Abstract
Lassa virus (LV) and Mopeia virus (MV) are closely related members of the Arenavirus genus, sharing 75% amino acid sequence identity. However, LV causes hemorrhagic fever in humans and nonhuman primates, whereas MV cannot induce disease. We have previously shown that antigen-presenting cells (APC)—macrophages (MP) and dendritic cells (DC)—sustain high replication rates of LV but are not activated, suggesting that they play a role in the immunosuppression observed in severe cases of Lassa fever. Here, we infected human APC with MV and analyzed the cellular responses induced. MV infection was productive in MP and even more so in DC. Apoptosis was not induced in either cell type. Moreover, unlike DC, MP were early and strongly activated in response to MV, as shown by the increased surface expression of CD86, CD80, CD54, CD40, and HLA-abc and by the production of mRNA encoding alpha interferon (IFN-α), IFN-β, tumor necrosis factor alpha and interleukin-6. In addition, MV-infected MP produced less of the virus than DC, which was related to the fact that these cells secreted IFN-α. Thus, the strong activation of MP is probably a major event in the control of MV infection and may be involved in the induction of an adaptive immune response in infected hosts. These results may explain the difference in pathogenicity between LV and MV.
Lassa fever, a hemorrhagic disease caused by Lassa virus (LV), is endemic in West Africa, where it causes between 5,000 and 6,000 deaths annually (29). LV, an Arenavirus belonging to the Arenaviridae family, is constituted of two segments of single-stranded RNA. The small segment, S, encodes the full-length envelope glycoprotein (GP) precursor, cleaved into GP1 and GP2 after translation (22), and the viral nucleoprotein (NP). The large segment, L, encodes the RNA polymerase (L) and a small zinc protein (Z) involved in the regulation of transcription (34). LV induces persistent and asymptomatic infections in its natural host, the peridomestic rodent Mastomys sp. (29). Humans are infected following cutaneous or mucosal contact with contaminated blood, feces, or urine from these rodents. Human-to-human transmission is often observed, particularly during nosocomial outbreaks (29). The severity of the illness in humans may range from asymptomatic infection to severe hemorrhagic fever. The first symptoms appear within 10 days: headache, fever, and asthenia are the most common. A few days later, diarrhea, vomiting, renal or hepatic disorders, and hemorrhages may appear. In the severe form, multisystem failure with hypotension and hypovolemia can lead to death (13). Symptoms disappear 15 days later in surviving patients, but deafness occurs in about one-third of patients (11). No vaccine is presently available, and treatment with ribavirin, the only antiviral drug able to cure patients, has to be initiated as soon as possible, within the first 7 days after infection to be efficient, limiting its use in countries of endemicity (28).
Immune responses to LV are not well understood. Macrophages (MP) and dendritic cells (DC) are the main targets of LV (2, 26), but hepatocytes (38) and endothelial cells (23) can also be infected. As MP and DC are essential to induce and regulate immune responses, their early infection may be crucial for the progression of the disease. Specific antibodies against LV are not correlated with survival in humans or nonhuman primates (15, 17). Instead, cellular immune responses against GP seem important to the control of infection (15). Conversely, immunosuppression could be at the origin of severe cases of Lassa fever, as described for mice infected with lymphocytic choriomeningitis virus (LCMV) (6). To improve our understanding of the immune mechanisms that occur during Lassa fever, we compared the responses of antigen-presenting cells (APC) infected with Mopeia virus (MV) to those recently described for LV-infected APC (2). Indeed, MV is closely related to LV, sharing 75% amino acid identity (7), and it is also isolated from the same reservoir. MV is not pathogenic for humans or nonhuman primates and can protect monkeys from a lethal challenge with LV (21). Moreover, unlike MV, LV suppresses the expression of the proinflammatory chemokine interleukin-8 (IL-8) (at the mRNA and protein levels) in endothelial cells and is a stronger down-regulator of IL-8 than MV in MP (23). Here, we analyzed the in vitro production of MV particles in MP and DC and the expression of cell surface molecules. We also determined the levels of mRNA encoding various cytokines and the amounts of the corresponding proteins secreted. Finally we studied the role of alpha/beta interferon (IFN-α/β) or their inducers in the control of MV replication. Comparison of the results obtained with MV and LV revealed differences in innate immune responses that could explain the different clinical outcomes observed with these two viruses.
MATERIALS AND METHODS
MV.
MV strain AN23166, isolated from Mastomys sp. in Mozambique (39), was kindly provided by C. Clegg and G. Lloyd (National Collection of Pathogenic Viruses, Centre for Applied Microbiology and Research, Porton Down, Salisbury, United Kingdom). MV was grown on Vero E6 cells cultured in Dulbecco's modified minimum Eagle's medium (DMEM) with 2% fetal calf serum (FCS), 1% penicillin-streptomycin, 1% nonessential amino acids (all from Invitrogen, Cergy Pontoise, France) (full DMEM-2% FCS) at 37°C in a 5% CO2 atmosphere. The culture supernatant was harvested 4 days after infection, and the viral titer was determined to be 108 focus-forming units/ml. Vero E6 cells and the MV strain were not contaminated with mycoplasma (data not shown).
Titration assays.
Serial 10-fold dilutions of MV-infected MP, DC, or Vero E6 cell supernatants (500 μl) were incubated in full DMEM-2% FCS for 1 h at 37°C on Vero E6 cells grown at confluence. Carboxymethyl cellulose (1.6%; BDH Laboratories, Poole, United Kingdom) in full DMEM-2% FCS was then added, and plates were incubated at 37°C in a 5% CO2 atmosphere for 6 days. Cells were fixed with 3.7% formaldehyde (Sigma, St Louis, Mo.) in phosphate-buffered saline (PBS; Invitrogen), washed, and permeabilized with 0.5% Triton X-100 (Sigma) in PBS. Monoclonal antibodies (MAbs), kindly provided by P. Jahrling (U.S. Army Medical Research Institute for Infectious Diseases, Fort Detrick, Md.), specific for MV GP2 (hybridoma L53-237-5), MV NP (hybridoma YQB06-AE05), or LV NP (hybridoma L52-54-A6, which also recognizes MV NP) were then added for 3 h at 37°C. Peroxidase-conjugated goat anti-mouse antibody (Sigma) was then added for 1 h at 37°C, and infected cell foci were revealed with diaminobenzidine.
Preparation of MP and DC.
Monocytes were isolated from the blood of healthy donors provided by the Etablissement Français du Sang (Lyon, France) as previously described (2). Purified monocytes were cultured for 6 days in RPMI with 1% penicillin-streptomycin, 1% nonessential amino acids, 1 M HEPES, and 10% FCS (full RPMI-10% FCS) containing recombinant human macrophage colony-stimulating factor (20 ng/ml) (PeproTech, Rocky Hill, N.J.) to obtain MP. To prepare immature DC, purified monocytes were cultured in full RPMI-10% FCS containing recombinant human granulocyte-macrophage colony-stimulating factor (2,000 IU/ml) and recombinant human IL-4 (rhIL-4; 1,000 IU/ml) (PeproTech). In both cases, 40% of the culture medium and the initial amount of cytokines were renewed every 48 h.
