Abstract
To investigate whether a DNA virus can evade passive immunotherapy with a polyclonal antiserum, we analyzed the protection of a neutralizing capsid antiserum against a lethal infection of the immunosuppressive strain of the parvovirus minute virus of mice (MVMi) in 42 immunodeficient mice over a period of 200 days. A few mice were effectively protected, but most developed a delayed lethal leukopenic syndrome during the treatment or weeks afterwards. Unexpectedly, viruses isolated from treated but also from control leukopenic mice showed no amino acid changes throughout the entire capsid coding region, although the viral populations were genetically heterogeneous, mainly in the second exon of the coding sequence of the NS2 nonstructural protein. The NS2 point amino acid changes (T88A, K96E, L103P, and L153 M) that were consistently selected in several mice clustered within the nuclear exportin CRM1 binding domain, in a reading frame that did not alter the overlapping NS1 coding region. These mutations endowed emerging viruses with an increased fitness that was demonstrable by their relative resistance to the neutralizing capsid antiserum in a postentry plaque-forming assay, the rapid overgrowth of a competing wild-type (wt) population in culture, and a larger yield of infectious particles. Mutant NS2 proteins interacted with a higher affinity and sequestered CRM1 in the perinuclear region of the cytoplasm more efficiently than the wt. Correspondingly this phenomenon, as well as the following timely ordered release of the NS1 nonstructural protein and the empty capsid from the nucleus to the cytoplasm, occurred markedly earlier in the infection cycle of the mutant viruses. We hypothesize that the enhanced cytoplasmic sequestration of CRM1 by the NS2 mutations selected in mice may trigger pleiotropic effects leading to an accelerated MVMi life cycle and thus to increased fitness. These results strengthen our earlier report on the rapid evolutionary capacity of this mammalian-specific DNA virus in vivo and indicate that the NS2-CRM1 interaction is an important determinant of parvovirus virulence that can be modulated in nature, hampering the effectiveness of passive antibody therapies in the long term.
Passive antibody administration is a common therapeutic measure that is in use or under evaluation for use against several viruses, including important human pathogens (34, 35, 38), and its efficacy is often evaluated in animal models of viral infections (24, 29, 46). The effectiveness of passive immunotherapy may be hampered by the extremely heterogeneous and dynamic distributions of mutant genomes in many RNA virus populations (21), a concept termed viral quasispecies (reviewed in references 19 and 23). Indeed, some RNA viruses can easily evade the action of neutralizing monoclonal antibodies (MAbs) through the rapid selection of MAb-resistant (Mar) mutants in vitro and in vivo (49). Thus, cocktails of MAbs targeting a wide repertoire of antigenic sites are being recommended in current protocols of passive immunotherapy against human RNA viruses (60).
It is generally believed that DNA viruses are no more variable than cellular genes (20, 58), as their genomes are replicated by cellular or their own polymerases, for which proofreading activities have been either proved (32) or assumed. However, there is increasing evidence of the capacity of some DNA viruses to rapidly evolve in nature under different selective pressures (31, 33, 56). Notably, rapid evolution in vitro was demonstrated for several members of the Parvoviridae (5, 15, 50), a family of viruses with a single-stranded DNA (5-kb) genome packaged into a 25-nm-diameter icosahedral capsid (41). The importance of the rapid evolutionary capacity of parvoviruses in nature is exemplified by the emergence of the canine parvovirus CPV (47), the host-range drift of CPV during epidemics, leading to an estimated rate of retained sequence substitution of 1.69 × 10−4/nucleotide (nt)/year in the VP gene (48, 56), and the isolation of heterogeneous populations of Aleutian mink disease parvovirus (ADV) in mink (30) and adeno-associated virus in monkeys (29). The association of parvoviruses with persistent and chronic diseases in animals and immunocompromised humans treated by passive immunotherapy (3, 35) may also be related to the selection of viruses that are adapted to those environments.
To study parvovirus evolution in a defined mammalian model, we adopted the immunosuppressive virulent strain of the parvovirus minute virus of mice (MVMi) (7) infecting adult mice with severe combined immunodeficiency (SCID) (8). In this host, MVMi causes acute leukopenia resulting from the capacity of the virus to target hemopoietic committed precursors and stem cells (51-53). A passive immunotherapy in SCID mice with a neutralizing MAb recognizing the MVMi capsid selected for Mar mutants harboring single radical amino acid changes at the threefold axes of the icosahedral capsid (36) and resulted in an unexpectedly high genetic heterogeneity in the populations of viruses emerging in vivo. Moreover, a high frequency of Mar mutants were found even in virus clonal stocks obtained without selection (36), suggesting a genetic composition for MVM populations that is typical of quasispecies, which would be a rare phenomenon when dealing with DNA viruses.
The MVM genome codes for two structural proteins (VP1 and VP2) that form a T=1 icosahedral capsid (2) and two nonstructural proteins (NS1 and NS2) that play diverse roles in virus multiplication. NS1 is a nuclear phosphoprotein that is required for viral DNA replication (16) and is cytotoxic for cells at certain transformation stages (11, 18). The smaller NS2 protein contains three isoforms (23 to 28 kDa) arising from alternate splicing (17) that can bind the cell cycle regulator 14-3-3 protein family (9) and the survival motor neuron (Smn) protein (59). NS2 shuttles from the nucleus to the cytoplasm by an interaction with the nuclear transport factor CRM1 (6, 45) mediated by a conventional nuclear export sequence (NES) mapped between amino acids 81 and 103 of the viral protein (22, 40, 45). A multiplicity of functions have been assigned to NS2, such as capsid assembly (15), messenger translation (43), DNA replication and virus production (42), and capsid egress from the nucleus (22, 40), even though its mode of action remains unclear. NS2 is not essential for the infection of some cell types in culture (13, 15, 42), but it is important in viral pathogenesis and tropism, since an NS2-defective virus was completely unable to infect newborn mice (10) and since an element of the MVM genome responsible for splicing regulation (nt 1884 to 2070) contributed to an infection of the prototype strain (MVMp) in lymphocytes (14).
For this report, continuing a previous study on the rapid selection of Mar mutants of MVMi in response to a passive MAb-based therapy (36), we used a neutralizing polyclonal capsid antibody (PAb) in SCID mice to challenge the structural plasticity of the MVM capsid in evading immune pressure targeted to multiple epitopes. Interestingly, viruses recovered from leukopenic mice were not resistant to the PAb by a conventional neutralization assay, in agreement with the lack of amino acid changes in the capsid proteins. However, point changes of amino acids were selected in the CRM1 binding domain of NS2 that increased the capacity, newly described here, of sequestering CRM1 in the perinuclear region, leading to an enhanced nuclear release of viral proteins and an accelerated life cycle. These results further support the evolutionary capacity of this DNA virus in vivo and identify NS2-CRM1 binding as a relevant determinant of parvovirus fitness in a natural host.
MATERIALS AND METHODS
Virus and cells.
Viral stocks of the immunosuppressive strain of the parvovirus MVMi were prepared from an infectious DNA clone through a minimal number of passages in mouse EL-4 lymphoma cells as previously described (36), purified by sucrose and cesium chloride gradient centrifugation (51, 53), and filter sterilized. Virus titers were determined by a PFU assay on monolayers of the permissive human simian virus 40-transformed NB324K cell line (55). Cells were cultured in Dulbecco's modified Eagle medium (GIBCO Laboratories) supplemented with 5% inactivated fetal calf serum (FCS). To block the reentry of produced virus capsids into cells, we added 0.05 U of neuraminidase (New England Biolabs)/ml to the infected cultures.
Viral neutralization and resistance.
A polyclonal antiserum against the MVM capsid (PAb) was raised in 8-week-old BALB/c female mice by intraperitoneal injection (20 μg per dose) of gradient-purified empty capsids emulsified in Freund's complete adjuvant the first time and by three further booster injections in incomplete adjuvant at 2-week intervals. Bleeding was done 10 days after the last injection. The preattachment neutralization titer of the PAb was determined by the incubation of serial dilutions of the serum with 150 PFU of MVMi in 400 μl for 30 min at 37°C, and the proportion of nonneutralized virus was determined by a plaque assay. One neutralization unit (NU) was defined as the amount of antibody required to neutralize 50% of the PFU in this assay. To determine the degree of viral resistance to the PAb in a postentry neutralization assay, we inoculated monolayers of NB324K cells seeded at the density of a standard plaque assay with serial dilutions of mouse organ homogenates or plaque-isolated viral clones diluted in phosphate-buffered saline (PBS). After 1 h of adsorption and removal of the inocula, the cells were incubated for 4 h in Dulbecco's modified Eagle medium-5% FCS to allow virus entry into the cells, and various PAb doses (1 to 20 NU/ml) were added to the overlaid semisolid medium. Plaques were counted from duplicates 6 days afterwards.
