Abstract
Avian leukosis virus (ALV) subgroup J is thought to have emerged through a recombination event between an unknown exogenous ALV and the endogenous retrovirus elements designated EAV-HP. All EAV-HP elements identified to date in the chicken genome show large deletions, including that of the entire pol gene. Here we report the identification of four segregating chicken EAV-HP proviruses with complete pol genes, one of which shows exceptionally high sequence identity and a close phylogenetic relationship with ALV-J with respect to the env gene. Embryonic expression of EAV-HP env has been suggested as a factor associated with immunological tolerance induction in a proportion of ALV-J-infected meat-type chickens. In support of this, env gene transcripts expressed from two of the four newly identified EAV-HP proviruses were demonstrated in chicken embryos. However, when ALV-J-infected outbred meat-type chickens were assessed, the presence of intact EAV-HP proviruses failed to directly correlate with ALV-J tolerance. This association was further examined using F2 progeny of two inbred lines of layer chicken that differed in EAV-HP status and immunological responses to ALV-J. Immunological tolerance developed in a small proportion of F2 progeny birds, reflecting the expected phenotypic ratio for inheritance of a double-recessive genotype; however, the status of tolerance did not show any direct correlation with the presence of the intact EAV-HP sequence. Nevertheless, identification of an intact chicken EAV-HP locus showing a uniquely close relationship to the ALV-J prototype clone HPRS-103 in the env region provides the strongest evidence of its contribution to the emergence of ALV-J by recombination.
The EAV-HP endogenous retroviruses are members of the ancient endogenous avian retrovirus (EAV) family identified in various species of the genus Gallus. The significance of EAV-HP elements was first recognized following the discovery of elements within the chicken genome with a high degree (>97%) of sequence identity to the env gene of the avian leukosis virus subgroup J (ALV-J), a new avian pathogen that emerged in the late 1980s (4). ALV-J was identified in the United Kingdom in 1988 as the causative agent of myeloid leukosis, which rapidly became a worldwide health and welfare problem in meat-type chickens (1, 21, 23, 24). The env genes of ALV subgroups A to E are closely related, sharing around 80 to 85% nucleotide sequence identity with each other; the ALV-J env is more distantly related, demonstrating only 40% identity to members of these other subgroups (4). The higher sequence similarity of the ALV-J env to that of EAV-HP suggested that ALV-J emerged as a result of a recombination event between EAV-HP transcripts and genomic RNA of an exogenous ALV (4, 35).
EAV-HP elements have a typical provirus structure consisting of 5′-long terminal repeat (LTR)-gag-pol-env-LTR-3′, and full-length EAV-HP proviruses have been detected in the Sonnerat's or gray jungle fowl (SJF; Gallus sonneratii) (31). In spite of the detection of such intact proviruses in the ancestral jungle fowl species, all EAV-HP proviruses identified to date in chickens show major deletions from the pol region that encodes the reverse transcriptase and integrase (IN), leaving the structural gag gene fused to the env sequences. While the deletion junctions in some of the proviruses give rise to in-frame gene fusions, most have single-base-pair insertions in or deletions from the gag region, preventing expression of any functional proteins. Southern blot hybridizations with env or LTR sequences have demonstrated that EAV-HP proviruses exist in the Gallus genus at approximately 10 to 15 copies per genome (30, 35). Studies on the distribution of three EAV-HP proviruses in several inbred layer-type chicken lines demonstrated that the loci were present only in some of the lines, suggesting that they are still segregating within the outbred layer-type chicken population from which these homozygous lines were derived (32). A single EAV-HP provirus termed ev/J clone 4-1, with a structure comprising a DNA copy of the subgenomic env transcript flanked by LTRs, from a white leghorn layer-type chicken line has been previously described (28). The ev/J 4-1 clone is the only chicken EAV-HP sequence described to date that provides the complete env gene sequence required to have served as a substrate for the generation of ALV-J by recombination. However, unlike what is expected of an ALV-J progenitor, the env sequence of this provirus shows a degree of similarity to ALV-J env that is no higher than that of any other EAV-HP provirus. Furthermore, the spliced subgenomic transcript structure makes it an unlikely candidate to be the progenitor sequence involved in the recombination event that led to the emergence ALV-J, since env transcripts are not packaged efficiently into avian retrovirus particles (5).
In addition to contributing to the emergence of ALV-J by recombination, EAV-HP elements are of interest because of their potential impact on adaptive immune responses of meat-type chickens to ALV-J infection (37). Ordinarily, infection of chicks with ALV at 1 day posthatch results in a transient viremia, with the infection subsequently cleared and accompanied by a neutralizing antibody response. In the case of ALV-J, a number of meat-type chicks infected posthatch by either natural exposure or experimental inoculation fail to induce a neutralizing antibody response and remain persistently viremic, although the numbers of birds developing this type of immunological tolerance to ALV-J infection in a flock can differ (1, 21, 37). Since the EAV-HP and ALV-J env sequences share a high degree of identity and since EAV-HP transcripts have been detected in chicken embryos, it has been postulated that the specific tolerance to ALV-J infection is due to the embryonic expression of the EAV-HP env peptides that share the epitopes with the ALV-J envelope glycoproteins that would lead to deletion or induction of anergy in ALV-J-responsive immune cells during ontogeny (21, 37). Some support for this hypothesis has been provided by the detection of ALV-J-specific antibody-forming cells without serum antibody in a portion of immunologically tolerant viremic meat-type chickens infected with ALV-J (29).
In this report, we describe the identification of intact EAV-HP provirus sequences that are segregating within the chicken population. Analysis of expression by reverse transcription (RT)-PCR showed that not all EAV-HP proviruses with complete env regions are expressed and capable of producing a spliced env transcript. One of these proviruses in which the env gene is expressed showed the closest sequence identity with the prototype ALV-J virus, HPRS-103. On the basis of sequence identity and phylogenetic analysis, we propose that this EAV-HP provirus is the endogenous element that contributed to the generation of the new ALV-J subgroup. However, in an experimental HPRS-103 virus infection model, this intact EAV-HP provirus did not show a direct correlation with the development of immunological tolerance, suggesting that the sequences contributing to the generation of ALV-J and the genetic determinants responsible for the induction of tolerance to ALV-J may be distinct.
MATERIALS AND METHODS
Chicken lines and cell culture.
Chicken lines 20 and 21 are outbred commercial meat-type chickens obtained from Aviagen Ltd. Specific pathogen-free layer-type chicken embryos were obtained from flocks maintained at the Institute for Animal Health and included inbred white leghorn lines 0, 15I, 6, and 7 (3) and a small closed population of brown leghorn chickens. For segregation analysis of ALV-J tolerance, inbred chicken lines 0 and 15I were crossed and F1 progeny were mated to produce F2 chicks that were used for viral challenge. Primary chicken embryo fibroblast cultures (CEF) prepared from 11-day-old embryos from chicken line 0, which lacks any endogenous ALV elements (2), were exclusively used for all the preparation of virus stocks and virological and serological assays. In addition, primary CEF prepared from 11-day-old embryos of various chicken lines were used for the isolation of RNA for subsequent RT-PCR assays.
Virus stocks and infection of chickens.
Stocks of ALV-J virus were prepared from tissue culture supernatants of CEF transfected with the prototype ALV-J clone HPRS-103 (4). For assessing the role of EAV-HP in ALV-J tolerance, 70 line 21 birds were infected at 1 day posthatch with 105 tissue culture infectious units of clone HPRS-103 by intra-abdominal injection of 100 μl of virus stock. Blood was collected from birds at 2-week intervals for 4 months for detection of virus and ALV-J-specific neutralizing antibodies and at one time point for isolation of DNA for PCR analysis. Virus isolation was carried out by inoculating 10-μl serum samples onto 4.5 × 104 CEF in duplicate wells of a 96-well tissue culture plate. The presence of virus in sera was monitored after 1 week by the use of enzyme-linked immunosorbent assay to assay culture supernatants for ALV p27 (34). Neutralizing antibodies were detected by microneutralization tests as previously described (22). Assays were carried out by preincubating duplicate samples of 10 μl of heat-inactivated serum with 100 tissue culture infectious units of HPRS-103 virus stock for 1 h under tissue culture incubation conditions (38.5°C, 5% CO2, 85% relative humidity) followed by infection of 4.5 × 104 CEF. Virus neutralization was determined using an ALV p27 enzyme-linked immunosorbent assay after 1 week.