Infection of MP and DC with MV.
After 6 days of culture, MP and DC were harvested and then infected with virus-free Vero E6 cell supernatants (mock) or with MV at different multiplicities of infection (MOI) for 1 h at 37°C. Low MOI (0.2) were used for titration assays to favor production of viral particles, whereas MOI of 2 were chosen for other studies. Cells were then washed three times and cultured at 106 cells/ml. In some experiments, cells were stimulated 2 h after infection with soluble CD40 ligand (sCD40L) (200 ng/ml) and enhancer (1 μg/ml) (Alexis Biochemical, Lausanne, Switzerland) or lipopolysaccharide (LPS) (0.5 μg/ml) from Escherichia coli (Sigma). In other cases, cells were cultured in the presence of rhIFN-α2b (5 ng/ml), IFN-β (10 ng/ml), IFN-γ (10 ng/ml) (PeproTech), neutralizing MAb anti-CD118 (4 μg/ml) (PBL; Biomedical Laboratories, Piscataway, N.J.), or poly(I-C) at 120 μg/ml (Pharmacia).
Flow cytometry.
To evaluate the expression of cell surface molecules, MP and DC were harvested, centrifuged, and incubated for 10 min in PBS containing 5% AB+ human serum. Cells were then incubated for 25 min at 4°C with different MAbs (0.1 μg/ml) conjugated to fluorescein (FITC) (HLA-DR, CD14, CD16 [Immunotech, Marseille, France], and CD86 [BD Pharmingen, San Jose, Calif.]), phycoerythrin (PE) (CD25, CD80, CD83 [Pharmingen], CD11c [BD], CD1a, and CD40 [Immunotech]), Cy5 chrome (Cy5) (HLA-abc, CD54, CD95, and CD40 [Pharmingen]), or PE-cyanin 5 (ILT3 [Immunotech]). Isotypic controls were performed with the following irrelevant MAbs: FITC-conjugated immunoglobulin G1(κ) [IgG1(κ)], PE-conjugated IgG2a(κ), and Cy5-conjugated IgG1(κ) (all from BD Pharmingen). The purity of monocytes was checked by using the following lineage-specific MAbs: PE-conjugated CD19 and PE-cyanin 5-conjugated CD3 (Immunotech). The differentiation of monocytes into DC was assessed with anti-CD1a (Immunotech). Stained cells were washed in 2.5% FCS in PBS and resuspended in 3% paraformaldehyde in PBS. To detect apoptosis, cells were double stained with annexin V-FITC and 7-amino-actinomycin D according to the manufacturer's instructions (BD Pharmingen). Finally, cells were analyzed with a three-color flow cytometer (FacsCalibur; BD). Data were analyzed with the Expo 32 ADC software (Applied Cytometry Systems, Dinnington, United Kingdom).
Phagocytosis.
The phagocytic capacity of DC was determined by measuring the internalization of dextran-FITC 3 days after infection. DC were incubated with 0.1 mg of dextran-FITC/ml for 1 h at 37°C or at 4°C as a control. Cells were then washed, resuspended in 3% paraformaldehyde in PBS, and analyzed by flow cytometry.
Analysis of mRNA by RT-PCR.
Total RNA was extracted from 5 × 105 MV-infected or control cells with the RNeasy kit (Qiagen, Hilden, Germany). Genomic DNA was eliminated by DNase I (RNase-free DNase set; Qiagen) digestion. To synthesize cDNA, reverse transcription (RT) was performed with 100 U of SuperScript II reverse transcriptase, 2.5 U of RNase inhibitor, 0.5 μg of oligo(dT), 0.1 M dithiothreitol, 5× first-strand buffer (all from Invitrogen), 10 mM deoxynucleotide triphosphate mix (Pharmacia), and one-fifth of the extracted RNA. The resulting cDNA was subsequently amplified by qualitative or quantitative PCR (TaqMan; Applied Biosystems, Courtaboeuf, France). Qualitative PCR was performed with 1 U of Taq polymerase, 10× PCR buffer (Invitrogen), 0.4 μM concentrations of each primer (Applied Biosystems), and 10 mM deoxynucleotide triphosphate mix (Pharmacia). The following primers were used: for β-actin, 5′-CAGGCACCAGGGCGTGAT-3′ and 5′-GCCAGCCAGGTCCAGACG-3′; for IFN-α, 5′-ACTTTGGATTTCCCCAGGA-3′ and 5′-CAGGCACAAGGGCTGTATT-3′; for IL-6, 5′-AGTTGCCTTCTCCCTGG-3′ and 5′-ATTTGCCGAAGAGCCCTCA-3′. Lack of contamination by genomic DNA was checked by amplifying RNA without RT. PCR products were visualized by electrophoresis on a 1.5% agarose gel. Real-time PCR was performed with commercial primers and TaqΜan probes for TNF-α, IL-1β, IL-10, IL-12p35, and CCR-7 (Applied Biosystems). We designed a specific probe and specific primers for IFN-β: probe, 5′-AACTTGCTTGGATTCCT-3′; forward primer, 5′-TCTCCACGACAGCTCTTTCCA-3′; reward primer, 5′-ACACTGACAATTGCTGCTTCTTTG-3′. The β-actin gene was amplified in duplex with a commercial probe and primers (Applied Biosystems) to allow normalization of the results. TaqMan Universal PCR master mix (Applied Biosystems) was used to amplify genes. Data were then analyzed as follows: ΔCt = gene Ct − β-actin Ct, where Ct represents the cycle threshold of the β-actin gene and the amplified gene. Means were calculated as follows: 2−(ΔCt1 + ΔCt2 +… + ΔCtn)/n.
Detection of cytokines in supernatants of MV-infected cells.
The following commercial kits were used to determine the amount of cytokines produced by MP or DC: hIFN-α enzyme-linked immunosorbent assay (ELISA) kit (PBL), human tumor necrosis factor alpha (hTNF-α) ELISA kit, hIL-10 ELISA kit, and hIL-1β ELISA kit (Sanquin Reagents, Amsterdam, The Netherlands). The concentration of cytokines is expressed in picograms per milliliter, except for IFN-α, which is expressed in international units per milliliter (with 0.3 IU/pg).
Statistical analysis.
The Student t test or the nonparametric Wilcoxon test was used to compare mRNA levels and virus titers. Differences between two sets of data were considered to be significant when the P value was <0.05 or <0.01. For some experiments, the standard deviation (SD) was also determined. Statistic tests were performed by using the Open Stat, version 3.4.1, software (developed by W. G. Miller).