Passive immunotherapy.
Eight-week-old females of the C.B-17 inbred strain of SCID mice (8) were used. Breeding pairs, originally obtained from Jackson Laboratories (Bar Harbor, Maine), were allowed autoclaved food and water ad libitum, were routinely handled under sterile conditions, and were housed in sterile microisolators with a 9 a.m.-to-9 p.m. light-dark cycle. Mice were inoculated oronasally with 106 PFU of a purified stock of the parvovirus MVMi/mouse in 10 μl of PBS, as previously described (53). At the indicated days postinfection (dpi), mice were treated by intravenous injection into the tail vein of 100 NU of PAb antiserum that had been diluted in 100 μl of PBS and filter sterilized. Infected control mice were either untreated or received periodical injections of a mouse preimmune serum with no detectable MVMi neutralizing capacity. Mice were euthanized as they became moribund, and their organs were extracted and homogenized in PBS to a concentration of 10% (wet wt/vol).
Genetic analysis of emerging viruses.
MVMi replicative and genomic DNA forms were isolated from mouse organs, culture cells, or viral plaques by a modified Hirt's procedure (39) as previously described (36). Virus DNAs were amplified by PCR according to published protocols by use of a Perkin-Elmer DNA Cetus (Gene Amp PCR system 9600) or Bio-Rad gene cycler. In order to encompass all of the coding region of the MVMi genome (4), we used the following four pairs of oligonucleotides for the specified nucleotide positions on the MVM genome: NSA (nt 222 to 238) and NSF (nt 2104 to 2077), NSD (nt 1686 to 1703) and VVPSEQ3 (nt 3366 to 3350), VVP1 (nt 2221 to 2237) and VVPSER388 (nt 3967 to 3950), and VVP6 (nt 3444 to 3460) and VPSEQ0 (nt 4706 to 4688). The amplified fragments were purified from agarose gels by use of the Concert rapid gel extraction system (Gibco-BRL and Promega) and were sequenced in a Perkin-Elmer 377 automated sequencer by use of the following oligonucleotides: NSA (nt 222 to 238), NSB (nt 948 to 966), NSC (nt 1061 to 1044), NSE (nt 2000 to 2017), NSF (nt 2104 to 2077), SMASIP3 (nt 2443 to 2422), VVP1 (nt 2221 to 2238), VVP2 (nt 2503 to 2518), VVP4 (nt 2945 to 2961), VVP6 (nt 3444 to 3460), VVP8 (nt 3901 to 3917), and VPSEQ0 (nt 4706 to 4688).
Immunological analyses.
For analyses of the subcellular distribution of proteins by indirect immunofluorescence (IF), NB3234K cells seeded onto glass coverslips and either growing or synchronized by isoleucine starvation and aphidicolin treatment (16) or by growth to confluence (E. Hernando and J. M. Almendral, unpublished data) were infected at a multiplicity of infection (MOI) of 1 to 2 and fixed in methanol-acetone (1:1) at −20°C for 7 min at the indicated times after infection. The cells were incubated with the following primary antibodies diluted in PBS-5% FCS: a MAb (B7) recognizing an epitope conformed at the MVM capsid surface (capsid) (36), a rabbit antiserum against the NS2 protein of MVM (α-NS2) (40), a rabbit polyclonal antiserum against NS1 (α-NS1) (6), a mouse MAb against NS1 (52), a rabbit serum against human CRM1 (α-CRM1) (27), and a goat polyclonal serum against CRM1 (C-20; Santa Cruz Biotech). Secondary antibodies (Jackson Immunoresearch Laboratories, Inc.), generally diluted 1/200, were conjugated to Texas red or fluorescein isothiocyanate. Phenotypes of subcellular distribution were scored under an Axiovert 2000 microscope (Zeiss) coupled to an image capture system (Spot RT slider; Diagnostic) and were confirmed by confocal microscopy (37) and nuclear counterstaining with To-Pro-3 iodide (Invitrogen), as shown in Fig. 5C.
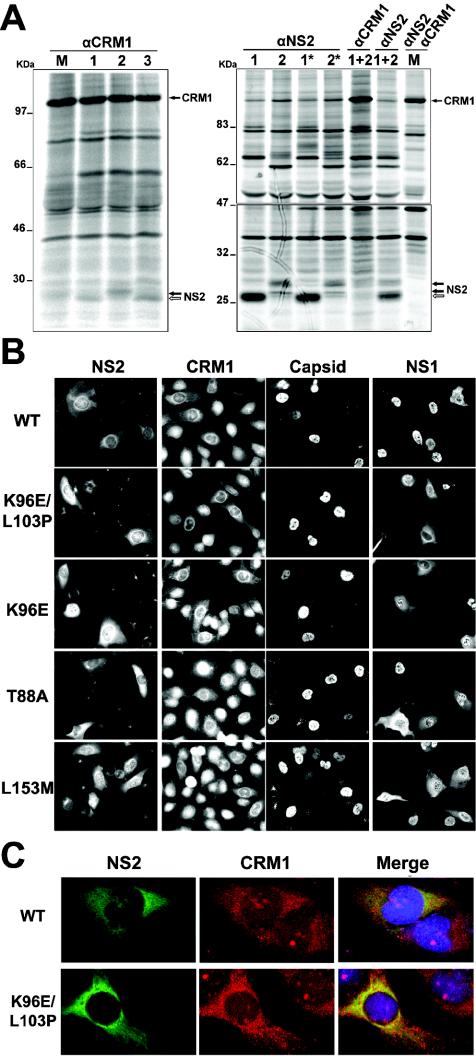
FIG. 5.
Properties of in vivo-selected NS2 mutant proteins. (A) wt and mutant NS2 proteins bind CRM1 with distinct affinities. Growing NB324K cells were infected with different MVMi viruses (lanes: M, mock; 1, wt; 2, K96E/L103P; 3, L153M) at an MOI of 5, labeled with 250 μCi of [35S]Met-Cys/ml in medium without methionine at either 18 to 20 hpi (left) or 18 to 19 hpi (right), and chased for 1 h in normal medium (two lanes with asterisks). NS2-CRM1 complexes were subjected to immunoprecipitation with specific antibodies under conditions that allowed protein-protein interactions, resolved in bipartite 10% and 12% acrylamide (left) or continuous 6 to 20% gradient acrylamide (right) SDS-PAGE gels, and developed by exposure for 1 or 5 days (lower part of the right gel) to a phosphorimager (Fuji). The positions of CRM1 and the wt (white arrow) and mutant (black arrows) NS2 protein species are indicated to the right of the gels. (B) Subcellular distribution of CRM1 and viral antigens in nonsynchronous NB324K cells. Cells were infected at an MOI of 1, and CRM1 and the viral antigens were stained for IF with specific antisera at 24 hpi. The CRM1 and capsid columns correspond to the same field of cells. (C) CRM1 and NS2 colocalize in the perinuclear region of MVMi-infected cells. The subcellular distribution of CRM1 (stained with goat anti-rabbit-FITC) and NS2 (stained with donkey anti-goat-Texas red) was visualized by confocal microscopy. The figure shows representative examples of the different localization of CRM1 in NS2-expressing cells infected with the indicated viruses versus nonexpressing cells.
For analyses of protein interactions, 35S-labeled cells were scraped into a solution containing 150 mM NaCl, 50 mM Tris (pH 8.0), 0.3% sodium dodecyl sulfate (SDS), 1% NP-40, 0.75% 2-mercaptoethanol, and protease and phosphatase inhibitors (1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 10 μg of pepstatin/ml, 10 μg of leupeptin/ml, 5 mM NaF, and 20 mM β-glycerophosphate). Samples were supplemented with 10% glycerol and disrupted by gentle sonication. Homogenates were incubated overnight at 4°C with the rabbit α-CRM1 and α-NS2 antisera at a 1/100 dilution, and the immune complexes were precipitated with protein A-Sepharose (10% [wt/vol]) and then washed with a mixture of cold PBS, 0.05% NP-40, and 1% bovine serum albumin. Bound proteins were eluted by heating of the samples for 5 min at 95°C in Laemmli buffer and then were subjected to SDS-polyacrylamide gel electrophoresis.