In a second experiment, parental line 0 and line 15I birds (30 each) and F2 progeny (100 birds) were infected and monitored as described above. United Kingdom Home Office guidelines were followed for animal experimentation. All the experimental birds were subjected to postmortem examination for gross and microscopic lesions.
PCR amplification and cloning of EAV-HP provirus sequences.
Genomic DNA from 11-day-old chicken embryos was isolated as previously described (30). Detection of EAV-HP pol IN-coding sequences was conducted with two embryos from several different chicken lines by PCR using primers EAV-IN1 and 103ER or EAV-IN4 (Table 1) in PCR buffer (20 mM Tris-HCl [pH 8.4], 50 mM KCl, 0.25 mM deoxynucleoside triphosphates, 2 mM MgCl2) with 0.5 U of Taq Gold polymerase (BioGene Ltd., Kimbolton, United Kingdom). The thermocycling was performed using a Mastercycler gradient thermocycler (Eppendorf) programmed for 1 cycle of 94°C for 2 min and 35 cycles of 94°C for 30 s, 50°C for 15 s, and 72°C for 30 s followed by 1 cycle of 72°C for 5 min. Spliced subgenomic structures were detected by the same PCR performed with primers EAV-SD2 and 103ER or H8 (Table 1). Microsatellite mapping to determine major histocompatibility complex (MHC) haplotypes of parental and F2 layer-type birds was carried out by PCR using LEI0258 locus-specific primers (17).
TABLE 1.
Oligonucleotide primers used for PCR amplification of overlapping fragments of intact EAV-HP proviruses and RT-PCR detection of transcripts
| Assay and primer | Position (nucleotides)a | Sequence (5′-3′) |
|---|---|---|
| 5′-end PCR | ||
| EVJFOR | 172-194b | TTCGTGATTGGAGGAAACACTTG |
| ARTU5F | 367-384c | ACAGAGACTACATGCAAC |
| 103ER | 5508-5491d | CACGTTTCCTGGTTGTTG |
| 3′-end PCR | ||
| EAV-IN1 | 4385-4403 | TTCCCGCCCCAAATTAAGAC |
| EAVREV | 6786-6806 | TAAGTGAGCTCAAATGGCGTTTAT TGCTATAGGCTACG |
| RT-PCR | ||
| H3 | 5659-5679b | AACAACACCGATTTAGCCAGC |
| H8 | 6019-5996b | TGGTGAATCCACAATATCTACGAC |
| EAV-SD2 | 291-208, 4894-4899 | ATGGACCAAGTCATTAAGGAAATG |
| EAV-IN4 | 4819-4800 | ATACAACCCTTCCCGATTCC |
| Anchor | NAe | GACCACGCGTATCGATATCGAC |
Primer position is given relative to prototype intact SJF provirus EAV-JF2 (accession number AJ292967) unless otherwise indicated.
Primers position is given relative to provirus clone EAV-HP1 (accession number AJ238124).
Primer sequence was derived from ART-CH clone LTR sequence (accession number L25262).
Primer position is given relative to prototype ALV-J clone HPRS-103 (accession number Z46390).
NA, not applicable.
Overlapping PCR products representing the proviral genome were amplified using the strategy previously described for cloning of intact EAV-HP elements from SJF (31). Oligonucleotide pairs used were specific for priming the LTR along with the IN-coding or env 5′ regions that are deleted from the predominant EAV-HP provirus types, allowing selective amplification of proviruses with intact pol genes (Table 1). PCR was performed on 100-ng genomic DNA samples by the use of 2.5 units Herculase Hotstart DNA polymerase (Stratagene Europe, Amsterdam, The Netherlands) and 4% dimethyl sulfoxide in 50-μl reaction mixtures as described by the manufacturer. Amplification was performed using a Mastercycler gradient thermocycler and the following program: 1 cycle of 95°C for 2 min and 35 cycles of 95°C for 15 s, 50°C for 30 s, and 72°C for 5 min followed by one cycle of 72°C for 5 min. PCR products were purified from proof-reading enzyme and primers by agarose gel separation and extraction from gel slices with a QIAquick gel extraction kit (QIAGEN Ltd., Crawley, United Kingdom) as described by the manufacturer. PCR products were A-tailed in A-tailing reaction buffer (20 mM Tris-HCl [pH 8.4], 50 mM KCl, 0.25 mM dATP, 2 mM MgCl2) with 0.5 U of Taq Gold polymerase (BioGene) for 20 min at 72°C for subsequent ligation into pGEM-T Easy vector (Promega UK, Southampton, United Kingdom) following manufacturers' instructions. Ligation reactions were transformed into Escherichia coli strain XL1-Blue (Stratagene), and inserts were sequenced using an Applied Biosystems 377 automated sequencing system as previously described (31). Sequence contigs were assembled using Pregap4 and Gap4 in the Staden 2002 package (8, 36). Pairwise comparisons and sequence alignments were performed using Wisconsin package version 10 (Genetics Computer Group, Madison, Wisconsin) or the BioEdit Sequence Alignment Editor, version 5.0.9 (14). Phylogenetic analysis was performed using DNAML, SEQBOOT, and CONSENSE in the PHYLIP package, version 3.5 (12).
Detection of EAV-HP transcripts by RT-PCR.
For detection of EAV-HP transcripts, RNA was isolated from primary CEF from different chicken lines by use of an RNeasy Mini kit (QIAGEN). Eluted RNA samples were digested using DNA-free DNase treatment and removal reagent (Ambion [Europe] Ltd., Huntingdon, United Kingdom). RT was performed following denaturation of 5 μg of total RNA at 65°C for 10 min and chilling immediately on ice with 30 pmol of oligo(dT) anchor primer (5′-GACCACGCGTATCGATATCGACTTTTTTTTTTTTTTTV-3′)-10 pmol of deoxynucleoside triphosphates. RNA was reverse transcribed at 42°C for 50 min using 15 U of Superscript RT II (Invitrogen Ltd., Paisley, United Kingdom) in 20-μl reaction volumes adjusted to 50 mM Tris-HCl (pH 8.3)-75 mM KCl-3 mM MgCl2-10 mM dithiothreitol by use of the 5× first-strand buffer and dithiothreitol supplied. RT reactions were stopped, and RNA:DNA hybrids were denatured by heating to 96°C for 10 min and immediately cooling on ice. PCR was performed in 20-μl reaction mixtures on 1 μl of RT reaction mixtures with the EAV-HP primers (Table 1) as described above for amplification of provirus sequences. Control reactions were performed for each RNA sample alongside RT reactions without the addition of Superscript RT II enzyme to allow for detection of contaminating DNA in any PCRs. For amplification of the spliced subgenomic transcript, which is estimated to be present at approximately 5% of the genomic transcript levels (19), nested PCR was performed using the EAV-SD2 and anchor primers, followed by amplification with EAV-SD2 and H8 primers. Primer EAV-SD2 spans the splice junction by including the first six gag gene codons and the first six nucleotides of the env gene.
Nucleotide sequence accession numbers.
The EMBL accession numbers for the sequences assembled from the overlapping PCR clones described here are AJ623289 (EAV-15I), AJ623290 (EAV-21-3), AJ623291 (EAV-21-1), and AJ623292 (EAV-6-3).
RESULTS
EAV-HP proviruses with pol sequences and complete env genes are segregating in chickens.