RESULTS
MV infection of MP and DC is productive.
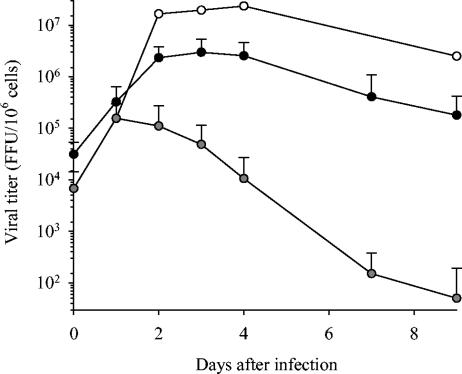
MOI of 0.01, 0.2, and 2 were tested for Vero E6 cells, DC, and MP, and each cell type was infected with the MOI that induced the most important release of MV particles (data not shown). Vero E6 cells were therefore infected with MV at an MOI of 0.01 while an MOI of 0.2 was used for MP and DC. No cytolytic effect was observed for any type of cells during the 9-day culture period. Infection of both MP and DC with MV was productive (Fig. 1). DC produced 10 to 100 times more viral particles than MP (P < 0.05); the titers almost reached the levels obtained with Vero E6 cells. In both cell types, virus release was detected as soon as 24 h after infection. Viral production peaked 24 to 48 h and 72 h after infection of MP and DC, respectively. Replication subsequently decreased more rapidly in MP than in DC. Immunofluorescence was performed 3 days after infection. Viral NP was detected in MV-infected DC and MP, confirming that most cells were infected (data not shown).
FIG. 1.
Production of viral particles by MV-infected MP and DC. The production of viral particles in supernatants of MP (gray circles) and DC (black circles) infected with MV (MOI = 0.2) is represented as the mean ± SD of the results from five and six independent experiments for MP and DC, respectively. The production of MV by Vero E6 cells (MOI = 0.01) is also indicated (white circles). Results are expressed as the number of viral particles (focus-forming units [FFU]) per million cells, and the day zero time point represents the virus titer 2 h after the infection of cells.
MV does not induce apoptosis of MP and DC.
To confirm that MV did not affect the viability of MP or DC, viable cells were enumerated after trypan blue staining. Cells were double stained with annexin V and 7-amino-actinomycin D to distinguish between early apoptotic cells, late apoptotic or necrotic cells, and viable cells. Two and four days after infection, the numbers of control and MV-infected MP were similar regarding viable cells (71 to 86% at day 2 and 78 to 84% at day 4 for control and MV-infected MP, respectively), early apoptotic cells (3 to 1% and 4 to 5%), and late apoptotic or necrotic cells (26 to 13% and 18 to 11%). MV infection seems to slightly inhibit the apoptosis of MP, but the difference was not significant. Similar data were obtained for DC for viable cells (80 to 84% at day 2 and 68 to 70% at day 4 for control and MV-infected DC, respectively), early apoptotic cells (10 to 8% and 14 to 13%), and late apoptotic or necrotic cells (10 to 8% and 18 to 17%). Thus, MV did not induce the apoptosis of MP and DC.
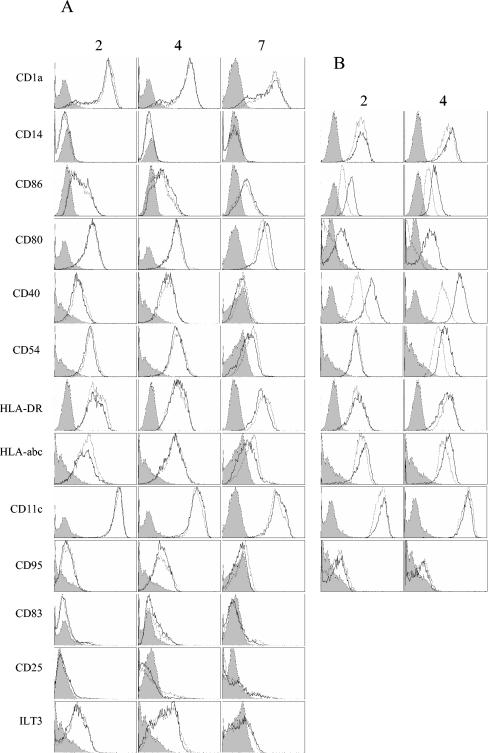
MV activates MP but not DC.
Given that MV replicates in both MP and DC, we evaluated their activation after MV infection. Thus, we used flow cytometry to determine the expression levels of several cell surface molecules. The differentiation of monocytes into MP or DC was checked by assessing the surface expression of CD14 and CD1a. DC were characterized by strong expression of CD1a and no CD14 (Fig. 2A), whereas MP strongly expressed CD14 (Fig. 2B). The expression of CD86, CD80, CD40, CD54, HLA-DR, HLA-abc, CD11c, CD95, CD25, and ILT-3 was not modified by MV infection of DC in the first 4 days after infection (Fig. 2A). A small increase in CD86, CD80, and CD11c expression and a slight decrease in HLA-DR, HLA-abc, CD54, and CD40 expression were observed 7 days after infection of DC compared to control cells, but these differences were not significant. Expression of CD83, a marker of mature DC, was similar in MV-infected and control DC. Thus, DC were not activated by MV and did not mature in response to infection. On the contrary, a marked increase in CD86, CD80, CD40, and HLA-abc expression was observed in MP just 2 days after infection and was even greater 2 days later, whereas the synthesis of CD54 was increased only 4 days after infection (Fig. 2B). These data indicate that MP are strongly and durably activated by MV and that this activation occurs early after infection.
FIG. 2.
Expression of surface molecules on MV-infected MP and DC. The expression of several molecules at the surface of DC (A) and MP (B) was analyzed by flow cytometry 2, 4, and 7 (DC only) days after infection. Control DC or MP are represented by gray lines, and MV-infected cells (MOI = 2) are represented by black lines. Isotype controls are shown as gray histograms. Data are representative of the results from five independent experiments for DC and four independent experiments for MP.
DC do not mature in response to MV.
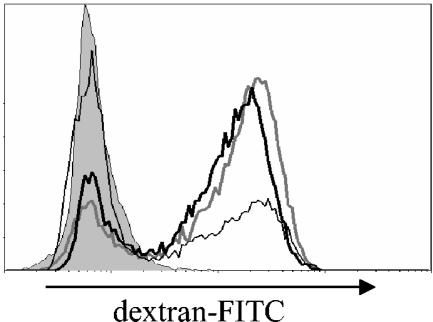
We studied the functional properties of DC by using flow cytometry to characterize their capacity to internalize dextran-FITC. Immature DC have a strong phagocytic capacity, whereas mature DC lose this ability. Three days after infection with MV (MOI of 2), DC internalized dextran-FITC in a similar manner to control DC (Fig. 3). This result was consistent with the lack of increase of CD83 and suggested that DC did not mature following infection with MV. When control DC were cultured with sCD40L, their phagocytic ability was strongly reduced compared with nonstimulated control DC (Fig. 3).
