Abstract
Current methods for determining the role of a given gene product in the gammaherpesvirus 68 (γHV68) life cycle require generation of a specific mutation by either homologous recombination in mammalian cells or bacterial artificial chromosome-mediated mutagenesis in Escherichia coli. The mutant virus is then compared to wild-type virus, and the role of the gene in the viral life cycle is deduced from its phenotype. This process is both time-consuming and labor intensive. Here we present the use of random, transposon-mediated signature-tagged mutagenesis for the identification of candidate viral genes involved in virus replication. Pools of viral mutants, each containing a random insertion of a transposon, were generated with a transposon donor library in which each transposon contains a unique sequence identifier. These pools were transfected into mammalian cells, and the ability of each mutant to replicate was assessed by comparing the presence of virus in the output pool to that present in the input pool of viral genomes. With this approach we could rapidly screen up to 96 individual mutants simultaneously. The location of the transposon insertion was determined by sequencing individual clones with a common primer specific for the transposon end. Here we present the characterization of 53 distinct viral mutants that correspond to insertions in 29 open reading frames within the γHV68 genome. To confirm the results of the signature-tagged mutagenesis screen, we quantitated the ability of each mutant to replicate compared to wild-type γHV68. From these analyses we identified 16 γHV68 open reading frames that, when disrupted by transposon insertions, score as essential for virus replication, and six other open reading frames whose disruption led to significant attenuation of virus replication. In addition, transposon insertion in five other γHV68 open reading frames did not affect virus replication. Notably, all but one of the candidate essential replication genes identified in this screen have been shown to be essential for the replication of at least one other herpesvirus.
The gammaherpesviruses include the human pathogens Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus. These viruses establish life-long infections of the host and are associated with a number of malignancies. To better understand gammaherpesvirus pathogenesis, we and others have begun to utilize infection of mice with murine gammaherpesvirus 68 (γHV68). γHV68 is a member of the gamma-2-herpesvirus subfamily based on genome sequence (13, 51). Sequence analysis of γHV68 identified 80 ATG-initiated open reading frames (ORFs) predicted to encode proteins of at least 100 amino acids in length (51). The majority of these ORFs were homologous to known genes present in other gammaherpesviruses (51). The requirement for most of the predicted ORFs during viral replication in vitro is unknown.
Traditional methods of generating mutations in gammaherpesviruses are time-consuming and labor intensive. Homologous recombination in mammalian cells relies on the use of a genetic marker (e.g., lacZ) to allow efficient screening for mutants. The presence of large expression cassettes in the γHV68 genome, such as lacZ, has been shown to generate phenotypes which are not specific to the gene in question (7). To generate subtler mutations by this method, insertion cassettes must be rescued to a mutant genotype. With this two-step approach, generation of a subtle mutation (e.g., introduction of a translation termination codon) in specific viral ORF can take several months.
With the cloning of the γHV68 genome as a bacterial artificial chromosome, the possibility of large-scale screens for mutant virus phenotypes has become a reality (1). The ease with which mutant viruses can be generated has removed a substantial block to genetic analyses of γHV68. Prior to the emergence of bacterial artificial chromosome technology, mutations which affected genes required for viral replication were difficult to generate. These mutants required cell lines expressing the function deleted in the mutant virus for growth in tissue culture. Bacterial artificial chromosome-mediated mutagenesis in Escherichia coli allows mutations to be introduced into the viral genome in a context which does not require viral gene function, and thus mutations that disrupt essential genes can be readily introduced into the viral genome. In addition to facilitating the production of individual mutant viruses, traditional bacterial genetic techniques have allowed the generation of large numbers of mutants in a single step in a random fashion. Transposon mutagenesis has been employed for large-scale mutagenesis of pseudorabies virus (47) and cytomegalovirus (5, 23, 30, 55). Specific mutant viruses have also been isolated from large pools of human cytomegalovirus transposon libraries (23). While these approaches are useful for the isolation of a large number of specific viral mutants, they are not amenable to assigning function to large numbers of viral genes simultaneously.
Simultaneous screening of large pools of mutants has been effectively addressed in bacterial pathogenesis by the development of signature-tagged mutagenesis technology. Signature-tagged mutagenesis was developed by Holden et al. (6, 21) to simultaneously analyze the phenotypes of multiple Salmonella enterica serovar Typhimurium transposon mutants. A pool of transposons, each containing a unique sequence identifier, was used to mutagenize S. enterica serovar Typhimurium, and the resultant mutants were screened for their ability to grow in mice. Mutants lost during growth in vivo were considered to have insertions in genes required for growth in the mouse. The absence of these mutants from the output pool was determined by Southern blot with the unique sequence identities contained in the transposon insertions as probes. With this technique, the authors were able to screen several hundred mutants in slightly more than 3 months. Nineteen new virulence genes were discovered on a previously unknown pathogenicity island (21).
Since the publication of the original screen, signature-tagged mutagenesis has been used in a variety of organisms to identify genes required for various aspects of bacterial and fungal growth (4). This technique has also been used to identify bacterial genes required for pathogenesis and colonization in vivo (4, 8, 29, 33). Here we report the first use of signature-tagged mutagenesis for the identification of viral genes required for replication in vitro. We report the generation and initial characterization of 53 distinct viral mutants, which correspond to transposon insertions in 29 γHV68 ORFs and several intergenic regions. In addition, we have quantitated the degree of attenuation of each of these viral mutants for growth in vitro.
MATERIALS AND METHODS
Plasmids and constructs.
The γHV68 bacterial artificial chromosome clone was a kind gift of U. Koszinowski (1). The transposon library containing unique sequence identifiers was a kind gift of David Holden (21). Strain GS500 is a RecA+ E. coli strain and was a gift from Greg Smith (47). E. coli S17λpir bacteria were provided by Andrew Darwin (8).
Transposition and isolation of γHV68 transposon mutants.