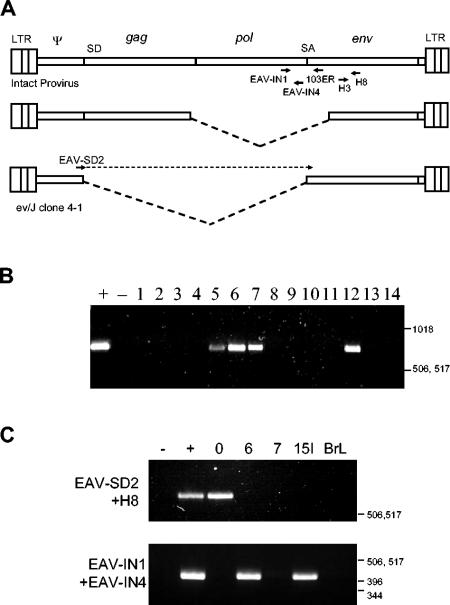
In our previous attempts to characterize EAV-HP proviruses from various chicken lines, particularly from meat-type chicken lines in which ALV-J may have arisen, we were unable to identify viruses that had intact provirus structures (5′-LTR-gag-pol-env-LTR-3′). Subsequent studies to find out the distribution of three specific provirus deletion loci revealed that these elements were segregating within the chicken population (32). This suggested that more-intact genomes may have eluded detection due to the chance selection of embryos from the outbred meat-type chicken lines. PCR was initiated to detect EAV-HP proviruses with the pol-env junction, a region that is deleted from all the chicken EAV-HP genomes previously identified, to determine whether more complete EAV-HP elements were segregating (Fig. 1A). Primer EAV-IN1, which was used in all PCRs including the EAV-HP IN region, was designed for pol sequences 508 bp 5′ to the splice acceptor site and 596 bp upstream of the EAV-HP 5′-end recombination point proposed for the recombination event that generated ALV-J (28). An examination of 14 individual line 21 embryos demonstrated that 4 birds were positive for the pol-env junction (Fig. 1B). A similar survey of embryos from another commercial breeding company also detected these sequences in 18 out of 21 embryos examined (data not shown).
FIG. 1.
Segregation of EAV-HP proviruses with complete env genes in the chicken population. (A) Schematic representation of PCR strategy used to detect env and pol IN-coding sequences or subgenomic transcript structures. Primers EAV-IN1 and 103ER are specific for pol and env gene sequences of the intact provirus, respectively, which reside in the region deleted from the predominant deleted provirus forms, as represented by the dashed lines of the middle genome (upper diagram). The lower diagram represents the location of the specific primer for amplification of the spliced subgenomic transcript provirus clone ev/J 4-1 or mRNA that spans the splice junction. Positions of the subgenomic transcript splice donor (SD) and splice acceptor (SA) are indicated. (B) PCR detection of pol-env junctions in meat-type chicken line 21. Test DNAs amplified by primers EAV-IN1 and 103ER were obtained from 14 line 21 embryos (lanes 1 to 14), with SJF clone EAV-JF2 and water used for positive and negative controls, respectively. (C) PCR detection of IN sequences in inbred layer lines. PCR was used to amplify segregating proviruses from line 0, 6, 7, and 15I chickens and from brown leghorns (BrL) (upper panel). The same controls were used as described for panel B for amplification of the pol-env junction (lower panel). Water and a previously cloned fragment amplified from the spliced subgenomic transcript provirus in red jungle fowl were used as negative (−) and positive (+) controls, respectively, for PCR with the EAV-SD2 and H8 primer pair. Positions of a 1-kb DNA ladder (Invitrogen) are indicated in base pairs.
Since the commercial meat-type chicken lines are outbred, they are not amenable to genetic studies designed to examine the interactions between ALV-J and endogenous retroviruses. To find more suitable birds for investigation of the potential of intact EAV-HP proviruses to cause immunological tolerance to ALV-J, chickens of inbred layer-type chicken lines that were not previously examined (lines 6, 7, and 15I) as well as brown leghorns were subjected to similar PCR tests. Line 0 chicken DNA, which previously tested negative by hybridization to a pol reverse transcriptase region probe (31), was also included for further analysis, as this line harbors the spliced subgenomic transcript provirus designated ev/J clone 4-1 (28, 32). Expression of this locus has not been examined, and the presence of this provirus may complicate analysis of the expression of spliced subgenomic transcripts derived from intact proviruses. Sequences encoding the EAV-HP IN gene up to a position 75 bp before the splice acceptor site were amplified from two layer lines, 6 and 15I (Fig. 1C), with primers EAV-IN1 and EAV-IN4. The ev/J clone 4-1 genome was detected in line 0 (Fig. 1C) and is segregating in the noninbred brown leghorn population (data not shown).
EAV-HP subgenomic transcripts are expressed in some chicken lines.
We have previously postulated that immunological tolerance to ALV-J results from the expression of envelope glycoproteins with epitopes shared between EAV-HP and ALV-J during embryonic development. To establish whether functional env expression occurs in embryonic cells, RNA was isolated from CEF for detection of the spliced subgenomic transcripts encoding the envelope glycoproteins. Since EAV-HP transcripts are not expressed at sufficient levels to be detected by Northern blot hybridization, a PCR strategy was used which allowed for specific amplification across the splice junction, as performed for detection of the spliced provirus genome (Fig. 1A). Previous examinations of EAV-HP expression have used RT-PCR to detect env sequences that are shared with all the EAV-HP provirus structures; however, this approach is not able to discriminate between spliced env and genomic transcripts expressed in chicken lines with many different provirus sequences.
Initial expression analysis was conducted on RNA samples isolated from meat-type line 21 chicken cells derived from embryos that were positive or negative by PCR for the IN sequence upstream of splice acceptor site or for the spliced subgenomic provirus (4-1), as indicated (Fig. 2A). PCR was performed on RT reactions that were set up in parallel with and without addition of reverse transcriptase enzyme to detect contaminating genomic DNA. PCR was carried out with primers H3 and H8 that amplify env sequences found in all the hitherto-known EAV-HP proviruses to demonstrate the integrity of the RNA used and that the RT reaction was successful (Fig. 2A, top panel). Nested PCR performed with the EAV-SD2 and anchor primer followed by EAV-SD2 with H8 amplified the spliced transcript from only one of the three CEF samples with provirus sequences that carried the spliced transcript genome and/or the IN-coding sequences.
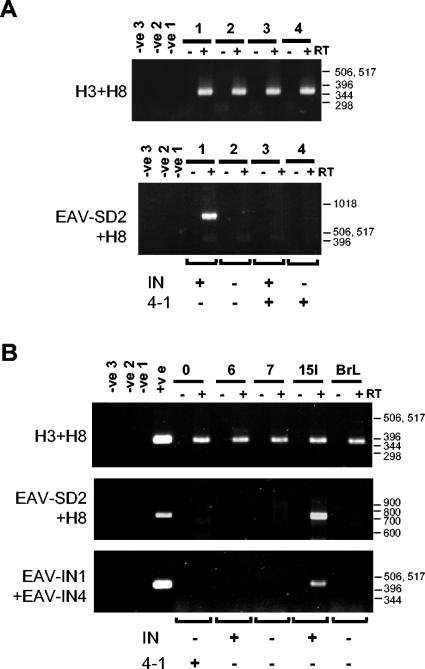
FIG. 2.
