMed] [Google Scholar]