RESULTS
Limited protection of passive therapy with capsid antiserum against MVMi infection in SCID mice.
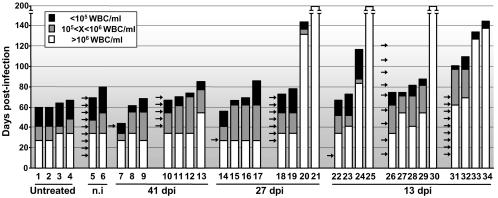
The therapeutic potential of passive immunotherapy with a PAb against the lethal leukopenia induced by MVMi in SCID mice (53) was evaluated over a long period. Adult SCID mice were oronasally inoculated with a lethal dose (106 PFU) of MVMi and subjected to systemic administration of the neutralizing MVM capsid PAb (5 × 104 NU/ml) raised in BALB/c mice. Groups of four female SCID mice were treated (100 NU/mouse per dose) under diverse protocols differing in the initiation and periodicity of the antibody therapy and were monitored for pathological signs and survival, and the number of white blood cells (WBC) was scored weekly for 200 days (Fig. 1). The PAb was injected as a single dose or multiple doses either weekly or every other week, and the treatment was initiated at three critical times during the disease (53): at 13 dpi, when viral DNA replication begins in the mouse bone marrow; at 27 dpi, when viral production in hemopoietic organs substantially increases and leukopenia is first demonstrable in the blood; and at 41 dpi, at the height of the disease, when viral production reaches a plateau. The immunotherapy protocol based on a single dose of the PAb was inefficient at protecting mice when the injection was administered at 41 or 27 dpi, as mice developed leukopenia at 30 to 35 dpi with concomitant weight loss and evident clinical signs leading to death around 60 dpi, as in control animals (no. 1 to 4). But a single dose did provide curative effects when it was administered at 13 dpi, since two of four treated mice showed a delayed onset of leukopenia and an increased life span. With the protocol of multiple doses, most animals gained survival and clinical benefits: protection was low and comparable to that of control mice receiving a nonimmune serum in animals treated starting at 41 dpi, but it was significant in two mice treated starting at 27 dpi and in all four mice given weekly injections starting at 13 dpi, as judged by a delayed leukopenic syndrome and extended life span. Finally, mice treated beginning at 13 dpi on a fortnightly basis showed, on average, less protection, suggesting that the neutralizing capacity of the PAb in mouse blood declines within 2 weeks. In summary, the MVMi capsid antiserum improved the clinical outcome of SCID mice when it was administered at early times postinfection, although most animals eventually succumbed to the disease, with the exception of three mice (no. 21, 25, and 30) who were apparently cured for up to 200 days.
FIG. 1.
Protection analysis of passive PAb therapy against lethal MVMi infection in SCID mice. Mice (designated by numbers) were oronasally infected with 106 PFU of MVMi and treated at the indicated dates (arrows) with an intravenous injection of 100 NU of PAb. Control mice were either untreated (mouse no. 1 to 4) or injected with nonimmune (n.i.) serum (mouse no. 5 and 6). Bars represent the life spans of the animals, and the shadowed and black portions illustrate two arbitrary degrees of the virally induced leukopenic syndrome. WBC, peripheral white blood cells. Surviving mice were sacrificed at 200 dpi.
Notably, several healthy mice (no. 20, 24, 33, and 34) developed leukopenia months after the antibody treatment was concluded. To gain insight into the mechanism of this late leukopenia arising in apparently cured mice, we treated a new group of mice (no. 35 to 42) with multiple doses of the PAb starting at 27 dpi and monitored the level of viral DNA replication in the bone marrow (BM) late in the infection. Consistent with the corresponding group at 27 dpi shown in Fig. 1, four mice died at about 65 dpi with a leukopenic syndrome while the rest remained healthy until sampling (90 to 140 dpi). As previously reported (53), all leukopenic mice showed active MVMi replication in the BM harvested at death, but interestingly the accumulation of viral DNA replicative intermediates was also high in the BM of one mouse (no. 39) that was sampled in the absence of pathological signs or WBC effects (data not shown). This result demonstrated that viral replication in the mouse BM may occur several weeks post-PAb therapy, which may account for the reappearance of infectious virus and a fatal leukopenic syndrome months afterwards.
Selection of precise amino acid changes within an NS2 domain during MVMi infection of SCID mice.
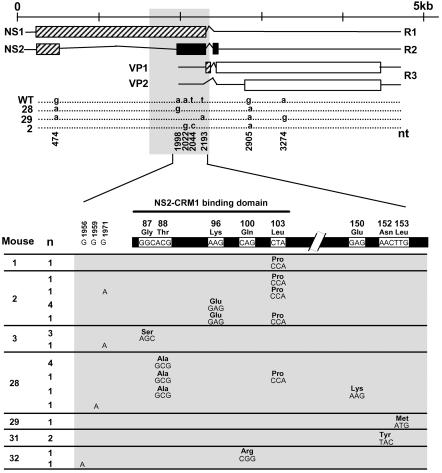
To investigate the genetic basis of the apparent resistance and reemergence of MVMi in PAb-treated mice, we undertook an extensive DNA sequence analysis of isolated infectious viruses. The genomes of viral clones isolated from two PAb-treated mice with acute leukopenia (no. 28 and 29) and from one untreated mouse (no. 2) were sequenced across the entire coding region (nt 253 to 4687). As shown in Fig. 2 (top), a few changes of nucleotides were found spread along the MVM genome compared to the reported wild-type (wt) sequence (4), but remarkably none of them introduced amino acid changes in the VP coding region. Nucleotide substitutions of G to A occurring at nt 474 (in clones from mouse no. 28 and 29) and nt 2905 (in all three viral clones), as well as substitution of A to G at nt 3274 (in a clone of mouse no. 29), were silent and did not change the amino acids with respect to the wt. However, the three clones harbored some mutations between nt 1998 and 2193 which did create amino acid changes within the NS coding region. The changes were as follows: A1998G (mouse no. 28), leading to the change T88A in the small nonstructural protein NS2; T2193A (mouse no. 29), leading to the NS2 change L153M; and A2022G and T2044C (control mouse no. 2), determining the NS2 changes K96E and L103P, respectively. Interestingly, although the central region of the MVM genome to which these four mutations map encodes amino acids of both NS1 and NS2 proteins, only one of these four mutations, T2044C, also altered the NS1 protein, changing tyrosine 595 to histidine (Y595H).
FIG. 2.
Sequence analysis of MVMi viruses isolated from SCID mice. (Top) Genetic organization of MVMi. For simplicity, only one of the three NS2 isoforms is shown. Shown are the nucleotide changes with respect to the wt in single viral clones isolated from mouse no. 2, 28, and 29 and sequenced across the nt 253 to 4687 region. The central region of the MVM genome (nt 1730 to 2298) that was sequenced in the viral populations and isolated clones outlined below is shaded. (Bottom) Region in which most selected genetic changes mapped. The figure shows the nucleotide positions of the mutations, triplet codes, and NS2 amino acid changes found in viral clones recovered from the indicated mouse spleens. n, number of clones with identical genotypes in this region. The wt sequence was confirmed with two plaque-isolated clones of the MVMi stock used to inoculate the mice.