Expression of EAV-HP env subgenomic transcripts in CEF from a subset of EAV-HP proviruses with IN sequences and complete env genes. (A) RT-PCR detection of EAV-HP transcripts in primary CEF from four line 21 embryos. Total RNA was used for RT with Superscript RT II (+), with duplicate reactions set up without Superscript (−) to ensure detection of contaminating DNA. A general EAV-HP env PCR (H3+H8) was performed on the same RT reaction mixtures to verify for cDNA synthesis. Detection of the spliced env transcript was performed by nested PCR with primer EAV-SD2 and an anchor primer followed by EAV-SD2 with H8 (EAV-SD2+H8). Negative controls correspond to replicated reactions set up with water instead of RNA template for the RT (-ve1) or instead of DNA template for the first (-ve2) and second (-ve3) rounds of PCR. PCR typing of DNA from the same CEF samples for intact IN gene or the spliced env provirus (4-1) is indicated below the lower panel. (B) RT-PCR detection of env subgenomic transcripts in layer line 15I. RT-PCR was analyzed as described above on total RNA isolated from chicken embryos from five different layer lines, with DNA isolated from the same CEF for detection of the intact (IN) or the spliced (4-1) env gene. A third RT-PCR was performed on the same cDNA by use of primers EAV-IN1 and EAV-IN4 to detect genomic transcripts expressed from the EAV-HP proviruses whose pol-env junctions are preserved because the EAV-SD2 complementary region is deleted from the provirus of interest in line 6 chickens (Fig. 3). Negative controls were as described above, and positive controls were DNA samples from line 0 (top and middle panels) or line 15I (bottom panel) chickens. Positions of a 1-kb DNA ladder (Invitrogen) or 100-bp DNA ladder (Invitrogen) are indicated in base pairs.
Similar RT-PCR analysis was later performed on the layer lines whose results are presented in Fig. 1C to determine whether spliced env transcripts were expressed from the line 0 spliced provirus or from the line 6 or 15I EAV-HP proviruses with IN sequences detected. As also seen with the line 21 CEF, transcripts from the spliced genome provirus could not be detected in the line 0 CEF (Fig. 2B). Mirroring the expression pattern results of the line 21 proviruses with IN sequences, subgenomic transcripts could be amplified from line 15I but not from line 6 CEF.
Cloning of four distinct EAV-HP proviruses with complete pol and env genes.
DNAs from layer chicken lines 6 and 15I, as well as DNAs from line 21 CEF 1 and 3 (21-1 and 21-3), were examined to further characterize the proviruses detected as described above. Analysis of the expression suggested that there could be only two different proviruses in the different chicken lines, only one of which is expressed. Alternatively, each of the chickens examined with IN-coding sequences may harbor distinct proviruses. Overlapping PCR products were produced from DNA from each of the four chickens by use of a forward LTR primer (EVJFOR) in combination with reverse env primer 103ER to obtain the 5′-end sequence and a reverse LTR primer (EAVREV) with forward IN primer EAV-IN1 for amplification of the 3′ ends. The 3′-end PCR amplified an approximately 2.4-kbp product from all four DNA samples. The 5′-end PCR products amplified from line 15I and embryo 21-3 were approximately 5.2 kbp in size, the size expected for an intact EAV-HP provirus. PCR from the line 6 embryo (embryo 6-3) generated a smaller product of approximately 3.4 kbp. This primer pair failed to amplify the EAV-HP provirus from embryo 21-1, but the 5′ end could be amplified using a forward LTR primer directed against the EAV-HP U5 region sequence (ARTU5F) that is shared with related EAV sequences previously named ART-CH instead of the U3 region sequence that is unique to EAV-HP.
The PCR products generated from the four chicken genomes were cloned, sequenced, and assembled for comparison and analysis of the genome structures. Assembled sequences were designated EAV-15I, EAV-6-3, and EAV-21-1 and EAV-21-3 to represent proviruses amplified from line 15I, line 6 embryo 3, and line 21 embryos 1 and 3, respectively. A diagram showing the overall structure of the proviruses in comparison to those of the intact EAV-HP from SJF described previously (30) and the subgenomic transcript provirus ev/J 4-1 is shown in Fig. 3. The smaller size of the EAV-6-3 5′ end is the result of a 1,749-bp deletion spanning the entire 5′-untranslated region (UTR) and most of the gag gene, leaving only 416 bp of protease coding sequence that overlaps the primer-binding site. While this deletion would render the provirus nonfunctional for expression of any gag-encoded sequences as well as rendering the env subgenomic transcript nonfunctional for envelope glycoprotein production, this provirus could potentially encode env sequences that were involved in the generation of ALV-J if it were expressed as a genomic transcript and randomly packaged into ALV particles despite the absence of a packaging signal. Since the deleted region includes the EAV-SD2 primer sequence at the splice donor, the RT-PCR analysis attempted with primers EAV-SD2 and 103ER could not amplify transcripts from this provirus. To determine whether this provirus is expressed, RT-PCR was conducted using primers for the IN region (EAV-IN1 and EAV-IN4). While this primer pair amplified the EAV-15I genomic RNA as expected, it failed to amplify the EAV-6-3 genomic RNA, indicating that this provirus is not expressed (Fig. 2B, lower panel).
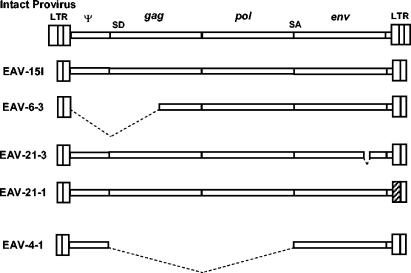
FIG. 3.
Schematic diagram showing the structures of the four chicken EAV-HP proviruses with complete pol and env genes. Diagrams depict the structures from assembled overlapping PCR fragments compared to the intact provirus previously described from SJF (top diagram) and the spliced subgenomic provirus, ev/J clone 4-1 (bottom diagram). Dashed lines indicate regions deleted from the defective proviruses, while the hatched area in EAV-21-1 shows the U3 region with a higher degree of sequence identity to the EAV-E51 clone than to the EAV-HP LTR region. SD, splice donor; SA, splice acceptor.
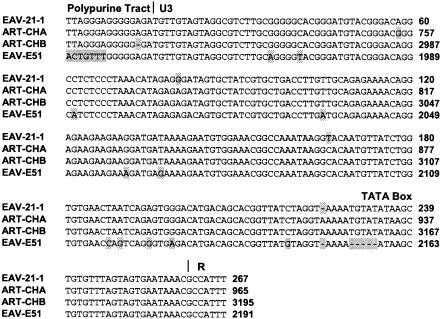
Sequence analysis of the 5′ ends of the PCR amplified proviruses revealed that the EAV-15I and the EAV-21-3 provirus sequences have complete and in-frame gag and pol genes. The EAV-21-1 provirus has a complete and in-frame pol gene, but the gag gene is disrupted by a single-base-pair deletion at a position three codons downstream of the conserved protease catalytic site DSG motif. Translation of the 3′ ends demonstrated that the proviruses EAV-15I, EAV-6-3, and EAV-21-1 have complete env genes with continuous open reading frames. A 30-bp deletion was found to have occurred in the EAV-21-3 env gene in the surface protein region of the gene that maintained the translational reading frame. Comparison of the EAV-HP clones with each other and with the prototype intact provirus sequence from SJF (EAV-JF2) indicated that the sequences are >97% identical in all regions, with the exception of EAV-21-1 retroviral unique 3′ (U3) region. The EAV-21-1 U3 sequence showed a high degree (98%) of identity with the related endogenous retrovirus EAV-E51-like sequences in ART-CH and EAV-E51 clones (Fig. 4).
FIG. 4.
Nucleotide sequence alignment of the U3 regions of the EAV-21-1- and the EAV-E51-related endogenous retrovirus LTRs. DNA sequences, including that of the polypurine tract, are shown aligned to the portion of the repeat (R) region included in the EAVREV primer used to amplify the EAV-21-1 clone. Divergent residues among the four sequences are indicated by shading.
Identification of the putative ALV-J env progenitor sequence.
