Rotaviruses are responsible for significant gastrointestinal disease, primarily in children <5 years of age and the young of other mammalian species. Each year rotaviruses cause approximately 111 million episodes of gastroenteritis in children, which result in 25 million visits to clinics, 2 million hospitalizations, and 352,000 to 592,000 deaths. On a worldwide basis, nearly every child experiences rotavirus gastroenteritis by age 5, 1 in 5 visits a clinic, 1 in 65 is hospitalized, and 1 in 293 dies. Children in the poorest countries account for 82% of rotavirus deaths (60). This disease burden underscores a need for interventions such as vaccines. A vaccine was developed and approved, but recommendation for its use was withdrawn because of vaccination-associated adverse events (57). Additional information about the molecular biology, immunology, and pathogenesis of rotavirus infection will inform ongoing vaccine development efforts.
Our understanding of rotavirus-induced diarrheal disease is incomplete compared to that of several other pathogens (e.g., cholera). Rotavirus diarrhea has been attributed to several different mechanisms, including malabsorption secondary to enterocyte destruction, a virus-encoded toxin, stimulation of the enteric nervous system (ENS), and villus ischemia (13, 45). Over the past several years, numerous studies have addressed mechanisms of diarrhea induction at the cellular and tissue levels, and a new understanding of the mechanisms is beginning to emerge. Here I will briefly outline the new data and present our current understanding of how rotaviruses induce diarrhea in the infected host.
Recent studies confirm sporadic case reports that rotavirus infection is not confined to the intestine as was generally assumed. Although systemic sequellae to rotavirus infection are apparently rare, reports have continually appeared in the literature, and recent work with animal models has begun to shed light on how the virus spreads to extraintestinal sites. Our current understanding of extraintestinal spread and infection will also be considered below.
GENERAL CHARACTERISTICS OF ROTAVIRUS INFECTION AND DISEASE IN HUMANS AND ANIMALS
Rotavirus.
Rotaviruses comprise a genus within the family Reoviridae. The rotavirion has a nonenveloped, complex, triple-layered capsid structure that surrounds a genome composed of 11 segments of double-stranded RNA. There are six structural proteins and six nonstructural proteins, each encoded in a unique genome segment except for nonstructural proteins 5 and 6 (NSP5 and NSP6), which are encoded in overlapping reading frames of a single segment. The rotavirus genus is divided into serological groups (A to E). Groups A to C infect humans, and all groups infect animals. All the information presented here is in regard to group A virus infections.
Pathophysiology of rotavirus diarrhea.
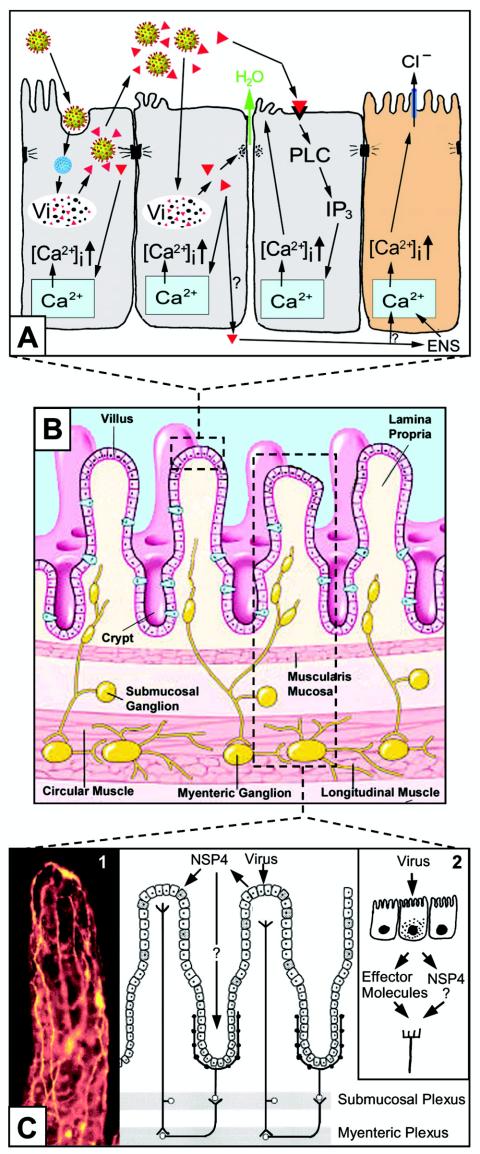
The enterocytes lining the small intestine are generally divided into two types: enterocytes and crypt cells (Fig. 1). Villus enterocytes are mature, nonproliferating cells covering the villi that are differentiated to digestive and absorptive functions. The absorptive enterocytes synthesize a number of disaccharidases, peptidases, and other enzymes that are expressed on the apical surface, where they carry out their digestive functions. Absorption across the enterocyte barrier occurs both by passive diffusion of solutes along electrochemical or osmotic gradients and by active transport. While the majority of water transport is passive along osmotic gradients, transporters such as the sodium-glucose cotransporter 1 (SGLT1) transport water along with solute (42). The crypt epithelium lines the crypts and is the progenitor of the villus enterocytes. Crypt cells lack the well-defined microvilli and absorptive functions of the enterocyte and actively secrete Cl− ions into the intestinal lumen. In the normal animal, the combined activity of the enterocytes and crypt cells results in a constant bidirectional flux of electrolytes and water across the epithelium. On the villi, the balance is toward absorption, and in the crypts, the balance favors secretion (51).
FIG. 1.
Model of rotavirus-induced diarrhea. Panel A depicts events in the infected epithelium. For clarity, not all events are shown in each cell. The following processes are shown in order from left to right across the four cells. (i) The initial cell is infected by luminal virus, with virus entry and uncoating, formation of a viroplasm (Vi), and the release of virus and viral proteins. NSP4 (red triangles) may be released via a nonclassical secretory pathway. Intracellular NSP4 also induces release of Ca2+ from the internal stores, primarily the endo-plasmic reticulum (blue), increasing [Ca2+]i. (ii) A cell is secondarily infected after virus release from the initial cell. NSP4 produced by the infection disrupts the tight junctions, allowing paracellular flow of water and electrolytes (green arrow). (iii) NSP4 binds to a specific receptor on a cell and triggers a signaling cascade through PLC and IP3 that results in release of Ca2+ and an increase in [Ca2+]i. Intracellular expression of NSP4 does not stimulate PLC. The increase in [Ca2+]i acts to disrupt the microvillar cytoskeleton. (iv) The brown cell represents a crypt cell. It can be acted on directly by NSP4, or NSP4 can stimulate the ENS, which in turn signals an increase in [Ca2+]i that induces Cl− secretion. Panel B shows the normal architecture of the small intestine, with the circulatory system removed for clarity. This panel emphasizes the ENS and its ganglia in the different submucosal levels. (Panel B is adapted from reference 25, with the permission of M. D. Gershon and the publisher.) Panel C shows a reflex arc in the ENS that can receive signals from the villus epithelium and activate the crypt epithelium. (Adapted from reference 44, with the permission of L. Svensson and the publisher.) Inset 1 shows a whole mount of an adult mouse small intestinal villus, stained with antibody to protein gene product 9.5 to reveal the rich innervation (yellow stain). (Inset 1 is used by courtesy of R. O. Heuckeroth and with permission.) Inset 2 shows that infected villus enterocytes may stimulate the ENS by the basolateral release of NSP4 or other effector molecules. (Inset 2 is adapted from reference 45 with permission of L. Svensson and the publisher.)