Transposition of the γHV68 bacterial artificial chromosome was performed as described by Smith et al. (47). Briefly, E. coli S17λpir bacteria containing the transposon donor vector pUTminiTn5Kn with unique sequence identifiers were mated to E. coli GS500containing the GHV68 bacterial artificial chromosome. Following a 1-h incubation in 10 mM MgSO4, bacteria were collected on sterile Millipore filters, and the filters were placed bacteria side up on Luria broth (LB) plates at 37°C for approximately 8 h. Filters were transferred to 100 ml of LB medium containing kanamycin and chloramphenicol and allowed to incubate overnight at 37°C. The γHV68 bacterial artificial chromosome contains the chloramphenicol acetyltransferase cassette, and kanamycin resistance is encoded in the transposon. The following day, 100 ml of fresh LB medium containing kanamycin and chloramphenicol cultures was inoculated, and the bacteria were allowed to grow for 6 h at 37°C with shaking. The bacteria were harvested, and DNA was prepared with a Qiagen Midi Prep kit as per the manufacturer's instructions. DNA was electroporated into fresh E. coli DH10B (Genehogs; Invitrogen) and plated on LB plates containing kanamycin and chloramphenicol. This step ensures that the Kanr phenotype is due to insertion of the transposon in the viral genome rather than the E. coli genome. The following day, each individual colony was picked and seeded into 100 μl of LB medium containing kanamycin and chloramphenicol in a single well of a 96-well plate. As the sequence of the unique sequence identifier is variable, each mutant was screened for efficient hybridization. Matings were arranged in 96-well plates, and the plates were screened by colony hybridization for mutants containing unique sequence identifiers with efficient hybridization profiles (see below for colony blot-hybridization protocol). Mutants displaying poor hybridization were removed from the pool, and the plates were reformatted to contain only mutants with unique sequence identifiers which hybridized efficiently.
Generation of radiolabeled sequence identifier probes.
γHV68 DNA prepared from either bacterial cultures (input pool) or purified virion DNA (output pool) was used to generate probes as described by Holden et al. (21). This method uses two rounds of PCR to generate probes of sufficient specific activity. In the first round, 5 μg of viral DNA was mixed with 20 mM Tris-Cl, pH 8.3, 50 mM KCl, 2 mM MgCl2, 0.01% Tween 20, 200 μM each deoxynucleoside triphosphate, 2.5 U of Taq (Promega), and 770 ng of primer P2 (5′-TAC CTA CAA CCT CAA GCT-3′) and P4 (5′-TAC CCA TTC TAA CCA AGC-3′). PCR conditions were as follows: 4 min at 95°C and 20 cycles of 50°C for 45 s, 72°C for 10 s, and 95°C for 30 s. PCR products were subsequently run on a 1.6% SeaPlaque low-melting-point agarose gel, and a fragment of approximately 80 bp was excised and diluted by the addition of 20 μl of water. The second-round PCR mixture was essentially the same as that in the first round with the following modifications: 50 mM each dATP, dTTP, and dGTP; 10 μl of [32P]dCTP (3,000 Ci/mmol, Amersham); 150 ng each of primers P2 and P4; and 3 μl of first-round PCR product in a total volume of 20 μl. PCR conditions were the same as for the first-round PCR. Following the second-round PCR, the reaction was diluted to 200 μl with 1× NEB#2 buffer and 40 U of HindIII restriction enzyme (New England Biolabs) to remove the primer hybridization sites. The reaction was incubated for at least 2 h at 37°C, denatured for 5 min at 95°C, chilled on ice, and then added directly to colony blots for hybridization overnight.
Colony blot hybridization and DNA preparation.
Colony blot hybridizations were performed as described in Hensel et al. (21). Colonies from 96-well plates were transferred to a nitrocellulose membrane on an LB plate containing kanamycin and chloramphenicol with a 96-prong replicator (Sigma). Plates with membranes were incubated overnight at 37°C. The following day, the membrane was lifted from the plate and placed onto Whatman paper soaked in 0.4 M NaOH for 8 min. Membranes were then neutralized for 5 min in a solution of 0.5 M Tris-Cl, pH 7.0, with shaking and then washed in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) for 5 min. Excess liquid was drained, and the DNA was fixed to the membrane by UV cross-linking (Stratalinker; Stratagene). Colony blots were hybridized in 0.25 M NaPO4 (pH 7.2)-7% sodium dodecyl sulfate (SDS)-1 mM EDTA (pH 8.0)-50% formamide in the presence of 250 μg of denatured salmon sperm DNA per ml. Following hybridization, the colony blots were washed twice for 30 min in 0.25 M NaPO4 (pH 7.2)-2% SDS-1 mM EDTA (pH 8.0) at 65°C. Next the blots were transferred to a solution of 0.05 M NaHPO4 (pH 7.2)-1% SDS-1 mM EDTA (pH 8.0) and washed twice for 30 min each at 65°C. Blots were exposed to phosphorimager screens and analyzed on a Typhoon Imager (Amersham).
Transfections.
Individual γHV68 bacterial artificial chromosome clones were transfected into Vero cells constitutively expressing Cre recombinase (Vero-Cre) with the Superfect transfection reagent (Qiagen). Two micrograms of DNA of each mutant clone was transfected in duplicate into 2 × 105 cells. Wild-type GHV68 bacterial artificial chromosome DNA was transfected in two separate wells in each experiment to control for transfection efficiency. Wells were harvested 4 days posttransfection for quantification of virus growth by plaque assay, as described below. Vero-Cre cells were a kind gift from David Leib.
For screening in bulk the mutants present in a 96-well master plate, DNA was isolated from bacterial cultures containing all clones from an individual row of the 96-well master plate. Two micrograms of DNA from each row was subsequently transfected in duplicate into Vero-Cre cells. Four to 5 days following transfection, cells were harvested. One hundred microliters from each transfection row (eight rows total) was mixed together into a total volume of 800 μl. This mixture was then used to inoculate four 162-cm2 tissue culture flasks for preparation of infectious DNA, as previously described (29). To confirm the results obtained following transfection of DNA prepared from pooled row cultures, DNA was also prepared from pooled bacterial cultures from each column of the 96-well master plate, and infectious DNA was prepared as described above. The results obtained following column pool DNA transfections were identical to those obtained following transfection of pooled row DNA.
Plaque assays.
Plaque assays were performed with Vero-Cre monolayers under a Noble agar overlay, as described previously (26), with the following alterations. Vero-Cre cells were plated in six-well plates at 2 × 105 cells per well the day prior to infection. Samples to be titered were thawed, and then 10-fold dilutions were made in complete Dulbecco's modified Eagle's medium and plated onto Vero-Cre cell monolayers. Infections were performed in a 100-μl volume, and plates were rocked every 15 min for 1 h at 37°C. Samples were overlaid with 3 ml of a 1:1 mixture of 1% Noble agar and 2× minimal essential medium supplemented with 10% fetal bovine serum and 2× concentration of antibiotic and l-glutamine and 300 μg of hygromycin B (Calbiochem) per ml. An additional 2 ml of Noble agar-2× minimal essential medium mixture was added between days 3 and 6 postplating. Monolayers were stained between days 6 and 8 by the addition of 2 ml of neutral red overlay (0.01% neutral red in 2× Dulbecco's modified Eagle's medium diluted 1:1 with 1% Noble agar). After 18 to 24 h, plaques were counted. The limit of detection for this assay is 10 PFU per sample.
