Abstract
We constructed a promoter mutation altering the immediate-early expression of the herpes simplex virus type 1 (HSV-1) ICP27 transcript and its cognate wild-type rescue viruses in order to assess the role of the ICP27 protein in the earliest stages of viral infection by global transcriptional analysis with a DNA microarray. This mutant, ICP27/VP16, replaces the whole ICP27 promoter/enhancer with the VP16 promoter. It demonstrates loss of immediate-early expression of ICP27 according to the criteria expression in the absence of de novo protein synthesis and earliest expression in the kinetic cascade. Significant differences in relative transcript abundances between the mutant and wild-type rescue viruses were limited at the earliest times measured and not evident at all by 4 h after infection. Consistent with this observation, levels of some critical proteins were reduced in the mutant as compared to rescue virus infections at the earliest times tested, but were equivalent by 8 h postinfection. Further, both single and multistep levels of virus replication were equivalent with both mutant and rescue viruses. Thus, altering the immediate-early kinetics of ICP27 leads to a suboptimal quantitative lag phase in gene expression but without consequence for replication fitness in vitro. Infections in vivo also revealed equivalent ability of mutant and rescue viruses to invade the central nervous system of mice following footpad injections. Limitations to an immediate-early role of ICP27 in the biology of HSV are discussed in light of these observations.
The early phase of the well-characterized herpes simplex virus type 1 (HSV-1) cascade of transcript abundance has two components: immediate-early (α) and early (β). The former, originally defined by expression in the absence of de novo protein synthesis and characterized by promoter/enhancer elements (TATGARAT boxes) activated by the interaction between the virion-associated VP16 activator and cellular “adaptor” DNA binding proteins (2, 3, 13, 21, 35, 41, 42), can be shown kinetically to be the earliest expressed in abundance by use of kinetic labeling and most completely by DNA microarray technology (39, 48). A requirement for very early expression of the HSV-1 α transcripts for efficient viral replication is buttressed by our recent use of DNA microarrays to demonstrate that a kinetically normal productive cascade can be induced in cells infected with a viral mutant lacking the VP16 activator of immediate-early transcription only when cells are stressed in such a manner as to lead to the expression of the immediate-early transcripts at the earliest stages of infection (43). The functions of most immediate-early transcripts are fully consistent with the timing of their expression; thus, expression of the extremely catholic transcriptional activator ICP4 is required for efficient expression of all other viral transcripts in the context of the viral genome (4, 9, 10, 24, 25). The requirement for ICP0 protein function is cell cycle and multiplicity of infection (MOI) dependent (8, 11) and has recently been shown to have a major role in HSV-1 genome circularization, potentially acting as a major switch in the productive/latent infection pathway in neurons (20). The function of the ICP22 protein also appears to be cell cycle dependent and have a role in the ability of virus to replicate efficiently in certain differentiated cell types (7, 26, 27, 30). Finally, the protein encoded by the ICP47 transcript interferes with major histocompatibility complex class I-mediated antigen presentation and thus can be envisioned as having a major role in the ability of HSV to establish long-term infections as well as augmenting reactivation from latency (12, 18, 47).
While transcriptional effects have been ascribed to the ICP27 protein, they have yet to be well characterized (28, 29, 31), and in light of the above discussion, the timing of expression of the immediate-early ICP27 protein stands as somewhat of a kinetic conundrum. Its well-characterized activities as a mediator of splicing inhibition and transport of unspliced transcripts from the nucleus to the cytoplasm are required throughout the replication cycle; however, while viral mutants lacking this gene express at least the majority of early transcripts at normal or above normal levels, the levels of many late transcripts are significantly reduced (15-17, 19, 23). In order to investigate functions of ICP27 requiring expression immediately upon infection, we generated an HSV-1 mutant in which the timing of expression of the transcript was altered. This mutant, ICP27/VP16, substitutes the leaky-late (βγ) VP16 promoter for the entire ICP27 promoter. While it failed to express the ICP27 transcript with immediate-early kinetics, accumulation of viral transcripts as measured by DNA microarrays was equivalent to that of rescue virus by 3 to 4 h following low-MOI infection of several differentiated cultured cell lines. Protein levels were somewhat affected at the earliest times measured, but both mutant and rescue viruses replicated to equivalent titers with equivalent kinetics in single- and multistep growth experiments on several different primary cell lines. Further, the mutant displayed no significant alteration in the course of infection in mice injected in the footpad according to several parameters of viral neuropathogenesis, including the ability to establish a latent infection in dorsal root ganglia (DRG) and the ability to efficiently recover virus from such ganglia upon explant cocultivation. This rather surprising set of results suggests a number of testable hypotheses, which are discussed along with other implications of these findings.
MATERIALS AND METHODS
Cells and viruses.
Human foreskin fibroblasts (HFFs) have been described in several previous publications (1, 36), and murine embryo fibroblasts (MEFs; NIH 3T3 cells) were obtained from the American Type Culture Collection. The cells were maintained at 37°C under 5% CO2 in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml. Cultures of 107 HFF or MEF cells in 150-cm2 flasks or 100-mm-diameter dishes were used for infections—usually at an MOI of 1 PFU/cell or less. Virus was adsorbed for 30 min prior to the addition of fresh overlay medium consisting of Dulbecco's modified Eagle's medium containing 5% fetal bovine serum.
The ICP27 promoter mutant and its rescue were constructed on a background of the 17syn+ strain of HSV-1. The modified promoter-containing fragment was cotransfected with infectious 17syn+ DNA into rabbit skin cells, and recombinants were identified and isolated by hybridization screening and plaque purification as described previously (22, 40). The basic approach was to introduce the modified promoters, each containing a short sequence of bacterial DNA to use as a screening marker into a KOS-derived SalI/EcoRI DNA fragment spanning bases 107379 to 110095 in which the BamHI site at 107536 had been converted into an XbaI site. This converted site lies ca. 270 bases upstream of the ICP27 transcript cap site and essentially 100 bases upstream of a 115-bp fragment of HSV-1 DNA bounded by SmaI sites containing the TATGARAT box (TATGTGATGT). The VP16/ICP27 promoter mutation was made by substituting the wild-type (WT) sequences from the converted BamHI site to an AgeI site at +72 relative to the ICP27 cap site with the VP16 promoter (−286 relative to the VP16 cap site to +6). This promoter construct has been described previously and contains a 360-bp fragment of the bacterial β-galactosidase gene as a screening marker (14). Once recombinant viruses were purified, infectious DNA was isolated, and a rescue was generated by recombining the original KOS-derived 4,425-bp SalI-to-EcoRI fragment containing the converted XbaI site.
In one set of control experiments, transcript abundance of a GFP/ICP27-null mutant constructed on a background of HSV-1 strain KOS was compared to that of WT KOS virus. The ICP27-null mutant virus 27-GFP was isolated by marker transfer of a DNA fragment containing the GFP (green fluorescent protein) gene from plasmid NES-27-GFP (32). The GFP coding sequence was cloned into a DrdI site upstream of the translational start site of the ICP27 gene in plasmid pSG130B/S (15) and an EcoRI site at the position of amino acid 504 in ICP27, which is eight residues from the translational stop codon. Therefore, the GFP coding sequence replaced the ICP27 coding sequence and is under the control of the ICP27 promoter and utilizes the ICP27 poly(A) site. Following transfection of complementing 2-2 cells (34) with intact HSV-1 KOS DNA and the ICP27-GFP DNA fragment, progeny were plated on 2-2 cells and recombinants were screened by observing green fluorescence under an epifluorescent microscope. The 27-GFP recombinant virus was plaque purified four times, and the presence of the GFP sequences was confirmed by Southern blot analysis. The mutant grows as efficiently as WT virus on 2-2 cells but has a titer that is 6 to 7 logs lower on Vero cells.
RNA preparation and generation of fluorescein- or biotin-tagged cDNA.
Infected cells were harvested at various times after infection, and total RNA was extracted with Trizol reagent (Molecular Research Center, Inc., Cincinnati, Ohio) as described previously (1, 43). Random hexamer-primed fluorescein (Enzo, Roche)- or biotin (Enzo, Life Sciences)-labeled cDNA was synthesized from 50- to 250-ng aliquots of purified poly(A)+ RNA by reverse transcription using Superscript II reverse transcriptase (Gibco-BRL). Fluorescein- or biotin-labeled cDNA was purified by ultrafiltration through a Microcon centrifugal filter device column (YM-30; Millipore).
Generation of microarrays, hybridization, and scanning.
The characteristics and construction of our HSV-1-specific oligonucleotide-based DNA microarray have been described previously (39, 43). In the present series of experiments, we used a two-color nucleic acid microarray resonance light-scattering-based (RLS) method (Genicon Sciences; http://www.invitrogen.com/content.cfm?pageid=9912) (44-46) and a MAUI hybridization system (BioMicro Systems, Inc.; http://www.biomicro.com/products/new_maui.html). This procedure, which is shown graphically in both the company's website and that of E.K.W. (http://darwin.bio.uci.edu/~faculty/wagner/hsv9fnew.html), utilizes nano-size gold and silver particles, which have the property of scattering polychromatic or white light, to tag the hybridization probe. The scattering is characterized by preferential radiation of a specific resonance wavelength for each metal tag. In comparative control experiments using RNA isolated from HSV-1-infected cells at various times after infection, we found that 50- to 100-ng samples of poly(A)-containing RNA provided hybridization sensitivity comparable to that seen with 2 to 3 μg of such RNA by Cy3/Cy5 fluorescent labeling. In RLS, signal intensity is a function of exposure time to white light, and we found, again using comparative controls, that under conditions of exposure in which all samples provided signal intensities within range of the detector (ca. 500 to 40,000 arbitrary units), the relative levels of hybridization to individual oligonucleotide probes were entirely equivalent by the two methods.
For RLS detection, microarrays were prehybridized and hybridized with cDNA probes for 18 h and rinsed for scanning with a proprietary HiLight dual-color kit (QIAGEN, Genicon Sciences) at 52°C in a MAUI hybrid mixer assembly. All procedures were as described in the instructions with the labeling kit. After hybridization, the slides were washed, blocked, bound by the gold and silver RLS method, and carefully dried in a dust-free environment, and the arrays were sealed by dipping in archiving solution also supplied with the kit. Microarrays were scanned with a GSD-501 HiLight reader (designed for the RLS system; QIAGEN).