Our understanding of rotavirus pathophysiology comes primarily from animal models. Rotaviruses replicate in the nondividing mature enterocytes near the tips of the villi, suggesting that differentiated enterocytes express factors required for efficient infection and replication (13). The severity and localization of rotavirus intestinal infection vary among animal species and between studies; however, the pathological changes are almost exclusively limited to the small intestine. In various animal models, rotavirus infection is associated with virtually no visible lesions; slight lesions, such as enterocyte vacuolization and loss; or larger changes, such as villus blunting and crypt hyperplasia. Inflammation is generally mild compared to that for other intestinal pathogens. This picture of pathology suggests that there is no absolute correlation between histological lesions and disease symptoms.
Rotavirus infection alters the function of the small intestinal epithelium, resulting in diarrhea. The diarrhea was generally considered to be malabsorptive, secondary to enterocyte destruction (34). In addition to enterocyte destruction, absorption of Na+, water, and mucosal disaccharidases are decreased (10, 28), while mucosal cyclic AMP appears not to be altered (16). Malabsorption results in the transit of undigested mono- and disaccharides, carbohydrates, fats, and proteins into the colon. The undigested bolus is osmotically active, and the colon is unable to absorb sufficient water, leading to an osmotic diarrhea (27). Another study suggested that the diarrhea was malabsorptive and resulted from epithelial damage caused by villus ischemia (58). A secretory component of the diarrhea was suggested, based on elevated levels of prostaglandin E2 (PGE2) in the infected gut and the stimulation of secretion by PGE2 (79). The fact that gut lesions often do not correlate with the presence of diarrhea stimulated the search for other mechanisms of diarrhea induction. The viral nonstructural protein NSP4, a secreted fragment of NSP4, or certain NSP4 peptides were found to have toxin-like activity and to induce diarrhea when inoculated into mice (3, 23, 77). The NSP4 enterotoxin activity provides a way to mediate diarrheagenic changes in the absence of significant damage or to mediate changes at uninfected sites. Recently, it was shown that several drugs that block the action of the ENS attenuate rotavirus-induced secretion in the intestine, suggesting a role for the ENS in rotavirus diarrhea (44, 45, 46). It was estimated that ∼67% of the fluid and electrolyte secretion in rotavirus diarrhea in experiments with mice was due to activation of the ENS (46). Thus, it is clear that rotavirus diarrhea is multifactoral, resulting from the direct effects of virus infection and the indirect effects of infection and the host response.
Some rotavirus infections are asymptomatic (10), which suggests that both viral and host factors can affect disease severity (13). Among the viral factors are the following. (i) Some alleles of VP4 may be associated with asymptomatic disease (24). (ii) Virus strains can be attenuated, particularly by passage in cell culture. Attenuation generally results in a restricted ability to replicate and cause disease in the host (32). (iii) Virus strains seem to be adapted for growth in particular host species (host range) (5). Several host factors have also been shown to affect the severity of rotavirus disease, including the following. (i) Malnutrition is documented to increase the severity of rotavirus diarrhea (71), where it delays small intestinal recovery (78) and modifies intestinal inflammatory responses (79). (ii) Rotavirus symptomatic infections are generally age restricted (13). The age dependence appears to be unrelated to receptor expression, as both the viral and the NSP4 receptors are expressed in adult animals. However, signaling downstream of the NSP4 receptor does appear to be age dependent (53), but age restriction may be related to immunity, as neutralizing antibodies increase with age and virus exposure. (iii) Rotavirus disease may be related to age-dependent protease expression, as viral infectivity requires protease cleavage of VP4 and newborns have low levels of protease in the gut (29). (iv) The expression of intestinal mucins and the rate of epithelial cell replacement and fluid absorption are both age dependent and have been shown to affect rotavirus infection and disease in the host (51, 76).
THE CURRENT MODEL OF DIARRHEA INDUCTION BY ROTAVIRUS
Many of the salient events in rotavirus induction of diarrhea are shown in Fig. 1. Most of the data used to construct this figure were derived from model animal and cell culture systems, although some items were demonstrated in humans as well. Numerous recent reviews have dealt with specific aspects of diarrhea induction (21-23, 43-45, 50, 52, 67). From these reviews and the discussion above, it is clear that a multitude of viral and host factors have the potential to influence rotavirus infection and disease production. Below, I have assembled what we currently understand about the interplay of these factors in the induction of rotavirus diarrhea.
The process leading to diarrhea is initiated when rotavirus binds to and infects enterocytes in the small intestine (Fig. 1A). Binding is mediated by sequential interaction with a series of sialic acid-containing and nonsialylated receptor molecules. The virus is internalized by an unknown mechanism, and the outer capsid is lost, activating the virion-associated transcriptase and viral macromolecular syntheses. Viral proteins and RNAs concentrate in cytoplasmic structures called viroplasms, where RNA replication and packaging take place. Intracellular events, probably involving NSP4, cause release of Ca2+ from the endoplasmic reticulum. The increase in intracellular Ca2+ concentration ([Ca2+]i) triggers a number of cellular processes, including disruption of the microvillar cytoskeletal network, lowered expression of disaccharidases and other enzymes at the apical surface, general inhibition of the Na+-solute cotransport systems, and necrosis. NSP4 appears to be released specifically by a Ca2+-dependent, nonclassical secretion pathway prior to cell lysis. These events lead to a malabsorption component of the diarrhea through reduction in absorptive capacity of the epithelium, reduced activity of Na+-solute cotransporters, and reduction of digestive enzyme expression on the epithelial surface.
The release of NSP4 from infected cells allows paracrine effects to occur on uninfected cells (Fig. 1A). NSP4 binds to these cells, using specific, unidentified receptor(s) (22), and triggers a phospholipase C-inositol 1,3,5-triphosphate (PLC-IP3) cascade that culminates in the release of Ca2+ from the endoplasmic reticulum, increasing [Ca2+]i. If NSP4 acts on enterocytes, one of the results is the disruption of tight junctions, resulting in paracellular permeability. If NSP4 acts on crypt cells, the resulting increase in [Ca2+]i leads to secretion in the crypt, mediated by activation of a Cl− transporter, resulting in an increased secretory component of the diarrhea. Secreted NSP4, or other effector molecules released from infected cells, may also stimulate the ENS (Fig. 1C). Indeed, experiments with agents that block function of the ENS showed that rotavirus infection induced secretion via stimulation of the ENS. This information begins to shed light on the mechanism(s) by which relatively few infected cells, causing little visible damage to the mucosa, can elicit a diarrheal response.