RESULTS AND DISCUSSION
Generation of a library of random transposon insertion mutant γHV68 bacterial artificial chromosome clones.
To generate the input pool of mutant viruses required for signature-tagged mutagenesis, we obtained from David Holden a library of transposon-containing vectors that have previously been used for signature-tagged mutagenesis screens in bacteria (8, 21, 29, 33). Each plasmid of the library contains a transposon with a unique sequence identifier and kanamycin resistance cassette (Kanr) located within the transposon ends (Fig. 1A). This vector also supplies a transposase expression cassette. The unique sequence identifier consists of a 40-bp variable sequence flanked by invariant arms (Fig. 1A). These arms are used as primer binding sites for P2 and P4 (see Materials and Methods) to allow generation of probes by PCR, as discussed below. Notably, this transposon donor vector is not capable of replicating in normal laboratory strains of E. coli (e.g., DH5α and DH10B) due to restrictions on the plasmid origin of replication.
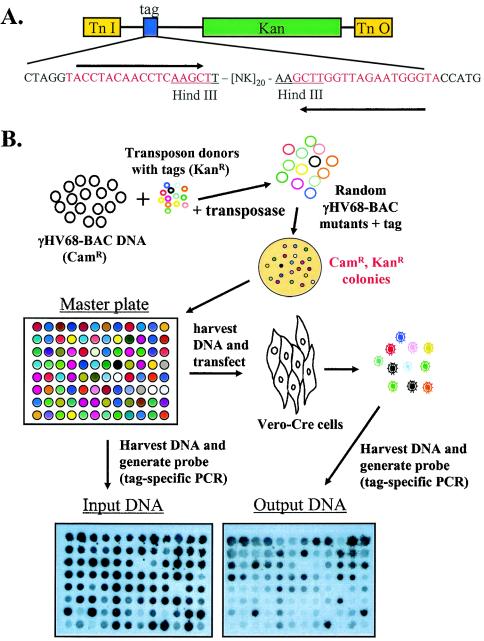
FIG. 1.
(A) Schematic illustration of the transposon used in the signature-tagged mutagenesis analysis (11). The Kanr cassette and a unique sequence identifier are contained within the transposon ends. The unique sequence identifier, or tag, consists of a 40-bp variable region flanked by invariant sequence arms. Primers specific to the invariant arms were used to generate tag-specific probes by PCR in the presence of [α-32P]dCTP. Following PCR amplification, the invariant arms were removed by digestion with HindIII. N = A, C, G, or T; K = G or T. (B) Illustration of the steps involved in generating and screening the γHV68 signature-tagged transposon mutant library. A library of transposon plasmids in which each plasmid contains a unique sequence identifier harbored in E. coli S17λpir was mated to E. coli GS500 containing the γHV68 bacterial artificial chromosome, and Camr Kanr colonies were isolated. Camr Kanr bacteria were used to prepare bacterial artificial chromosome DNA, which was electroporated into E. coli DH10B, ensuring that the Kanr phenotype was due to transposon insertion into the γHV68 bacterial artificial chromosome rather than the E. coli genome. From each mating, 12 transposon insertion mutants were isolated and used to seed one row of a 96-well plate (i.e., each row was derived from a separate mating). Bacterial artificial chromosome DNA preparations were made from pooled row cultures and transfected in duplicate into Vero-Cre cells. This DNA is referred to as input DNA. The supernatants from each row transfection were harvested 5 days later, mixed, and amplified on fresh Vero-Cre cells. The resultant virus was used to generate virion DNA. The virion DNA is referred to as the output DNA. Tag-specific probes were generated from input and output DNAs, and probes were hybridized to duplicate colony blots of the master plate. Changes in hybridization intensity for a given mutant between the input and output blots were indicative of the ability of that mutant to grow in Vero-Cre cells. Shown are representative hybridizations of input and output probes to colony blots of one of the master plates of γHV68 transposon mutants.
E. coli strain S17λpir, containing the transposon library, was mated to E. coli DH10B, containing the γHV68 bacterial artificial chromosome, and the resulting colonies were selected on LB plates containing kanamycin and chloramphenicol (Fig. 1B). In this system, chloramphenicol resistance (Camr) is supplied by the γHV68 bacterial artificial chromosome. As the transposon vector is incapable of replicating in E. coli DH10B, only E. coli DH10B in which a transposition event has taken place will be Camr Kanr.
As the transposon can incorporate into either the E. coli genome or the γHV68 bacterial artificial chromosome, bacterial artificial chromosome DNA was extracted from the exconjugates and electroporated into fresh E. coli DH10B. Camr Kanr colonies from this transfection were transferred to a well of a 96-well plate and grown overnight in LB medium containing kanamycin and chloramphenicol (Fig. 1B). Each mating is predicted to give rise to 8 to 12 unique insertion events. Therefore, each row of mutants in a 96-well plate was derived from a separate mating.
To determine whether or not a given clone is present following a selection event, it is necessary to be able to distinguish one clone from another. In the signature-tagged mutagenesis approach, the unique sequence identifiers are used for this step. DNA was prepared from a pooled culture of all the mutants on a given 96-well plate (Fig. 1B). PCR with primers specific for the invariant arms flanking the unique sequence identifier was performed in the presence of α[32P]cytosine (Fig. 1A). The invariant arms were cleaved from the resulting PCR product, and the radiolabeled PCR product was used to probe colony blots prepared from the master plate (Fig. 1B; also see Materials and Methods). Not all of the mutants generated in the initial mating process were suitable for use. This is due to the nature of the variable sequence; some unique sequence identifiers contain relatively few cytosine nucleotides and therefore incorporate label poorly in the PCR step. In addition, some transposon inserts may not contain unique sequence identifiers or may complement one another if transfected into the same cell. The latter process may lead to low-level replication, a concern when screening for essential viral genes. However, despite the possibility of complementation, comparison of the pre- and postscreen colony blots revealed clear differences in the intensity of signals for specific mutants (Fig. 1B).