Net signals were calculated in a Microsoft Excel spreadsheet by subtracting the signal from a ring of area equal to each spotted probe immediately surrounding the probe data spot from the corresponding individual experimental spots. The median net value of each probe (spotted in triplicate) was taken as the experimental value. To compare data from the various experimental conditions, the net hybridization values were used in two ways. First, each experimental condition was repeated at least three times, the median values from those experiments were determined, and the 75th percentile rank for the total viral hybridization was calculated as described previously (39, 43). To compare the abundance of specific transcripts present in cells infected with either mutant or control (rescue) virus, relative transcript abundance for each experiment was determined for the conditions being considered. These were then compared by Student's two-tailed t test, assuming unequal variance and with the null hypothesis being that the true values under those two conditions are identical. The original data as well as selected data not shown in the manuscript will be available (accession no. GXE-00030, GXE-00032, GXE-00033, and GXE-00034) in the MIAME compliant GTI expression database (GPXdb; http://mendel.gti.ed.ac.uk:8080/GPX/cgi_bin/gpx.cgi).
Analysis of the levels of representative viral proteins in infected cells.
Samples of total protein extracted from 3T3 cells infected at 1 PFU per cell with the appropriate mutant and/or WT virus described were fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes as described previously (34). The blots were then probed for the proteins indicated (ICP4, ICP0, ICP27, gD, and gC) with monoclonal antibodies from the Goodwin Institute. Bands were visualized by enhanced chemiluminescence).
Assay of in vivo replication of the ICP27 recombinants and rescue viruses following footpad inoculation of mice.
Four- to 6-week-old ND4 mice (Harlan) were infected with 105 PFU (total per mouse) of each recombinant on both rear footpads as previously described (5, 6). The feet were injected subepithelially with 50 μl of a sterile 10% saline solution 4 h prior to infection. At 1, 3, and 5 days postinfection (p.i.), four mice per recombinant per time point were euthanized and the feet, spinal ganglia, and spinal cords were dissected and snap-frozen in liquid nitrogen. Total infectious virus present in each tissue was determined as described previously (6). Briefly, the combined feet, spinal ganglia, and spinal cords for each time point were homogenized as 10% (wt/vol) suspensions in minimal essential medium (MEM) and centrifuged at 3,000 × g for 5 min to clear cell debris. Infectious virus present in each sample was determined by a standard plaque assay on rabbit skin cells (RSC). The MEM used for homogenates of the feet was supplemented with 2× antibiotics (500 U of penicillin and 500 μg of streptomycin/ml) and amphotericin B (5 μg/ml).
Latent infection and explant cocultivation of murine DRG.
For latent infection of DRG with the ICP27 recombinants and their rescuants, 6-week-old ND4 mice (Harlan) were inoculated with 500 PFU of each recombinant on both rear footpads. The feet were pretreated with 10% saline (as described above). One month p.i., the mice were euthanized and DRG were dissected (positions L4 to L6) and transferred to 24-well tissue culture dishes containing a monolayer of rabbit skin cells. The explant cultures were scored daily for reactivation as assessed by cytopathic effect of the rabbit skin cells. The cultures were fed every other day, and maintained for 21 days postexplant. Explant cultures that failed to demonstrate reactivation were scored as negative at this time point.
Virus replication.
Both single- and multiple-round virus replication experiments were carried out as follows. The required number of replicate cultures of 106 3T3 cells were grown to confluence, fed overnight with culture medium containing 5% serum, and then infected with either mutant or rescue virus. Cell count was confirmed with a control culture. For single-cycle growth, replicate cultures were infected with 103 PFU of virus and two separate plates for each virus were harvested for titration at 12 and 24 h following this. For multicycle replication, cultures were infected at an MOI of 0.1 PFU/cell, and two cultures each were harvested at 6, 24, 30, 48, and 54 h p.i. Finally, single-step growth experiments were carried out by infecting cultures at an MOI of 10 PFU/cell and harvesting and measuring virus yields 20 h later. In all cases, cells were overlaid with MEM containing 10% newborn calf serum following a 40-min virus adsorption period. Cells were freeze-thawed three times and sonicated following harvest, and titrations were carried out on Vero cells. Each experiment was repeated three times.
RESULTS
Promoter modifications result in loss of immediate-early kinetics of expression of the ICP27 transcript.
As a preliminary experiment, we used Northern blots to confirm the loss of expression of ICP27 under conditions of cycloheximide blockage of de novo protein synthesis and the recovery of this expression with the cognate rescue viruses (data not shown). In order to carry out a global, quantitative analysis of the role of immediate-early ICP27 expression in all viral transcript levels, we used HSV-1 DNA microarrays to compare transcript abundance in cells infected with the ICP27/VP16 promoter mutants with that in their cognate rescue viruses. Table 1 includes data obtained following infections in PFU per cell for relative transcript abundance at 3 h p.i. in the presence of 60-μg/ml cycloheximide as well as at 1 h p.i. in untreated cells. Under conditions of inhibition of de novo protein synthesis, of the five immediate-early transcripts only ICP27 was significantly reduced in expression in mutant infections, ranging from undetectable to at least fivefold less in individual experiments. Also shown in Table 1, the expression of the early unique long (UL) transcripts UL23, UL29, UL30, and UL50 was essentially undetectable in both infections. The levels of UL39/40 were somewhat more variable, consistent with the known leakiness of this transcript under marginally complete conditions of inhibition of protein synthesis, and this leakiness (especially in the VP16 promoter substitution) serves as an internal reference to the greatly reduced or absent expression of ICP27 under these conditions.
TABLE 1.
Loss of immediate-early expression of ICP27 with the ICP27/VP16 kinetic mutant
| Classa | Transcript | ICP27 expression (PFU/cell)
|
||||
|---|---|---|---|---|---|---|
| ICP27/VP16
|
ICP27/VP16R
|
Pc | ||||
| Median value | SDb | Median value | SDb | |||
| Condition 1 | ||||||
| IE | ICP27 | 0 | 400 | 24,000 | 7,600 | 0.039 |
| IE | ICP0 | 21,800 | 2,000 | 15,400 | 7,900 | 0.365 |
| IE | ICP4 | 28,100 | 3,600 | 33,200 | 8,800 | 0.870 |
| IE | ICP22 | 28,300 | 11,400 | 27,200 | 23,600 | 0.828 |
| IE/E | ICP47/US10-12 | 22,200 | 5,800 | 16,700 | 13,300 | 0.771 |
| E | UL23 | 800 | 700 | 500 | 400 | 0.663 |
| E | UL29 | 300 | 500 | 500 | 100 | 0.681 |
| E | UL30 | 800 | 200 | 500 | 1,100 | 0.813 |
| E | UL39/40 | 7,200 | 6,400 | 4,900 | 11,700 | 0.925 |
| Condition 2 | ||||||
| IE | ICP27 | 1,700 | 900 | 4,100 | 1,100 | 0.066 |
| IE | ICP0 | 1,800 | 200 | 1,000 | 100 | 0.007 |
| IE | ICP4 | 3,700 | 1,300 | 2,800 | 1,100 | 0.466 |
| IE | ICP22 | 6,100 | 1,900 | 4,200 | 1,300 | 0.227 |
| IE/E | ICP47/US10-12 | 4,600 | 1,300 | 3,900 | 1,300 | 0.291 |
| E | UL23 | 900 | 500 | 600 | 200 | 0.250 |
| E | UL29 | 600 | 100 | 300 | 100 | 0.057 |
| E | UL30 | 1,000 | 300 | 500 | 100 | 0.053 |
| E | UL39/40 | 2,400 | 600 | 1,400 | 200 | 0.060 |
| E | UL50 | 1,100 | 400 | 700 | 200 | 0.121 |
Cells were preincubated for 60 min in the presence of cycloheximide prior to infection, and the drug was present during virus adsorption. IE, immediate early; E, early. Condition 1, 3 h p.i. in the presence of 60-μg/ml cycloheximide; condition 2, 1 h p.i. at an MOI of 1 PFU/cell. Infection was initiated at a multiplicity of 1 PFU per cell. Only selected transcripts are shown. The original data (accession no. GXE-00030, GXE-00032, GXE-00033, and GXE-00034) is available in the MIAME-compliant GTI expression database, GPXdb (http://mendel.gti.ed.ac.uk:8080/GPX/cgi_bin/gpx.cgi).
SD, standard deviation.
Absolute (nonnormalized) values of transcript levels for three separate infections at the various time points were compared by Student's t test (MS-Excel based) as described in Materials and Methods. The null hypothesis is that the true values for the WT and mutant viruses are identical.
After 1 h without inhibitor present, the infection with the WT rescue viruses showed transcript abundance patterns typical for this time after infection (39): i.e., a preponderance of the five immediate-early transcripts, levels of the early ribonucleotide reductase (UL39/40) nearly as high as those of the immediate-early ones, and readily detectable levels of a number of other early transcripts, including that encoding thymidine kinase (UL23). In the cells infected with the ICP27/VP16 mutant, only ICP27 of the immediate-early transcripts appeared reduced in relative abundance as compared to the rescue virus. Interestingly, levels of UL29, UL30, and UL39/40 mRNAs were higher in the mutant than in the rescue virus, with marginal significance, while the overexpression of ICP0 in the mutant was significantly higher in the mutant than in the rescue virus infections (P = 0.007).
Lack of immediate-early kinetics in ICP27 expression had minimal effects on the accumulation of HSV-1 transcripts by 2 h and none at later times p.i.
The complete absence of expression of the ICP27 protein is known to have profound effects on the accumulation of viral transcripts (cf. reference 33). As a control, we confirmed these effects in the context of the cell types currently used and the ability of the extremely high-sensitivity RLS detection of hybrids on DNA microarrays to provide an accurate quantitative measure of these changes by comparing the relative abundances of viral transcripts at 4 and 8 h p.i. with a full ICP27-null mutant (GFP/ICP27) to those in a WT infection (Table 2). Since the reciprocity between exposure time and signal strength is not linear as it is with fluorescent tags (39), we utilized relative transcript abundance at any given time as a measure of deviations from the WT patterns. We have highlighted those table entries where differences in these relative abundances are statistically highly significant (P ≤ 0.05 by t test). Values for those transcripts whose fractional abundance in the cells infected by mutant virus is increased by greater than a factor of 0.8 (mainly early) are shown in italic, while those whose relative abundance is decreased by this factor (mainly late) are shown in boldface. Since we are using a different chip, different cells, and polyadenylated versus total RNA in the present experiments, we did not attempt to fully correlate the transcript abundances seen with those reported in the earlier report, especially since the methods we are currently using are not applicable to measuring large differences in absolute transcript levels. Given these provisos, the transcript abundance patterns reported here are consistent with those in HeLa cells infected with a null mutant (ICP27lacz) at a different MOI (36). This new null mutant has a significantly lower reversion frequency since the whole open reading frame is deleted from the viral genome.