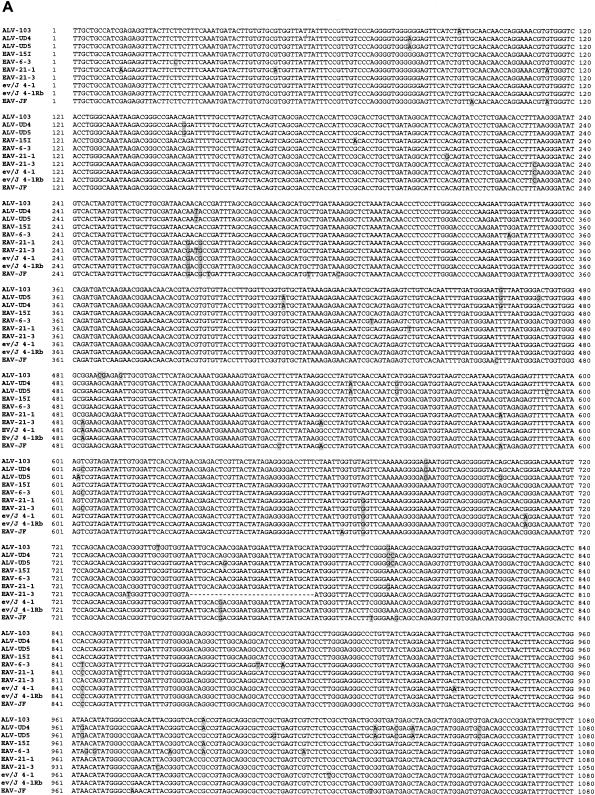
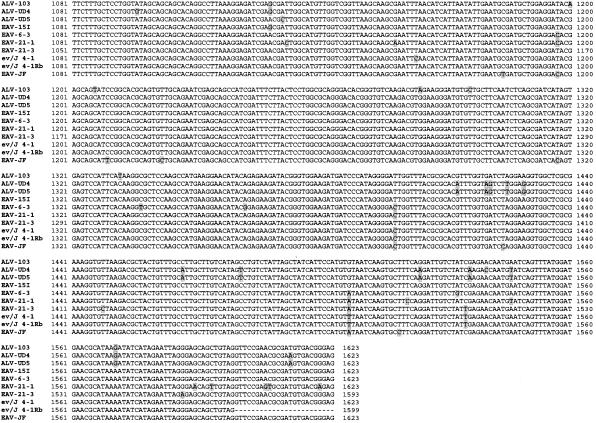
Comparison of the EAV-HP env sequences with those of the ALV-J prototype clone HPRS-103 was subsequently performed to obtain evidence of whether one of the proviruses identified may have contributed to the origin of the ALV-J subgroup. Clones EAV-6-3, EAV-21-1, and EAV-21-3 shared >97% sequence identity with the HPRS-103 env gene, a degree of identity similar to that previously shown (7, 28, 31, 35). Remarkably, clone EAV-15I demonstrated >99% sequence identity to the HPRS-103 env gene, the highest so far for any of the EAV-HP env sequences (Fig. 5A).
FIG. 5.
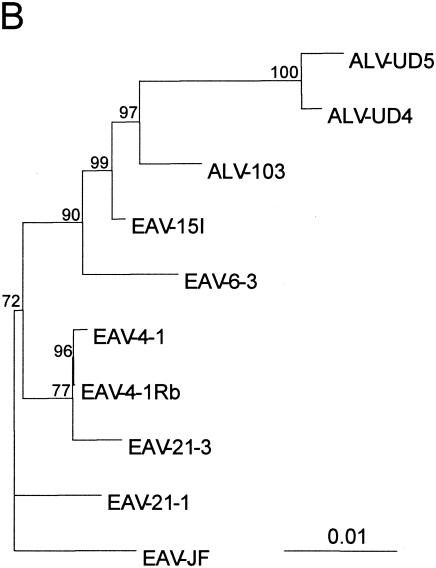
Sequence comparison and phylogenetic analysis of the relationships between EAV-HP proviruses with intact env genes and ALV-J isolates. (A) Sequence comparison of EAV-HP and ALV-J env genes. Sequences used for alignment spanned the region of the env gene from the putative 5′-end recombination site involved in generation of the ALV-J subgroup to the first 24 bp of the 3′ UTR (HPRS-103 nucleotides 5395 to 7017), with the exception of that of ev/J 4-1Rb, for which no UTR sequence was available. The sequence alignment was generated using the BioEdit Sequence Alignment Editor, version 5.0.9 (14), and divergent residues are indicated by shading. (B) Phylogenetic analysis of EAV-HP and ALV-J env sequences. The unrooted tree was generated by maximum-likelihood analysis performed using the SEQBOOT, DNAML, and CONSENSE programs in the PHYLIP package, version 3.5 (12) on the sequence alignment described above. Branch lengths are proportional to genetic divergence, with the scale bar shown to indicate 0.01 nucleotide substitutions per site. Numbers at the nodes indicate the percentages of bootstrap values obtained from 500 replicates of the data, shown for values over 50%. ALV-J sequences used for alignment were derived from prototype clone HPRS-103 (ALV-103; accession number Z46390), isolate UD4 (ALV-UD4; accession number AF307951), and isolate UD5 (ALV-UD5; accession number AF307952). Additional EAV-HP sequences used in the analysis were the SJF clone EAV-JF2 (accession number AJ292967), the original ev/J clone 4-1 (accession number AY157601), and the more recently published ev/J clone 4-1Rb (accession number AY157601).
The relationship between the EAV-15I env and ALV-J was further investigated by phylogenetic analysis using the maximum-likelihood method to construct a phylogenetic tree of the intact EAV-HP and ALV-J env genes (Fig. 5B). Sequences used for alignment spanned the env region, beginning with the putative ALV-J recombination site and 24 bp of the 3′ UTR shared among three ALV-J clones and the EAV-HP proviruses (1,623 bp), except for the sequence for an EAV-HP clone designated ev/J 4-1Rb, a recently published sequence variant of the ev/J 4-1 clone (11), which ends at the stop codon (1,599 bp). ALV-J sequences derived from United States isolates UD4 (ALV-UD4) and UD5 (ALV-UD5) clustered with the HPRS-103 (ALV-103) clone derived from the earlier United Kingdom ALV-J isolate and with the EAV-15I sequence, with strong bootstrap support for a common ancestor (Fig. 5B). Our own pair-wise analysis demonstrated that the original EAV-HP sequence designated clone ev/J 4-1 (28), which was amplified from the DF-1 cell line derived from endogenous ALV-free line 0 (15), differs from the ev/J 4-1Rb sequence variant amplified from a meat-type chicken line at only three different residues (data not shown), despite Denesvre et al. having indicated four codon differences (11). We also found that the published sequence of a 1,518-bp PCR product amplified from endogenous ALV-J sequences from line 0 chicken embryo DNA (accession number AF247392) (33) is 100% identical to the corresponding sequence in the ev/J 4-1Rb variant sequence (data not shown). These observations strongly suggest that the three clones represent the same provirus locus and that the nucleotide differences arose during PCR amplification or cloning.
Tolerance to ALV-J does not show correlation with the intact EAV-HP locus in line 15I.
Our working hypothesis that EAV-HP env gene expression during embryogenesis is associated with the phenomenon of immunological tolerance to ALV-J was first tested in line 21 birds. The HPRS-103 prototype virus was used to challenge 70 birds by intraperitoneal injection at 1 day posthatch. DNA was isolated from blood samples collected at the time of infection to detect the presence of EAV-HP proviruses with PCR primers for IN sequences (EAV-IN1 and EAV-IN4) or for the spliced env provirus (EAV-SD2 and 103ER). These two PCR tests revealed that over 50% of the birds were positive for each of the two provirus types. These two proviruses segregated independently, however, as evidenced by identification of birds that had no proviruses or had one or both proviruses (Table 2).
TABLE 2.
Analysis of intact EAV-HP distribution and immunological tolerance development in line 21 chickens injected with ALV-J at 1 day posthatch after 4 months
| No. of birds (n = 61) | Result
|
||||
|---|---|---|---|---|---|
| ev/J 4-1 PCR (n = 34) | EAV-HP IN PCR (n = 37) | No. of Ab− V+ (n = 33)a | No. of Ab+ V− (n = 28)a | No. of tumors (n = 7) | |
| 9 | − | − | 4 | 5 | 1 |
| 18 | − | + | 9 | 9 | 1 |
| 15 | + | − | 8 | 7 | 1 |
| 19 | + | + | 12 | 7 | 4 |
Ab−V+, neutralizing antibody is negative, and virus is positive; Ab+V−, neutralizing antibody is positive, and virus is negative.