Analysis of in vitro replication screen results.
To confirm the predictions made from the signature-tagged mutagenesis screening of the library of transposon mutants, DNA from individual mutants was transfected into mammalian cells. Wild-type DNA was transfected in parallel to control for transfection efficiency. Four days posttransfection, wells were harvested and titered on Vero-Cre cells. The results of 53 such independent analyses are presented in Tables 1, 2, and 3. In general, the results obtained from the colony blots accurately predicted the ability of a given mutant to replicate. Of 15 initial colonies whose hybridization profile indicated a defect in viral replication, 13 were found to be severely attenuated for growth when analyzed individually. In addition, of 14 mutants predicted to be nonattenuated for replication, 12 grew to titers comparable to that of the wild type following independent transfection. When colonies were randomly screened (e.g., all colonies in a column were tested regardless of hybridization profile), approximately 75% of the mutants analyzed were found to be severely attenuated for growth in mammalian cells. Therefore, our ability to successfully predict the nonattenuated phenotype confirms that the hybridization profile observed for a given mutant was strongly predictive of the ability of that mutant to replicate in tissue culture.
TABLE 1.
Transposon insertions in candidate essential genes
| Mutant | Insertion point (bp) | Gene | Coordinates (bp) | Proposed functionc | Attenuation | Phenotype of targeted null mutants (reference) |
|---|---|---|---|---|---|---|
| C5 | 13227 | ORF6 | 11215-14523 | ssDNABPd | >10e6 | HSVa (53), EBVa (15), HCMVa (55) |
| 3E5 | 14101 | ORF6 | ssDNABPd | >10e6 | ||
| F6 | 16508 | ORF8 | 16505-19051 | gB | >10e6 | HSVa (41), EBVa (21), HCMVa (55) |
| 2B6 | 16508 | ORF8 | gB | >10e6 | ||
| E9 | 36104 | ORF22 | 34833-37022 | gH | >10e6 | HSVa (16), EBVa (38), HCMVa (55) |
| E4 | 36139 | ORF22 | gH | >10e6 | ||
| 2E2 | 46675 | ORF29b | 47434-46395 | Packaging protein | >10e6 | HSVa (42), HCMVa (55) |
| 2B7 | 46591 | ORF29b | Packaging protein | >10e6 | ||
| 2B5 | 48089 | ORF31 | 47710-48309 | >10e6 | HCMVa (55) | |
| 2C5 | 48089 | ORF31 | >10e6 | |||
| 3C8 | 49822 | ORF33 | 49588-50568 | >10e6 | HSVb (2), HCMVb (55) | |
| 2C7 | 49831 | ORF33 | >10e6 | |||
| 2C8 | 51195 | ORF29a | 51466-50549 | Packaging protein | >10e6 | HSVa (42), HCMVa (55) |
| 2C3 | 51643 | ORF34 | 51465-52460 | >10e6 | HCMVa (55) | |
| H9 | 51643 | ORF34 | >10e6 | |||
| 2E8 | 51678 | ORF34 | >10e6 | |||
| 2C9 | 56113 | ORF39 | 56950-55802 | gM | >10e6 | HSVb (32), HCMVa (23, 55) |
| G7 | 56910 | ORF39 | gM | >10e6 | PRVb (12), EBVb (27) | |
| F5 | 56901 | ORF39 | gM | >10e6 | ||
| 2B3 | 60253 | ORF43 | 61334-59634 | Capsid protein | >10e6 | HSVa (28), HCMVa (55) |
| 3D10 | 61215 | ORF43 | Capsid protein | >10e6 | ||
| 2F2 | 63427 | ORF44 | 61303-63630 | Helicase-primase | >10e6 | |
| E12 | 68345 | ORF50 | 67907-69373 | Rta homolog | >10e6 | EBVa (14), GHV68a (40) |
| 3E2 | 68430 | ORF50 | Rta homolog | >10e6 | ||
| 2E5 | 73917 | ORF56 | 73289-75793 | DNA replication? | >10e6 | HSVa (18), HCMVa (55) |
| G5 | 73908 | ORF56 | DNA replication? | >10e6 | ||
| 3A11 | 73500 | ORF56 | DNA replication? | >10e6 | ||
| 3A12 | 73589 | ORF56 | DNA replication? | >10e6 | ||
| 3B12 | 74382 | ORF56 | DNA replication? | >10e6 | ||
| 3G10 | 75127 | ORF56 | DNA replication? | >10e6 | ||
| 3D9 | 75232 | ORF56 | DNA replication? | >10e6 | ||
| 3A9 | 75479 | ORF56 | DNA replication? | >10e6 | ||
| 3F6 | 75580 | ORF56 | DNA replication? | >10e6 | ||
| C4 | 89812 | ORF64 | 86567-93937 | Tegument protein? | >10e6 | HSVa (9), HCMVb (55) |
| 3F1 | 90134 | ORF64 | Tegument protein? | >10e6 | ||
| H12 | 95396 | ORF66 | 95741-94515 | >10e6 | HCMVa (55) | |
| 2E6 | 95396 | ORF66 | >10e6 |
Viral gene product is essential for virus replication in vivo.
The mutant is attenuated for replicated in vivo.
HSV, herpes simplex virus; EBV, Epstein-Barr virus; HCMV, human cytomegalovirus; PRV, pseudorabies virus.
SSDNABP, single-stranded DNA-binding protein.
TABLE 2.
Transposon insertions that attenuate virus replication in vitro
| Mutant | Insertion point (bp) | Gene | Coordinates (bp) | Proposed function | Attenuation | Phenotype of targeted null mutants (reference) |
|---|---|---|---|---|---|---|
| E11 | 42564 | ORF25 | 40263-44381 | Major capsid protein | 10e4 | HSVa (10), HCMVa (55) |
| 2A1 | 55307 | ORF37 | 54129-55586 | Alkaline exonuclease | 10e5 | HSVb (19)a, HCMVa (55) |
| B1 | 55919 | ORF39 | 56950-55802 | gM | 10e5 | See Table 1 |
| 2F8 | 66695 | Insertion between ribonucleotide ORFS 48 and 49 | 10e2 | |||
| 2E10 | 67145 | ORF49 | 67643-66741 | 10e2 | ||
| 2D6 | 67156 | ORF49 | 10e3 | |||
| 2B2 | 73351 | ORF56 | 73289-75793 | 10e1 | ||
| 3D6 | 80425 | ORF60 | 80479-79565 | Ribonucleotide reductase, S | 10e3 | HSVb (20) |
| 2C1 | 80950 | ORF61 | 82865-80517 | Ribonucleotide reductase, L | 10e3 | HSVb (20) HCMVb (39) |
| F2 | 94117 | M9 | 94519-93962 | Glycoprotein | 10e3 | |
| 2H1 | 95393 | ORF66 | 95741-94515 | 10e3 | HCMVa (55)a |
Viral gene product is essential for virus replication in vivo.