FIG. 3.
Phagocytosis of dextran-FITC by MV-infected DC. Flow cytometry was used to analyze the internalization of dextran-FITC by DC 3 days after infection. Control cells incubated at 4°C are represented by the gray histogram, mock-infected DC are represented by the gray line, and MV-infected DC (MOI = 2) are represented by the thick black line. Control cells stimulated with sCD40L 2 h after infection are also shown (thin black line).
MV-infected MP, and to a lesser extent DC, produce mRNA coding for inflammatory mediators.
Qualitative RT-PCR showed that IFN-α and IL-6 mRNA levels were elevated in MV-infected MP 24 h after infection (MOI of 2) (data not shown). IL-6 mRNA levels peaked at this time, whereas IFN-α mRNA was still present 72 h after infection. Production by MV-infected DC of mRNA encoding IFN-α was increased from 48 h after infection.
Real-time RT-PCR data were consistent with these results. We showed that MV-infected MP produced substantially more IFN-β mRNA than control MP whatever the time after infection was (Table 1). The production peaked at 24 h postinfection (h.p.i.). MV-infected MP also produced significantly more mRNA encoding TNF-α and IL-12p35 than mock-infected MP, notably on the first day after infection. No difference was found between the production of IL-1β and IL-10 mRNA by MV-infected and control MP. MV-infected DC produced significantly more IFN-β mRNA than control DC but 10 times less than MP. Mock- and MV-infected DC produced similar levels of mRNA encoding TNF-α, IL-1β, IL-12p35, IL-10, and CCR-7. Thus, MV-infected MP appear to produce more mRNA encoding inflammatory cytokines in response to MV infection than DC, which only produce IFN-α/β mRNA in small amounts. This confirms that MP are strongly activated by MV while DC are only slightly activated.
TABLE 1.
Expression of mRNAs in MV-infected MP and DCa
| Cell type | Time (h.p.i.) | Infection type | Expression of:
|
|||||
|---|---|---|---|---|---|---|---|---|
| IFN-β (10−4) | TNF-α (10−2) | IL-1β (10−1) | IL-10 (10−2) | IL-12p35 (10−4) | CCR-7 (10−2) | |||
| MP | 6 | Mock | 1.9 (0.9-4) | 10 (3.3-25) | 8.7 (1.7-150) | 4.7 (1.7-11) | NDb | ND |
| MV | 7.4 (1.5-28) | 20 (2.4-103) | 15 (1.4-170) | 8.2 (2.5-19) | ND | ND | ||
| 12 | Mock | 3.2 (2.6-4) | 5.8 (3.3-11) | 16 (6.2-40) | 22 (8.8-58) | ND | ND | |
| MV | 52 (20-140) | 22 (5.4-93) | 9.3 (3.5-26) | 14 (5.4-38) | ND | ND | ||
| 24 | Mock | 0.6 (0.2-1.9) | 2.4 (0.3-7.2) | 0.9 (0.5-2.9) | 3.8 (0.7-14) | 0.6 (0.3-2.1) | ND | |
| MV | 170*c (73-630) | 15** (5.4-54) | 0.8 (0.2-3.5) | 6.7 (3.3-10) | 26* (4.6-55) | ND | ||
| 48 | Mock | 0.7 (0.2-2.4) | 3.4 (3.3-3.6) | 0.8 (0.3-2.9) | 3.1 (2.9-3.3) | 14 (14-14) | ND | |
| MV | 130* (96-180) | 10 (7.7-13) | 1.4 (1.1-1.9) | 8.2 (3.6-20) | 16 (16-16) | ND | ||
| DC | 6 | Mock | 4.6 (1.9-12) | 76 (47-132) | 1.6 (1.4-2) | 0.6 (0.2-2.1) | ND | ND |
| MV | 7.9 (7.4-8.5) | 81 (38-174) | 2 (0.8-5) | 1.2 (1.2-1.3) | ND | ND | ||
| 12 | Mock | 2.1 (0.5-9.8) | 54 (20-152) | 1.3 (0.8-1.9) | 0.5 (0.1-1.9) | ND | ND | |
| MV | 5.6 (3.7-9.1) | 47 (25-87) | 1.1 (0.5-2.3) | 1.1 (1-1.2) | ND | ND | ||
| 24 | Mock | 0.3 (0.2-0.6) | 2.2 (1.3-3.1) | 0.2 (0.02-0.9) | 1.1 (0.4-9.5) | 0.9 (0.05-6) | 4.4 (0.8-25) | |
| MV | 4.9* (1.4-14) | 3.1 (1.7-6.7) | 0.2 (0.01-1.3) | 1.4 (0.2-9.5) | 1.4 (0.08-9.1) | 8.2 (1.6-38) | ||
| 48 | Mock | 0.9 (0.4-4.9) | 4.7 (0.8-107) | 0.4 (0.1-1.3) | 1.3 (0.5-2.4) | 4.9 (4-7.9) | 5.4 (1.8-9.5) | |
| MV | 11* (4.3-28) | 6.7 (1-152) | 0.7 (0.2-6.2) | 2.5 (0.7-12) | 4.3 (1.2-2) | 11 (7.7-15) | ||
Averages of the results from six or five independent experiments at 24 and 48 h.p.i. are given for DC and MP, respectively. Averages of the results from two independent experiments at 6 and 12 h.p.i. are also given for both MP and DC. Results represent the number of copies of the respective mRNA per the number of copies of β-actin mRNA. The ranges of the results from the independent experiments are indicated in parentheses.
ND, not determined.
Significant differences between control cells and MV-infected cells at 24 and 48 h.p.i. are indicated by an asterisk (*) (P < 0.01) or a double asterisk (**) (P < 0.05). Statistics were not calculated at 6 and 12 h.p.i. due to the low number of experiments.
MV-infected MP, but not DC, secrete IFN-α.
As several mRNAs encoding inflammatory cytokines were produced by MV-infected APC, we determined whether these mRNAs were translated into proteins. We therefore determined the quantities of IFN-α, TNF-α, IL-1β, and IL-10 in cell supernatants. IL-1β, IL-10 (data not shown), and TNF-α were not secreted by MV-infected MP, whereas moderate levels of IFN-α were detected from 24 h after infection in the supernatants. Higher levels were detected 48 and 72 h after infection (Table 2). Mock- and MV-infected DC secreted neither IL-1β protein nor IFN-α protein (data not shown). Levels of TNF-α and IL-10 were lower or equivalent in MV-infected DC supernatants than in mock-infected cell supernatants, whatever the time after infection was (Table 2).