THE MOLECULAR BASIS OF DIARRHEA INDUCTION
Rotavirus diarrhea is multifactoral, has malabsorption and secretion components, and may have other components suggested to be related to villus ischemia and intestinal motility. Here I present the relevant data on the induction of each of these components (references are selective and for illustrative purposes).
Malabsorption.
A malabsorptive component of rotavirus diarrhea appears to be related to the primary infection with the virus. Infection of villus enterocytes leads to a cascade of events involving Ca2+ (Fig. 1A). This disruption of Ca2+ homeostasis appears to be mediated by synthesis of viral proteins (17, 49). Increased Ca2+ permeability at both the plasma membrane and the endoplasmic reticulum leads to an increase in [Ca2+]i, triggering a chain of events that leads to cell lysis (61). The fact that NSP4 expressed in cells also leads to increases in [Ca2+]i implicates it as the mediator of virus-induced [Ca2+]i dysregulation (74). A fragment of NSP4 (amino acids 112 to 175) is secreted via a nonclassical pathway early after infection (77), and this fragment added exogenously to cells also causes an increase in [Ca2+]i (73). The increase in [Ca2+]i follows NSP4 binding to a specific apical receptor (22) that triggers a PLC-IP3 cascade resulting in release of Ca2+ from intracellular stores (19). In contrast, the increase in [Ca2+]i induced by intracellular NSP4 is independent of PLC stimulation (73). The NSP4-mediated effects may amplify the diarrheagenic effect of infection in the absence of significant visible tissue damage. However, the ability of inactivated rotavirus particles to induce diarrhea (68) suggests that viral structural proteins may also play a role in the dysregulation leading to diarrhea.
Rotavirus infection has other effects on enterocytes that may contribute to malabsorption (Fig. 1A). Infection leads to an increase in [Na+]i and a decrease in [K+]i, which appear to be related to increased plasma membrane permeability and not inhibition of the Na+/K+ pump (17). Changes in intracellular levels of Na+ and K+ could impair electroneutral NaCl absorption and Na+-linked nutrient absorption, resulting in a loss of fluid (50). [Na+]i dysregulation may be related to a general inhibition of the Na+-solute cotransport systems (30). NSP4 may also be involved, as the NSP4114-135 peptide is a specific and noncompetitive inhibitor of SGLT1 (31). Infection also reduces the expression of digestive enzymes at the apical surface of infected enterocytes. For example, the activities of alkaline phosphatase, lactase, sucrase, and maltase are reduced (6, 12, 16). The expression of sucrase and isomaltase in cultured human intestinal epithelium was also reduced, probably as a result of perturbation of protein targeting and the microvillar cytoskeleton (33). Rotavirus infection alters the structure of polarized enterocytes in a number of ways. The increase in [Ca2+]i induced by rotavirus infection affects the Ca2+-sensitive proteins F-actin, villin, and tubulin, damaging the microvillar cytoskeleton, whereas rearrangements of other cytoskeletal proteins (cytokeratin-18) are independent of changes in [Ca2+]i (6, 7). Both rotavirus infection and NSP4 promote functional changes in tight junctions between enterocytes that maintain the epithelial barrier (18, 72). The drop in transepithelial resistance induced by either the virus or NSP4 suggests that infection can cause paracellular leakage. Rotavirus also induces intestinal epithelial cells to secrete CXC and CC chemokines, suggesting that enterocyte chemokine secretion plays a role in initiating the immune response to infection (9, 66, 69). Interleukin-8 (IL-8), GRO-α, RANTES, interferon (IFN)-stimulated protein 10, and granulocyte-macrophage colony-stimulating factor (GM-CSF) are stimulated, whereas other chemokines (tumor necrosis factor alpha, IL-1α, IFN-α, IFN-γ, MIP-α, MCP-1, and IL-6) are unchanged. Induction of IL-8 and RANTES is noteworthy because these are the most potent chemoattractants for intestinal intraepithelial lymphocytes (20). It is unclear if virus replication is required for induction of chemokine secretion (9, 66). In addition, the levels of PGE2 are increased in infected intestine (79). The chemokines may activate the immune response rather than directly contribute to diarrhea. Thus, rotavirus infection causes a number of changes in the villus epithelium that contribute to malabsorption.
Secretion.
The secretory component of rotavirus diarrhea appears to be secondary to virus-induced functional changes at the villus epithelium. The central players in secretion appear to be NSP4 and the ENS. The precise role and targets of secreted NSP4 are unknown. NSP4 may simply amplify the effects of infection in the enterocyte epithelium. However, NSP4 may also act at the crypt epithelium (Fig. 1A), where it would induce increases in crypt cell [Ca2+]i, activate Cl− secretion, and lead to an outflow of water. This Cl− secretion is known to be unrelated to the cAMP-dependent Cl− channel of crypt cells because CFTR-knockout mice are susceptible to rotavirus or NSP4-induced diarrhea (2, 53). The identity of the Cl− channel involved in rotavirus diarrhea in CFTR-knockout mice remains unknown. It is proposed that NSP4 itself may form a channel or that NSP4 activates a dormant Ca2+-activated anion channel (53). Interestingly, these studies also showed that the age dependence of rotavirus diarrhea is not due to age-dependent expression of a NSP4 receptor or age-dependent Ca2+ mobilization but rather to the age dependence of Cl− permeability (53). Another possible target of secreted NSP4 is the ENS, which is also a target in classic cholera toxin-induced diarrhea (45). Indeed, the ENS is rich immediately under the villus epithelium, and it is situated to receive stimuli from the rotavirus-damaged epithelium (Fig. 1C, inset 1). Although NSP4 stimulation of the ENS has not been shown experimentally, it has been shown that the ENS is involved in rotavirus diarrhea (46). The application of a number of pharmacologic agents that block ENS stimulation (lidocaine, tetrodotoxin, and mecamylamide) significantly lowered the transmembrane potential difference in a virus dose-dependent manner in Ussing chamber or organ bath experiments using rotavirus-infected intestinal tissues. In organ bath experiments, blocking the ENS could change net secretion to net absorption (45). In live infected animals, repeated administration of lidocaine significantly prevented fluid losses. Thus, it is clear that the ENS is activated during rotavirus infection, and this activation could explain how relatively few infected cells at the villus tips could stimulate crypt cells to secrete electrolytes and water (45). While it is unknown if NSP4 directly stimulates the ENS, the ENS is known to respond to a number of molecules released from enterocytes. Cholera toxin induces the release of 5-hydroxytryptamine (5-HT) from enterochromaffin cells in the villus epithelium, and 5-HT is a stimulator of the ENS (44). It is possible that secreted NSP4 binds to enterochromaffin cells, inducing a release of 5-HT and stimulation of the ENS (44) (Fig. 1C, inset 2). Likewise, the secretion of chemokines and prostaglandins by infected enterocytes may serve to stimulate the ENS. Currently, the ENS and NSP4 appear to have the major roles in the secretory response to infection.