Since mutations altering MVM coding regions clustered in the second NS2 exon, the region flanked by nt 1730 to 2298, encompassing the entire second NS2 exon and some NS1-specific coding sequence, was sequenced for 25 plaque-isolated viral clones recovered from several PAb-treated (no. 28, 29, 31, and 32) and control (no. 1 to 3) mice (Fig. 2, bottom). With the exception of one wt clone found in mouse no. 29 (not shown), every viral clone analyzed from any of the MVMi-infected SCID mice showed one or two point mutations. Three of the 11 mutations found (G1956A, G1959A, and G1971A) mapped outside the NS2 coding region and were silent for NS1, but the other eight mutations (transitions, G1995A, A1998G, A2022G, A2035G, T2044C, and G2184A; and transversions, A2190T and T2193A) fell inside a region in which the coding sequences of NS1 and NS2 overlap in different reading frames. All eight mutations introduced mostly radical amino acid changes in NS2 which were clustered in two separate regions: the first spanned the NS2-CRM1 binding domain (22, 40, 45) and included five changes (G87S, T88A, K96E, Q100R, and L103P) present in 18 viral clones recovered from five different mice, and the second included three nearly contiguous amino acid changes (E150K, N152Y, and L153 M) appearing in four viral clones recovered from three distinct mice. Again, consistent with the results described above, only two (A2035G and T2044C) of the eight mutations affected the NS1 amino acid composition (changes S592G and Y595H, respectively). Moreover, the T2044C mutation, selected in a parallel MVMi evolution in three mice, targets the second and only nucleotide of the triplet codon that can introduce a radical amino acid change (L103P) in NS2 but a conservative (Y595H) change in NS1. These results suggested an important role of this region in the adaptation process developing in the mice and the presence of a selective pressure targeted to NS2 while preserving essentially unaltered NS1.
Genetic heterogeneity in MVMi populations derived from leukopenic mice.
To analyze the global evolutionary process of MVMi adaptation to SCID mice, we determined the consensus PCR sequence of seven different viral populations recovered from the organs of four treated (no. 28, 29, 31, and 32) and three control (no. 1 to 3) mice, focusing on the region from nt 1730 to 2298, where most mutations mapped in the isolated viral clones. Only one of the viral populations (from the spleen of mouse no. 28) showed a fixed mutation (A1998G) as a predominant single peak in the consensus sequence, in agreement with the presence of this mutation in seven of the eight analyzed clones (Fig. 2), whereas the consensus sequence of the population of mouse no. 1 remained wt and the other five populations showed a variable polymorphism ranging from 20 to 60% at the mutated nucleotide positions in the respective viral clones (Fig. 2) of each population (data not shown). These sequence analyses suggested that there are heterogeneous pools of genomes in most viral populations, and indeed consensus sequences did not offer information about less common substitutions that are easily found in some individual viral clones analyzed in this region of the MVM genome (e.g., E150K and L103P in mouse no. 28). This genetic heterogeneity of most MVMi populations recovered from PAb-treated as well as control mice is consistent with a previous report on the evasion of MAb neutralization in SCID mice by a high mutation frequency (36), further supporting the rapid evolution of MVMi in the mouse host.
Mutations selected in vivo in the NS2-CRM1 binding domain increase MVMi fitness.
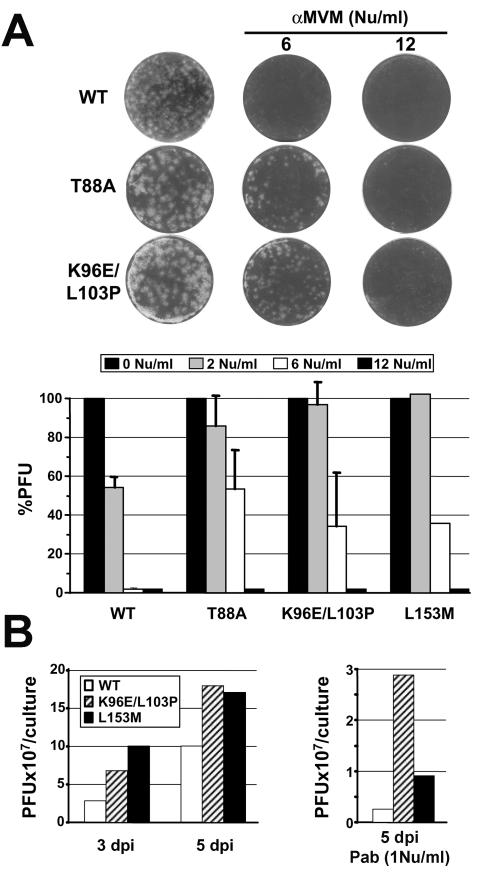
The delayed leukopenia developed in some mice undergoing neutralizing PAb treatment for months after the last injection prompted us to analyze the phenotypes of representative MVMi mutants selected in control and treated mice. Viral clones whose genomes were entirely sequenced in the coding region (Fig. 2, top) and which carried single (T88A) or double (K96E and L103P) mutations in the NS2-CRM1 binding domain or in another NS2 region localized 50 amino acids away (L153M) were studied by different tests of antibody sensitivity and multiplication in vitro. In agreement with the lack of amino acid changes in the VP gene (Fig. 2) and unlike the Mar mutants appearing in MAb-treated mice (36), all of these viruses were as sensitive to the PAb as the original MVMi stock in a conventional preattachment test of neutralization (not shown). However, these viruses formed plaques in NB324K cells that were significantly larger than those produced by the wt, and furthermore they showed significant resistance to the PAb in a postentry neutralization test (see Materials and Methods), as judged by the number and size of the plaques obtained upon the addition of antiserum to the semisolid medium (Fig. 3A). Plaque sizes were progressively reduced with increasing concentrations of the neutralizing PAb, but at 2 NU/ml the wt plaque-forming capacity was reduced 50%, whereas mutant viruses maintained plaque formation at a normal level, and moreover at 6 NU/ml mutant viruses formed still small but countable plaques at a 30 to 50% efficiency, while wt plaques were not demonstrable at all (Fig. 3A, bottom). This postentry resistance was also observed for the neutralizing B7 capsid MAb (not shown). To determine a second phenotypic property of the selected virus mutants, we infected NB324K cells at a low MOI and measured the titer of infectious virus (PFU) produced in the cultures in the presence or absence of the PAb over time. As shown in Fig. 3B, mutant viruses isolated from SCID mice displayed growth rates that were significantly higher than those of the wt at 3 and 5 dpi, and furthermore the production of larger pools of infectious virus was also demonstrated in the presence of 1 NU of PAb added to the medium/ml at 4 h postadsorption.
FIG. 3.
Phenotypic analysis of MVMi mutants selected in SCID mice. (A) Viruses selected in mice formed larger plaques and showed postentry resistance to PAb neutralization. Representative plaque sizes of NS2 mutants recovered from spleen homogenates of SCID mice are shown. Note the different sizes compared to that of the wt and the reduction in size upon PAb addition to the overlaid medium. The bottom panel shows the degree of postentry resistance. Shown are percentages of residual PFU after the addition of the indicated NU of PAb to the overlay medium. Bars show averages with standard errors for three independent experiments (the results for the L153M mutant are from one experiment). (B) Growth rates of NS2 mutants. NB324K cells were infected at an MOI of 0.01 with the indicated viruses in the absence (left) or presence (right) of the neutralizing PAb added to the medium, and the production of infectious virus in the cultures was monitored by a PFU assay. The results are representative of two experiments with similar outcomes.
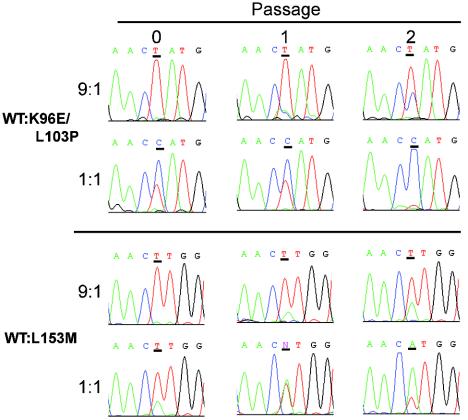
To gain insight into the dynamic mechanism in mice leading to the selection of emerging variant MVMi genotypes, we compared the multiplication rate of two mutant viruses (K96E/L103P and L153M) with that of the original wt virus in a competition assay in culture. NB324K cells were inoculated at wt/mutant PFU ratios of 9:1 and 1:1, the cultures were subjected to two serial passages, and the viral populations were sequenced across the region encompassing the tested mutations. As shown in the DNA sequence profiles in Fig. 4, the proportion of NS2 mutant genomes increased gradually in the 9:1 competition and became the dominant genomes in the cultures upon only two passages in 1:1 competitions with the wt. These experiments demonstrated a growth advantage of the viruses harboring NS2 mutations over the wt, which may explain their efficient selection in mice.
FIG. 4.