TABLE 2.
Production of inflammatory cytokines by MV-infected MP and DCa
| Cell type | Time (h.p.i.) | Infection type | Amt of IFN-α or IL-10b | Amt of TNF-α |
|---|---|---|---|---|
| MP | 6 | Mock | <3 | 7 |
| MV | <3 | 6 | ||
| 12 | Mock | <3 | <6 | |
| MV | <3 | <6 | ||
| 24 | Mock | 30 | <6 | |
| MV | 45 | <6 | ||
| 48 | Mock | 36 | <6 | |
| MV | 76 | <6 | ||
| 72 | Mock | <3 | <6 | |
| MV | 120 | <6 | ||
| DC | 6 | Mock | 10 | 110 |
| MV | 10 | 70 | ||
| 12 | Mock | 10 | 147 | |
| MV | 8 | 47 | ||
| 24 | Mock | 34 | 46 | |
| MV | 12 | 14 | ||
| 48 | Mock | 8 | 6 | |
| MV | <6 | 10 | ||
| 72 | Mock | 12.8 | 24 | |
| MV | 12 | <6 |
Results are expressed in picograms per milliliter, except for those for IFN-α, which are expressed in international units per milliliter. Results are averages of those from two independent experiments.
IFN-α was measured for MP, and IL-10 was measured for DC.
Role of IFN-α/β in control of MV replication.
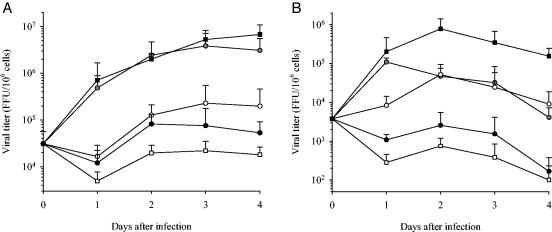
To determine whether IFN-α/β could be involved in the control of viral replication, DC and MP were infected with MV (MOI of 0.2) or mock infected and were stimulated 2 h after infection with LPS, rhIFN-α, IFN-β, IFN-γ, poly(I-C), or neutralizing MAb anti-CD118. The production of viral particles by DC and MP was strongly inhibited when cells were cultured with IFN-α (Fig. 4) or IFN-β (data not shown) (P < 0.05). The drop in virus production was even greater when DC and MP were stimulated with poly(I-C) (P < 0.05). In contrast, virus titers were not altered when DC and MP were infected in the presence of IFN-γ (data not shown). LPS reduced the release of viral particles by DC (P < 0.05) but had only a transient effect at 24 h on MP. The neutralization of IFN-α/β receptors did not modify the release of the virus by DC, whereas it significantly (P < 0.05) enhanced viral production by MP. These results confirmed that IFN-α/β is not secreted by MV-infected DC despite the synthesis of the respective mRNAs; this differs from the situation for MV-infected MP. This experiment highlights the role of endogenous IFN-α/β produced by MV-infected MP in the control of virus replication.
FIG. 4.
Replication of MV in stimulated DC and MP. The production of MV in DC (A) and MP (B) (MOI = 0.2) is represented as a function of time after infection. DC and MP were stimulated 2 h after infection with medium (gray circles), LPS (white circles), rhIFN-α2b (black circles), poly(I-C) (white squares), or neutralizing anti-CD118 (black squares). Results are expressed as the number of viral particles (focus-forming units [FFU]) per million cells (mean ± SD of the results from three independent experiments). The day zero time point represents the virus titer 2 h after the infection of cells.
Production of mRNA encoding cytokines and a chemokine receptor by MV-infected MP and DC activated by different stimuli.
To assess whether the reduction of viral replication observed with some stimuli was linked to the production of IFN-α/β, MP and DC were infected at an MOI of 2 and stimulated with the same molecules used in the previous experiments. The level of IFN-α mRNA was determined by qualitative RT-PCR (data not shown). Only DC, infected or not, produced consistent amounts of IFN-α mRNA after stimulation with poly(I-C). MV-infected MP produced more IFN-α mRNA than control cells in the presence of each stimulus except poly(I-C), which induced strong levels of mRNA in both control and MV-infected MP (data not shown). Data obtained with MP and DC by quantitative RT-PCR demonstrated that LPS was a very strong inducer of TNF-α, IL-1β, and IL-10 for MP, as early as 6 h.p.i. (Table 3). Then the production of these mRNAs rapidly decreased. The same results were obtained for DC, even if the production of these mRNAs was always less important than that of MP. poly(I-C) and, to a lesser extent, IFN-α were very strong inducers of IFN-β, IL-12p35, and TNF-α mRNAs in MP and DC, without a decrease after 6 h.p.i. for MP. It seems important that, concerning a majority of cytokines, the production of mRNA was higher in mock-infected APC at 6 h.p.i., whereas production was higher in MV-infected APC at 24 h after infection (Table 3). Thus, MV infection could probably interact early with the stimuli, and this effect slowly disappears, being negligible at 24 h after infection. At this time, stimuli and MV infection may act in synergy to produce mRNA encoding inflammatory cytokines.
TABLE 3.