Villus ischemia.
Although damage to the intestinal epithelium is minimal in rotavirus-infected mice, villus ischemia was observed in some studies (58, 70). It was proposed that diarrhea could result from virus-induced release of an unknown vasoactive agent from infected epithelium, causing a local villus ischemia and subsequent functional damage to enterocytes (59). However, villus ischemia has not been observed in other animal models, so the significance of this observation remains unknown.
Intestinal motility.
In some diarrheal infections, intestinal motility is significantly increased. The intestinal transit time is decreased in rotavirus infection, implying increased motility (50). The ENS generally controls motility, but the molecular stimulator of motility is not known. It could be any of the ENS stimulators discussed above.
To summarize, rotavirus diarrhea is clearly a multicomponent disease. Good evidence exists for malabsorptive and secretory components. The mediators of these disease components range from primary cellular damage to a secreted viral enterotoxic peptide and a virus-induced interaction with the ENS.
SYSTEMIC INFECTION WITH ROTAVIRUS
L. M. Kraft performed some of the early studies of rotavirus infection, using epidemic diarrhea of infant mice virus (EDIM) before it was recognized as a rotavirus (1). An important but under-appreciated aspect of this work is that rotavirus spread throughout the bodies of the infected mice following oral infection (39, 40, 41). By 72 h postinfection, infectious EDIM was found in the lungs, liver, spleen, kidney, bladder, brain, and blood. Since most of the organs where EDIM was detected are highly vascular, it was generally assumed that virus in the tissues reflected the presence of blood.
Subsequently, a large number of clinical case reports suggested that rotaviruses could be found at extraintestinal sites following infection. It was not clear if the virus was replicating and infectious at these sites or passively present in the blood or if it resulted from contamination during sample collection. Examples include the finding of virus in the liver following fatal disease (8), the finding of elevated liver enzymes associated with virus infection (38), and the demonstration of viral replication in the liver and kidneys of an immunodeficient child (26). Rotavirus involvement in biliary atresia was suggested, although the best data involve group C rotaviruses (65). Neurological involvement was suggested by several reports of children with concomitant convulsions and rotavirus diarrhea (for examples, see references 35 and 56). Whether putative central nervous system (CNS) infection results from contamination is not known, but one study strongly suggested infection of the CNS (48). In recent studies, rotavirus antigens were detected in myocardium from patients who died unexpectedly (11), and in vitro studies demonstrated the ability of rotavirus to replicate in primary islet cells, an observation that correlates with the temporal association of infection with development of pancreatic islet autoantibodies (14). These case reports strongly indicate that rotavirus infection may have rare systemic sequellae, although one must bear in mind that infectious viruses were not isolated and replication was shown only once (26).
In animal models, rotaviruses have also been documented to spread beyond the intestine after oral infection. In the mouse model, sites of spread include the lamina propria, Peyer's patches, mesenteric lymph nodes, lung, liver, kidney, and bile duct (13). Group A rotaviruses have been shown to induce biliary atresia in mice, but this system requires intraperitoneal, not oral, inoculation of virus (15). The most detailed studies of extraintestinal spread in the mouse examined spread to the liver following oral inoculation.
The liver as a site of systemic infection.
Spread of virus in orally infected mice was shown in persistently infected SCID mice where a diffuse hepatitis was noted. Infectious virus was isolated from the liver, and surviving mice developed chronic liver disease (64, 75). Infectious virus was also found in the livers of normal mice, although the hepatitis was less severe and resolved spontaneously (75). Spread of virus to the liver and development of hepatitis were described as virus strain dependent. These results were supported by studies of cultured human hepatoma (HepG2) cells, where some viruses were capable of complete infectious cycles and others were not (37, 63). The strain-specific ability to infect HepG2 cells segregated with viral genome segment 4, encoding the outer capsid spike protein VP4 (36, 63). Subsequent studies indicated that viruses unable to replicate in HepG2 cells entered less efficiently, but the absolute block to infection occurred at a late step in the intracellular replication cycle (S. Jafar and R. F. Ramig, unpublished data).
Recent studies utilized the strain difference in extraintestinal spread to the liver as the basis for a genetic approach to identifying viral determinants of the spread phenotype. A collection of reassortants made from virus strains RRV (spread competent) and SA11-Cl4 (spread incompetent) were inoculated orally into suckling mice, and the presence of infectious virus in the liver was used as a proxy for extraintestinal spread. A statistical analysis of the data indicated that genome segment 7, encoding the nonstructural protein NSP3, was significantly linked to virus spread to the liver (54). However, replication of virus to high titer in the intestine was not significantly linked to the ability to spread. Thus, a virus must replicate to spread, but it need not replicate to high titer to spread to the liver. A follow-up study showed that virus escaped the intestine via a lymphatic route, appearing sequentially in the Peyer's patch, the mesenteric lymph node, and, finally, the peripheral tissues (55). These studies also showed that genome segment 6, encoding VP6, was a secondary determinant of spread. The combination of segment 6 from a spreading virus with segment 7 from a nonspreading virus allowed escape from the intestine but only to the mesenteric lymph node and no farther (55). The association of a nonstructural protein, NSP3, with escape of virus from the intestine suggested that virus transits in infected cells. Indeed, preliminary experiments indicate that virus in the mesenteric lymph node is internalized by some cells of the lymphocytic or myloid lineage (E. C. Mossel and R. F. Ramig, unpublished data). NSP3 is described as a protein that functions in the regulation of protein synthesis by binding to a specific sequence at the 3′ termini of the nonpolyadenylated viral mRNAs and interacts with the translation initiation complex to favor viral protein synthesis in the infected cell (62). How NSP3 functions as the primary determinant of spread to the liver is not understood.
Potential role for viremia in rotavirus spread and pathogenesis.
The numerous clinical reports of rotavirus at systemic sites and work with mice that showed systemic spread prompted a systematic search for evidence of widespread rotavirus viremia in children and animal model systems (4). In a retrospective study, 22 of 33 (66%) serum samples from rotavirus-infected children were positive for rotavirus antigen by enzyme immunoassay (EIA), whereas 0 of 35 rotavirus-negative children had antigens in their sera. In a subset of six children with paired acute and convalescent-phase sera, viral antigen was present in all acute-phase sera and none of the convalescent-phase sera, indicating that the antigenemia is transient (4). Only three of six serum samples from rotavirus-infected children were positive by PCR, the lower positivity probably reflecting the lower sensitivity of the PCR assay used (4). In another study, three children who died of rotavirus-associated disease were examined retrospectively. Two of the three children had rotavirus RNA in extraintestinal tissues (spleen, heart, lung, kidney, testes, bladder, adrenal gland, and pancreas), as determined by reverse transcription-PCR. Confirmation of positive PCR products by hybridization with serotype-specific probes indicated that the two children with extraintestinal rotavirus were infected with different G serotypes (47). These findings show that viral antigen and/or virus particles enter the circulation.