TABLE 2.
Effect of the absence of ICP27 expression on HSV-1 transcript abundance at 4 and 8 h p.i.
| Classa | Transcript | HSV-1 transcript abundanceb
|
Pd | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h p.i.
|
8 h p.i.
|
||||||||||||||
| WT
|
ICP27(−)
|
Pd | WT
|
ICP27(−)
|
|||||||||||
| Median | SD | Fractionc | Median | SD | Fractionc | Median | SD | Fractionc | Median | SD | Fractionc | ||||
| IE | ICP27 | 25,000 | 2,500 | 0.031 ± 0.017 | 800 | 1,600 | 0.002 ± 0.004 | 0.011 | 13,900 | 1,600 | 0.034 ± 0.003 | 200 | 300 | 0.001 ± 0.001 | 0.002 |
| IE | ICP0 | 6,600 | 5,600 | 0.006 ± 0.002 | 2,500 | 2,100 | 0.006 ± 0.005 | 0.591 | 4,400 | 2,200 | 0.011 ± 0.005 | 1,300 | 700 | 0.005 ± 0.003 | 0.214 |
| IE | ICP4 | 8,400 | 2,000 | 0.011 ± 0.006 | 7,900 | 8,300 | 0.017 ± 0.017 | 0.256 | 5,400 | 800 | 0.012 ± 0.002 | 6,000 | 500 | 0.021 ± 0.004 | 0.035 |
| IE | ICP22 | 25,900 | 2,600 | 0.032 ± 0.017 | 5,100 | 2,700 | 0.011 ± 0.006 | 0.015 | 15,800 | 4,500 | 0.037 ± 0.009 | 1,100 | 800 | 0.004 ± 0.002 | 0.014 |
| IE/E | ICP47/ US10-12 | 22,300 | 4,900 | 0.028 ± 0.015 | 13,600 | 5,100 | 0.029 ± 0.011 | 0.669 | 10,700 | 4,500 | 0.025 ± 0.010 | 9,600 | 1,300 | 0.030 ± 0.004 | 0.558 |
| E | UL4-5′ | 5,500 | 6,500 | 0.007 ± 0.004 | 4,600 | 4,600 | 0.010 ± 0.010 | 0.652 | 1,500 | 1,000 | 0.004 ± 0.002 | 3,200 | 1,600 | 0.012 ± 0.006 | 0.242 |
| E | UL4/5 | 12,200 | 3,200 | 0.010 ± 0.005 | 12,200 | 6,600 | 0.026 ± 0.015 | 0.242 | 4,300 | 600 | 0.010 ± 0.002 | 7,100 | 1,100 | 0.025 ± 0.006 | 0.047 |
| E | UL8/9 | 10,000 | 4,200 | 0.011 ± 0.005 | 11,400 | 1,500 | 0.024 ± 0.003 | 0.013 | 3,900 | 100 | 0.009 ± 0.000 | 4,100 | 700 | 0.016 ± 0.001 | 0.019 |
| E | UL8-5′ | 700 | 3,800 | 0.001 ± 0.002 | 900 | 2,000 | 0.002 ± 0.004 | 0.823 | 300 | 200 | 0.001 ± 0.000 | 300 | 100 | 0.001 ± 0.001 | 0.308 |
| E | UL21 | 10,800 | 4,300 | 0.012 ± 0.006 | 4,400 | 4,500 | 0.009 ± 0.009 | 0.717 | 7,800 | 1,500 | 0.018 ± 0.004 | 2,800 | 400 | 0.010 ± 0.000 | 0.098 |
| E | UL23 | 18,000 | 3,600 | 0.020 ± 0.010 | 21,700 | 4,700 | 0.045 ± 0.011 | 0.028 | 2,000 | 900 | 0.005 ± 0.002 | 10,000 | 6,400 | 0.036 ± 0.017 | 0.059 |
| E | UL29 | 1,000 | 5,800 | 0.001 ± 0.003 | 2,500 | 4,400 | 0.005 ± 0.009 | 0.496 | 200 | 400 | 0.000 ± 0.001 | 1,400 | 1,500 | 0.006 ± 0.005 | 0.180 |
| E | UL30 | 11,700 | 3,700 | 0.011 ± 0.005 | 12,800 | 6,100 | 0.027 ± 0.014 | 0.255 | 2,800 | 1,100 | 0.006 ± 0.002 | 9,600 | 1,500 | 0.037 ± 0.008 | 0.018 |
| E | UL37 | 14,100 | 6,100 | 0.007 ± 0.006 | 8,100 | 3,100 | 0.017 ± 0.007 | 0.964 | 4,300 | 1,300 | 0.010 ± 0.003 | 6,000 | 1,900 | 0.021 ± 0.008 | 0.145 |
| E | UL39-5′ | 8,700 | 4,900 | 0.008 ± 0.003 | 11,300 | 5,100 | 0.025 ± 0.011 | 0.289 | 2,800 | 1,600 | 0.007 ± 0.004 | 5,500 | 2,600 | 0.021 ± 0.010 | 0.196 |
| E | UL39/40 | 15,800 | 6,000 | 0.020 ± 0.012 | 16,600 | 5,400 | 0.036 ± 0.013 | 0.172 | 6,800 | 1,800 | 0.016 ± 0.004 | 10,600 | 2,100 | 0.038 ± 0.003 | 0.001 |
| E | UL42 | 20,500 | 1,900 | 0.026 ± 0.014 | 7,400 | 1,800 | 0.016 ± 0.004 | 0.043 | 11,700 | 1,300 | 0.027 ± 0.002 | 5,100 | 100 | 0.018 ± 0.002 | 0.004 |
| E | UL43 | 5,400 | 7,700 | 0.007 ± 0.002 | 3,300 | 1,400 | 0.007 ± 0.003 | 0.465 | 3,600 | 1,000 | 0.009 ± 0.003 | 1,100 | 700 | 0.004 ± 0.002 | 0.250 |
| E | UL50 | 19,400 | 5,100 | 0.024 ± 0.011 | 15,900 | 4,600 | 0.034 ± 0.011 | 0.102 | 5,100 | 1,900 | 0.012 ± 0.004 | 9,600 | 900 | 0.032 ± 0.001 | 0.008 |
| E | UL52-5′ | 100 | 1,700 | 0.000 ± 0.001 | 0 | 400 | 0.000 ± 0.001 | 0.671 | 0 | 0 | 0.000 ± 0.000 | 0 | 0 | 0.000 ± 0.000 | 0.877 |
| E | UL55 | 9,000 | 3,800 | 0.009 ± 0.004 | 2,800 | 2,200 | 0.006 ± 0.005 | 0.275 | 2,000 | 500 | 0.005 ± 0.001 | 1,700 | 400 | 0.006 ± 0.002 | 0.167 |
| E | UL56 | 11,100 | 7,600 | 0.007 ± 0.004 | 2,000 | 900 | 0.004 ± 0.002 | 0.139 | 11,000 | 3,400 | 0.025 ± 0.009 | 1,500 | 200 | 0.005 ± 0.001 | 0.051 |
| E | US2 | 16,000 | 5,300 | 0.019 ± 0.009 | 2,200 | 1,300 | 0.005 ± 0.003 | 0.003 | 12,500 | 4,100 | 0.030 ± 0.010 | 1,000 | 200 | 0.003 ± 0.001 | 0.057 |
| L | UL1 | 13,000 | 2,400 | 0.019 ± 0.009 | 15,700 | 1,700 | 0.034 ± 0.004 | 0.014 | 10,200 | 2,600 | 0.024 ± 0.005 | 9,600 | 3,200 | 0.034 ± 0.007 | 0.197 |
| L | UL3 | 19,100 | 4,600 | 0.017 ± 0.009 | 9,200 | 300 | 0.019 ± 0.001 | 0.660 | 12,100 | 400 | 0.028 ± 0.001 | 6,000 | 600 | 0.021 ± 0.004 | 0.104 |
| L | UL10 | 15,400 | 3,300 | 0.016 ± 0.009 | 5,800 | 1,700 | 0.012 ± 0.003 | 0.052 | 9,100 | 900 | 0.022 ± 0.002 | 5,900 | 600 | 0.022 ± 0.001 | 0.642 |
| L | UL16/17 | 16,500 | 2,500 | 0.018 ± 0.010 | 3,800 | 2,100 | 0.008 ± 0.004 | 0.070 | 9,000 | 900 | 0.020 ± 0.003 | 2,100 | 1,200 | 0.008 ± 0.003 | 0.006 |
| L | UL15 | 11,200 | 2,300 | 0.011 ± 0.006 | 6,800 | 2,000 | 0.015 ± 0.005 | 0.816 | 5,500 | 600 | 0.013 ± 0.001 | 5,100 | 700 | 0.018 ± 0.002 | 0.045 |
| L | UL18/20 | 23,000 | 4,100 | 0.029 ± 0.014 | 15,600 | 2,900 | 0.033 ± 0.007 | 0.525 | 12,700 | 1,300 | 0.031 ± 0.003 | 8,700 | 900 | 0.032 ± 0.001 | 0.309 |
| L | UL19/20 | 15,600 | 8,500 | 0.010 ± 0.007 | 7,900 | 4,000 | 0.017 ± 0.009 | 0.996 | 9,400 | 4,000 | 0.022 ± 0.010 | 6,100 | 2,500 | 0.024 ± 0.010 | 0.825 |
| L | UL19-5′ | 800 | 6,900 | 0.001 ± 0.004 | 200 | 1,000 | 0.000 ± 0.002 | 0.529 | 500 | 900 | 0.001 ± 0.002 | 200 | 200 | 0.001 ± 0.001 | 0.531 |
| L | UL22 | 18,000 | 1,000 | 0.022 ± 0.012 | 12,700 | 4,100 | 0.028 ± 0.008 | 0.361 | 7,300 | 300 | 0.018 ± 0.001 | 5,300 | 600 | 0.019 ± 0.002 | 0.340 |