Production of mRNA by MV-infected MP or DC stimulated with various molecules
| Cell type and stimulatory molecule | Infection type | Expression of mRNA at time (h.p.i.)a
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IFN-β (10−4)
|
TNF-α (10−2)
|
IL-1β (10−1)
|
IL-10 (10−2)
|
IL-12 (10−4), 24 | CCR-7, 24 | ||||||||||
| 6 | 12 | 24 | 6 | 12 | 24 | 6 | 12 | 24 | 6 | 12 | 24 | ||||
| MP | |||||||||||||||
| Medium | Mock | 1.9 | 3.2 | 0.6 | 10 | 5.8 | 2.4 | 8.7 | 16 | 0.9 | 4.7 | 22 | 3.8 | 0.6 | NDb |
| MV | 7.4 | 52 | 170 | 20 | 22 | 15 | 15 | 9 | 0.8 | 8.2 | 14 | 6.7 | 26 | ND | |
| LPS | Mock | 110 | 11 | 2.8 | 650 | 54 | 18 | 43,900 | 7,240 | 70 | 8,450 | 750 | 31 | 2.4 | ND |
| MV | 55 | 20 | 160 | 430 | 44 | 40 | 27,000 | 20,500 | 110 | 1,970 | 860 | 100 | 42 | ND | |
| IFN-α | Mock | 17 | 2.4 | 7.4 | 300 | 7.7 | 44 | 2.3 | 0.7 | 1.9 | 160 | 13 | 13 | 34 | ND |
| MV | 28 | 330 | 310 | 90 | 57 | 66 | 1.5 | 1.5 | 7.6 | 13 | 27 | 31 | 90 | ND | |
| poly(I-C) | Mock | 5,000 | 13,200 | 24,600 | 920 | 750 | 985 | 65 | 4.1 | 37 | 81 | 27 | 62 | 2,330 | ND |
| MV | 8,120 | 16,300 | 40,000 | 370 | 860 | 610 | 140 | 3.5 | 98 | 87 | 17 | 22 | 2,330 | ND | |
| DC | |||||||||||||||
| Medium | Mock | 4.6 | 2.1 | 0.3 | 76 | 54 | 2.2 | 1.7 | 1.3 | 0.2 | 0.6 | 0.5 | 1.1 | 0.9 | 0.04 |
| MV | 7.9 | 5.6 | 4.9 | 81 | 47 | 3.1 | 2 | 1.1 | 0.2 | 1.2 | 1.1 | 1.4 | 1.4 | 0.08 | |
| LPS | Mock | 310 | 28 | 1.1 | 1,300 | 57 | 6.3 | 520 | 35 | 0.9 | 22 | 11 | 1.3 | 9.8 | 1.1 |
| MV | 130 | 42 | 4.9 | 430 | 110 | 10 | 600 | 150 | 2 | 15 | 25 | 0.7 | 16 | 2 | |
| IFN-α | Mock | 45 | 3.7 | 6.4 | 265 | 62 | 15 | 3.8 | 0.3 | 0.2 | 6.3 | 3.1 | 2.4 | 2.4 | 0.2 |
| MV | 110 | 59 | 20 | 430 | 41 | 13 | 3.3 | 0.5 | 0.2 | 7.7 | 4.1 | 6.3 | 9.8 | 0.3 | |
| Poly(I-C) | Mock | 6,600 | 1,170 | 1,090 | 1,600 | 160 | 123 | 40 | 1.9 | 1.8 | 5.1 | 1.8 | 9.5 | 96 | 5.3 |
| MV | 1,650 | 880 | 1,650 | 350 | 130 | 57 | 7.1 | 1.5 | 4 | 1.5 | 1.8 | 2.7 | 136 | 1.4 | |
Results are expressed as means of the results from two independent experiments and represent the number of copies of the respective mRNA per the number of copies of β-actin mRNA.
ND, not determined.
DISCUSSION
This in vitro model of human MP and DC allowed us to analyze the responses of these cells to infection with MV, an Arenavirus that is not pathogenic for humans or nonhuman primates. Comparison of these results with those obtained in similar experiments with LV (2), a highly pathogenic Arenavirus, offered an opportunity to understand the mechanisms associated with immunosuppression and pathogenesis in Lassa fever.
MP and DC were highly permissive to MV infection which was productive but not cytolytic. These results are consistent with those of previous experiments demonstrating the ability of MV to infect MP and endothelial cells in vitro (23). MV replicated more rapidly in DC than in MP. Virus production rapidly decreased in MP, whereas viral release remained almost constant in DC over time. These results are similar to the kinetics of viral particle production observed during the infection of MP and DC by LV (2). MV-infected MP and DC produced as many viral particles as their LV-infected counterparts (2), indicating that the attenuation of MV is not linked to the intensity of the initial viral replication or to the tropism for APC.
We showed that MV, like LV (2), did not induce the apoptosis of MV-infected MP or DC, even if we could note a slight nonsignificant inhibition of MP apoptosis. This is coherent with the suggested role of the Z protein of the arenaviruses to inhibit apoptosis by interacting with the proapoptotic promyelocytic leukemia protein (4, 5). These results are also consistent with the lack of up-regulation of surface expression of CD95 in MV-infected cells.
The cutaneous or mucosal location of MP and DC, together with the probable cutaneous or mucosal mode of contamination, means that MP and DC are likely to be the first cells encountered by the virus. The elevated production of MV particles by DC and MP combined with the lack of cell death suggests that these cells are early and crucial reservoirs, permitting the replication and spread of the virus from the initial site of infection to the whole body.
DC were only weakly activated by MV; we observed a low but nonsignificant increase in the expression of CD86 and CD80 7 days after infection. MV-infected DC did not mature, as demonstrated by the strong internalization of dextran-FITC and the lack of expression of CD83. The production of cytokines by DC is in accordance with these results. No difference was found between mock- and MV-infected DC concerning the mRNA and protein contents of TNF-α, IL-1β, IL-6, and IL-12. These results are consistent with those obtained with LV, as DC were not activated in response to LV infection and did not produce inflammatory cytokines (2). In contrast, the synthesis of mRNA encoding IFN-α/β was induced in response to MV infection, demonstrating that these cells are weakly activated during the first days after infection.
In striking contrast, the MP surface expression of CD86, CD80, CD54, and HLA-abc is rapidly and strongly up-regulated by MV. MP activation was confirmed by the induction in response to MV of the synthesis of moderate levels of mRNA coding for TNF-α and IL-6 (23). Nevertheless, this low amount of mRNA did not lead to a significant release of TNF-α in the supernatant. More importantly, MV-infected MP produce large amounts of mRNA coding for IFN-α/β, and significant levels of IFN-α were detected in the supernatant. Thus, the response of MP to MV infection differs strikingly from the response of MP to LV. Indeed, LV infection of MP induces neither cell activation nor the expression of inflammatory cytokines, except for a modest synthesis of IFN-α/β mRNA (2; S. Baize, D. Pannetier, C. Faure, P. Marianneau, I. Marendat, M.-C. Georges-Courbot, and V. Deubel, submitted for publication). The activation of MP and their synthesis of inflammatory cytokines could be primordial in the rapid clearance of MV infection, as activated MP are known to possess strong microbicidal functions (32). Indeed, a study on the new world Arenavirus Pichinde, a model for Lassa fever, suggested that virulence is negatively correlated with the activation of MP (14): two strains of this virus were able to infect MP but only the less virulent strain activated these cells. These results suggest that during Arenavirus infection, viruses that are not pathogenic for humans may activate MP. The difference in MP responses to LV and MV infections is thus probably crucially involved in the control of viral infection. MV and other arenaviruses, such as LV, Mobala virus, Oliveros virus, and LCMV, infect host cells, notably DC, via α-dystroglycan (αDG) (8, 37). In LCMV-infected mice, the virulence of the different strains of LCMV is related to their affinity for αDG. In fact, strains that have a high affinity for αDG present a tropism for splenic white pulp DC, leading to immunosuppression and viral persistence. In contrast, strains with low affinity replicate in splenic red pulp MP, leading to the control of infection (36). However, the binding affinity for αDG seems to be unrelated to human pathogenicity, since LV and MP are both high-affinity binders (8, 37). Before reaching distinct splenic compartments, all LCMV strains replicate primarily in splenic marginal zone MP (36), which play a crucial role in the control of the infection. In fact, depletion of metallophilic and marginal zone MP in LCMV-infected mice leads to uncontrolled infection (35). Thus, we can speculate that both LV and MV also have an initial tropism for marginal zone MP before infecting DC of the white pulp. The ability of MP to be strongly activated and to release significant amounts of IFN-α/β in response to MV infection probably allows an early control of MV release. This may finally result in less extensive infection of splenic white pulp DC. Moreover, in the environment of secondary lymphoid organs, the weak activation of DC observed early after infection could be enhanced either by contact with MP, which are strongly activated by MV, or by the inflammatory cytokines produced by these MP. Indeed, IFN-α strongly activated MV-infected DC, as shown by the synthesis of mRNA coding for IFN-β, IL-1β, IL-6, IL-12p35, and CCR-7. This may result in mature and activated DC, the most efficient cells to induce primary T lymphocyte responses (3). Conversely, antigens presented by immature DC can induce a tolerance state, impeding the activation of T lymphocytes (16). Thus, our results agree with previous experiments suggesting that the absence of activation of LV-infected DC could explain the immunosuppressive nature of Lassa fever (2). Interestingly, during human Lassa fever, survival is correlated with the presence of inflammatory cytokines in plasma (25).