The mutant is attenuated for replication in vivo.
TABLE 3.
Transposon insertions that do not affect virus replication in vitro
| Mutant | Insertion point (bp) | Gene | Coordinates (bp) | Proposed function | Attenuation | Phenotype of targeted null mutants (reference) |
|---|---|---|---|---|---|---|
| 2H2 | BAC vector | NAb | 0 | NAb | ||
| 2F10 | BAC vector | NAb | 0 | |||
| 3E3 | 1441 | NAb | Insertion between t.4 & t.5c | 0 | NAb | |
| 2D10 | 4258 | M2 | 4627-4031 | 0 | GHV68a (24) | |
| 2D3 | 4915 | NAb | Insertion between M2 & M3 | 0 | NAb | |
| 2B4 | 5186 | NAb | Insertion between M2 & M3 | 0 | ||
| 2E4 | 8772 | M4 | 8409-9785 | Secreted protein | 0 | γHV68a (unpublished data) |
| 3G12 | 9421 | M4 | Secreted protein | 0 | ||
| H3 | 22963 | ORF 10 | 22269-23522 | dUTPase? | 0 | HSVa (43), PRVa (25), VZVa (45) |
| F9 | 22963 | ORF 10 | dUTPase? | 0 | ||
| G6 | 45794 | ORF 27 | 45329-46090 | dUTPase? | 0 | |
| 2C4 | 46337 | NAb | Insertion between ORFS 27 & 29b | 0 | NAb | |
| 2G11 | 112891 | ORF 75b | 113901-110077 | FGARATd | 0 | |
| 2A12 | 118325e | M12/M13 | 117992-118681/ | 0 | ||
| 118149-118784 |
No growth defect of mutant virus in vivo.
NA, not applicable.
γHV68 encodes eight tRNA-like genes which are clustered near the left end of the viral genome. The genome coordinates for t.4 are bp 1182 to 1254, and for t.5 they are bp 1588 to 1659.
FGRAT, N-formylglycinamide ribotide amidotransferase.
The transposon insertion site lies inside the terminal repeat, while the M12 and M13 ORFS are initiated within the unique sequence at the right end of the virus. The current insertion assignment assumes that the transposon is inserted in the first copy of the terminal repeat adjacent to the unique sequence at the right-hand end of the γHV68 genome.
As shown in Tables 1 to 3, transposon insertions were found in 27 different ORFs, as identified in Virgin et al. (51). Sites of transposon insertion in each bacterial artificial chromosome mutant were determined by sequencing with a primer, EBAC1, specific for the transposon (5′-ACA GCC GGA TCC TCT AGA GTC-3′) (Fig. 2). The majority of disrupted ORFs were found to be required for viral replication in mammalian cells, as would be predicted by their homology to genes in other herpesviruses (Table 1). Importantly, all candidate essential gene mutants were examined by restriction endonuclease digestion with EcoRI to assess the presence of rearrangements or deletions in the viral genome. Notably, no deletions or rearrangements were detected (data not shown), indicating the absence of any gross structural changes, although subtle changes in viral genome structure cannot be ruled out. However, with the exception of two candidate essential gene disruptions (ORFs 29a and 44), multiple independent transposon mutants were recovered (Table 1). In addition, as discussed below, nearly all the genes identified by transposon insertion as being involved in virus replication in vitro (essential and attenuated replication phenotypes; Tables 1 and 2) have also been identified as being involved in the replication of other herpesviruses (Tables 1 and 2).
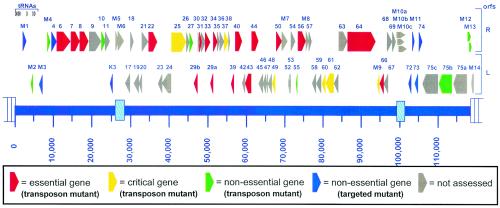
FIG. 2.
Schematic illustration of the γHV68 genome indicating the locations of predicted viral genes as originally described by Virgin et al. (51). Shown in color are the genes that were assessed by the signature-tagged mutagenesis analysis presented here, which denotes genes that were identified as essential for virus replication (red) as well as those that exhibited an attenuated phenotype (orange) and those which appeared to be dispensable for virus replication (green). Also shown for reference are the viral genes that have been specifically knocked out by targeted homologous recombination and shown to be nonessential for virus replication in tissue culture (blue). The last group included the unique genes M1 (7), M2 (24), M3 (49), M11 (17), gene 4 (26), K3 (48), gene 72 (v-cyclin) (50), gene 73 (LANA) (35), and gene 74 (v-GPCR) (34). v-GPCR, viral G-protein-coupled receptor.
Transposon disruption of ORF6 indicates that this is an essential gene, as would be predicted by its homology to the single-stranded-DNA-binding protein of other herpesviruses. Along these lines, the gH, gB, and gM homologues (ORFs 22, 8, and 39, respectively), proteins associated with viral DNA replication (ORFs 40, 44, and 56), and genes involved in transport and packaging of viral genomes (ORFs 29b, 29a, and 7) were all identified as being essential for viral replication. Insertions in ORF43, predicted to encode part of the viral capsid, also abolished virus replication. A mutation in ORF50 was found to abolish virus replication in culture, consistent with previously published data demonstrating an essential role for the ORF50 gene product, Rta, in virus replication (40, 54). In addition, several ORFs (ORFs 31, 34, and 66) of unknown function were identified as being required for efficient viral replication in vitro. ORFs homologous to all three are found in both beta- and gammaherpesviruses, but there are no known cellular homologues of these viral genes to aid in predicting the functions of these viral gene products.