The development of an immune response and recovery from ALV-J was determined by detection of ALV-J-neutralizing antibodies and the inability to isolate virus from blood serum. Due to development of symptoms unrelated to the virus infection, nine birds were excluded from the experiment before data could be gathered. Of the surviving 61 birds studied until the termination of the experiment, 33 remained immunologically tolerant to ALV-J while 28 were able to mount an adaptive immune response in the form of neutralizing antibodies and clear the virus (Table 2). A small proportion (7 out of 33) of immunologically tolerant birds also developed tumors before the termination of the experiment. The development of immunological tolerance or tumors in the line 21 birds failed to correlate with the presence of intact EAV-HP proviruses represented by clones EAV-21-1 and EAV-21-3, as revealed in particular by the identification of persistently viremic birds with genomes that were negative by PCR for the pol gene IN region.
In the absence of a direct correlation between the presence of specific EAV-HP loci and tolerance to ALV-J infection, further characterization to identify the line 21 birds harboring the expressed EAV-HP proviruses with complete pol and env genes was not carried out since the outbred nature of these birds would make further genetic analysis difficult. Instead, two inbred layer chicken lines were used to generate F2 progeny that could be tested for segregation of the immunological tolerance phenotype. Line 15I was chosen as one of the parental lines, since it carries the intact provirus that is transcribed and spliced and has a putatively functional env gene that is the most likely endogenous progenitor of the ALV-J env sequences. The selection of this line for genetic analysis of tolerance to ALV-J was also strongly supported by the results of earlier studies in which line 15I birds infected with ALV-J at 1 day posthatch had detectable ALV Gag protein expressed in various tissues after 4 weeks, demonstrating an ALV-J virus tropism similar to that of the line 21 meat-type chickens (1). Furthermore, line 15I has been selected for susceptibility to Marek's disease and lymphoid leukosis by ALVs (3). The second line used (line 0) was chosen for the absence of proviruses that express the spliced env transcript, as observed by RT-PCR (see above). This line was bred to select for the absence of endogenous ALV-E loci (2) and has been shown to be highly susceptible to ALV-J infection (25).
As previously observed, ALV-J challenge of parental line 0 birds at 1 day posthatch resulted in an immune response and clearing of virus (Table 3). After 5 months, 100% of the line 15I parental birds were persistently viremic, with no detectable neutralizing antibodies. Because of the homozygous nature of the parental chicken lines, the expected number of birds developing immunological tolerance to ALV-J in the F2 progeny would be 3:1 if the presence of an endogenous retrovirus from one of the parental lines were the determinant, when expressed as a dominant trait. Since defective EAV-HP proviruses may encode peptides that could induce immunological tolerance to ALV-J when the required epitopes are expressed during development of immune cell repertoire, these elements were also considered along with the EAV-15I provirus. When the F2 progeny were examined, only 7 of the 85 birds tested were immunologically tolerant to ALV-J, as indicated by the isolation of virus and absence of neutralizing antibodies (Table 3). This tolerance also failed to correlate with the presence of the intact EAV-HP provirus detected by PCR and was not restricted by the MHC (or B complex) haplotype, since immunologically tolerant birds were heterozygous or homozygous for either of the parental MHC haplotypes. The low proportion of birds developing tolerance in the layer-type chicken F2 progeny indicates that tolerance is not likely caused by inheritance of a single dominant element, such as an expressed intact or defective EAV-HP provirus with ALV-J epitopes. Interestingly, the number of birds developing tolerance fit closely to the 15:1 phenotypic ratio required for tolerance to be manifested only in the progeny of a dihybrid cross with both genes homozygous and recessive. Chi-square testing on these data showed no statistically significant difference (P < 0.05) between the observed and expected numbers of birds for immunological tolerance to correlate with a genotype with both genes recessive. None of the immunologically tolerant F2 or line 15I parental birds developed tumors, as determined by postmortem examination for gross lesions or by microscopic inspection of histologically stained tissue sections (data not shown).
TABLE 3.
Analysis of intact EAV-HP distribution and immunological tolerance development in line 0, line 151, and F2 progeny challenged with ALV-J at 1 day posthatch
| Line or generation (no. of birds) and MHC haplotype | EAV-HP IN PCR | No. Ab− V+ (n = 7)a | No. Ab+ V− (n = 78)a |
|---|---|---|---|
| Line | |||
| 0 (19) | |||
| B2B2 | − | 0 | 19 |
| 151 (28) | |||
| B15B15 | + | 28 | 0 |
| Generation | |||
| F2 (85) | |||
| B15B15 | − | 2 | 13 |
| B15B15 | + | 0 | 4 |
| B2B2 | − | 1 | 15 |
| B2B2 | + | 0 | 0 |
| B2B15 | − | 4 | 44 |
| B2B15 | + | 0 | 2 |
Ab− V+, neutralizing antibody is negative, and virus is positive; Ab+ V−, neutralizing antibody is positive, and virus is negative.
DISCUSSION
The emergence of ALV-J has resulted in the identification of a previously unknown group of endogenous retroviruses designated EAV-HP that appear to have contributed to its origin. Starting from the initial attempts to characterize the EAV-HP endogenous retroviruses, an important aim of the research has been to identify a provirus that could have contributed to the generation of the ALV-J subgroup by recombination. Until now, all EAV-HP proviruses characterized from chickens showed large deletions that rendered them replication defective and none of the clones showed a degree of identity to ALV-J that would unequivocally distinguish it as the source of the ALV-J env sequences. The two previously reported clones, designated ev/J 4-1 and ev/J 4-1Rb, representing the EAV-HP provirus locus with the subgenomic transcript structure had demonstrated the closest similarity to the ALV-J env, due to its approximately 98% identical env gene sequence remaining intact at the putative ALV-J recombination point (11, 28). However, this provirus appears to be silent since transcripts are not detectable by RT-PCR, making it an unlikely candidate of the source of ALV-J env sequences. The EAV-HP provirus found in line 15I chickens (EAV-15I) has a remarkably high degree of sequence identity with the ALV-J prototype HPRS-103 env gene and represents the first intact and expressed provirus that could have contributed sequences to the recently emerged J subgroup viruses.
It is possible that proviruses that were not expressed in embryonic cells may be transcribed in other chicken cell types where the recombination event that generated the ALV-J subgroup occurred. However, the relationship between EAV-15I and HPRS-103 was further supported by phylogenetic analysis of the env genes of the four proviruses described here and three ALV-J clone sequences. The sequence of the ALV-J prototype clone HPRS-103 was generated from an early United Kingdom isolate of ALV-J in meat-type chickens and logically should be more closely related to the original ALV-J generated by recombination than the sequences derived from more recent American isolates (UD4 and UD5). By this assumption, the HPRS-103 env should also be more closely related to the endogenous elements from which the env was derived. This is indeed reflected by the phylogenetic trees generated, as the ALV-J clones appear to descend from a common ancestor of EAV-15I, with ALV-J clones UD4 and UD5 being more distantly related than the HPRS-103 sequence. The branch length for EAV-15I from the common ancestor it shares with the ALV-J sequences is nearly zero, further supporting the idea that EAV-15I is the putative endogenous retrovirus coprogenitor of ALV-J. However, compared to this, the silent EAV-HP locus represented by ev/J 4-1 clones was more distantly related, reinforcing the possibility that this element was unlikely to be involved in the emergence of ALV-J.