IFN-α is well known for its major antiviral properties (20, 31). This IFN-α/β production could be linked to the recognition of double-stranded RNA by Toll-like receptor 3 or by protein kinase receptor (1, 18). On the other hand, Toll-like receptor 7 may also be crucially involved, considering its ability to recognize single-stranded RNA, as recently described (24). We demonstrated that MV is sensitive to IFN-α/β and their inducers. In fact, the addition of IFN-α or IFN-β to MV-infected MP and DC strongly reduced virus production. The synthetic double-stranded RNA poly(I-C), a strong inducer of IFN-α (19, 27), was even more efficient than IFN-α at inhibiting viral production. poly(I-C) probably induces a strong synthesis of IFN-β and of many IFN-α subclasses able to act on virus replication.
MV induced stronger IFN-α/β responses in DC and MP than did LV. MV-infected DC produced IFN-α mRNA, unlike LV-infected DC (Baize et al., submitted). However, the production of IFN-α/β by MV-infected DC was very weak, as evidenced by the absence of detection of IFN-α in supernatants and by the lack of increase of viral titers when the IFN-α/β receptor was neutralized. Similarly, MV-infected MP produced large amounts of IFN-α/β mRNA and protein, whereas LV-infected MP only produced modest amounts of IFN-α mRNA after some delay, with low levels of the protein in the supernatant. Moreover, the important production of IFN-α/β by MV-infected cells is reminiscent of the observed differences in the responses of mice infected with LCMV and murine cytomegalovirus. In fact, unlike murine cytomegalovirus, a massive release of IFN-α/β occurred during LCMV infection (9, 12). This response favors the induction of cytotoxic T-cell responses and induces proliferation and cytotoxic functions of NK cells. Thus, the important IFN-α/β release by MV-infected MP may contribute to the induction of protective CD8+ T cells.
The strong decrease in virus replication in MP from the second day after infection was probably linked to the production of IFN-α/β. In fact, neutralization of the IFN-α/β receptor increases virus release to almost the level detected in MV-infected DC. This confirms the presence of endogenous IFN-α/β in supernatants from MV-infected MP and highlights their functional role in the control of MV replication. Conversely, the very low level of IFN-α produced by DC in response to MV infection may explain the strong and durable viral replication in these cells. These data are in agreement with the role of IFN-α/β during LCMV infection. In fact, a correlation between the resistance of LCMV strains to IFN-α/β and their capacity to establish persistent infections in mice has been described previously (30). Other studies have demonstrated that IFN-α and IFN-β may reduce the LCMV load during the initial stages of infection (10, 33). Thus, IFN-α and IFN-β seem to play a fundamental role in the control of MV infection, as they do during LCMV infection, and are probably involved in the attenuation of MV in comparison to LV.
MV-infected MP are probably not involved in the initiation of primary T-cell responses, but they may play a crucial role in the early control of virus replication by their strong activation and production of inflammatory cytokines. Even if DC are weakly activated in vitro after MV infection, their ability to mature in response to the inflammatory cytokines produced by MP probably allows them to induce T-cell responses in secondary lymphoid organs. The relevance of this hypothesis will be further assessed by using an in vitro model of T cells, DC, and MP coculture.
Thus, in contrast to LV, the activation of MP by MV could favor the control of the virus and prevents the immunosuppression observed in Lassa fever. It seems, therefore, that APC and, more particularly, MP are crucially involved in the difference in pathogenicity between the two viruses and that the IFN-α/β response is essential in the control of Arenavirus infections.
Acknowledgments
D.P. is a fellow recipient of the Délégation Générale de l'Armement.
We thank Christopher Clegg and Graham Lloyd for the gift of MV and Peter Jahrling for providing MAbs specific for MV and LV. We also thank the Etablissement Français du Sang for providing blood.