| L | UL24 | 15,700 | 6,900 | 0.007 ± 0.007 | 6,200 | 900 | 0.014 ± 0.002 | 0.761 | 5,400 | 2,100 | 0.013 ± 0.005 | 6,200 | 2,100 | 0.022 ± 0.009 | 0.227 |
| L | UL25 | 18,800 | 4,100 | 0.020 ± 0.011 | 10,200 | 4,000 | 0.022 ± 0.008 | 0.567 | 9,600 | 3,600 | 0.023 ± 0.009 | 5,800 | 2,000 | 0.022 ± 0.008 | 0.662 |
| L | UL27/8 | 15,200 | 2,100 | 0.019 ± 0.011 | 13,400 | 2,700 | 0.029 ± 0.006 | 0.392 | 5,800 | 1,800 | 0.013 ± 0.004 | 9,700 | 1,300 | 0.032 ± 0.004 | 0.003 |
| L | UL27-5′ | 0 | 300 | 0.000 ± 0.000 | 0 | 100 | 0.000 ± 0.000 | 0.547 | 0 | 0 | 0.000 ± 0.000 | 0 | 0 | 0.000 ± 0.000 | 0.043 |
| L | UL31/34 | 12,900 | 5,000 | 0.016 ± 0.011 | 7,800 | 4,100 | 0.017 ± 0.008 | 0.791 | 2,700 | 4,500 | 0.006 ± 0.010 | 2,200 | 1,900 | 0.008 ± 0.005 | 0.890 |
| L | UL35 | 24,200 | 3,600 | 0.033 ± 0.015 | 10,300 | 3,600 | 0.022 ± 0.008 | 0.141 | 11,900 | 5,100 | 0.029 ± 0.011 | 6,900 | 2,000 | 0.026 ± 0.004 | 0.401 |
| L | UL38 | 16,100 | 3,200 | 0.019 ± 0.011 | 2,900 | 1,400 | 0.006 ± 0.003 | 0.009 | 9,700 | 600 | 0.023 ± 0.001 | 2,700 | 400 | 0.010 ± 0.001 | 0.000 |
| L | UL41 | 12,800 | 3,100 | 0.013 ± 0.007 | 1,700 | 800 | 0.004 ± 0.002 | 0.002 | 6,400 | 1,200 | 0.016 ± 0.003 | 3,800 | 900 | 0.012 ± 0.004 | 0.293 |
| L | UL44-5′ | 9,800 | 9,000 | 0.010 ± 0.006 | 1,000 | 600 | 0.002 ± 0.001 | 0.256 | 7,500 | 5,000 | 0.018 ± 0.012 | 400 | 400 | 0.002 ± 0.001 | 0.212 |
| L | UL44/45 | 26,900 | 6,100 | 0.034 ± 0.017 | 4,400 | 3,100 | 0.010 ± 0.007 | 0.082 | 16,800 | 6,600 | 0.039 ± 0.014 | 4,000 | 2,300 | 0.014 ± 0.006 | 0.046 |
| L | UL46/47 | 18,900 | 4,900 | 0.024 ± 0.012 | 16,400 | 7,800 | 0.035 ± 0.018 | 0.317 | 8,900 | 4,400 | 0.022 ± 0.009 | 10,900 | 8,000 | 0.039 ± 0.022 | 0.215 |
| L | UL48 | 26,300 | 6,200 | 0.033 ± 0.015 | 14,600 | 3,800 | 0.031 ± 0.007 | 0.898 | 11,800 | 4,900 | 0.027 ± 0.010 | 8,800 | 3,500 | 0.031 ± 0.008 | 0.787 |
| L | UL51 | 18,300 | 2,000 | 0.023 ± 0.012 | 7,300 | 7,500 | 0.016 ± 0.015 | 0.917 | 9,900 | 2,800 | 0.023 ± 0.006 | 6,500 | 1,400 | 0.025 ± 0.003 | 0.739 |
| L | RLXY | 6,100 | 4,200 | 0.004 ± 0.003 | 1,800 | 1,300 | 0.004 ± 0.003 | 0.547 | 8,300 | 3,200 | 0.019 ± 0.008 | 1,200 | 500 | 0.005 ± 0.001 | 0.078 |
| L | RLX | 1,700 | 3,400 | 0.002 ± 0.002 | 2,000 | 1,200 | 0.004 ± 0.002 | 0.717 | 2,200 | 1,300 | 0.005 ± 0.003 | 600 | 600 | 0.002 ± 0.002 | 0.470 |
| L | RICP34.5 | 3,400 | 1,700 | 0.002 ± 0.002 | 600 | 500 | 0.001 ± 0.001 | 0.375 | 900 | 600 | 0.002 ± 0.002 | 300 | 100 | 0.001 ± 0.001 | 0.366 |
| L | US5-5′ | 300 | 1,600 | 0.000 ± 0.001 | 100 | 100 | 0.000 ± 0.000 | 0.462 | 200 | 100 | 0.001 ± 0.000 | 0 | 0 | 0.000 ± 0.000 | 0.211 |
| L | US8-5′ | 800 | 2,600 | 0.001 ± 0.001 | 300 | 400 | 0.001 ± 0.001 | 0.512 | 300 | 200 | 0.001 ± 0.001 | 400 | 200 | 0.001 ± 0.001 | 0.493 |
| L | US8/9 | 20,800 | 9,200 | 0.026 ± 0.018 | 21,300 | 13,100 | 0.047 ± 0.027 | 0.326 | 12,200 | 3700 | 0.028 ± 0.007 | 9,800 | 5,500 | 0.035 ± 0.014 | 0.399 |
| Latent | RLAT-5′ | 0 | 100 | 0.000 ± 0.000 | 100 | 100 | 0.000 ± 0.000 | 0.115 | 0 | 0 | 0.000 ± 0.000 | 0 | 0 | 0.000 ± 0.000 | 0.752 |
| Latent | RHA6 | 1,100 | 1,000 | 0.001 ± 0.000 | 500 | 1,500 | 0.001 ± 0.003 | 0.606 | 3,300 | 2,200 | 0.008 ± 0.005 | 1,200 | 700 | 0.005 ± 0.002 | 0.267 |
| Latent | RLAT-I | 100 | 300 | 0.002 ± 0.002 | 200 | 100 | 0.003 ± 0.004 | 0.955 | 200 | 100 | 0.012 ± 0.003 | 0 | 100 | 0.008 ± 0.003 | 0.283 |
| Latent | RLATX | 1,600 | 4,200 | 0.007 ± 0.003 | 1,200 | 1,800 | 0.004 ± 0.031 | 0.549 | 4,900 | 1,300 | 0.021 ± 0.003 | 2,300 | 1,200 | 0.016 ± 0.006 | 0.344 |
| Latent | RLAT-3′ | 6,300 | 3,200 | 0.001 ± 0.000 | 1,800 | 14,900 | 0.004 ± 0.004 | 0.349 | 9,200 | 1,000 | 0.002 ± 0.001 | 4,300 | 2,400 | 0.002 ± 0.004 | 0.566 |
| ?? | U1X | 900 | 1,700 | 0.020 ± 0.011 | 1,800 | 2,000 | 0.020 ± 0.002 | 0.991 | 600 | 700 | 0.021 ± 0.005 | 500 | 1,300 | 0.017 ± 0.006 | 0.607 |
| Mixed | U6/7 | 16,000 | 2,600 | 0.024 ± 0.013 | 9,700 | 900 | 0.017 ± 0.006 | 0.300 | 8,900 | 1,900 | 0.016 ± 0.002 | 4,800 | 1,300 | 0.020 ± 0.005 | 0.511 |
| Mixed | U11/13 | 18,900 | 400 | 0.011 ± 0.006 | 7,800 | 2,800 | 0.016 ± 0.007 | 0.525 | 7,200 | 800 | 0.020 ± 0.000 | 5,100 | 1,200 | 0.016 ± 0.004 | 0.162 |
| Mixed | U36 | 13,400 | 5,000 | 0.002 ± 0.001 | 7,400 | 3,400 | 0.001 ± 0.001 | 0.498 | 8,800 | 300 | 0.003 ± 0.001 | 4,000 | 1,100 | 0.002 ± 0.000 | 0.919 |
| ?? | U43.5-5′ | 2,000 | 1,700 | 0.029 ± 0.015 | 600 | 400 | 0.029 ± 0.019 | 0.631 | 1,200 | 600 | 0.038 ± 0.009 | 700 | 100 | 0.028 ± 0.005 | 0.273 |
| Mixed | U49 | 23,200 | 4,200 | 0.008 ± 0.007 | 13,300 | 9,200 | 0.020 ± 0.004 | 0.558 | 15,900 | 4,400 | 0.014 ± 0.006 | 7,200 | 2,200 | 0.024 ± 0.008 | 0.234 |
| Mixed | U52/53 | 16,300 | 7,400 | 0.000 ± 0.000 | 9,000 | 1,900 | 0.000 ± 0.000 | 0.480 | 5,900 | 2,700 | 0.000 ± 0.000 | 6,700 | 1,900 | 0.000 ± 0.000 | 0.440 |
| ?? | ROP | 500 | 500 | 0.001 ± 0.000 | 200 | 100 | 0.000 ± 0.000 | 0.219 | 1,000 | 200 | 0.002 ± 0.001 | 200 | 0 | 0.001 ± 0.000 | 0.036 |
| ?? | US3-5′ | 1,600 | 4,500 | 0.002 ± 0.002 | 100 | 400 | 0.000 ± 0.001 | 0.379 | 300 | 400 | 0.001 ± 0.001 | 0 | 0 | 0.000 ± 0.000 | 0.255 |
| Mixed | US3/4 | 13,100 | 5,300 | 0.016 ± 0.007 | 1,000 | 900 | 0.002 ± 0.002 | 0.010 | 4,700 | 500 | 0.011 ± 0.002 | 200 | 200 | 0.001 ± 0.000 | 0.005 |
| Mixed | US5/6/7 | 17,900 | 4,900 | 0.014 ± 0.008 | 12,300 | 800 | 0.027 ± 0.002 | 0.086 | 7,000 | 2,100 | 0.017 ± 0.005 | 8,500 | 1,600 | 0.033 ± 0.007 | 0.066 |
IE, immediate early; E, early; L, late.