Recently, envelope proteins expressed from an EAV-HP env gene derived from the variant ev/J 4-1Rb clone under the control of a strong viral promoter were shown to interfere with exogenous ALV-J in vitro (11). While this interference is not biologically relevant, since the clone corresponds to a provirus that is not expressed as a transcript that is detectable by RT-PCR, this study indicates that EAV-HP env genes encode sequences that could function in ALV-J receptor interference. We have previously shown by reporter gene assays that the EAV-HP LTR functions as a weak promoter manifested by the low level of EAV-HP transcript accumulation detectable only by RT-PCR (28, 30). Furthermore, the ability of ALV-J to infect line 15I and meat-type birds clearly demonstrates that the low-level expression of putatively functional EAV-HP env subgenomic transcripts from the native promoter is insufficient to interfere with ALV-J in vivo.
PCR amplification of EAV-HP sequences with complete pol and env genes identified a provirus in line 21 chickens that had a U3 region of the LTR that was more similar to this region in endogenous retroviruses described as ART-CH or EAV-E51 (9, 13, 20). ART-CH has previously been shown to share a stretch of gag gene sequences with EAV-HP (28, 30). Recombination between EAV-HP and other EAV family endogenous retroviruses and subsequent integration into germ line cells appears to have occurred frequently for these chimeric genomes to be inherited. A more extensive analysis of the relationship between EAV-HP, other EAV family members, and their chimeric proviruses will be presented elsewhere.
To examine the potential correlation between an intact EAV-HP provirus and tolerance to ALV-J, two inbred layer lines that differed in their ability to respond to ALV-J infection in newly hatched chicks (1) were mated, and the F1 progeny were used for a dihybrid cross. If the observed immunological tolerance were due to expression of the endogenous retrovirus as a dominant trait from one inbred parental line, it would be expected to appear in 75% of the F2 progeny. Interestingly, when the F2 progeny were challenged, tolerance to ALV-J developed only in a small proportion of birds. This small proportion (approximately 8%) is suggestive of a genotype with both genes recessive and provides the first indication that ALV-J tolerance might be due to two unlinked yet interacting gene products. This result contradicts the previous hypothesis that tolerance develops from the expression of EAV-HP env peptides that provide epitopes for MHC presentation that are shared with ALV-J during lymphocyte negative selection (30-32, 37).
From a comparison of the two animal experiments described here, the development of immunological tolerance and development of the myeloid leukosis tumors typically induced by ALV-J appear to be determined by distinct genetic elements. None of the immunologically tolerant line 15I layer birds developed tumors throughout the duration of the experiment despite the fact that this line was originally selected for its susceptibility to lymphoid leukosis induction by other ALV subgroups (3).
In previous studies using congenic birds that differed only at the MHC locus (or B complex), the MHC class I (B to F) haplotype had been shown to be the key determinant in the regression of tumors resulting from Rous sarcoma virus infection (26) (27). Given the prime role of the MHC in the regulation of self-tolerance, we also attempted to assess whether the MHC played a clear role in determining the immunocompetence of layer-line birds infected with ALV-J. Tolerance appeared to be unrestricted by MHC haplotype, given that persistent viremia was observed in birds with either or both parental haplotypes. MHC haplotypes were not determined for the outbred line 21 birds because of the existence of many poorly characterized haplotypes in these flocks (16); therefore, no correlation between MHC haplotype and tumor development could be examined.
In comparison to the progeny of (15I × 0)F2 birds, the proportion of the outbred meat-type line 21 birds immunologically tolerant to ALV-J was very high (54%). This indicates that the genotype responsible for ALV-J tolerance does not ordinarily have a detrimental phenotype and has no negative effect on commercial productivity. If two unlinked genes are responsible for immunological tolerance, as suggested by the data presented here, a high frequency of tolerance may be observed in the meat-type birds when the recessive allele of one of the determinants is fixed within the flock and the other is present at a high frequency. This is possible if one of the tolerance determinants is genetically linked to a locus that has undergone selection to homogeneity, such as the recessive ALV-A receptor (TVA) allelic variant that provides resistance to this subgroup (6). Selecting for such recessive receptor alleles by chorioallantoic membrane testing with pseudotyped Rous sarcoma virus is used by primary breeding companies for improvement of genetic resistance to ALV (18). Alternatively, other genes involved in tolerance may be linked to a quantitative trait locus that is being positively selected by commercial breeding companies.
Although the intact EAV-HP proviruses examined here do not interact immunologically with ALV-J, there has been a clear involvement in ALV-J emergence, as shown by the sequence identity and expression characteristics of the provirus locus represented by clone EAV-15I. Although the present schemes to eradicate ALV-J from many infected flocks have been effective, the existence of intact EAV-HP loci capable of generating env transcripts could pave the way for the future reemergence of this subgroup, which has fiercely impacted broiler breeder flocks. Our studies indicate that such loci are segregating in the chicken population. Elimination of birds from breeding stock carrying such EAV-HP loci could also be used as another important measure to prevent the possibility of a future reemergence of ALV-J through such recombination events. While locus-specific PCR can be used to target the expressed intact loci described here, the integration sites of these proviruses would need to be determined first to design such assays. Use of the general PCR primers (EAV-IN1 and EAV-IN4 or 103ER) would unnecessarily eliminate the silent intact loci; however, it would bypass the need to clone integration sites of expressed loci. More importantly, intact proviruses that are expressed may exist within the outbred broiler breeder lines other than those described here, and use of a more general PCR detection assay would also allow these uncharacterized and potentially detrimental loci to be eliminated also. The possibility also remains that EAV-HP proviruses are present within the chicken genome that have complete env and truncated pol genes that would not be detected by the PCR primers designed for the IN region. Additionally, sequence divergence in some EAV-HP proviruses within the primer sequence region would also prevent detection of such elements. A thorough eradication scheme may require additional assays to ensure complete elimination of EAV-HP proviruses with complete env genes. This can be done by using alternative PCR primers or by Southern blot hybridization with a probe specific for the env 5′ region that is deleted from all of the defective chicken EAV-HP elements except that of the ev/J 4-1.
The characterization of line 15I in this report provides an important new role for this layer line as a model for the tolerance observed in outbred meat-type chickens that are not amenable to genetic studies. This line will be valuable for determination of the kinetics of tolerance in chicks, since ALV-J challenge in older birds results in virus clearance and the production of neutralizing antibodies. Previously, any attempt to define the point when birds become immunocompetent after hatch was doomed in meat-type birds, where the proportion of birds developing tolerance is variable. Moreover, the generation of F2 birds with the immunologically tolerant phenotype will allow for map-based cloning of the determinants for ALV-J tolerance by the use of microsatellite markers.
Since the only major nucleotide sequence differences between ALV-J and other ALV subgroups lies within the env gene (4), it is possible that the genetic elements involved in tolerance encode proteins that interact with the ALV-J surface glycoprotein (gp85), possibly even the ALV-J cellular receptor itself. It can be envisioned that tolerance develops in birds with the genotype with both genes recessive when the ALV-J gp85 interaction with its receptor in some immune cell type induces a signal that results in compromised adaptive immunity. Precedence for this proposed ALV gp85-receptor signaling has been demonstrated for the B and D ALV subgroups that use a tumor necrosis factor-related death receptor, with binding causing activation of the caspase pathway leading to cell death (10). It will be interesting to determine the identity of the two genes to reveal whether they encode two components of a multimeric protein or a cognate receptor and ligand.
Acknowledgments
The work was funded by the British Biotechnology and Biological Sciences Research Council (BBSRC).
We thank S. Baigent, A. Brown, P. Chesters, S. Evans, and L. Petherbridge for assistance with animal experiments, N. Salmon for MHC typing, and W. Mwangi for PCR detection of EAV-HP in F2 birds.