REFERENCES
- 1.Alexopoulou, L., A. Czopik Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 2.Baize, S., J. Kaplon, C. Faure, D. Pannetier, M.-C. Georges-Courbot, and V. Deubel. 2004. Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J. Immunol. 172:2861-2869. [DOI] [PubMed] [Google Scholar]
- 3.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 4.Borden, K. L. B., E. J. Campbell Dwyer, and M. S. Salvato. 1998. An arenavirus RING (zinc-binding) protein binds the oncoprotein promyelocyte leukemia protein (PML) and relocates PML nuclear bodies to the cytoplasm. J. Virol. 72:758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borden, K. L. B., E. J. Campbell Dwyer, and M. S. Salvato. 1997. The promyelocytic leukemia protein PML has a pro-apoptotic activity mediated through its RING domain. FEBS Lett. 418:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borrow, P., C. F. Evans, and M. B. A. Oldstone. 1995. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69:1059-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bowen, M. D., C. J. Peters, and S. T. Nichol. 1997. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for cospeciation between Arenavirus and their rodent hosts. Mol. Phylogenet. Evol. 8:301-316. [DOI] [PubMed] [Google Scholar]
- 8.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. A. Oldstone. 1998. Identification of α-dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 9.Cousens, L. P., J. S. Orange, H. C. Su, and C. A. Biron. 1997. Interferon-α/β inhibition of interleukin 12 and interferon-γ production in vitro and endogenously during viral infection. Proc. Natl. Acad. Sci. USA 94:634-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cousens, L. P., R. Peterson, S. Hsu, A. Dorner, J. D. Altman, R. Ahmed, and C. A. Biron. 1999. Two roads diverged: interferon α/β- and interleukin 12-mediated pathways in promoting T cell interferon γ responses during viral infection. J. Exp. Med. 189:1315-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummins, D., J. B. McCormick, D. Bennett, J. Samba, B. Farrar, S. Machin, and S. P. Fisher-Hoch. 1990. Acute sensorineural deafness in Lassa fever. JAMA 264:2093-2096. [PubMed] [Google Scholar]
- 12.Dalod, M., T. P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Brière, G. Trinchieri, and C. A. Biron. 2002. Interferon α-β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drew, R., G. M. Edington, and H. A. White. 1972. The pathology of Lassa fever. Trans. R. Soc. Trop. Med. Hyg. 66:381-389. [DOI] [PubMed] [Google Scholar]
- 14.Fennewald, S. M., J. F. Aronson, L. Zhang, and N. K. Herzog. 2002. Alterations in NF-κB and RBP-Jk by arenavirus infection of macrophages in vitro and in vivo. J. Virol. 76:1154-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher-Hoch, S. P., L. Hutwagner, B. Brown, and J. B. McCormick. 2000. Effective vaccine for Lassa fever. J. Virol. 74:6777-6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hawiger, D., K. Inaba, Y. Dorsett, M. Guo, K. Mahnke, M. Rivera, J. V. Ravetch, R. M. Steinman, and M. C. Nussenzweig. 2001. Dendritic cells induce peripheral T cell unresponsiveness under steady state in vivo. J. Exp. Med. 194:769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, K. M., J. B. McCormick, P. A. Webb, E. S. Smith, L. H. Elliott, and I. J. King. 1987. Clinical virology of Lassa fever in hospitalized patients. J. Infect. Dis. 155:456-464. [DOI] [PubMed] [Google Scholar]
- 18.Kadereit, S., H. Xu, T. M. Engeman, Y. L. Yang, R. L. Fairchild, and B. R. Williams. 2000. Negative regulation of CD8+ T cell function by the IFN-induced and double-stranded RNA-activated kinase PKR. J. Immunol. 165:6896-6901. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki, N., S. Antonenko, and Y. J. Liu. 2001. Distinct CpG DNA and polyinosinic-polycytidilic acid double-stranded RNA, respectively, stimulate CD11c- type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J. Immunol. 166:2291-2295. [DOI] [PubMed] [Google Scholar]
- 20.Katze, M. G., Y. He, and M. Gale, Jr. 2002. Viruses and interferon: a fight for supremacy. Nature 2:675-687. [DOI] [PubMed] [Google Scholar]
- 21.Kiley, M. P., J. V. Lange, and K. M. Johnson. 1979. Protection of rhesus monkeys from Lassa virus by immunisation with closely related Arenavirus. Lancet ii:738. [DOI] [PubMed] [Google Scholar]
- 22.Lenz, O., J. Ter Meulen, H. Feldmann, H. D. Klenk, and W. Garten. 2000. Identification of a novel consensus sequence at the cleavage site of the Lassa virus glycoprotein. J. Virol. 74:11418-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukashevich, I. S., R. Maryankova, A. S. Vladyko, N. Nashkevich, S. Koleda, M. Djavani, D. Horejsh, N. N. Voitenok, and M. S. Salvato. 1999. Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-α gene expression. J. Med. Virol. 59:552-560. [PMC free article] [PubMed] [Google Scholar]
- 24.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahanty, S., D. G. Bausch, R. L. Thomas, A. Goba, A. Bah, C. J. Peters, and P. E. Rollin. 2001. Low levels of interleukin-8 and interferon-inducible protein 10 in serum are associated with fatal infections in acute Lassa fever. J. Infect. Dis. 183:1713-1721. [DOI] [PubMed] [Google Scholar]
- 26.Mahanty, S., K. Hutchinson, S. Agarwal, M. McRae, P. E. Rollin, and B. Pulendran. 2003. Cutting edge: impairment of dendritic cells and adaptive immunity by Ebola and Lassa viruses. J. Immunol. 170:2797-2801. [DOI] [PubMed] [Google Scholar]
- 27.Manetti, R., F. Annunziato, L. Tomasevic, V. Gaianno, P. Parrochi, S. Romagnani, and E. Maggi. 1995. Polyinosinic acid: polycytidylic acid promotes T helper type 1-specific immune responses by stimulating macrophage production of interferon-alpha and interleukin-12. Eur. J. Immunol. 25:2656-2660. [DOI] [PubMed] [Google Scholar]
- 28.McCormick, J. B., I. J. King, P. A. Webb, C. Scribner, R. Craven, K. Johnson, L. H. Elliott, and R. Belmont-Williams. 1986. Lassa fever: effective therapy with ribavirin. N. Engl. J. Med. 314:20-26. [DOI] [PubMed] [Google Scholar]
- 29.McCormick, J. B., P. A. Webb, J. Krebs, K. Johnson, and E. S. Smith. 1987. A prospective study of the epidemiology and ecology of Lassa fever. J. Infect. Dis. 155:437-444. [DOI] [PubMed] [Google Scholar]
- 30.Moskophidis, D., M. Battegay, M. A. Bruendler, E. Laine, I. Gresser, and R. Zinkernagel. 1994. Resistance of lymphocytic choriomeningitis virus to alpha/beta interferon and to gamma interferon. J. Virol. 68:1951-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 32.Oswald, I. P., T. A. Wynn, A. Sher, and S. L. James. 1992. Interleukin 10 inhibits macrophage microbicidal activity by blocking the endogenous production of tumor necrosis factor a required as a costimulatory factor for interferon γ-induced activation. Immunology 89:8676-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ou, R., S. Zhou, L. Huang, and D. Moskophidis. 2001. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75:8407-8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters, C. J., M. Buchmeier, P. E. Rollin, and T. G. Ksiazek. 1996. Arenavirus, p. 1521-1551. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
- 35.Seiler, P., P. Aichele, B. Odermatt, H. Hengartner, R. M. Zinkernagel, and R. A. Schwendener. 1997. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur. J. Immunol. 27:2626-2633. [DOI] [PubMed] [Google Scholar]
- 36.Smelt, S. C., P. Borrow, S. Kunz, W. Cao, A. Tishon, H. Lewicki, K. P. Campbell, and M. B. A. Oldstone. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strain to the cellular receptor α-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75:448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spiropoulou, C. F., S. Kunz, P. E. Rollin, K. P. Campbell, and M. B. Oldstone. 2002. New world arenavirus clade C, but not clade A and B viruses, utilizes α-dystroglycan as its major receptor. J. Virol. 76:5140-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Winn, W. C. J., T. P. Monath, F. A. Murphy, and S. G. Whitfield. 1975. Lassa virus hepatitis. Observations on a fatal case from the 1972 Sierra Leone epidemic. Arch. Pathol. 99:599-604. [PubMed] [Google Scholar]
- 39.Wulff, H., B. M. McIntosh, D. B. Hamner, and K. M. Johnson. 1977. Isolation of an arenavirus closely related to Lassa virus from Mastomys natalensis in south-east Africa. Bull. W. H. O. 55:441-444. [PMC free article] [PubMed] [Google Scholar]