Infection was initiated at a multiplicity of 0.1 PFU/cell. All data are based on three replicate experiments. SD, standard deviation. Boldface means that the fractional values in mutant infections is reduced by 0.8 or greater as compared to WT, where the differences are significant at P ≤ 0.05. Italic means that fractional values in WT infections are reduced by 0.8 or greater as compared to the mutant, where the differences are significant at P ≤ 0.05.
Fractional values are the medians of the individual fractional values calculated from the total viral signal for each experiment as described in Materials and Methods; the standard deviation is shown.
Relative values of transcript levels were compared by Student's t test as described in Materials and Methods. The null hypothesis is that the true values for the WT and mutant viruses are identical.
As shown in Table 3, at 2 h after infection at an MOI of 0.1 PFU/cell, the relative abundance of only the UL42 transcript was significantly different in a comparison between infections with the ICP27/VP16 mutant and its WT rescue virus, while differences in relative levels of ICP27, ICP4, ICP47/US10-12 (unique short region 10-12), and UL4/5 are of marginal significance. Interestingly, while ICP27 is still reduced in relative abundance in the mutant infections, the relative levels of the other transcripts noted are increased. At later times following infection with an MOI of 0.1 PFU/cell, there were no statistical differences seen in relative transcript abundance in comparisons between the kinetic mutant and its cognate rescue virus; furthermore, no consistent differences in the overall transcript abundance seen in infections with mutant versus WT rescue virus were observed.
TABLE 3.
Relative abundance of HSV-1 transcripts expressed by the ICP27/VP16 mutants at 2 and 4 h p.i.
| Classa | Transcript | HSV-1 transcript abundanceb
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 h p.i.
|
4 h p.i.
|
||||||||||||||
| ICP27/VP16
|
ICP27/VP16R
|
ICP27/VP16
|
ICP27/VP16R
|
||||||||||||
| Median | SD | Fractionc | Median | SD | Fractionc | Pd | Median | SD | Fractionc | Median | SD | Fractionc | Pd | ||
| IE | ICP27 | 7,500 | 800 | 0.031 ± 0.003 | 4,400 | 1,700 | 0.054 ± 0.014 | 0.069 | 24,500 | 4,800 | 0.036 ± 0.008 | 8,400 | 2,200 | 0.063 ± 0.016 | 0.128 |
| IE | ICP0 | 5,300 | 1,000 | 0.023 ± 0.004 | 1,700 | 300 | 0.018 ± 0.005 | 0.280 | 4,100 | 3,300 | 0.007 ± 0.004 | 1,800 | 1,100 | 0.011 ± 0.006 | 0.552 |
| IE | ICP4 | 8,900 | 1,100 | 0.038 ± 0.005 | 2,300 | 400 | 0.025 ± 0.005 | 0.051 | 4,000 | 1,900 | 0.006 ± 0.002 | 1,700 | 900 | 0.010 ± 0.005 | 0.282 |
| IE | ICP22 | 22,300 | 5,700 | 0.096 ± 0.024 | 6,700 | 2,400 | 0.072 ± 0.030 | 0.326 | 29,800 | 2,900 | 0.051 ± 0.007 | 7,000 | 1,100 | 0.042 ± 0.004 | 0.331 |
| IE/E | ICP47/ US10-12 | 18,400 | 1,300 | 0.076 ± 0.007 | 4,100 | 1,400 | 0.050 ± 0.011 | 0.067 | 20,700 | 3,000 | 0.036 ± 0.007 | 4,100 | 900 | 0.025 ± 0.010 | 0.509 |
| E | UL4-5′ | 2,100 | 1,000 | 0.009 ± 0.004 | 1,000 | 700 | 0.012 ± 0.009 | 0.470 | 3,200 | 6,500 | 0.005 ± 0.009 | 800 | 700 | 0.005 ± 0.004 | 0.809 |
| E | UL4/5 | 4,400 | 1,200 | 0.018 ± 0.005 | 800 | 200 | 0.009 ± 0.002 | 0.097 | 11,200 | 3,000 | 0.019 ± 0.004 | 2,700 | 300 | 0.017 ± 0.001 | 0.956 |
| E | UL8/9 | 2,500 | 5,300 | 0.011 ± 0.022 | 1,000 | 1,400 | 0.011 ± 0.018 | 0.902 | 6,400 | 4,400 | 0.010 ± 0.005 | 1,600 | 400 | 0.010 ± 0.002 | 0.502 |
| E | UL8-5′ | 2,000 | 400 | 0.008 ± 0.002 | 600 | 500 | 0.007 ± 0.006 | 0.913 | 1,200 | 1,700 | 0.002 ± 0.002 | 500 | 400 | 0.003 ± 0.002 | 0.777 |
| E | UL21 | 500 | 700 | 0.002 ± 0.003 | 800 | 400 | 0.009 ± 0.005 | 0.208 | 3,900 | 1,200 | 0.006 ± 0.001 | 1,000 | 300 | 0.006 ± 0.001 | 0.636 |
| E | UL23 | 11,000 | 1,800 | 0.046 ± 0.008 | 1,400 | 1,600 | 0.018 ± 0.016 | 0.198 | 22,000 | 2,300 | 0.034 ± 0.004 | 5,300 | 1,400 | 0.040 ± 0.009 | 0.543 |
| E | UL29 | 2,200 | 1,300 | 0.010 ± 0.005 | 800 | 400 | 0.009 ± 0.004 | 0.954 | 2,800 | 3,400 | 0.004 ± 0.005 | 1,400 | 700 | 0.009 ± 0.004 | 0.824 |
| E | UL30 | 5,800 | 800 | 0.024 ± 0.004 | 1,400 | 400 | 0.018 ± 0.004 | 0.082 | 10,800 | 4,500 | 0.019 ± 0.006 | 2,100 | 900 | 0.014 ± 0.005 | 0.987 |
| E | UL37 | 600 | 500 | 0.002 ± 0.002 | 400 | 200 | 0.005 ± 0.002 | 0.966 | 4,000 | 3,700 | 0.007 ± 0.005 | 1,000 | 500 | 0.006 ± 0.003 | 0.894 |
| E | UL39-5′ | 4,500 | 2,200 | 0.019 ± 0.009 | 1,800 | 400 | 0.022 ± 0.006 | 0.432 | 4,700 | 3,600 | 0.008 ± 0.005 | 900 | 800 | 0.006 ± 0.005 | 0.853 |
| E | UL39/40 | 11,600 | 3,300 | 0.048 ± 0.015 | 2,900 | 1,700 | 0.038 ± 0.016 | 0.422 | 20,900 | 13,600 | 0.036 ± 0.022 | 6,400 | 2,400 | 0.048 ± 0.014 | 0.663 |
| E | UL42 | 2,100 | 100 | 0.009 ± 0.001 | 1,000 | 100 | 0.013 ± 0.001 | 0.002 | 15,100 | 8,700 | 0.026 ± 0.012 | 2,400 | 1,900 | 0.018 ± 0.011 | 0.690 |
| E | UL43 | 4,400 | 4,000 | 0.019 ± 0.017 | 1,900 | 1,300 | 0.024 ± 0.019 | 0.864 | 6,600 | 3,300 | 0.010 ± 0.005 | 1,400 | 1,500 | 0.009 ± 0.009 | 0.714 |
| E | UL50 | 7,500 | 1,400 | 0.032 ± 0.006 | 2,000 | 900 | 0.026 ± 0.010 | 0.269 | 24,100 | 7,400 | 0.042 ± 0.013 | 7,700 | 1,700 | 0.046 ± 0.014 | 0.627 |
| E | UL52-5′ | 200 | 100 | 0.001 ± 0.000 | 200 | 100 | 0.002 ± 0.001 | 0.124 | 100 | 800 | 0.000 ± 0.001 | 300 | 300 | 0.002 ± 0.002 | 0.390 |
| E | UL55 | 700 | 500 | 0.003 ± 0.002 | 600 | 200 | 0.006 ± 0.003 | 0.339 | 7,000 | 1,600 | 0.011 ± 0.001 | 1,900 | 400 | 0.012 ± 0.002 | 0.649 |
| E | UL56 | 1,600 | 500 | 0.007 ± 0.002 | 700 | 200 | 0.008 ± 0.002 | 0.572 | 4,200 | 6,900 | 0.007 ± 0.009 | 900 | 1,100 | 0.006 ± 0.007 | 0.873 |
| E | US2 | 200 | 600 | 0.001 ± 0.003 | 200 | 100 | 0.003 ± 0.001 | 0.990 | 6,900 | 400 | 0.011 ± 0.001 | 1,200 | 400 | 0.007 ± 0.002 | 0.143 |
| L | UL1 | 2,000 | 600 | 0.008 ± 0.002 | 700 | 100 | 0.010 ± 0.001 | 0.534 | 14,600 | 11,200 | 0.025 ± 0.015 | 3,100 | 2,400 | 0.023 ± 0.015 | 0.922 |
| L | UL3 | 1,400 | 800 | 0.006 ± 0.003 | 1,000 | 600 | 0.012 ± 0.007 | 0.510 | 9,500 | 2,700 | 0.016 ± 0.004 | 2,000 | 200 | 0.013 ± 0.002 | 0.639 |
| L | UL10 | 2,500 | 1,000 | 0.010 ± 0.004 | 1,600 | 600 | 0.020 ± 0.008 | 0.290 | 4,800 | 6,200 | 0.008 ± 0.008 | 1,600 | 900 | 0.009 ± 0.005 | 0.797 |