The in vitro screen also identified a number of viral mutants that were capable of replication in tissue culture (Tables 2 and 3). While some of these mutations resulted in decreased replication compared to the wild type (Table 2), several replicated to levels equivalent to that of wild-type virus (Table 3). Viral mutants containing insertions in ORFs 25 and M9 were found to be compromised for replication, with defects of 100- to 1,000-fold, respectively. M9 is a candidate latency-associated gene based on the observed expression of a transcript corresponding to the M9 ORF in latently infected splenocytes (44, 52).
Several ORFs of unknown function were found to tolerate transposon insertions. ORFs 10, 27, 49, 56, and 66 fall into this category. While no cellular homolog has been identified for these ORFs, viral homologues are found in gamma- and betaherpesviruses for all of these ORFs, indicating that their function has been conserved and thus is likely important for some aspect of herpesvirus pathogenesis. The M designation for some of the ORFs present in the γHV68 genome indicates that these ORFs are unique to γHV68 (i.e., no obvious homologues in the other herpesviruses). Insertions in ORFs M2 and M4 did not affect the ability of the virus to replicate in vitro. The phenotype observed for the M2 mutant corresponds to that observed with an M2 mutant virus containing a premature translational stop codon, which was also found to replicate as well as the wild-type virus did in vitro (24). In addition, we have recently characterized an M4 knockout virus and shown that it is wild type for replication in vitro but exhibits a defect in the early establishment of latency in vivo (N. Moorman, A. Evan, and S. H. Speck, unpublished data).
Identification of functional domains by signature-tagged mutagenesis.
Several open reading frames characterized in this screen were identified multiple times. Five pairs of mutants were found in which the transposon had inserted at the same site. Two of these pairs of mutants (2B5 and 2C5 and H3 and F9) were found on the same master plate and may therefore be siblings from the same mating reaction. The other three pairs of mutants (F6 and 2B6, 2C3 and H9, and H12 and 2E6), which arose from separate mating reactions, appear to reflect transposon “hot spotting.” While the Tn5 transposon inserts in many sites in the γHV68 genome, site preference for this transposon has been documented in other systems (31). This is presumably the result of site bias in transposon insertion events. Therefore, these pairs of mutants most likely represent separate transposon insertions into the same locus.
Another region of the viral genome that was densely targeted was ORF56, where 10 transposon insertions were mapped (Tables 1 and 2). For each of these mutants, the transposon integration site was unique. Nine of these mutations were found to result in an essential phenotype (>106-fold attenuation of replication compared to wild-type γHV68) (Table 1). However, a transposon insertion near the 5′ end of the predicted ORF caused only a modest decrease in viral replication (ca. 10-fold) (mutant 2B2 in Table 2). This result was surprising in light of the results with the other mutants. The ability of this mutant to replicate efficiently may indicate that the actual translation start site of ORF56 is downstream of the predicted start site or that ORF56 is spliced, with the transposon insertion in mutant 2B2 occurring within an intron.
Analysis of the insertion in ORF39 revealed hints about functional domains of the protein. ORF39 encodes the gM glycoprotein of γHV68, which is predicted to be 409 amino acids in length and span the membrane multiple times, with its N terminus located in the cytoplasm. All members of the herpesvirus family encode gM homologues, although the precise role of gM in the viral life cycle remains unclear. Several reports have suggested that gM is required for efficient viral egress from infected cells (3, 37, 46). Mutants of pseudorabies virus and equine herpesvirus lacking gM were found to be capable of replicating in vitro but had profound defects in their ability to replicate and cause disease in vivo (11, 36). In the case of Epstein-Barr virus, disruption of the gene encoding gN results in the absence of both gN and gM and a severe defect in virus egress (27).
A similarly severe defect was observed with transposon insertions into the γHV68 gM gene. Three different insertion mutants of ORF39 were found in the course of our screen. Two of these mutants, containing transposon insertions at amino acids 37 and 278, were found not to replicate to detectable levels (Table 1). An additional mutant containing an insertion at amino acid 370 was capable of replicating in culture, though at greatly reduced levels compared to the wild-type virus (Table 2). The transposon insertion in this mutant is predicted to disrupt the coding sequence after the final membrane-spanning domain of the protein, presumably resulting in the production of a partially functional gM mutant.
Conclusion.
While the data presented here do not represent an exhaustive analysis of the γHV68 genome, they do assess the contribution of 27 ORFs to γHV68 replication in tissue culture. There are several important caveats to signature-tagged mutagenesis analyses that should be considered when interpreting these results. First, the insertion of a transposon into the viral genome could affect the expression of surrounding genes as well as of the disrupted gene. As such, the essential genes identified here should be considered candidate essential genes. In addition, some transposon insertions into essential genes could result in the production of a partially functional protein (e.g., see discussion above of transposon insertions in the gM gene). Confirmation of their essential role in virus replication will require the introduction of subtler mutations into the viral genome. Conversely, some transposon insertions into essential genes could result in the production of a partially functional protein (see discussion above of transposon insertions in the gM gene). Finally, the assignment of function to a given ORF was based solely on the sequence analysis of the γHV68 genome outlined in Virgin et al. (51), in which predicated viral genes based on ORFs encoding proteins of greater than 100 amino acids in length and/or the presence of homology to known cellular or viral genes.
The transposon insertions analyzed here would be predicted to disrupt transcription on both strands of the viral genome, and therefore the phenotypes described may actually be due to disruption of an unknown ORF. Additionally, this analysis only takes into account disruption of open reading frames and not disruption of cis-acting DNA elements that may be required for virus replication. Notwithstanding these concerns, the majority of candidate essential genes identified in our signature-tagged mutagenesis screen correspond to gene products whose functions are very likely required for virus replication and/or have been shown to be essential for replication of other herpesviruses (see discussion above and Tables 1 and 2).
In summary, the cloning of the γHV68 genome as a bacterial artificial chromosome has greatly increased the speed with which specific viral mutants can be obtained and initially analyzed. With the techniques described here, we have been able to tentatively assign an in vitro replication phenotype to 27 viral genes. While this analysis does not assign a phenotype to all the known γHV68 ORFs, this approach should make that goal feasible. Additionally, this approach should be applicable to screening pools of transposon mutants in vivo. Experiments are currently under way to determine the role of γHV68 genes required for efficient in vivo replication as well as those involved in establishment and/or reactivation from latency.
Acknowledgments
This research was supported by NIH grants CA58524, CA87650, and CA95318 to S.H.S.
