Abstract
Lipid rafts are enriched in cholesterol and sphingomyelin and are isolated on the basis of insolubility in detergents, such as Brij 98 and Triton X-100. Recent work by Holm et al. has shown that rafts insoluble in Brig 98 can be found in human immunodeficiency virus type 1 (HIV-1) virus-like particles, although it is not known whether raft-like structures are present in authentic HIV-1 and it is unclear whether a virion-associated raft-like structure is required for HIV replication. Independently, it was previously reported that virion-associated cholesterol is critical for HIV-1 infectivity, although the specific requirement of virion cholesterol in HIV-1 was not examined. In the present study, we have demonstrated that infectious wild-type HIV-1 contains Brij 98 rafts but only minimal amounts of Triton X-100 rafts. To directly assess the functional requirement of virion-associated rafts and various features of cholesterol on HIV-1 replication, we replaced virion cholesterol with exogenous cholesterol analogues that have demonstrated either raft-promoting or -inhibiting capacity in model membranes. We observed that variable concentrations of exogenous analogues are required to replace a defined amount of virion-associated cholesterol, showing that structurally diverse cholesterol analogues have various affinities toward HIV-1. We found that replacement of 50% of virion cholesterol with these exogenous cholesterol analogues did not eliminate the presence of Brij 98 rafts in HIV-1. However, the infectivity levels of the lipid-modified HIV-1s directly correlate with the raft-promoting capacities of these cholesterol analogues. Our data provide the first direct assessment of virion-associated Brij 98 rafts in retroviral replication and illustrate the importance of the raft-promoting property of virion-associated cholesterol in HIV-1 replication.
Lipid rafts are ordered plasma membrane (PM) subdomains that have high levels of cholesterol, sphingomyelin, and glycophospholipids compared to the overall membrane (5, 6, 56). Lipid rafts are isolated from the cell on the basis of their cholesterol-dependent insolubility in cold detergent (6, 7, 53), which is a direct consequence of the physical association of sphingomyelin and cholesterol (5, 55, 57). Recently, lipid rafts obtained with different detergents have been shown to vary extensively in their protein and lipid contents (53). Lipid rafts have been implicated as important for the replication of human immunodeficiency virus type 1 (HIV-1) (8, 23, 33, 39, 41, 48, 64). The precise role of lipid rafts in HIV-1 replication is, however, unknown. In particular, it is unclear how virion-associated lipids, such as cholesterol, contribute to HIV-1 infectivity and whether the HIV-1 lipids must be in a raft-like arrangement in the virion. While recent work has shown that rafts insoluble in Brij 98 can be found in HIV-1 virus-like particles (VLPs) (23), the presence of raft-like structures in wild-type (WT) HIV-1 has not been determined. A number of other enveloped viruses also rely on cholesterol-rich lipid rafts to support their replication (3, 25, 34, 35, 37, 52, 60), and it is unclear whether these different viral pathogens share a similar mechanistic requirement of cholesterol to support their replication.
During HIV-1 assembly, viral proteins are transported from the cytoplasm to the PM for budding. The translocation of membrane-associated HIV-1 Gag complexes to membrane rafts is thought to be driven by the multimerization of Gag via its interacting domains (33, 41). These membrane raft structures exhibit properties that are distinct from those of classical rafts (13, 33), and the term “barges” was coined to reflect their altered flotation properties during raft isolation (33). The notable lack of raft markers and the detergent insolubility after cholesterol removal reinforce the notion that these Gag complexes are distinct from classical Triton X-100 (Triton) rafts (13). In contrast to Gag, the viral envelope proteins (Env) are directly targeted to PM lipid rafts by palmitoylation of the cytoplasmic domain of Env (48). The difference between the route of raft association of Env and that of Gag may play a subtle role in virion assembly and release from specific PM raft environments, although Gag is known to associate efficiently with rafts (13, 23, 33, 41) and undergo viral budding in the absence of Env expression (18, 24).
As WT HIV-1 buds from an infected cell, it incorporates host cell raft molecules (such as Thy-1, GM1, CD14, CD48, CD55, and CD59) (39, 42) yet excludes the nonraft membrane protein CD45 (39), supporting the notion that HIV-1 particles are released from lipid rafts. The HIV-1 lipid envelope becomes enriched in cholesterol and sphingomyelin (2), a phenomenon that is most probably attributable to the high concentrations of these lipids in rafts (6, 56). The enrichment of cholesterol and incorporation of raft proteins into HIV-1, while highly suggestive, does not prove that a Brij 98 raft-like lipid arrangement is present in the HIV-1 envelope, nor whether such a structure is required for the subsequent infection of target cells. Like HIV-1, fowl plague virus (FPV), vesicular stomatitis virus, and Semliki Forest virus are enveloped viruses with cholesterol-enriched viral membranes (30, 36, 47, 51). In comparison with the virion membranes of vesicular stomatitis virus and Semliki Forest virus, which do not contain Triton rafts, cholesterol within the viral envelope of FPV is strongly associated with the virion particle, and the FPV envelope contains Triton raft-like structures (51).