| L | UL16/17 | 600 | 300 | 0.003 ± 0.001 | 500 | 400 | 0.005 ± 0.005 | 0.493 | 7,900 | 10,400 | 0.014 ± 0.014 | 1,500 | 1,900 | 0.012 ± 0.011 | 0.937 |
| L | UL15 | 1,100 | 100 | 0.005 ± 0.000 | 400 | 0 | 0.005 ± 0.001 | 0.539 | 4,800 | 5,100 | 0.008 ± 0.007 | 1,500 | 800 | 0.009 ± 0.004 | 0.978 |
| L | UL18/20 | 6,700 | 1,800 | 0.0290.008 | 1,600 | 600 | 0.020 ± 0.005 | 0.487 | 23,300 | 8,400 | 0.037 ± 0.015 | 3,800 | 1,700 | 0.023 ± 0.015 | 0.880 |
| L | UL19/20 | 2,500 | 600 | 0.011 ± 0.003 | 900 | 200 | 0.011 ± 0.002 | 0.923 | 13,400 | 7,700 | 0.023 ± 0.012 | 2,200 | 900 | 0.015 ± 0.007 | 0.603 |
| L | UL19-5′ | 800 | 500 | 0.003 ± 0.002 | 600 | 300 | 0.008 ± 0.004 | 0.248 | 500 | 1,900 | 0.001 ± 0.003 | 500 | 200 | 0.003 ± 0.001 | 0.922 |
| L | UL22 | 2,400 | 200 | 0.010 ± 0.001 | 900 | 300 | 0.011 ± 0.004 | 0.387 | 11,300 | 4,700 | 0.019 ± 0.008 | 3,200 | 500 | 0.021 ± 0.004 | 0.916 |
| L | UL24 | 1,800 | 900 | 0.007 ± 0.004 | 900 | 100 | 0.011 ± 0.002 | 0.322 | 10,200 | 2,700 | 0.014 ± 0.005 | 1,900 | 400 | 0.012 ± 0.001 | 0.390 |
| L | UL25 | 1,900 | 1,500 | 0.008 ± 0.006 | 600 | 700 | 0.008 ± 0.008 | 0.878 | 13,700 | 6,800 | 0.022 ± 0.013 | 2,200 | 1,200 | 0.013 ± 0.011 | 0.852 |
| L | UL27/8 | 4,200 | 2,400 | 0.018 ± 0.010 | 1,000 | 600 | 0.011 ± 0.008 | 0.380 | 14,500 | 7,800 | 0.025 ± 0.013 | 3,100 | 900 | 0.023 ± 0.004 | 0.514 |
| L | UL27-5′ | 1,000 | 700 | 0.004 ± 0.003 | 400 | 200 | 0.005 ± 0.003 | 0.775 | 100 | 100 | 0.000 ± 0.006 | 100 | 500 | 0.000 ± 0.003 | 0.376 |
| L | UL31/34 | 900 | 1,000 | 0.004 ± 0.004 | 400 | 800 | 0.005 ± 0.011 | 0.510 | 13,000 | 4,000 | 0.018 ± 0.006 | 2,200 | 600 | 0.014 ± 0.002 | 0.194 |
| L | UL35 | 1,400 | 600 | 0.006 ± 0.002 | 1,400 | 600 | 0.015 ± 0.006 | 0.139 | 18,400 | 4,400 | 0.032 ± 0.004 | 5,400 | 1,500 | 0.032 ± 0.007 | 0.929 |
| L | UL38 | 500 | 700 | 0.002 ± 0.003 | 400 | 200 | 0.006 ± 0.003 | 0.354 | 5,400 | 4,900 | 0.009 ± 0.006 | 1,800 | 700 | 0.011 ± 0.004 | 0.672 |
| L | UL41 | 300 | 100 | 0.001 ± 0.000 | 200 | 300 | 0.003 ± 0.004 | 0.467 | 2,500 | 3,700 | 0.004 ± 0.005 | 1,400 | 700 | 0.008 ± 0.004 | 0.880 |
| L | UL44-5′ | 200 | 100 | 0.001 ± 0.000 | 400 | 300 | 0.005 ± 0.003 | 0.278 | 1,400 | 1,600 | 0.002 ± 0.002 | 400 | 300 | 0.003 ± 0.002 | 0.864 |
| L | UL44/45 | 1700 | 600 | 0.007 ± 0.002 | 1,000 | 300 | 0.011 ± 0.003 | 0.413 | 22,700 | 5,900 | 0.039 ± 0.008 | 4,200 | 2,200 | 0.032 ± 0.013 | 0.943 |
| L | UL46/47 | 13,200 | 2,400 | 0.055 ± 0.009 | 2,600 | 1,500 | 0.031 ± 0.019 | 0.441 | 20,200 | 7,700 | 0.035 ± 0.011 | 3,600 | 1,300 | 0.027 ± 0.008 | 0.732 |
| L | UL48 | 6,400 | 3,300 | 0.028 ± 0.014 | 3,300 | 2,000 | 0.040 ± 0.022 | 0.629 | 23,900 | 10,700 | 0.041 ± 0.018 | 7,400 | 3,100 | 0.055 ± 0.020 | 0.986 |
| L | UL51 | 1,100 | 700 | 0.004 ± 0.003 | 500 | 300 | 0.006 ± 0.003 | 0.804 | 8,800 | 4,300 | 0.013 ± 0.007 | 1,700 | 800 | 0.011 ± 0.004 | 0.351 |
| L | RLXY | 100 | 300 | 0.001 ± 0.001 | 200 | 200 | 0.003 ± 0.003 | 0.204 | 900 | 400 | 0.001 ± 0.000 | 400 | 300 | 0.002 ± 0.002 | 0.305 |
| L | RLX | 12,000 | 2,600 | 0.050 ± 0.010 | 4,400 | 1,300 | 0.057 ± 0.018 | 0.866 | 600 | 700 | 0.001 ± 0.001 | 200 | 600 | 0.001 ± 0.004 | 0.533 |
| L | RICP34.5 | 1,500 | 400 | 0.006 ± 0.002 | 1,100 | 400 | 0.014 ± 0.005 | 0.230 | 900 | 600 | 0.002 ± 0.001 | 300 | 200 | 0.002 ± 0.001 | 0.294 |
| L | US5-5′ | 800 | 200 | 0.003 ± 0.001 | 100 | 200 | 0.001 ± 0.002 | 0.394 | 500 | 200 | 0.001 ± 0.000 | 200 | 300 | 0.001 ± 0.002 | 0.392 |
| L | US8-5′ | 1,000 | 400 | 0.004 ± 0.002 | 300 | 300 | 0.004 ± 0.004 | 0.799 | 400 | 1,000 | 0.001 ± 0.001 | 300 | 200 | 0.002 ± 0.001 | 0.844 |
| L | US8/9 | 7,800 | 3,700 | 0.034 ± 0.015 | 2,600 | 1,300 | 0.028 ± 0.018 | 0.595 | 31,300 | 17,800 | 0.054 ± 0.029 | 8,100 | 3,000 | 0.048 ± 0.022 | 0.935 |
| Latent | RLAT-5′ | 1,300 | 700 | 0.006 ± 0.003 | 800 | 200 | 0.010 ± 0.003 | 0.195 | 100 | 500 | 0.000 ± 0.001 | 100 | 500 | 0.000 ± 0.003 | 0.466 |
| Latent | RHA6 | 500 | 500 | 0.002 ± 0.002 | 400 | 300 | 0.005 ± 0.004 | 0.417 | 200 | 300 | 0.000 ± 0.000 | 100 | 400 | 0.000 ± 0.002 | 0.460 |
| Latent | RLAT-1 | 2,600 | 700 | 0.011 ± 0.003 | 1,000 | 100 | 0.012 ± 0.002 | 0.988 | 100 | 700 | 0.000 ± 0.001 | 100 | 500 | 0.001 ± 0.003 | 0.544 |
| Latent | RLATX | 200 | 0 | 0.001 ± 0.000 | 200 | 100 | 0.003 ± 0.001 | 0.240 | 900 | 700 | 0.001 ± 0.001 | 200 | 500 | 0.002 ± 0.003 | 0.863 |
| Latent | RLAT-3′ | 600 | 2,200 | 0.003 ± 0.009 | 100 | 700 | 0.002 ± 0.010 | 0.986 | 1,500 | 1,300 | 0.002 ± 0.002 | 600 | 300 | 0.004 ± 0.002 | 0.871 |
| ?? | U\?\1X | 600 | 800 | 0.003 ± 0.003 | 100 | 500 | 0.001 ± 0.006 | 0.977 | 1,900 | 1,700 | 0.003 ± 0.002 | 600 | 500 | 0.004 ± 0.003 | 0.647 |
| Mixed | U6/7 | 2,400 | 1,300 | 0.010 ± 0.005 | 1,100 | 700 | 0.012 ± 0.009 | 0.553 | 5,500 | 3,700 | 0.009 ± 0.005 | 1,400 | 900 | 0.010 ± 0.006 | 0.636 |
| Mixed | U11/13 | 9,800 | 6,900 | 0.040 ± 0.030 | 2,800 | 2,300 | 0.034 ± 0.024 | 0.916 | 18,900 | 14,900 | 0.033 ± 0.024 | 5,300 | 2,700 | 0.040 ± 0.018 | 0.992 |
| Mixed | U36 | 1,000 | 600 | 0.004 ± 0.002 | 200 | 200 | 0.002 ± 0.002 | 0.618 | 5,600 | 7,700 | 0.010 ± 0.010 | 1,400 | 1,000 | 0.010 ± 0.006 | 0.892 |
| ?? | U43.5-5′ | 200 | 2,000 | 0.001 ± 0.008 | 0 | 600 | 0.000 ± 0.007 | 0.852 | 600 | 600 | 0.001 ± 0.001 | 100 | 100 | 0.001 ± 0.001 | 0.874 |
| Mixed | U49 | 5,200 | 400 | 0.022 ± 0.002 | 1,800 | 200 | 0.022 ± 0.000 | 0.763 | 25,100 | 15,400 | 0.043 ± 0.025 | 6,000 | 3,600 | 0.045 ± 0.022 | 0.068 |
| Mixed | U52/53 | 200 | 1,200 | 0.001 ± 0.005 | 1,100 | 600 | 0.015 ± 0.005 | 0.061 | 300 | 900 | 0.000 ± 0.001 | 1,800 | 1,200 | 0.011 ± 0.007 | 0.467 |
| ?? | ROP | 2,200 | 700 | 0.009 ± 0.003 | 800 | 600 | 0.010 ± 0.007 | 0.336 | 200 | 0 | 0.000 ± 0.000 | 100 | 300 | 0.000 ± 0.002 | 0.398 |
| ?? | US3-5′ | 1,800 | 1,200 | 0.007 ± 0.005 | 700 | 400 | 0.009 ± 0.006 | 0.841 | 900 | 1,700 | 0.002 ± 0.002 | 800 | 400 | 0.005 ± 0.002 | 0.557 |
| Mixed | US3/4 | 1,500 | 600 | 0.006 ± 0.002 | 300 | 300 | 0.003 ± 0.004 | 0.724 | 4,600 | 5,000 | 0.007 ± 0.006 | 1,300 | 400 | 0.008 ± 0.002 | 0.534 |
| Mixed | US5/6/7 | 2,400 | 900 | 0.031 ± 0.003 | 1,000 | 600 | 0.013 ± 0.006 | 0.669 | 8,600 | 1,800 | 0.014 ± 0.004 | 1,900 | 300 | 0.014 ± 0.002 | 0.648 |
IE, immediate early; E, early; L, late.