NS2 viral mutants replace the wt in culture. The wt and the outlined NS2 mutants were inoculated onto 105 NB324K cells (MOI, 0.01) at PFU ratios of 9:1 and 1:1. Samples of total low-molecular-weight DNA were collected at 3 dpi (passage 1) or at 6 dpi (passage 2) from 105 cells inoculated with 1/10 of the virus harvested in the previous passage. Viral DNAs were amplified by PCR and then sequenced. Histograms show the sequence profiles of the viral genomes for the input virus used for initial infection and at each of the passages, with the consensus sequence shown above. The nucleotide position at which the competing genomes differ is underlined.
CRM-1 interaction and phenotypic effects of NS2 mutants selected in vivo.
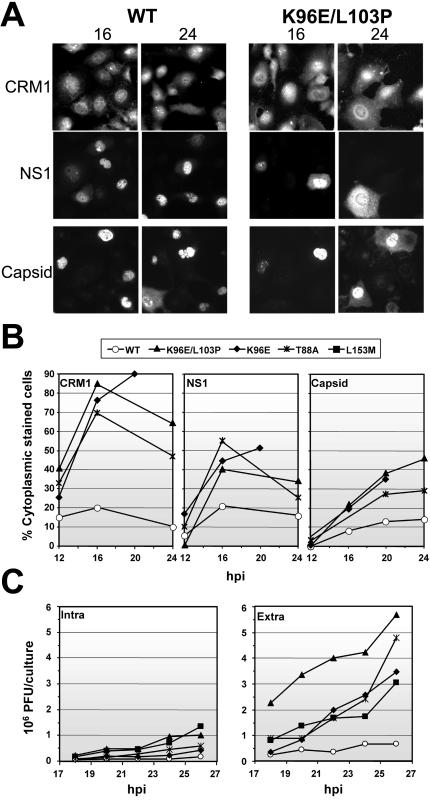
The region in which most viral clones harbored amino acid substitutions mapped within the CRM1 binding domain of NS2 previously described for the MVMp strain, which allows this protein to shuttle across the nuclear membrane (22, 40). This finding led us to investigate the interaction between mutant NS2 proteins of MVMi and CRM1 in infected NB324K cells and its role in increased viral fitness. As shown in Fig. 5A (left panel), the wt and two mutant NS2 proteins were immunoprecipitated with the α-CRM1 antibody, with the K96E/L103P mutant NS2 protein showing a lower electrophoretic mobility than the other proteins, as previously reported for the single mutation K96S in the NS2 protein of MVMp (18). This pattern of interaction demonstrated that NS2-CRM1 binding is not abolished even by the most disruptive mutations selected in mice. Moreover, monitoring of this interaction by the use of pulse-labeling experiments (Fig. 5A, right panel) showed that with respect to the amount of synthesized NS2 protein, a significantly larger proportion of CRM1 protein was precipitated by the α-NS2 antibody in the K96E/L103P versus wt infection. This result suggested a higher affinity of the mutant NS2 proteins for interactions with CRM1, which was further reinforced by a direct competition assay in which the mutant NS2 species were predominantly precipitated by the α-CRM1 antibody in coinfections with the wt and K96E/L103P viruses.
The phenotypic effects of NS2-CRM1 interactions on the subcellular distribution of CRM1 and viral proteins were analyzed by IF with infected growing NB324K cells (Fig. 5B). The NS2 subcellular distribution was not significantly altered by the mutations that were selected in vivo, as it showed a previously described mixed phenotype in wt and mutant viruses (17). This phenotype was consistent with the CRM1 interactive capacity described above for the NS2 mutants and with the nuclear retention of other NS2 mutants of this domain that lacked CRM1 binding properties (22, 40). Notably, NS2 staining was generally more intense in the mutants, with a large amount of accumulation in the cytoplasmic perinuclear region of most infected cells (Fig. 5B and C). The cellular transporter CRM1 showed a characteristic, predominant, though not exclusive, nuclear localization in uninfected cells (1), but remarkably its subcellular distribution was drastically and specifically altered in capsid-expressing cells, indicating an ongoing MVMi infection (Fig. 5B). As shown in Fig. 5C, CRM1 was localized to the cytoplasm of cells that were infected by the wt as well as the mutant viruses, with clear nuclear exclusion, as it mainly accumulated in the perinuclear region and colocalized with NS2. These results indicated cytoplasmic sequestration of the shuttling CRM1 transport receptor by the interaction with the NS2 protein.
Interestingly, some differences induced by the selected NS2 mutations with respect to the wt were found for the subcellular distribution of MVMi proteins that did not carry amino acid changes, mainly for the karyophilic NS1 protein, and to a lesser extent, for the assembled empty capsid (Fig. 5B, right panels). The subcellular distribution of the NS1 protein in cells infected by the mutant viruses (T88A, K96E, K96E/L103P, and L153M) showed a mixed phenotype, with predominant cytoplasmic staining in 35 to 60% of the cells (n > 300). This result was in clear contrast to the general nuclear accumulation of NS1 found in infections with the wt MVMi and MVMp strains, in which only 10 to 15% of the cells (n > 300) showed significant cytoplasmic staining. The effect of NS2 mutations on capsid nuclear release was less evident in nonsynchronized cells at 24 h postinfection (hpi) and is described below. This result suggested an indirect alteration of the MVM infection cycle induced by the selected NS2 mutations localized in the CRM1 binding domain.
Mutant NS2 proteins determine early cytoplasmic sequestration of CRM1 and enhanced nuclear release of viral proteins.
The subcellular localization of viral antigens and CRM1 was quantitatively analyzed over time with a single round of infection of synchronized NB324K cells (Fig. 6A and B). For the wt infection, a low to moderate level of cytoplasmic sequestration of CRM1 was demonstrable at 12 hpi in cells showing NS1 nuclear staining. The wt NS1 protein was found localized inside the nucleus at 12 hpi, but definite cytoplasmic staining in a small proportion of cells was noticed by 16 hpi and remained at similar levels for the rest of the infection (Fig. 6B). The well-defined staining for CRM1 and NS1 in the infected cells was lost concomitantly with the general disruption of cell morphology at 28 hpi. The capsid antigen, however, remained mostly nuclear for a longer time in the wt infection cycle, staining the cytoplasm in a significant proportion of synchronized cells by 20 hpi (Fig. 6B), although it was eventually excluded from the nucleus by 28 hpi. Incubation of the infected cells with neuraminidase, which was added to the cultures after aphidicolin release, eliminated the characteristic punctate cytoplasmic staining of reentering particles, but it did not suppress the homogeneous staining of the empty capsids being released from the nucleus (not shown).
FIG. 6.
Features of the single infection cycle of NS2 viral mutants. (A) Subcellular distribution of CRM1 and viral antigens. NB324K cells synchronized with aphidicolin were infected (MOI, 1), and the localization of CRM1 and the viral proteins during the infection cycle was determined by IF staining with specific antibodies. The panels show some representative fields of subcellular localization for each antigen. The CRM1 and NS1 (anti-NS1 MAb) columns correspond to the same fields of cells. (B) Quantitative analysis of subcellular distribution. The plots show estimated percentages of cells infected with the indicated viruses showing a mixed nuclear-cytoplasmic phenotype for NS1, CRM1 perinuclear accumulation, or patent invasion of the cytoplasm by the capsid antigen (n = 200 for two experiments). (C) Viral production. NB324K cells synchronized by growth to confluence were infected (MOI, 1) with the indicated viruses, and the infectious particles associated with the cells (intra) or released into the extracellular medium (extra) were measured as PFU at several times postinfection. Titers are the averages from two experiments.