REFERENCES
- 1.Arshad, S. S., K. Howes, G. S. Barron, L. M. Smith, P. H. Russell, and L. N. Payne. 1997. Tissue tropism of the HPRS-103 strain of J subgroup avian leukosis virus and of a derivative acutely transforming virus. Vet. Pathol. 34:127-137. [DOI] [PubMed] [Google Scholar]
- 2.Astrin, S. M., E. G. Buss, and W. S. Haywards. 1979. Endogenous viral genes are non-essential in the chicken. Nature 282:339-341. [DOI] [PubMed] [Google Scholar]
- 3.Bacon, L. D., H. D. Hunt, and H. H. Cheng. 2000. A review of the development of chicken lines to resolve genes determining resistance to diseases. Poult. Sci. 79:1082-1093. [DOI] [PubMed] [Google Scholar]
- 4.Bai, J., L. N. Payne, and M. A. Skinner. 1995. HPRS-103 (exogenous avian leukosis virus, subgroup J) has an env gene related to those of endogenous elements EAV-0 and E51 and an E element found previously only in sarcoma viruses. J. Virol. 69:779-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banks, J. D., B. O. Kealoha, and M. L. Linial. 1999. An Mψ-containing heterologous RNA, but not env mRNA, is efficiently packaged into avian retroviral particles. J. Virol. 73:8926-8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates, P., L. Rong, H. E. Varmus, J. A. Young, and L. B. Crittenden. 1998. Genetic mapping of the cloned subgroup A avian sarcoma and leukosis virus receptor gene to the TVA locus. J. Virol. 72:2505-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson, S. J., B. L. Ruis, A. M. Fadly, and K. F. Conklin. 1998. The unique envelope gene of the subgroup J avian leukosis virus derives from ev/J proviruses, a novel family of avian endogenous viruses. J. Virol. 72:10157-10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonfield, J. K., K. Smith, and R. Staden. 1995. A new DNA sequence assembly program. Nucleic Acids Res. 23:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyce-Jacino, M. T., K. O'Donoghue, and A. J. Faras. 1992. Multiple complex families of endogenous retroviruses are highly conserved in the genus Gallus. J. Virol. 66:4919-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brojatsch, J., J. Naughton, H. B. Adkins, and J. A. Young. 2000. TVB receptors for cytopathic and noncytopathic subgroups of avian leukosis viruses are functional death receptors. J. Virol. 74:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denesvre, C., D. Soubieux, G. Pin, D. Hue, and G. Dambrine. 2003. Interference between avian endogenous ev/J 4.1 and exogenous ALV-J retroviral envelopes. J. Gen. Virol. 84:3233-3238. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein, J. 1993. PHYLIP (Phylogeny Inference Package) version 3.5c. Department of Genetics, University of Washington, Seattle, Wash.
- 13.Gudkov, A. V., E. A. Komarova, M. A. Nikiforov, and T. E. Zaitsevskaya. 1992. ART-CH, a new chicken retroviruslike element. J. Virol. 66:1726-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 15.Himly, M., D. N. Foster, I. Bottoli, J. S. Iacovoni, and P. K. Vogt. 1998. The DF-1 chicken fibroblast cell line: transformation induced by diverse oncogenes and cell death resulting from infection by avian leukosis viruses. Virology 248:295-304. [DOI] [PubMed] [Google Scholar]
- 16.Iglesias, G. M., L. A. Soria, R. M. Goto, A. M. Jar, M. C. Miquel, O. J. Lopez, and M. M. Miller. 2003. Genotypic variability at the major histocompatibility complex (B and Rfp-Y) in Camperos broiler chickens. Anim. Genet. 34:88-95. [DOI] [PubMed] [Google Scholar]
- 17.McConnell, S. K., D. A. Dawson, A. Wardle, and T. Burke. 1999. The isolation and mapping of 19 tetranucleotide microsatellite markers in the chicken. Anim. Genet. 30:183-189. [DOI] [PubMed] [Google Scholar]
- 18.McKay, J. C. 1998. A poultry breeder's approach to avian neoplasia. Avian Pathol. 27:S74-S77. [Google Scholar]
- 19.Miller, C. K., J. E. Embretson, and H. M. Temin. 1988. Transforming viruses spontaneously arise from nontransforming reticuloendotheliosis virus strain T-derived viruses as a result of increased accumulation of spliced viral RNA. J. Virol. 62:1219-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nikiforov, M. A., and A. V. Gudkov. 1994. ART-CH: a VL30 in chickens? J. Virol. 68:846-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Payne, L. N. 1998. HPRS-103: a retrovirus strikes back. The emergence of subgroup J avian leukosis virus. Avian Pathol. 27:S36-S45. [Google Scholar]
- 22.Payne, L. N., S. R. Brown, N. Bumstead, K. Howes, J. A. Frazier, and M. E. Thouless. 1991. A novel subgroup of exogenous avian-leukosis virus in chickens. J. Gen. Virol. 72:801-807. [DOI] [PubMed] [Google Scholar]
- 23.Payne, L. N., A. M. Gillespie, and K. Howes. 1991. Induction of myeloid leukosis and other tumors with the HPRS-103 strain of ALV. Vet. Rec. 129:447-448. [DOI] [PubMed] [Google Scholar]
- 24.Payne, L. N., A. M. Gillespie, and K. Howes. 1992. Myeloid leukaemogenicity and transmission of the HPRS-103 strain of avian-leukosis virus. Leukemia 6:1167-1176. [PubMed] [Google Scholar]
- 25.Payne, L. N., A. M. Gillespie, and K. Howes. 1993. Recovery of acutely transforming viruses from myeloid leukosis induced by the HPRS-103 strain of avian-leukosis virus. Avian Dis. 37:438-450. [PubMed] [Google Scholar]
- 26.Plachy, J., and V. Albrecht. 1981. Genetic control of Rous sarcoma regression in inbred lines of chickens. Folia Biol. (Prague) 27:289-300. [PubMed] [Google Scholar]
- 27.Plachy, J., K. Hala, J. Hejnar, J. Geryk, and J. Svoboda. 1994. src-specific immunity in inbred chickens bearing v-src DNA- and RSV-induced tumors. Immunogenetics 40:257-265. [DOI] [PubMed] [Google Scholar]
- 28.Ruis, B. L., S. J. Benson, and K. F. Conklin. 1999. Genome structure and expression of the ev/J family of avian endogenous viruses. J. Virol. 73:5345-5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell, P. H., K. Ahmad, K. Howes, and L. N. Payne. 1997. Some chickens which are viraemic with subgroup J avian leukosis virus have antibody-forming cells but no circulating antibody. Res. Vet. Sci. 63:81-83. [DOI] [PubMed] [Google Scholar]
- 30.Sacco, M. A., D. M. J. Flannery, K. Howes, and K. Venugopal. 2000. Avian endogenous retrovirus EAV-HP shares regions of identity with avian leukosis virus subgroup J and the avian retrotransposon ART-CH. J. Virol. 74:1296-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacco, M. A., K. Howes, and K. Venugopal. 2001. Intact EAV-HP endogenous retrovirus in Sonnerat's jungle fowl. J. Virol. 75:2029-2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sacco, M. A., and K. Venugopal. 2001. Segregation of EAV-HP ancient endogenous retroviruses within the chicken population. J. Virol. 75:11935-11938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva, R. F., A. M. Fadly, and H. D. Hunt. 2000. Hypervariability in the envelope genes of subgroup J avian leukosis viruses obtained from different farms in the United States. Virology 272:106-111. [DOI] [PubMed] [Google Scholar]
- 34.Smith, E. J., A. Fadly, and W. Okazaki. 1979. An enzyme-linked immunosorbent assay for detecting avian leukosis-sarcoma viruses. Avian Dis. 23:698-707. [PubMed] [Google Scholar]
- 35.Smith, L. M., A. A. Toye, K. Howes, N. Bumstead, L. N. Payne, and K. Venugopal. 1999. Novel endogenous retroviral sequences in the chicken genome closely related to HPRS-103 (subgroup J) avian leukosis virus. J. Gen. Virol. 80:261-268. [DOI] [PubMed] [Google Scholar]
- 36.Staden, R. 1996. The Staden sequence analysis package. Mol. Biotech. 5:233-241. [DOI] [PubMed] [Google Scholar]
- 37.Venugopal, K. 1999. Avian leukosis virus subgroup J: a rapidly evolving group of oncogenic retroviruses. Res. Vet. Sci. 67:113-119. [DOI] [PubMed] [Google Scholar]