Prospective studies with animal models confirmed the antigenemia seen in humans and also demonstrated viremia. Rotavirus antigen could be detected by EIA in 100% of the sera from infant and adult mice, rats, rabbits, and calves that were experimentally infected (4). Three of three infected infant mouse serum samples yielded infectious virus when assayed by oral inoculation of naive mice with sera, and 9 of 11 sera from adult mice yielded infectious virus in the same assay (4). Studies were subsequently carried out with infant rats infected with the heterologous virus strains rhesus RRV and human HAL1166 (S. E. Crawford, D. G. Patel, E. Cheng, Z. Berkova, J. M. Hyser, A. A. McKinstry, and M. K. Estes, Abstr. 8th International Symposium on Double-Stranded RNA Viruses, abstr. W6.4, 2003). In these studies, the sacrificed animals were perfused with buffer to wash blood out of the tissues. Rotavirus antigen was detected in small intestine, stomach, liver, kidney, and spleen in some animals after perfusion, suggesting that the virus present was specifically associated with those tissues and not passively present in the circulation. Infectious virus could be detected in 100% of sera from infected rat pups by fluorescent focus assay on MA104 cells, at titers up to 103 focus-forming units/ml (Crawford et al., 8th International Symposium on Double-Stranded RNA Viruses). Finally, in a study with pigs, no viremia was detected in gnotobiotic piglets infected with attenuated human rotavirus Wa, but viremia was detected in 100% of gnotobiotic piglets infected with virulent human strain Wa. Inoculating additional gnotobiotic piglets with the viremic sera and observing diarrhea confirmed the presence of infectious virus in the serum (L. J. Saif, M. S. P. Azevedo, L. Yuan, K. I. Nguyen, S. M. Pouly, and M. Gochnauer, Abstr. 8th International Symposium on Double-Stranded RNA Viruses, abstr. W6.3, 2003). It is interesting that viremia was not observed routinely in the studies of rotavirus intestinal escape to the liver (55). In these studies, infectious virus was detected in the blood of only 2 of 60 (3%) animals infected with strain RRV, the strain that induced antigenemia in 100% of mice (4) and viremia in 100% of rats (Crawford et al., 8th International Symposium on Double-Stranded RNA Viruses). The reason for this difference is unclear, although it may indicate that virus moves from the intestine to the liver by a mechanism that does not involve viremia.
The results presented here indicate that antigenemia is a nearly universal event in children and animal models (Table 1). In animals, antigenemia was accompanied by a transient viremia, as demonstrated by the presence of infectious virus in the sera of virtually all animals tested. While viremia is not yet proven in children for lack of demonstration of infectious virus in the serum, it seems likely that viremia also occurs in children. This viremia may provide a mechanism for seeding peripheral tissues with virus during rotavirus infection and may account for the reports of systemic sequellae associated with rotavirus infection. However, these data also suggest that systemic sequellae to viremia are rare, since, for example, 100% of mice and rats are viremic (4; Crawford et al., 8th International Symposium on Double-Stranded RNA Viruses) but only about 25% of mice experience liver infection after oral infection with the same virus strain (54, 55).
TABLE 1.
Rotavirus antigenemia and viremia in various hosts
| Host | % Infected animals with:
|
|
|---|---|---|
| Serum antigenemia | Serum infectivity | |
| Childrena | 66 | NTd |
| Mice (infant)a | 100 | 100 |
| Mice (adult)a | 100 | NTd |
| Rat (infant)a,b | 100 | 100 |
| Rabbit (adult)a | 100 | NTd |
| Bovine (adult)a | 100 | NTd |
| Pig (infant)c | 100 | 100 |
The children had natural infections, the mice were challenged with homologous or heterologous virus, the rats were challenged with heterologous virus, the rabbits were challenged with homologous virus, and the bovines were naturally infected.
Identical results were obtained with rhesus or human challenge virus.
Challenged with virulent human virus (Saif et al., 8th International Symposium on Double-Stranded RNA Viruses).
NT, not tested.
CLOSING COMMENTS
Rotaviruses naturally infect the enteric tract and cause diarrheal disease in children and young animals. The pathophysiology of rotavirus diarrhea is clearly multifactoral. There is a malabsorptive component of the diarrhea that seems related to primary damage to intestinal epithelium by virus infection and the action of a secreted viral enterotoxin (NSP4). The effects observed on the epithelium can be traced primarily to dysregulation of Ca2+ in the epithelium. A secretory component of rotavirus diarrhea appears to result from stimulation of the ENS. The mode of activation of the ENS is not clear, but it may be through secreted NSP4 or the chemokines and other factors released from infected epithelial cells. The secretory component of the diarrhea appears to result from Ca2+ dysregulation of the secretory crypt cells. We have learned a great deal of the molecular biology of rotavirus diarrheal disease components, but a great deal of work remains to be done before rotavirus diarrhea induction is completely understood.
In the past several years, interest in rotavirus spread to extraintestinal tissues has been renewed. It appears that some of this spread depends on viral factors, but other components of the spread may be related to viremic hosts. It remains unclear if viremia is, in any way, related to diarrheal disease. It is important to determine if systemic infection with rotavirus is responsible for, or plays a role in, clinical syndromes not currently associated with rotavirus.
The studies reviewed here indicate that we have made significant progress in understanding the molecular basis for rotavirus intestinal pathogenesis and spread to peripheral sites. However, there remain a large number of questions to be answered. For example, we need to know if NSP4 is the key viral protein or if other viral structural and/or nonstructural proteins play a role in induction of diarrhea. How is a NSP4 fragment specifically secreted from infected cells early in infection? How is the ENS activated by rotavirus infection, and what are the roles of NSP4, chemokines, or other stimulatory molecules released by infected cells? Do rotavirus strains with NSP4 polymorphisms differ in activation of the ENS? What is the nature of the age-dependent, Ca2+-regulated Cl− channel activated by infection? Does the host innate or adaptive immune response play any role in the development of intestinal disease or systemic spread? How does a viral nonstructural protein known to regulate translation play a central role in the spread of virus from the intestine to the liver? How does rotavirus gain access to the circulation, and does it leave circulation to cause clinically significant infections at systemic sites? Finally, it will be important to determine if viremia and/or systemic spread contribute to pathogenesis in the average rotavirus infection where extraintestinal sequellae are not seen. Answers to these and other questions are exciting to anticipate and will increase our understanding of this ubiquitous disease of childhood.
Acknowledgments
I thank Robert O. Heuckeroth for allowing use of his unpublished work (Fig. 1C, inset 1) and M. D. Gershon (Fig. 1B) and L. Svensson (Fig. 1C and inset 2) for use of their published figures. I also thank my colleagues at Baylor College of Medicine, S. Crawford, S. Blutt, M. Conner, M. Estes, E. Mossel, and B. V. V. Prasad, for helpful discussions and for critically reviewing the manuscript.
The work from the author's laboratory was supported by National Institutes of Health grants RO1-AI16687 and T32-AI07471.