Infection was initiated at a multiplicity of 0.1 PFU per cell. All data are based on three replicate experiments. SD, standard deviation. Italic means that fractional values in WT infections are reduced by 0.8 or greater as compared to the mutant, where the differences are significant at P ≤ 0.05.
Fractional values are the medians of the individual fractional values calculated from the total viral signal for each experiment as described in Materials and Methods; the standard deviation of these values is shown.
Relative values of transcript levels were compared by Student's t test as described in Materials and Methods. The null hypothesis is that the true values for the WT and mutant viruses are identical.>
We also carried out comparisons of RNA abundance at 6 and 8 h after infection of human HFFs and SK-N-SH (neuroblastoma) cells as well as mouse neural bulb cells at a multiplicity of 1 to 5 PFU/cell and also observed no significant differences in relative (or absolute) transcript abundance (data not shown).
Levels of viral proteins are consistent with the transcription abundance measurements for both ICP27 kinetic mutants.
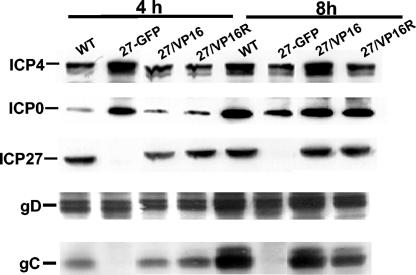
We measured levels of ICP4, ICP0, ICP27 (WT or mutant), gC, and gD at 4 and 8 h following infections with the mutant, its rescue, ICP27-null, and WT viruses. These data are shown in Fig. 1. Following infections at 1 PFU/cell, the 27-GFP (null) virus overexpresses ICP4 and ICP0 at 4 h as compared to WT virus, but as seen in Table 2, the relative abundance of the transcripts expressing these proteins is similar to that of the WT virus. At this MOI, the ICP27/VP16 mutant and its rescue virus behaved identically to the WT control at both 4 and 8 h, ICP4 and ICP0 were equal in the mutant and its rescue virus, and these levels were equivalent to WT levels.
FIG. 1.
Western blot analysis of HSV protein synthesis following infection with ICP27 mutants. Extracts from cells with the indicated viruses were fractionated by SDS-PAGE, the proteins were transferred to nitrocellulose, and the blots were probed with antibodies specific for ICP4, ICP0, ICP27, gB, and gC. Infections were performed at an MOI of 1 for the indicated times.
Lack of the ability of ICP27 RNA to accumulate in the absence of de novo protein synthesis has no effect upon levels of virus replication in cultured cells.
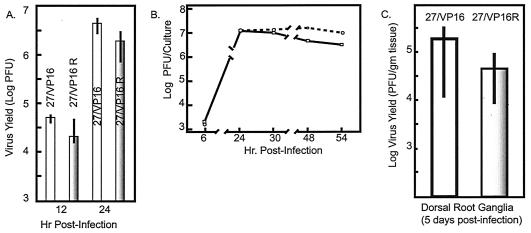
We examined the relative efficiency of replication of the ICP27 promoter mutant with its rescue virus after both single and multiple replication cycles in order to assess the effect of the transcriptional effects seen; these data are shown in Fig. 2. For single-cycle replication, aliquots of 1,000 PFU of the appropriate virus were used to infect replicate cultures of confluent MEFs, and virus titers were determined 12 and 24 h after virus adsorption. Based upon three replicate experiments, we found there was no significant difference in virus yield between the mutant and rescue viruses (Fig. 2A). In a second set of experiments, cultures were infected at an MOI of 0.1 PFU/cell and virus yields were determined 6, 24, 30, 48, and 54 h after virus adsorption. Here too, no significant difference in yield was seen between the mutant and rescue viruses (Fig. 2B). Finally, we confirmed the equivalent replication of the mutant as compared to its rescue and WT virus in a classic single-step growth experiment in which cells were infected at 10 PFU/cell and harvested 20 h later. No statistically significant differences in virus “burst” size was seen between any of the infections tested (data not shown).
FIG. 2.
Replication of the ICP27/VP16 kinetic mutant in vitro and in vivo. (A) Cultures of confluent MEFs were infected with 1,000 PFU of virus as described in the text, and the titer of the virus yield was determined 12 and 24 h p.i. The experiment is described in the Results section. (B) Multistep replication of the ICP27/VP16 kinetic mutant in confluent MEFs. Circles represent the rescue values, and squares represent the mutant values. The vertical size of the symbols is a measure of the standard deviation of each experimental determination (see Results and Materials and Methods for details). (C) Recovery of the ICP27/VP16 kinetic mutant in mouse DRG at 5 days following footpad infection (see Results and Materials and Methods for details).
The viral recombinants with altered temporal expression of ICP27 showed similar patterns of replication and spread in the mouse following footpad inoculation.
Footpad inoculation of mice provides a sensitive means of assessing even subtle differences in virulence and viral replication (5). In order to assess whether the ICP27 promoter mutant under study displayed alterations in replication in vivo, mice were inoculated on both rear footpads with 105 PFU. Four mice per viral recombinant (or its rescue virus) per time point were infected, and mice were sacrificed at 1, 3, and 5 days p.i.. Feet, DRG, and spinal cords from the mice were dissected, and amounts of infection virus were determined. These analyses revealed that there were no significant differences in amounts of infectious virus detected when the ICP27 promoter mutants were compared with their cognate WT rescue viruses or the parental 17syn+ virus. All viruses showed similar yields of infectious virus in the feet throughout the course of the infection (data not shown). This was not surprising given the demonstrated ability of the virus to replicate normally in cultured fibroblasts. Measurement of virus yields in DRG and spinal cords provides an indication of whether the ICP27 kinetic mutants exhibited any alteration in their ability to replicate within neurons in vivo. Typically, viruses with alterations in the ability to replicate within neurons initially show a reduction in viral yields within the sensory neurons of the DRG (6, 37). As shown in Fig. 2C, the viruses tested yielded similar amounts of virus in the DRG assayed at 5 days p.i. and there was no statistically significant difference between the ICP27/VP16 recombinant and its rescue virus (P = 0.3112, respectively, two-tailed t test). Similarly, at day 5 p.i., all viruses were detected in similar amounts in the spinal cord (data not shown), demonstrating that the mutants were both capable of normal patterns of spread through the nervous system.
ICP27/VP16 both reactivate efficiently from mouse DRG following explant cocultivation.
In order to assess whether the altered temporal expression of ICP27 might exert an influence on the relative ability of these recombinants to reactivate from latency, mice were infected with 500 PFU of the mutant and rescue viruses in order to establish a latent infection. Thirty days p.i., four mice per virus were sacrificed and individual DRG (six per mouse) were explanted (see Materials and Methods). Ganglia from both mice infected with ICP27/VP16 and its rescue virus began to show evidence of reactivation by day 5 and reactivated as efficiently (>90% of ganglia were explant positive by day 18) as their rescue viruses (data not shown).
DISCUSSION
The overall goal of the study described herein was to begin an analysis of possible functions of the immediate-early HSV-1 ICP27 transcript correlated with its kinetics of expression. It should be noted that these kinetics are somewhat different from those displayed by the other HSV-1 immediate-early transcripts. This has been measured both globally for relative levels of transcript abundance and directly by pulse-labeling (39, 48). Although ICP27 is expressed at the very outset of the infection cycle, rates of expression and relative transcript abundance remain high during the first 3 h or so following infections at moderate MOIs, while rates of synthesis and relative abundances of ICP4 and ICP0 are highest at the earliest times measured and rapidly decline thereafter, consistent with the known shutoff functions of the ICP4 protein. The continued expression of the ICP27 transcript fits well with the protein's function in RNA transport.
While our results clearly demonstrate that abrogation of the immediate-early expression of ICP27 has an effect on the earliest patterns of transcript abundance in the viral replication cascade, it is not clear how vital the precise timing of this expression is to the virus in a biological sense. Thus, the kinetic mutant of ICP27 demonstrate altered patterns of transcript abundance at the earliest times studied (Tables 1 and 3), but unlike the situation with a deletion mutant, delayed expression has no measurable effect on virus replication in vitro or in vivo. The observation of “robustness” in the interleaving of viral functions in the early stages of the replication cycle has been documented in recent studies in which the entire immediate-early cascade is disrupted due to loss of activation by VP16 (43), but the complete lack of any discernible biological manifestation was unexpected.
Finally, we should note that the evolutionary stability of the immediate-early kinetic signature for the ICP27 transcript is a strong argument in favor of the timing of expression of this viral protein being critical and essential during the earliest window of viral gene expression in at least some tissue important in the natural history of HSV-1. Naively, we assumed that this window was part of the latency/reactivation system of the virus, given the potential for tissue-specific restriction during either establishment of or reactivation from the latent state (cf. 38). The results presented here suggest that any such restriction is subtler than measurable in several well-established pathogenicity models for HSV-1 infections. Importantly, the observed phenotype of time-based differences in quantitative transcript expression but neutral fitness strongly suggests a threshold model for activation of viral expression that incorporates a high level of regulatory network robustness. Again, further kinetic modification of ICP27 expression in combination with other viral mutants as well as utilization of more demanding models may well illuminate finer detail; at the very least, however, the data here suggests critical limitations to the pathogenicity models utilized.
Acknowledgments
This work was supported by PHS grants CA11861 and CA90287 to E.K.W.; AI48633 to D.C.B; AI21515 to R.M.S-G.; and the British Biotechnology Science Research Council, Wellcome Trust and Scottish Higher Education Funding Council to P.G.