A similar pattern of sequential alteration in the subcellular localization of viral antigens was found for infections with two NS2 single (K96E and T88A) and one double (K96E/L103P) mutant. However, in all cases, the course of events occurred earlier in the life cycle of the mutant viruses than in that of the wt (Fig. 6A and B). A significant proportion of infected cells (showing NS1 expression) showed predominantly cytoplasmic sequestration of CRM1 by 12 hpi, and moreover this phenotype occurred markedly in most infected cells by 16 hpi, even in cells showing clear NS1 nuclear staining. As outlined above for nonsynchronous infections, CRM1 perinuclear accumulation colocalized with NS2 in synchronized cells (not shown). NS1 cytoplasmic staining in cells infected with mutant viruses also occurred in some cells after 12 hpi, and this effect sharply increased with time, reaching about half of the infected cell population by 16 hpi. By 24 hpi, however, the well-defined CRM1 sequestration and NS1 cytoplasmic staining were less evident in many cells showing cytopathic effects. As described above, nuclear release of the capsid antigen followed delayed kinetics in mutant viruses, reaching a mixed nucleus-cytoplasm phenotype in a high proportion of infected cells by 24 hpi, with the eventual general loss of intracellular capsid staining in the cultures by 28 hpi. Parallel results were obtained with cultures that were synchronized by growth to confluence (data not shown). These results indicated that the mutations selected in the CRM1 binding domain of NS2 efficiently sequestrate CRM1 in the perinuclear region earlier in the infection cycle of viral mutants and that this phenomenon is followed by an ordered release of karyophilic viral antigens from the nucleus, occurring first for NS1 and in a delayed manner for the capsid. A final analysis of the effect of NS2 mutations on the virus yield (Fig. 6C) showed that all of the mutants produced large numbers of PFU in synchronized cultures in various amounts, ranging from two to five times the wt production by 26 hpi. This moderate though consistent higher yield of mutant infectious particles was observed in the culture medium and in the cell-associated viruses, indicating an overall benefit in virus production rather than a specifically facilitated cellular egress. Indeed, as recently described (37), MVM virions actively exit from the nucleus several hours prior to empty capsid egress by using a signal localized at the VP2 N terminus.
DISCUSSION
For this report, we attempted a comprehensive study of the capacity of a single-stranded DNA parvovirus to evade a passive therapy in immunodeficient mice with an antiserum raised against the capsid and of the molecular mechanisms involved. SCID mice that were lethally infected with MVMi were injected with a neutralizing antiserum according to several therapeutic protocols, and a large collection of viruses recovered from the mice were subjected to genetic and phenotypic analyses. Extensions of this survey should help us to understand the mechanisms developed by other rapidly evolving viruses to prevail against antibody therapies in humans and animals.
Failed long-lasting protection of SCID mice against MVMi by passive therapy with polyclonal antiserum.
A systemic passive therapy with a capsid polyclonal antiserum administered to SCID mice that were lethally infected with MVMi (Fig. 1) was not curative but increased the average survival time when it was administered at 13 dpi, increased survival in only half of the mice if treatment began at 27 dpi, and gave no health benefit in mice treated at 41 dpi. The therapeutic potential of the PAb was poor compared with the clinical outcome and lasting survival of SCID mice that were similarly infected with MVMi and subjected to transplantation of immunocompetent BM (52), suggesting that cell-mediated immunity is important for the control of the infection and for virus clearance. An interesting related phenomenon observed in some apparently cured mice was the onset of acute leukopenia at several weeks post-PAb treatment (mouse no. 20, 24, 33, 34, and 39). The long-term reemergence of MVMi indicates that the PAb therapy restricted the infection but did not completely eliminate virus reservoirs in host tissues. This observation is consistent with the reported capacity of MVMi to persist in quiescent hemopoietic stem cells (52), which may also act as a reservoir of virus in PAb-treated mice. Persistence in quiescent hemopoietic stem cells leading to virus activation in response to proliferative stimuli may be a major issue for the long-term efficacy of immune therapies in humans (35).
Evolution and fitness of MVMi in mice.
MVMi viruses recovered from leukopenic mice showed a high level of genetic heterogeneity (Fig. 2). Three viral genomes isolated from either PAb-treated (mouse no. 28 and 29) or nontreated (mouse no. 2) mice and sequenced almost entirely showed three or four nucleotide changes per genome compared to the wt, giving an average of 11 mutations per 13,272 nt sequenced. This finding results in a mutation frequency of 8.2 × 10−4 mutations/nt in the MVMi genome during the 2 to 4 months of viral growth in mice, which is in the same range as that found for a single-stranded plant geminivirus (33) and slightly higher than the 1.69 × 10−4 substitutions/year estimated for the VP gene of canine parvovirus (48, 57). The genetic heterogeneity of MVMi best exemplified by the heterogeneous population harvested from one mouse (mouse no. 2 or 28) was previously noticed in some viral populations of MAb-treated mice (36) and further reinforces the notion that MVMi multiplication in the mouse follows a quasispecies distribution (19, 23).
The rapid MVMi evolution in several mice led to the selection of a short collection of mutations at specific nucleotide positions that introduced eight radical amino acid changes in NS2 but that, in most cases, did not affect the overlapping NS1 amino acid sequence. The remarkable selection of NS2 mutations occurred in every infected mouse (Fig. 2), even in previously isolated Mar mutants (36) that were sequenced across this NS2 region (data not shown). Viruses obtained from hemopoietic organs carrying NS2 mutations were phenotypically distinct from the wt in plaque size, postentry resistance to the PAb administered to mice, and multiplication rate in coinfected cultures (Fig. 3 and 4). These features, which likely reflect the larger virus yield found for a single infection cycle (Fig. 6C), were shared by the viral populations recovered from control and PAb-treated animals, indicating that the increased fitness was not selected by the immune pressure elicited by the PAb, but rather developed over the weeks that were required for MVMi adaptation to mice. Further research may explain whether a restriction in the size of viral populations imposed by the antiserum led to a faster selection of viral clones with higher replication rates. Surprisingly, and unlike the Mar mutants, which were easily isolated from SCID mice at short postinfection times and harbored amino acid substitutions at the spike of the MVMi capsid (36), no amino acid changes in the VP coding region occurred in viruses isolated from PAb-treated mice, regardless of the survival time (Fig. 2), even though both antibodies showed similar therapeutic potentials when administered weekly starting at 25 to 27 dpi. These results suggest that structural constraints of the MVMi capsid cannot tolerate the large changes in amino acids that would likely be required to evade the reactivity of an antibody directed to multiple epitopes. In support of this hypothesis, it was recently described that the loops of the MVM capsid cannot tolerate peptide insertions without compromising stability (12).
CRM1-NS2 interaction in MVMi life cycle and virulence.
The NS2 mutations selected in different mice (Fig. 2) clustered mainly within the 81- to 103-amino-acid region previously proposed as the CRM1 binding domain (22, 40, 45). Changes G87S and T88A (selected in mouse no. 3 and 28) mapped next to L89, a highly conserved hydrophobic residue that has been shown to be essential for CRM1 binding (22, 40). The mutation L103P, which was highly selected in mice (mouse no. 1, 2, and 28), targets a leucine that is strictly conserved among parvoviruses (45) and is generally an amino acid that is commonly critical for this type of leucine-rich NES (27), and the mutation K96E (mouse no. 2) occurred at a position that was recently shown to be important for MVMp cytotoxicity when NS1 was also affected (18). The L153M virus mutant showed increased fitness (Fig. 3) and phenotypic features similar to the other mutants (Fig. 5B and 6C), suggesting that the region from positions 150 to 153 of NS2 placed outside of the consensus NES, in which amino acid changes were also selected in some mice (no. 28, 29, and 31), may also modulate NS2-CRM1 binding. The NS2 changes selected in different mice not only preserved CRM1 binding but, moreover, endowed NS2 with a CRM1 interactive capacity with a higher affinity (Fig. 5A). Correspondingly, the capacity of NS2 to sequester CRM1 in the perinuclear region of the cytoplasm was increased in the mutants (Fig. 5C and 6A and B).
Other mutations engineered in the CRM1 binding domain of NS2 in the MVMp genome actually disrupted binding, severely impairing virus spreading and the nuclear egress of capsids in mouse fibroblasts (22, 40), and to a lesser extent, in human transformed NB324K cells (40). Those reports are consistent with the increased fitness in NB324K cells described here for the MVMi viruses carrying NS2 mutations. However, several independent pieces of evidence strongly suggest that capsid egress from the nucleus is a nonspecific effect that is neither directly related to NS2 shuttling or CRM1 transport activity nor involved in the active egress of the mature virus. Indeed, capsid egress from the nucleus occurred late in the infection cycle after NS1 cytoplasmic invasion with a large nonstructural polypeptide lacking a consensus NES and several hours after the cytoplasmic sequestration of CRM1 became general in infected cells (Fig. 6). Infectious viruses exit the nucleus several hours prior to the general nuclear release of viral particles by an active transport driven by a short peptide sequence that is not exposed in empty capsids, as described elsewhere (37). In agreement with this, no reciprocal interaction of CRM1 or NS2 with viral structural proteins (22) or with the intact capsid or mature virus (our unpublished observations) could be demonstrated after extensive trials. Therefore, the nuclear release of viral antigens (NS1 and capsid) should be regarded as a late event in the infection cycle that, like the overall virus yield, may indirectly be enhanced or accelerated by the CRM1-NS2 cytoplasmic interaction.