REFERENCES
- 1.Adams, W. R., and L. M. Kraft. 1963. Epizootic diarrheas of infant mice: identification of the etiologic agent. Science 141:359-360. [DOI] [PubMed] [Google Scholar]
- 2.Angel, J., B. Tang, N. Feng, H. B. Greenberg, and D. Bass. 1998. Studies of the role for NSP4 in the pathogenesis of homologous murine rotavirus diarrhea. J. Infect. Dis. 177:455-458. [DOI] [PubMed] [Google Scholar]
- 3.Ball, J. M., P. Tian, C. Q-Y. Zeng, A. P. Morris, and M. K. Estes. 1996. Age-dependent diarrhea induced by a rotavirus nonstructural glycoprotein. Science 272:101-104. [DOI] [PubMed] [Google Scholar]
- 4.Blutt, S. E., C. D. Kirkwood, V. Parreno, K. L. Warfield, M. Ciarlet, M. K. Estes, K. Bok, R. F. Bishop, and M. E. Conner. 2003. Rotavirus antigenaemia and viraemia: a common event? Lancet 362:1445-1449. [DOI] [PubMed] [Google Scholar]
- 5.Broome, R. L., P. T. Vo, R. L. Ward, H. F. Clark, and H. B. Greenberg. 1993. Murine rotavirus genes encoding outer capsid proteins VP4 and VP7 are not major determinants of host range restriction and virulence. J. Virol. 67:2448-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunet, J. P., J. Cotte-Laffitte, C. Linxe, A. M. Quero, M. Geniteau-Legendre, and A. L. Servin. 2000. Rotavirus infection induces an increase in intracellular calcium concentration in human intestinal epithelial cells: role in microvillar actin alteration. J. Virol. 74:2323-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet, J. P., N. Jourdan, J. Cotte-Laffitte, C. Linxe, M. Geniteau-Legendre, A. L. Servin, and A. M. Quero. 2000. Rotavirus infection induces cytoskeleton disorganization in human intestinal epithelial cells: implication of an increase in intracellular calcium concentration. J. Virol. 74:10801-10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson, J. A., P. J. Middleton, M. T. Szymanski, J. Huber, and M. Petric. 1978. Fatal rotavirus gastroenteritis: an analysis of 21 cases. Am. J. Dis. Child. 132:477-479. [DOI] [PubMed] [Google Scholar]
- 9.Casola, A., M. K. Estes, S. E. Crawford, P. L. Ogra, P. B. Ernst, R. P. Garfalo, and S. E. Crowe. 1998. Rotavirus infection of cultured intestinal epithelial cells induces secretion of CXC and CC chemokines. Gastroenterology 114:947-955. [DOI] [PubMed] [Google Scholar]
- 10.Chrystie, I. L., B. M. Totterdell, and J. E. Banatvala. 1978. Asymptomatic endemic rotavirus infections of the newborn. Lancet i:1176-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cioc, A. M., and G. J. Nuovo. 2002. Histologic and in situ viral findings in the myocardium in cases of sudden unexpected death. Mod. Pathol. 9:914-922. [DOI] [PubMed] [Google Scholar]
- 12.Collins, J., W. G. Starkey, T. S. Wallis, G. J. Clarke, K. J. Worton, A. J. Spencer, S. J. Haddon, M. P. Osborne, C. D. Candy, and J. Stephen. 1988. Intestinal enzyme profiles in normal and rotavirus-infected mice. J. Pediatr. Gastroenterol. Nutr. 7:264-272. [DOI] [PubMed] [Google Scholar]
- 13.Conner, M. E., and R. F. Ramig. 1997. Viral enteric diseases, p. 713-743. In N. Nathanson (ed.), Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.
- 14.Coulson, B. S., P. D. Witterick, Y. Tan, M. J. Hewish, J. N. Mountford, L. C. Harrison, and M. C. Honeyman. 2002. Growth of rotaviruses in primary pancreatic cells. J. Virol. 76:9537-9544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Czech-Schmidt, G., W. Verhagen, P. Szavay, J. Leonhardt, and C. Petersen. 2001. Immunological gap in the infectious animal model for biliary atresia. J. Surg. Res. 101:62-67. [DOI] [PubMed] [Google Scholar]
- 16.Davidson, G. P., D. G. Gall, M. Petric, D. G. Butler, and J. R. Hamilton. 1977. Human rotavirus enteritis induced in conventional piglets: intestinal structure and transport. J. Clin. Investig. 60:1402-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.del Castillo, J. R., J. E. Ludert, A. Sanchez, M. C. Ruiz, F. Michelangeli, and F. Liprandi. 1991. Rotavirus infection alters Na+ and K+ homeostasis in MA104 cells. J. Gen. Virol. 72:541-547. [DOI] [PubMed] [Google Scholar]
- 18.Dickman, K. G., S. J. Mempson, J. Anderson, S. Lippe, L. Shao, and R. D. Shaw. 2000. Rotavirus alters paracellular permeability and energy metabolism in Caco-2 cells. Am. J. Physiol. 279:G757-G766. [DOI] [PubMed] [Google Scholar]
- 19.Dong, Y., C. Q. Zeng, J. M. Ball, M. K. Este, and A. P. Morris. 1997. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc. Natl. Acad. Sci. USA 94:3960-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebert, E. 1995. Human intestinal intraepithelial lymphocyres have potent chemotactic activity. Gastroenterology 109:1154-1159. [DOI] [PubMed] [Google Scholar]
- 21.Estes, M. K., G. Kang, C. Q. Zeng, S. E. Crawford, and M. Ciarlet. 2001. Pathogenesis of rotavirus gastroenteritis, p. 82-96. In D. J. Chadwick and J. A. Goode, (ed.), Gastroenteritis viruses. Novartis Foundation Symposium 238. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed]
- 22.Estes, M. K. 2003. The rotavirus NSP4 enterotoxin: current status and challenges, p. 207-224. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis. Elsevier Science BV, Amsterdam, The Netherlands.
- 23.Estes, M. K. and A. P. Morris. 1999. A viral enterotoxin: a new mechanism of virus induced pathogenesis, p. 73-82. In P. S. Paul and D. H. Francis (ed.), Mechanisms in the pathogenesis of enteric diseases 2. Kluwer Academic/Plenum Publishers, New York, N.Y. [PubMed]
- 24.Flores, J., K. Midthun, Y. Hoshino, K. Green, M. Gorziglia, A. Z. Kapikian, and R. M. Chanock. 1986. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J. Virol. 60:972-979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gershon, M. D. 1999. The enteric nervous system: a second brain. Hosp. Pract. 34:31-39. [DOI] [PubMed] [Google Scholar]
- 26.Gilger, M. A., D. O. Matson, M. E. Conner, H. M. Rosenblatt, M. J. Finegold, and M. K. Estes. 1992. Extraintestinal rotavirus infections in children with immunodeficiency. J. Pediatr. 120:912-917. [DOI] [PubMed] [Google Scholar]
- 27.Graham, D. Y. and M. K. Estes. 1988. Viral infections of the intestine, p. 566-578. In G. Gitnick (ed.), Gastroenterology. Medical Examination Publishing Company, New Hyde Park, N.Y.