We thank David Holden for permission to use signature-tagged mutagenesis technology and Andrew Darwin and Virginia Miller for providing the library of transposon donor vectors. We also thank Skip Virgin and David Leib for helpful discussions. Special thanks to Eric Clambey for conceptual contributions.
REFERENCES
- 1.Adler, H., M. Messerle, M. Wagner, and U. H. Koszinowski. 2000. Cloning and mutagenesis of the murine gammaherpesvirus 68 genome as an infectious bacterial artificial chromosome. J. Virol. 74:6964-6974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines, J. D., and B. Roizman. 1991. The open reading frames UL3, UL4, UL10, and UL16 are dispensable for the replication of herpes simplex virus 1 in cell culture. J. Virol. 65:938-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brack, A. R., J. M. Dijkstra, H. Granzow, B. G. Klupp, and T. C. Mettenleiter. 1999. Inhibition of virion maturation by simultaneous deletion of glycoproteins E, I, and M of pseudorabies virus. J. Virol. 73:5364-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown, J. S., A. Aufauvre-Brown, and D. W. Holden. 1998. Insertional mutagenesis of Aspergillus fumigatus. Mol. Gen. Genet. 259:327-335. [DOI] [PubMed] [Google Scholar]
- 5.Brune, W., C. Menard, U. Hobom, S. Odenbreit, M. Messerle, and U. H. Koszinowski. 1999. Rapid identification of essential and nonessential herpesvirus genes by direct transposon mutagenesis. Nat. Biotechnol. 17:360-364. [DOI] [PubMed] [Google Scholar]
- 6.Chiang, S. L., J. J. Mekalanos, and D. W. Holden. 1999. In vivo genetic analysis of bacterial virulence. Annu. Rev. Microbiol. 53:129-154. [DOI] [PubMed] [Google Scholar]
- 7.Clambey, E. T., H. W. Virgin, and S. H. Speck. 2000. Disruption of the murine gammaherpesvirus 68 M1 open reading frame leads to enhanced reactivation from latency. J. Virol. 74:1973-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darwin, A. J., and V. L. Miller. 1999. Identification of Yersinia enterocolitica genes affecting survival in an animal host using signature-tagged transposon mutagenesis. Mol. Microbiol. 32:51-62. [DOI] [PubMed] [Google Scholar]
- 9.Desai, P. J. 2000. A null mutation in the UL36 gene of herpes simplex virus type 1 results in accumulation of unenveloped DNA-filled capsids in the cytoplasm of infected cells. J. Virol. 74:11608-11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai, P., N. A. DeLuca, J. C. Glorioso, and S. Person. 1993. Mutations in herpes simplex virus type 1 genes encoding VP5 and VP23 abrogate capsid formation and cleavage of replicated DNA. J. Virol. 67:1357-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dijkstra, J. M., V. Gerdts, B. G. Klupp, and T. C. Mettenleiter. 1997. Deletion of glycoprotein gM of pseudorabies virus results in attenuation for the natural host. J. Gen. Virol. 78:2147-2151. [DOI] [PubMed] [Google Scholar]
- 12.Dijkstra, J. M., N. Visser, T. C. Mettenleiter, and B. G. Klupp. 1996. Identification and characterization of pseudorabies virus glycoprotein gM as a nonessential virion component. J. Virol. 70:5684-5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Efstathiou, S., Y. M. Ho, S. Hall, C. J. Styles, S. D. Scott, U. A. Gompels. 1990. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J. Gen. Virol. 71:1365-1372. [DOI] [PubMed] [Google Scholar]
- 14.Feederle, R., M. Kost, M. Baumann, A. Janz, E. Drouet, W. Hammerschmidt, and H. J. Delecluse. 2000. The Epstein-Barr virus lytic program is controlled by the co-operative functions of two transactivators. EMBO J. 19:3080-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fixman, E. D., G. S. Hayward, and S. D. Hayward. 1995. Replication of the Epstein-Barr virus oriLyt:lack of a virally encoded origin-binding protein and dependence on Zta in cotransfection assays. J. Virol. 69:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangappa, S., L. F. van Dyk, T. J. Jewett, S. H. Speck, and H. W. Virgin. 2002. Identification of the in vivo role of a viral bcl-2. J. Exp. Med. 195:931-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein, D. J., and S. K. Weller. 1998. An ICP6::lacZ insertional mutagen is used to demonstrate that the UL52 gene of herpes simplex virus type 1 is required for virus growth and DNA synthesis. J. Virol. 62:2870-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein, J. N., and S. K. Weller. 1998. The exonuclease activity of HSV-1 UL12 is required for in vivo function. Virology 244:442-457. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein, D. J., and S. K. Weller. 1988. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6-lacZ insertion mutant. J. Virol. 62:196-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hensel, M., J. E. Shea, C. Gleeson, M. D. Jones, E. Dalton, and D. W. Holden. 1995. Simultaneous identification of bacterial virulence genes by negative selection. Science 269:400-403. [DOI] [PubMed] [Google Scholar]
- 22.Herrold, R. E., A. Marchini, S. Fruehling, and R. Longnecker. 1996. Glycoprotein 110, the Epstein-Barr virus homolog of herpes simplex virus glycoprotein B, is essential for Epstein-Barr virus replication in vivo. J. Virol. 70:2049-2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacoby, M. A., H. W. Virgin, and S. H. Speck. 2002. Disruption of the M2 gene of murine gammaherpesvirus 68 alters splenic latency following intranasal, but not intraperitoneal, inoculation. J. Virol. 76:1790-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jons, A., and T. C. Mettenleiter. 1996. Identification and characterization of pseudorabies virus dUTPase. J. Virol. 70:1242-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapadia, S. B., B. Levine, S. H. Speck, and H. W. Virgin. 2002. Critical role of complement and viral evasion of complement in acute, persistent, and latent gamma-herpesvirus infection. Immunity 17:143-155. [DOI] [PubMed] [Google Scholar]
- 27.Lake, C. M., and L. M. Fletcher. 2000. Epstein-Barr Virus that Lacks Glycoprotein gN is Impaired in assembly and Infection. J. Virol. 74:11162-11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamberti, C., and S. K. Weller. 1996. The herpes simplex virus type 1 UL6 protein is essential for cleavage and packaging but not for genomic inversion. Virology 226:403-407. [DOI] [PubMed] [Google Scholar]