The mechanisms by which the enhanced CRM1 cytoplasmic sequestration by NS2 benefit MVMi fitness and virulence in mice remain unclear and deserve further research. An interruption of the CRM1 nucleus-cytoplasm shuttling required for the normal functioning of this cellular transporter may unleash cytotoxic effects leading to a progressive leakiness of the nuclear envelope for viral antigens (Fig. 6). NS2 may deregulate the control of nuclear-cytoplasmic transport, facilitating the transport of CRM1-independent viral messengers, or may recruit CRM1 to an efficient transport pathway, as described for other viral factors (44, 54), resulting in both cases in a shortening of the parvovirus infection cycle. An activity of this type could mechanistically account for most of the multiple roles in the regulation of the MVM life cycle that have been ascribed to NS2 (15, 22, 40, 42, 43). At the organism level, an increased virus yield in an accelerated virus cycle may facilitate viral spreading and evasion of host defenses that could underlie the role of NS2 in MVM pathogenesis (10). The sequestration of CRM1 by NS2 may occur in tissues, as demonstrated for other nuclear viruses (25-27), affecting important processes in the pathogenesis of parvoviruses.
Acknowledgments
We are indebted to J. C. Ramírez (Hospital 12 de Octubre, Madrid, Spain) and J. Dean (MIRT Program, UCI, Irvine, Calif.) for experimental support, N. Salomé and J. Rommelaere (DKFZ, Heidelberg, Germany) for the polyclonal α-NS1 antibody and immunoprecipitation advice, M. Fornerod (EMBL, Heidelberg, Germany) for the α-hCRM-1 antibody, D. Pintel (Columbia, Mo.) for the α-NS2 antibody, and C. Parrish (Cornel, N.Y.) for the hybridoma producing the B7 capsid MAb.
This work was supported by grant SAF 2001-1325 CICYT from the Spanish Ministry of Science, EU contract QLK3-CT-2001-01010, grant 07B/0020/2002 from the Comunidad de Madrid, and an institutional grant from Fundación Ramón Areces to the Centro de Biología Molecular “Severo Ochoa.” J.P. was financed by the MIRT program with NIH grant TW00023.
REFERENCES
- 1.Adachi, Y., and M. Yanagida. 1989. Higher order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kD protein preferentially localized in the nucleus and at its periphery. J. Cell Biol. 108:1195-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agbandje-McKenna, M., A. LLamas-Saiz, F. Wang, P. Tattersall, and M. G. Rossmann. 1998. Functional implications of the structure of the murine parvovirus minute virus of mice. Structure 6:1369-1381. [DOI] [PubMed] [Google Scholar]
- 3.Alexandersen, S., S. Larsen, A. Cohn, A. Uttenthal, R. E. Race, B. Aasted, M. Hansen, and M. E. Bloom. 1989. Passive transfer of antiviral antibodies restricts replication of Aleutian mink disease parvovirus in vivo. J. Virol. 63:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Astell, C. R., M. E. Gardinerd, and P. Tattersall. 1986. DNA sequence of the lymphotropic variant of minute virus of mice, MVM(i), and comparison with the DNA sequence of the fibrotropic prototype strain. J. Virol. 57:656-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badgett, M. R., A. Auer, L. E. Carmichael, C. R. Parrish, and J. J. Bull. 2002. Evolutionary dynamics of viral attenuation. J. Virol. 76:10524-10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodendorf, U., C. Cziepluch, J.-C. Jauniaux, J. Rommelaere, and N. Salomé. 1999. Nuclear export factor CRM1 interacts with nonstructural proteins NS2 from parvovirus minute virus of mice. J. Virol. 73:7769-7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnard, G. D., E. K. Manders, D. A. Campbell, R. B. Herberman, and M. J. Collins. 1976. Immunosuppressive activity of a subline of the mouse EL-4 lymphoma. J. Exp. Med. 143:187-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosma, G. C., R. P. Custer, and M. J. Bosma. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301:527-530. [DOI] [PubMed] [Google Scholar]
- 9.Brockhaus, K., S. Plaza, D. J. Pintel, J. Rommelaere, and N. Salomé. 1996. Nonstructural protein NS2 of minute virus of mice associates in vivo with 14-3-3 protein family members. J. Virol. 70:7527-7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brownstein, D. G., A. L. Smith, E. A. Johnson, D. J. Pintel, L. K. Naeger, and P. Tattersall. 1992. The pathogenesis of infection with minute virus of mice depends on expression of the small nonstructural protein NS2 and on the genotype of the allotropic determinants VP1 and VP2. J. Virol. 66:3118-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caillet-Fauquet, P., M. Perros, A. Brandenburger, P. Spegelaere, and J. Rommelaere. 1990. Programmed killing of human cells by means of an inducible clone of parvoviral genes encoding non-structural proteins. EMBO J. 9:2989-2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carreira, A., M. Menendez, J. Reguera, J. M. Almendral, and M. G. Mateu. 2004. In vitro disassembly of a parvovirus capsid and effect on capsid stability of heterologous peptide insertions in surface loops. J. Biol. Chem. 279:6517-6525. [DOI] [PubMed] [Google Scholar]
- 13.Cater, J. E., and D. J. Pintel. 1992. The small non-structural protein NS2 of the autonomous parvovirus minute virus of mice is required for virus growth in murine cells. J. Gen. Virol. 73:1839-1843. [DOI] [PubMed] [Google Scholar]
- 14.Colomar, M. C., B. Hirt, and P. Beard. 1998. Two segments in the genome of the immunosuppressive minute virus of mice determine the host-cell specificity, control viral DNA replication and affect viral RNA metabolism. J. Gen. Virol. 79:581-586. [DOI] [PubMed] [Google Scholar]
- 15.Cotmore, S. F., J. D. Abramo, L. F. Carbonell, J. Bratton, and P. Tattersall. 1997. The NS2 polypeptide of parvovirus MVM is required for capsid assembly in murine cells. Virology 231:267-280. [DOI] [PubMed] [Google Scholar]
- 16.Cotmore, S. F., and P. Tattersall. 1987. The autonomously replicating parvoviruses of vertebrates. Adv. Virus Res. 33:91-174. [DOI] [PubMed] [Google Scholar]
- 17.Cotmore, S. F., and P. Tattersall. 1990. Alternate splicing in a parvoviral nonstructural gene links a common amino-terminal sequence to downstream domains which confer radically different localization and turnover characteristics. Virology 177:477-487. [DOI] [PubMed] [Google Scholar]
- 18.Daeffler, L., R. Horlein, J. Rommelaere, and J. P. F. Nuesch. 2003. Modulation of minute virus of mice cytotoxic activities through site-directed mutagenesis within the NS coding region. J. Virol. 77:12466-12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domingo, E., and J. J. Holland. 1997. RNA virus mutations and fitness for survival. Annu. Rev. Microbiol. 51:151-178. [DOI] [PubMed] [Google Scholar]
- 20.Drake, J. W. 1991. A constant rate of spontaneous mutations in DNA-based microbes. Proc. Natl. Acad. Sci. USA 88:7160-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drake, J. W., and J. J. Holland. 1999. Mutation rates among RNA viruses. Proc. Natl. Acad. Sci. USA 96:13910-13913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eichwald, V., L. Daeffler, M. Klein, J. Rommelaere, and N. Salome. 2002. The NS2 proteins of parvovirus minute virus of mice are required for efficient nuclear egress of progeny virions in mouse cells. J. Virol. 76:10307-10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eigen, M., and C. K. Biebricher. 1988. Sequence space and quasispecies distribution, p. 211-245. In E. Domingo, J. J. Holland, and P. Ahlquist (ed.), RNA genetics, vol. III. CRC Press, Boca Raton, Florida.