- 28.Graham, D. Y., J. W. Sackman, and M. K. Estes. 1984. Pathogenesis of rotavirus-induced diarrhea: preliminary studies in miniature swine piglet. Dig. Dis. Sci. 29:1028-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg, H. B., H. F. Clark, and P. A. Offit. 1994. Rotavirus pathology and pathophysiology. Curr. Top. Microbiol. Immunol. 185:255-283. [DOI] [PubMed] [Google Scholar]
- 30.Halaihel, N., V. Lievin, F. Alvarado, and M. Vasseur. 2000. Rotavirus infection impairs intestinal brush-border membrane Na+-solute cotransport activities in young rabbits. Am. J. Physiol. Gastrointest. Liver Physiol. 279:G587-G596. [DOI] [PubMed] [Google Scholar]
- 31.Halaihel, N., V. Lievin, J. M. Ball, M. K. Estes, F. Alvarado, and M. Vasseur. 2000. Direct inhibitory effect of rotavirus NSP4(114-135) peptide on the Na+-d-glucose symporter of rabbit intestinal brush border membrane. J. Virol. 74:9464-9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hall, G. A., J. C. Bridger, K. R. Parsons, and R. Cook. 1993. Variation in rotavirurus virulence: a comparison of pathogenesis in calves between two rotaviruses of different virulence. Vet. Pathol. 30:223-233. [DOI] [PubMed] [Google Scholar]
- 33.Jourdan, N., J. P. Brunet, C. Sapin, A. Blais, J. Cotte-Laffitte, F. Forestier, A. M. Quero, G. Trugnan, and A. L. Servin. 1998. Rotavirus infection reduces sucrase-isomaltase expression in human intestinal epithelial cells by perturbing protein targeting and organization of microvillar cytoskeleton. J. Virol. 72:7228-7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapikian, A. Z., Y. Hoshino, and R. M. Chanock. 2001. Rotaviruses, p. 1787-1833. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus, Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 35.Keidan, I., I. Shif, G. Keren, and J. H. Passwell. 1992. Rotavirus encephalopathy: evidence of central nervous system involvement during rotavirus infection. Pediatr. Infect. Dis. J. 11:773-775. [PubMed] [Google Scholar]
- 36.Kitamoto, N., N. M. Mattion, and M. K. Estes. 1993. Alterations in the sequence of the gene 4 from a human rotavirus after multiple passages in HepG2 cells. Arch. Virol. 130:179-185. [DOI] [PubMed] [Google Scholar]
- 37.Kitamoto, N., R. F. Ramig, D. O. Matson, and M. K. Estes. 1991. Comparative growth of different rotavirus strains in differentiated cells (MA104, HepG2, and CaCo-2). Virology 184:729-737. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs, A., L. Chan, C. Hotrakitya, G. Overturf, and B. Portnoy. 1986. Serum transaminase elevations in infants with rotavirus gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 5:873-877. [DOI] [PubMed] [Google Scholar]
- 39.Kraft, L. M. 1958. Observations on the control and natural history of epidemic diarrhea of infant mice (EDIM). Yale J. Biol. Med. 31:121-137. [PMC free article] [PubMed] [Google Scholar]
- 40.Kraft, L. M. 1962. Two viruses causing diarrhea in infant mice, p.115-127. In R. J. C. Harris (ed.), The problems of laboratory animal disease. Academic Press, New York, N.Y.
- 41.Kraft, L. M. 1982. Viral diseases of the digestive system, p. 159-191. In H. L. Foster, J. G. Fox and D. J. Small (ed.), The mouse in biomedical research, vol. 2. Academic Press, New York, N.Y. [Google Scholar]
- 42.Loo, D. D. F., E. M. Wright, and T. Zeuthen. 2002. Water pumps. J. Physiol. 542:53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lopez, S., and C. F. Arias. 2003. Attachment and post-attachment receptors for rotavirus, p. 143-163. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis. Elsevier Science BV, Amsterdam, The Netherlands.
- 44.Lundgren, O., and L. Svensson. 2001. Pathogenesis of rotavirus diarrhea. Microbes Infect. 3:1145-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundgren, O., and L. Svensson. 2003. The enteric nervous system and infectious diarrhea, p. 51-68. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis. Elsevier Science BV, Amsterdam, The Netherlands.
- 46.Lundgren, O., A. Timar-Peregrin, K. Persson, S. Kordasti, I. Uhnoo, and L. Svensson. 2000. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287:491-495. [DOI] [PubMed] [Google Scholar]
- 47.Lynch, M., W.-J. Sheih, K. Tatti, J. R. Gentsch, T. Ferebee-Harris, B. Jiang, J. Guarner, J. S. Bresee, M. Greenwald, S. Cullen, H. D. Davies, C. Trevens, S. R. Zaki, and R. I. Glass. 2003. The pathology of rotavirus-associated deaths using new molecular diagnostics. Clin. Infect. Dis. 37:1327-1333. [DOI] [PubMed] [Google Scholar]
- 48.Lynch, M., B. Lee, P. Azimi, J. Gentsch, C. Glaser, S. Gilliam, H. G-H. Chang, R. Ward, and R. I. Glass. 2001. Rotavirus and central nervous system symptoms: cause or contaminant? Case reports and review. Clin. Infect. Dis. 33:932-938. [DOI] [PubMed] [Google Scholar]
- 49.Michelangeli, F., M. C. Ruiz, J. R. del Castillo, J. E. Ludert, and F. Liprandi. 1991. Effect of rotavirus infection on intracellular calcium homeostasis in cultured cells. Virology 181:520-527. [DOI] [PubMed] [Google Scholar]
- 50.Michelangeli, F., and M. C. Ruiz. 2003. Physiology and pathophysiology of the gut in relation to viral diarrhea, p. 23-50. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis. Elsevier Science BV, Amsterdam, The Netherlands. [DOI] [PMC free article] [PubMed]
- 51.Moon, H. W. 1994. Pathophysiology of viral diarrhea, p. 27-52. In A. Z. Kapikian (ed.), Viral infections of the gastrointestinal tract. Marcel Dekker, Inc., New York.