- 29.Lau, G. W., S. Haataja, M. Lonetto, S. E. Kensit, A. Marra, A. P. Bryant, D. McDevitt, D. A. Morrison, and D. W. Holden. 2001. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol. Microbiol. 40:555-571. [DOI] [PubMed] [Google Scholar]
- 30.Lee, M., G. Abenes, X. Zhan, W. Dunn, E. Haghjoo, T. Tong, A. Tam, K. Chan, and F. Liu. 2002. Genetic analyses of gene function and pathogenesis of murine cytomegalovirus by transposon-mediated mutagenesis. J. Clin. Virol. Suppl. 2:S111-S122. [DOI] [PubMed] [Google Scholar]
- 31.Lodge, J. K., and D. E. Berg. 1990. Mutations that affect Tn5 insertion into pBR322: importance of local DNA supercoiling. J. Bacteriol. 172:5956-5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLean, C. A., L. M. Robertson, and F. E. Jamieson. 1993. Characterization of the UL10 gene product of herpes simplex virus type 1 and investigation of its role in vivo. J. Gen. Virol. 74:975-983. [DOI] [PubMed] [Google Scholar]
- 33.Mei, J. M., F. Nourbakhsh, C. W. Ford, and D. W. Holden. 1997. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol. Microbiol. 26:399-407. [DOI] [PubMed] [Google Scholar]
- 34.Moorman, N. J., H. W. Virgin, and S. H. Speck. 2003. Disruption of the gene encoding the γHV68 v-GPCR leads to decreased efficiency of reactivation from latency. Virology 307:179-190. [DOI] [PubMed] [Google Scholar]
- 35.Moorman, N. J., D. O. Willer, and S. H. Speck. 2003. The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J. Virol. 77:10295-10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neubauer, A., M. Beer, C. Brandmuller, O. R. Kaaden, and N. Osterrieder. 1997. Equine herpesvirus 1 mutants devoid of glycoprotein B or M are apathogenic for mice but induce protection against challenge infection. Virology 239:36-45. [DOI] [PubMed] [Google Scholar]
- 37.Nixdorf, R., B. G. Klupp, and T. C. Mettenleiter. 2001. Role of the cytoplasmic tails of pseudorabies virus glycoproteins B, E and M in intracellular localization and virion incorporation. J. Gen. Virol. 82:215-226. [DOI] [PubMed] [Google Scholar]
- 38.Oda, T., S. Imai, S. Chiba, and K. Takada. 2000. Epstein-Barr virus lacking glycoprotein gp85 cannot infect B cells and epithelial cells. Virology 276:52-58. [DOI] [PubMed] [Google Scholar]
- 39.Patrone, M., E. Percivalle, M. Secchi, L. Fiorina, Pedrali- G. Noy, M. Zoppe, F. Baldanti, G. Hahn, U. H. Koszinowski, G. Milanesi, and A. Gallina. 2003. The human cytomegalovirus UL45 gene product is a late, virion-associated protein and influences virus growth at low multiplicities of infection. J. Gen. Virol. 84:3359-3370. [DOI] [PubMed] [Google Scholar]
- 40.Pavlova, I. V., H. W. Virgin, and S. H. Speck. 2003. Disruption of the gammaherpesvirus 68 gene 50 demonstrates that Rta is essential for virus replication. J. Virol. 77:5731-5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira, L. 1994. Function of the glycoprotein B homologues of the family herpesviridae. Infect. Agents Dis. 3:9-28. [PubMed] [Google Scholar]
- 42.Przech, A. J., D. Yu, and S. K. Weller. 2003. Point mutations in exon I of the herpes simplex virus putative terminase subunit, UL15, indicate that the most conserved residues are essential for cleavage and packaging. J. Virol. 77:9613-9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pyles, R. B., N. M. Sawtell, and R. L. Thompson. 1992. Herpes simplex virus type 1 dUTPase mutants are attenuated for neurovirulence, neuroinvasiveness, and reactivation from latency. J. Virol. 66:6706-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rochford, R., M. L. Lutzke, R. S. Alfinito, A. Clavo, and R. D. Cardin. 2001. Kinetics of murine gammaherpesvirus 68 gene expression following infection of murine cells in culture and in mice. J. Virol. 75:4955-4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ross, J., M. Williams, and J. I. Cohen. 1997. Disruption of the varicella-zoster virus dUTPase and the adjacent ORF9A gene results in impaired growth and reduced syncytia formation in vitro. Virology 234:186-195. [DOI] [PubMed] [Google Scholar]
- 46.Rudolph, J., and N. Osterrieder. 2002. Equine herpesvirus type 1 devoid of gM and gp2 is severely impaired in virus egress but not direct cell-to-cell spread. Virology 293:356-367. [DOI] [PubMed] [Google Scholar]
- 47.Smith, G. A., and L. W. Enquist. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol. 73:6405-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevenson P. G., J. S. May, X. G. Smith, S. Marques, H. Adler, U. K. Koszinowski, J. P. Simas, and S. Efstathiou. 2002. K3-mediated evasion of CD8(+) T cells aids amplification of a latent gamma-herpesvirus. Nat. Immunol. 3:733-740. [DOI] [PubMed] [Google Scholar]
- 49.van Berkel, V., B. Levine, S. H. Speck, and H. W. Virgin. 2002. Critical role for a high affinity chemokine-binding protein in γ-herpesvirus-induced meningitis. J. Clin. Investig. 109:905-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Dyk, L. F., H. W. Virgin, and S. H. Speck. 2000. The murine gammaherpesvirus 68 v-cyclin is a critical regulator of reactivation from latency. J. Virol. 74:7451-7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Virgin, H. W., P. Latreille, P. Wamsley, K. Hallsworth, K. E. Weck, A. J. Dal Canto, and S. H. Speck. 1997. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 71:5894-5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Virgin, H. W., R. M. Presti, X. Y. Li, C. Liu, and S. H. Speck. 1999. Three distinct regions of the murine gammaherpesvirus 68 genome are transcriptionally active in latently infected mice. J. Virol. 73:2321-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weller, S. K., K. J. Lee, D. J. Sabourin, and P. A. Schaffer. 1983. Genetic analysis of temperature-sensitive mutants which define the gene for the major herpes simplex virus type 1 DNA-binding protein. J. Virol. 45:354-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu, T. T., L. M. Tong, T. Rickabaugh, S. Speck, and R. Sun. 2001. Function of Rta is essential for lytic replication of murine gammaherpesvirus 68. J. Virol. 75:9262-9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 100:12396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]