- 24.Engle, M., and M. S. Diamond. 2003. Antibody prophylaxis and therapy against west Nile virus infection in wild-type and immunodeficient mice. J. Virol. 77:12941-12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forgues, M., A. J. Marrogi, E. A. Spillare, C.-G. Wu, Q. Yang, M. Yoshida, and X. W. Wang. 2001. Interaction of the hepatitis B virus X protein with the Crm1-dependent nuclear export pathway. J. Biol. Chem. 276:22797-22803. [DOI] [PubMed] [Google Scholar]
- 26.Forgues, M., M. J. Difilippantonio, S. P. Linke, T. Ried, K. Nagashima, J. Feden, K. Valerie, K. Fukasawa, and X. W. Wang. 2003. Involvement of Crm1 in hepatitis B virus X protein-induced aberrant centriole replication and abnormal mitotic spindles. Mol. Cell. Biol. 23:5282-5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornerod, M., M. Ohno, M. Yoshida, and I. W. Mattaj. 1997. Crm1 is an export receptor for leucine-rich nuclear export signals. Cell 90:1051-1060. [DOI] [PubMed] [Google Scholar]
- 28.Gao, G., M. R. Alvira, S. Somanathan, Y. Lu, L. H. Vandenberghe, J. J. Rux, R. Calcedo, J. Sanmiguel, Z. Abbas, and J. M. Wilson. 2003. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc. Natl. Acad. Sci. USA 100:6081-6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gauduin, M. C., P. W. H. I. Parren, R. Weir, C. F. Barbas III, D. R. Burton, and R. A. Koup. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3:1389-1393. [DOI] [PubMed] [Google Scholar]
- 30.Gottschalck, E., S. Alexandersen, A. Cohn, L. A. Poulsen, M. E. Bloom, and B. Aasted. 1991. Nucleotide sequence analysis of Aleutian mink disease parvovirus shows that multiple virus types are present in infected mink. J. Virol. 65:4378-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland, T. C., S. D. Marlin, M. Levine, and J. Glorioso. 1983. Antigenic variants of herpes simplex virus selected with glycoprotein-specific monoclonal antibodies. J. Virol. 45:672-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang, Y. T., B. Y. Liu, D. M. Coen, and C. B. C. Hwang. 1997. Effects of mutations in the Exo III motif of the herpes simplex virus DNA polymerase gene on enzyme activities, viral replication, and replication fidelity. J. Virol. 71:7791-7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isnard, M., M. Granier, R. Frutos, B. Reynaud, and M. Peterschmitt. 1998. Quasispecies nature of three maize streak virus isolates obtained through different modes of selection from a population used to assess response to infection of maize cultivars. J. Gen. Virol. 79:3091-3099. [DOI] [PubMed] [Google Scholar]
- 34.Keller, M. A., and E. R. Stiehm. 2000. Passive immunity in prevention and treatment of infectious diseases. Clin. Microbiol. Rev. 13:602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kurtzman, G. J., K. Ozawa, B. Cohen, G. Hanson, R. Oseas, and N. S. Young. 1987. Chronic bone marrow failure due to persistent B19 parvovirus infection. N. Engl. J. Med. 317:287-294. [DOI] [PubMed] [Google Scholar]
- 36.López-Bueno, A., M. G. Mateu, and J. M. Almendral. 2003. High mutant frequency in populations of a DNA virus allows evasion from antibody therapy in an immunodeficient host. J. Virol. 77:2701-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maroto, B., N. Valle, R. Saffrich, and J. M. Almendral. 2004. Nuclear export of the nonenveloped parvovirus virion is directed by an unordered protein signal exposed on the capsid surface. J. Virol. 78:10685-10694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mascola, J. R., G. Stiegler, T. C. Van Cott, H. Katinger, C. B. Carpenter, C. E. Hansos, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 39.McMaster, G. K., P. Beard, H. D. Engers, and B. Hirt. 1981. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J. Virol. 38:317-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, C. L., and D. J. Pintel. 2002. Interaction between parvovirus NS2 protein and nuclear export factor Crm1 is important for viral egress from the nucleus of murine cells. J. Virol. 76:3257-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muzyczka, N., and K. I. Berns. 2001. Parvoviridae: the viruses and their replication. In D. M. Knipe et al. (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 42.Naeger, L. K., J. Cater, and D. J. Pintel. 1990. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J. Virol. 64:6166-6175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naeger, L. K., N. Salomé, and D. J. Pintel. 1993. NS2 is required for efficient translation of viral mRNA in minute virus of mice-infected murine cells. J. Virol. 67:1034-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neumann, G., M. T. Hughes, and Y. Kawaoka. 2000. Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1. EMBO J. 19:6751-6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohshima, T., T. Nakajima, T. Oishi, N. Imamoto, Y. Moneda, A. Fukamizu, and K. Yagami. 1999. CRM1 mediates nuclear export of nonstructural protein 2 from parvovirus minute virus of mice. Biochem. Biophys. Res. Commun. 264:144-150. [DOI] [PubMed] [Google Scholar]
- 46.Palladino, G., K. Mozdzanowska, G. Washko, and W. Gerhard. 1995. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J. Virol. 69:2075-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parrish, C. R. 1990. Emergence, natural history, and variation of canine, mink, and feline parvoviruses. Adv. Virus Res. 38:403-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parrish, C. R., C. F. Aquadro, M. L. Strassheim, J. F. Evermann, J.-Y. Sgro, and H. O. Mohammed. 1991. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J. Virol. 65:6544-6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poignard, P., R. Sabbe, G. R. Picchio, M. Wang, R. J. Gulizia, H. Katinger, P. W. H. I. Parren, D. E. Mosier, and D. R. Burton. 1999. Neutralizing antibodies have limited effects on the control of established HIV-1 infection in vivo. Immunity 10:431-438. [DOI] [PubMed] [Google Scholar]
- 50.Ron, D., and J. Tal. 1985. Coevolution of cells and virus as a mechanism for the persistence of lymphotropic minute virus of mice in L cells. J. Virol. 55:424-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segovia, J. C., A. Real, J. A. Bueren, and J. M. Almendral. 1991. In vitro myelosuppressive effects of the parvovirus minute virus of mice (MVMi) on hematopoietic stem and committed progenitor cells. Blood 77:980-988. [PubMed] [Google Scholar]
- 52.Segovia, J. C., G. Guenechea, J. M. Gallego, J. M. Almendral, and J. A. Bueren. 2003. Parvovirus infection suppresses long-term repopulating hematopoietic stem cells. J. Virol. 77:8495-8503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Segovia, J. C., J. M. Gallego, J. A. Bueren, and J. M. Almendral. 1999. Severe leukopenia and dysregulated erythropoiesis in SCID mice persistently infected with the parvovirus minute virus of mice. J. Virol. 73:1774-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soliman, T. M., and S. J. Silverstein. 2000. Herpesvirus mRNAs are sorted for export via Crm1-dependent and -independent pathways. J. Virol. 74:2814-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tattersall, P., and J. Bratton. 1983. Reciprocal productive and restrictive virus-cell interaction of immunosuppressive and prototype strains of minute virus of mice. J. Virol. 46:944-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Truyen, U., A. Gruemberg, S.-F. Chang, B. Obermaier, P. Veijalainen, and C. R. Parrish. 1995. Evolution of the feline-subgroup parvoviruses and the control of canine host range in vivo. J. Virol. 69:4702-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villarreal, L. P., and V. R. De Filippis. 2001. Virus evolution. In D. M. Knipe et al. (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 58.Wichman, H. A., M. R. Badgett, L. A. Scott, C. M. Boulianne, and J. J. Bull. 1999. Different trajectories of parallel evolution during viral adaptation. Science 285:422-424. [DOI] [PubMed] [Google Scholar]
- 59.Young, P. J., K. T. Jensen, L. R. Burger, D. J. Pintel, and C. L. Lorson. 2002. Minute virus of mice small nonstructural protein NS2 interacts and colocalizes with the Smn protein. J. Virol. 76:6364-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zwick, M. B., M. Wang, P. Poignard, G. Stiegler, H. Katinger, D. R. Burton, and P. W. H. I. Parren. 2001. Neutralization synergy of human immunodeficiency virus type 1 primary isolates by cocktails of broadly neutralizing antibodies. J. Virol. 75:12198-12208. [DOI] [PMC free article] [PubMed] [Google Scholar]