J. Sunabe, Qian Dai, and Carole Dehmel provided excellent technical assistance.
REFERENCES
- 1.Aguilar, J. S., D. Roy, P. Ghazal, and E. K. Wagner. 2002. Dimethyl sulfoxide blocks herpes simplex virus-1 productive infection in vitro acting at different stages with positive cooperativity. Application of micro-array analysis. BMC Infect. Dis. 2:9. [Online.] http://www.biomedcentral.com. [DOI] [PMC free article] [PubMed]
- 2.Arnosti, D. N., C. M. Preston, M. Hagmann, W. Schaffner, R. G. Hope, G. Laughlan, and B. F. Luisi. 1993. Specific transcriptional activation in vitro by the herpes simplex virus protein VP16. Nucleic Acids Res. 21:5570-5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey, A. C., and R. Thompson. 1992. A sequence-specific DNA-binding protein recognising a GA-rich element cooperates with Oct-1 at the herpes simplex virus type 1 IE3 promoter. Intervirology 34:74-85. [DOI] [PubMed] [Google Scholar]
- 4.Beard, P., S. Faber, K. W. Wilcox, and L. I. Pizer. 1986. Herpes simplex virus immediate early infected-cell polypeptide 4 binds to DNA and promotes transcription. Proc. Natl. Acad. Sci. USA 83:4016-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bloom, D. C. 1999. HSV vectors for gene therapy, p. 369-386. In S. M. Brown and A. R. MacLean (ed.), Methods in molecular medicine. Humana Press, Inc., Totowa, N.J. [DOI] [PubMed]
- 6.Bloom, D. C., and J. G. Stevens. 1994. Neuron-specific restriction of a herpes simplex virus recombinant maps to the UL5 gene. J. Virol. 68:3761-3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruni, R., and B. Roizman. 1998. Herpes simplex virus 1 regulatory protein ICP22 interacts with a new cell cycle-regulated factor and accumulates in a cell cycle-dependent fashion in infected cells. J. Virol. 72:8525-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai, W., and P. A. Schaffer. 1992. Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J. Virol. 66:2904-2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLuca, N. A., M. A. Courtney, and P. A. Schaffer. 1984. Temperature-sensitive mutants in herpes simplex virus type 1 ICP4 permissive for early gene expression. J. Virol. 52:767-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLuca, N. A., and P. A. Schaffer. 1985. Activation of immediate-early, early, and late promoters by temperature-sensitive and wild-type forms of herpes simplex virus type 1 protein ICP4. Mol. Cell. Biol. 5:1997-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett, R. D. 1989. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J. Gen. Virol. 70:1185-1202. [DOI] [PubMed] [Google Scholar]
- 12.Früh, K., K. Ahn, H. Djaballah, P. Sempé, P. M. Van Endert, R. Tampé, P. A. Peterson, and Y. Yang. 1995. A viral inhibitor of peptide transporters for antigen presentation. Nature 375:415-418. [DOI] [PubMed] [Google Scholar]
- 13.Gelman, I. H., and S. J. Silverstein. 1987. Herpes simplex virus immediate-early promoters are responsive to virus and cell trans-acting factors. J. Virol. 61:2286-2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guzowski, J. F., and E. K. Wagner. 1993. Mutational analysis of the herpes simplex virus type 1 strict late UL38 promoter/leader reveals two regions critical in transcriptional regulation. J. Virol. 67:5098-5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardwicke, M. A., P. J. Vaughan, R. E. Sekulovich, R. O'Conner, and R. M. Sandri-Goldin. 1989. The regions important for the activator and repressor functions of herpes simplex virus type 1 α protein ICP27 map to the C-terminal half of the molecule. J. Virol. 63:4590-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardy, W. R., and R. M. Sandri-Goldin. 1994. Herpes simplex virus inhibits host cell splicing, and regulatory protein ICP27 is required for this effect. J. Virol. 68:7790-7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hibbard, M. K., and R. M. Sandri-Goldin. 1995. Arginine-rich regions succeeding the nuclear localization region of the herpes simplex virus type 1 regulatory protein ICP27 are required for efficient nuclear localization and late gene expression. J. Virol. 69:4656-4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 19.Hill, J. M., F. Sedarati, R. T. Javier, E. K. Wagner, and J. G. Stevens. 1990. Herpes simplex virus latent phase transcription facilitates in vivo reactivation. Virology 174:117-125. [DOI] [PubMed] [Google Scholar]
- 20.Jackson, S. A., and N. A DeLuca. 2003. Relationship of herpes simplex virus genome configuration to productive and persistent infections. Proc. Natl. Acad. Sci. USA 100:7871-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kristie, T. M., and P. A. Sharp. 1990. Interactions of the Oct-1 POU subdomains with specific DNA sequences and with the HSV α-trans-activator protein. Genes Dev. 4:2383-2396. [DOI] [PubMed] [Google Scholar]
- 22.Lieu, P. T., and E. K. Wagner. 2000. The kinetics of VP5 mRNA expression is not critical for viral replication in cultured cells. J. Virol. 74:2770-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLauchlan, J., A. Phelan, C. Loney, R. M. Sandri-Goldin, and J. B. Clements. 1992. Herpes simplex virus IE63 acts at the posttranscriptional level to stimulate viral mRNA 3′ processing. J. Virol. 66:6939-6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michael, N., and B. Roizman. 1989. Binding of the herpes simplex virus major regulatory protein to viral DNA. Proc. Natl. Acad. Sci. USA 86:9808-9812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael, N., D. Spector, P. Mavromara-Nazos, T. M. Kristie, and B. Roizman. 1988. The DNA-binding properties of the major regulatory protein alpha 4 of herpes simplex viruses. Science 239:1531-1534. [DOI] [PubMed] [Google Scholar]
- 26.Poffenberger, K. L., A. D. Idowu, E. B. Fraser-Smith, P. E. Raichlen, and R. C. Herman. 1994. A herpes simplex virus type 1 ICP22 deletion mutant is altered for virulence and latency in vivo. Arch. Virol. 139:111-119. [DOI] [PubMed] [Google Scholar]
- 27.Poffenberger, K. L., P. E. Raichlen, and R. C. Herman. 1993. In vitro characterization of a herpes simplex virus type 1 ICP22 deletion mutant. Virus Genes 7:171-186. [DOI] [PubMed] [Google Scholar]
- 28.Rice, S. A., and D. M. Knipe. 1988. Gene-specific transactivation by herpes simplex virus type 1 alpha protein ICP27. J. Virol. 62:3814-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rice, S. A., and D. M. Knipe. 1990. Genetic evidence for two distinct transactivation functions of the herpes simplex virus α protein ICP27. J. Virol. 64:1704-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rice, S. A., M. C. Long, V. Lam, P. A. Schaffer, and C. A. Spencer. 1995. Herpes simplex virus immediate-early protein ICP22 is required for viral modification of host RNA polymerase II and establishment of the normal viral transcription program. J. Virol. 69:5550-5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacks, W. R., C. C. Greene, D. P. Aschman, and P. A. Schaffer. 1985. Herpes simplex virus type 1 ICP27 is an essential regulatory protein. J. Virol. 55:796-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandri-Goldin, R. M. 1998. ICP27 mediates HSV RNA export by shuttling through a leucine-rich nuclear export signal and binding viral intronless RNAs through an RGG motif. Genes Dev. 12:868-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandri-Goldin, R. M., and G. E. Mendoza. 1992. A herpesvirus regulatory protein appears to act post-transcriptionally by affecting mRNA processing. Genes Dev. 6:848-863. [DOI] [PubMed] [Google Scholar]
- 34.Smith, I. L., M. A. Hardwicke, and R. M. Sandri-Goldin. 1992. Evidence that the herpes simplex virus immediate early protein ICP27 acts post-transcriptionally during infection to regulate gene expression. Virology 186:74-86. [DOI] [PubMed] [Google Scholar]
- 35.Stern, S., and W. Herr. 1991. The herpes simplex virus trans-activator VP16 recognizes the Oct-1 homeo domain: evidence for a homeo domain recognition subdomain. Genes Dev. 5:2555-2566. [DOI] [PubMed] [Google Scholar]
- 36.Stingley, S. W., J. J. G. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tran, R. K., P. T. Lieu, J. S. Aguilar, E. K. Wagner, and D. C. Bloom. 2004. Altering the expression kinetics of VP5 results in altered virulence and pathogenesis of herpes simplex virus type 1 in mice. J. Virol. 76:2199-2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagner, E. K., and D. C. Bloom. 1997. Experimental investigation of herpes simplex virus latency. Clin. Microbiol. Rev. 10:419-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner, E. K., R. J. J. Garcia, S. W. Stingley, J. S. Aguilar, L. Buehler, G. B. Devi-Rao, and P. Ghazal. 2002. Practical approaches to long oligonucleotide-based DNA microarrays: lessons from herpesvirues. Prog. Nucleic Acid Res. Mol. Biol. 71:445-491. [DOI] [PubMed] [Google Scholar]
- 40.Wagner, E. K., M. D. Petroski, N. T. Pande, P. T. Lieu, and M. K. Rice. 1998. Analysis of factors influencing the kinetics of herpes simplex virus transcript expression utilizing recombinant virus. Methods 16:105-116. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, A. C., M. A. Cleary, J.-S. Lai, K. LaMarco, M. G. Peterson, and W. Herr. 1993. Combinatorial control of transcription: the herpes simplex virus VP16-induced complex. Cold Spring Harbor Symp. Quant. Biol. 58:167-178. [DOI] [PubMed] [Google Scholar]
- 42.Xiao, P., and J. P. Capone. 1990. A cellular factor binds to the herpes simplex virus type 1 transactivator Vmw65 and is required for Vmw65-dependent protein-DNA complex assembly with Oct-1. Mol. Cell. Biol. 10:4974-4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang, W. C., G. V. Devi-Rao, P. Ghazal, E. K. Wagner, and S. J. Triezenberg. 2002. General and specific alterations in programming of global viral gene expression during infection by VP16 activation-deficient mutants of herpes simplex virus type 1. J. Virol. 76:12758-12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yguerabide, J., and E. E. Yguerabide. 1998. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. Anal. Biochem. 262:137-156. [DOI] [PubMed] [Google Scholar]
- 45.Yguerabide, J., and E. E. Yguerabide. 1998. Light-scattering submicroscopic particles as highly fluorescent analogs and their use as tracer labels in clinical and biological applications. Anal. Biochem. 262:157-176. [DOI] [PubMed] [Google Scholar]
- 46.Yguerabide, J., and E. E. Yguerabide. 2001. Resonance light scattering particles as ultrasensitive labels for detection of analytes in a wide range of applications. J. Cell Biochem. Suppl. 37:71-81. [DOI] [PubMed] [Google Scholar]
- 47.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CH8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]
- 48.Zhang, Y.-F., and E. K. Wagner. 1987. The kinetics of expression of individual herpes simplex virus type 1 transcripts. Virus Genes 1:49-60. [DOI] [PubMed] [Google Scholar]