- 52.Morris, A. P., and M. K. Estes. 2001. Microbes and microbial toxins: paradigms for microbial-mucosal interactions. VIII. Pathological consequences of rotavirus infection and its enterotoxin. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G303-G310. [DOI] [PubMed] [Google Scholar]
- 53.Morris, A. P., J. K. Scott, J. M. Ball, C. Q. Zeng, W. K. O'Neal, and M. K. Estes. 1999. NSP4 elicits age-dependent diarrhea and Ca2+ mediated I− influx into intestinal crypts of CF mice. Am. J. Physiol. 277:G431-G444. [DOI] [PubMed] [Google Scholar]
- 54.Mossel, E. C., and R. F. Ramig. 2002. Rotavirus genome segment 7 (NSP3) is a determinant of extraintestinal spread in the neonatal mouse. J. Virol. 76:6502-6509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mossel, E. C., and R. F. Ramig. 2003. A lymphatic mechanism of rotavirus extraintestinal spread in the neonatal mouse. J. Virol. 77:12352-12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nishimura, S., H. Ushijima, and H. Shiraishi. 1993. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcriptions polymerase chain reaction. Brain Dev. 15:457-459. [DOI] [PubMed] [Google Scholar]
- 57.Offit, P. A., H. F. Clark, and R. L. Ward. 2003. Current state of development of human rotavirus vaccines, p. 345-356. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis. Elsevier Science BV, Amsterdam, The Netherlands.
- 58.Osborne, M. P., S. J. Haddon, A. J. Spencer, J. Collings, W. G. Starkey, T. S. Wallis, G. J. Clarke, K. J. Worton, D. C. Candy, and J. Stephen. 1988. An electron microscopic investigation of time-related changes in the intestine of neonatal mice infected with murine rotavirus. J. Pediat. Gastroenterol. Nutr. 7:236-248. [DOI] [PubMed] [Google Scholar]
- 59.Osborne, M. P., S. J. Haddon, K. J. Worton, A. J. Spencer, W. G. Starkey, D. Thornber, and J. Stephen. 1991. Rotavirus-induced changes in the microcirculation of intestinal villi of neonatal mice in relation to the induction and persistence of diarrhea. J. Pediatr. Gastroenterol. Nutr. 12:111-120. [DOI] [PubMed] [Google Scholar]
- 60.Parashar, U. M., E. G. Hummelman, J. S. Bresee, M. A. Miller, and R. I. Glass. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9:565-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez, J. F., M. E. Chemello, F. Liprandi, M. C. Ruiz, and F. Michelangeli. 1998. Oncosis in MA104 cells is induced by rotavirus infection through an increase in intracellular Ca2+ concentration. Virology 252:17-27. [DOI] [PubMed] [Google Scholar]
- 62.Poncet, D. 2003. Translation of rotavirus mRNAs in the infected cell, p. 185-205. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis. Elsevier Science BV, Amsterdam, The Netherlands.
- 63.Ramig, R. F., and K. L. Galle. 1990. Rotavirus genome segment 4 determines viral replication phenotype in cultured liver cells (HepG2). J. Virol. 64:1044-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riepenhoff-Talty, M., T. Dharakul, E. Kowalski, S. Michalak, and P. L. Ogra. 1987. Persistent rotavirus infection in mice with severe combined immunodeficiency. J. Virol. 61:3345-3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Riepenhoff-Talty, M., V. Gouvea, M. J. Evans, L. Svensson, E. Hoffenberg, R. J. Sokol, I. Uhnoo, S. J. Greenberg, K. Schakel, G. Zhaori, J. Fitzgerald, S. Chong, M. El-Yousef, A. Nemeth, M. Brown, D. Piccoli, J. Hyans, D. Ruffin, and T. Rossi. 1996. Detection of group C rotavirus in infants with extrahepatic biliary atresia. J. Infect. Dis. 174:8-15. [DOI] [PubMed] [Google Scholar]
- 66.Rollo, E. E., K. P. Kumar, N. C. Reich, J. Cohen, J. Angel, H. B. Greenberg, R. Sheth, J. Anderson, B. Oh, S. J. Hempson, E. R. Mackow, and R. D. Shaw. 1999. The epithelial cell response to rotavirus infection. J. Immunol. 163:4442-4452. [PubMed] [Google Scholar]
- 67.Ruiz, M., J. Cohen, and F. Michelangeli. 2000. Role of Ca2+ in the replication and pathogenesis of rotavirus and other viral infections. Cell Calcium 28:137-149. [DOI] [PubMed] [Google Scholar]
- 68.Shaw, R. D., S. J. Hempson, and E. R. Mackow. 1995. Rotavirus diarrhea is caused by nonreplicating viral particles. J. Virol. 69:5946-5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheth, R., J. Anderson, T. Sato, B. Oh, S. J. Hempson, E. Rollo, E. R. Mackow, and R. D. Shaw. 1996. Rotavirus stimulates IL-8 secretion from cultured epithelial cells. Virology 221:251-259. [DOI] [PubMed] [Google Scholar]
- 70.Starkey, W. G., J. Collins, T. S. Wallis, G. J. Clarke, A. J. Spencer, S. J. Haddon, M. P. Osborne, D. C. Candy, and J. Stephen. 1986. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J. Gen. Virol. 67:2625-2634. [DOI] [PubMed] [Google Scholar]
- 71.Steel, R. B., and A. Torres-Medina. 1984. Effects of environmental and dietary factors on human rotavirus infection in gnotobiotic piglets. Infect. Immun. 43:906-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tafazoli, F., C. Q. Zeng, M. K. Estes, K.-E. Magnusson, and L. Svensson. 2001. NSP4 enterotoxin of rotavirus induces paracellular leakage in polarized epithelial cells. J. Virol. 75:1540-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian, P., M. K. Estes, Y. Hu, J. M. Ball, C. Q-Y. Zeng, and W. P. Schilling. 1995. The rotavirus nonstructural glycoprotein NSP4 mobilizes Ca2+ from the endoplasmic reticulum. J. Virol. 69:5763-5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian, P., Y. Hu, W. P. Schilling, D. A. Lindsay, J. Eiden, and M. K. Estes. 1994. The nonstructural glycoprotein of rotavirus affects intracellular calcium levels. J. Virol. 68:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Uhnoo, I., M. Riepenhoff-Talty, T. Dharakul, P. Chegas, J. E. Fisher, H. B. Greenberg, and P. L. Ogra. 1990. Extramucosal spread and development of hepatitis in immunodeficient and normal mice infected with rhesus rotavirus. J. Virol. 64:361-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yolken, R. H., L. A. Peterson, S. L. Vondrfecht, E. T. Fouts, K. Midthun, and D. S. Newburg. 1992. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J. Clin. Investig. 90:1984-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang, M., C. Q.-Y. Zeng, A. P. Morris, and M. K. Estes. 2000. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells. J. Virol. 74:11663-11670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zijlstra, R. T., S. M. Donovan, J. Odle, H. B. Gelberg, B. W. Petschow, and H. R. Gaskins. 1997. Protein-energy malnutrition delays small-intestinal recovery in neonatal pigs infected with rotavirus. J. Nutr. 127:1118-1127. [DOI] [PubMed] [Google Scholar]
- 79.Zijlstra, R. T., B. A. McCracken, J. Odle, S. M. Donovan, H. B. Gelberg, B. W. Petschow, F. A. Zuckermann, and H. R. Gaskins. 1999. Malnutrition modified pig small intestinal inflammatory responses to rotavirus. J. Nutr. 129:838-843. [DOI] [PubMed] [Google Scholar]