During HIV-1 entry, gp120 Env binds CD4 and undergoes a conformational change that promotes the binding of gp120 Env to cell surface coreceptors (29). In the event of fusion between HIV-1 and the target cell membrane, the pretransmembrane region of gp41 Env protein is thought to aggregate on the virion surface and expose a fusion-active peptide domain, which facilitates virion entry into the target cell (4). The conformational rearrangement of the viral fusion peptide draws the viral and cellular lipid bilayers into close proximity and leads to the formation of the hemifusion stalk and the eventual creation of the fusion pore (10, 14). It is conceivable that virion-associated lipid may play an active role in the rearrangement of the viral envelope proteins to facilitate virion fusion.
In assessing the functional role of virion-associated Brij 98 rafts and structural features of cholesterol on HIV-1 replication, we replaced virion-associated cholesterol with exogenous cholesterol analogues that have demonstrated raft-promoting or -inhibiting properties and measured the resultant virion particle density and viral infectivity. We found that replacement of virion-associated cholesterol with these exogenous cholesterol analogues did not eliminate the presence of Brij 98 raft-like structures in HIV-1. However, we show that HIV-1 infection directly correlates with the raft-promoting capacity of the sterol analogues. Our findings highlight the importance of the HIV-1 lipid composition for viral infectivity and suggest that the raft-promoting property of virion-associated cholesterol is an important contributor to viral function.
MATERIALS AND METHODS
Virus production.
WT HIV-1 particles were produced via calcium phosphate transfection of 293T cells as previously described (9, 22, 54). Cells were maintained in Dulbecco's modified Eagle's medium (Gibco/BRL, Gaithersburg, Md.) containing 10% heat-inactivated fetal calf serum (P.A. Biologicals Co., Melbourne, Australia), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Cells were transfected with 10 μg of HIV-1 proviral DNA (pNL-4.3) (1) or 10 μg of HIV-1 luciferase reporter proviral DNA pNL4-3.Luc.R-E- (11) (obtained from the AIDS Reagent Program, National Institute of Allergy and Infectious Diseases, National Institutes of Health, from N. Landau) and 5 μg of the HIV-1 envelope, Vpr and Nef expression vector pNLA1 (gift of D. Purcell, University of Melbourne), as previously described (9).
Methyl-β-cyclodextrin (CD) treatment of HIV-1.
Viral particles were purified from the supernatant and concentrated by ultracentrifugation through 20% sucrose, by using an L-90 ultracentrifuge (SW28 rotor; Beckman, Fullerton, Calif.) at 93,000 × g for 1 h at 4°C. Virus pellets were eluted in phosphate-buffered saline (PBS). CD preferentially removes cholesterol over phospholipids (28). Concentrated virus was treated with 0.5 mM CD (Sigma, St. Louis, Mo.) in PBS at 37°C for 1 h to remove cholesterol from the HIV-1 lipid envelope as previously described (9).
Cholesterol and cholesterol analogue replenishment of CD-treated HIV-1.
4-Cholestenone (4-cholesten-3-one), cholesterol (cholest-5-en-3β-ol), coprostanol (5β-cholestan-3β-ol), 7-dehydrocholesterol (5,7-cholestadien-3β-ol), and dihydrocholesterol (5α-cholestan-3β-ol) were obtained from Sigma. 3-Epicholesterol was obtained from Steraloids (Newport, R.I.). The exchange of virion-associated cholesterol with exogenous cholesterol requires the initial removal of cholesterol from the HIV-1 particle by CD and the subsequent replenishment of the cholesterol-depleted virus with exogenous cholesterol or cholesterol analogues. Briefly, 1.3 μg of p24 capsid (CA) equivalent concentrated virus was treated with 0.5 mM CD for 1 h at 37°C. This step was followed by the addition of a 12, 25, 50, or 100 μM concentration of exogenous cholesterol or cholesterol analogue to the CD and virus suspension in a total volume of 400 μl for another 1 h of incubation at 37°C. As all exogenous sterols were first dissolved in ethanol and subsequently introduced to CD-treated HIV-1, the same amounts of ethanol were added to the mock- and CD-treated HIV-1 without sterol replenishment as the control.
Sucrose equilibrium density gradient analysis.
To examine alterations to the viral particle buoyant density induced by lipid modification of the virus structure, we employed 30 to 55% sucrose (Gibco/BRL) density gradients prepared in 2.5% increments as previously described (9). Untreated, CD-treated, and cholesterol analogue-replenished HIV-1 preparations were layered onto separate gradients and centrifuged for 16 h at 87,000 × g (SW41 rotor; L-90 ultracentrifuge; Beckman). Fractions were collected from the top of the gradient and analyzed for reverse transcriptase (RT) activity and density as previously described (9).
Infectivity assay.
To ensure the removal of free CD and CD-sterol complexes, the peak virus-containing fractions (by RT activity) were pooled from sucrose density gradients, diluted with PBS and repelleted through a 20% sucrose cushion at 93,000 × g for 1 h at 4°C. After eluting the virion pellet in 100 μl of PBS, equivalent p24 CA amounts, determined by an HIV-1 antigen (p24 CA) MicroELISA assay (Vironostika; Organon Teknika, Boxtel, The Netherlands), of untreated and cholesterol-depleted (and sterol-replenished) HIV-1 were used to infect MT2 cells as previously described (9). The success of infection was determined by the level of luciferase activity in the MT2 cells by using a Luciferase Assay System (Promega, Madison, Wis.). The impact of CD treatment and replenishment by cholesterol or cholesterol analogues on HIV-1 infectivity is represented as a percentage (mean ± standard error [SE] for duplicate samples) of untreated controls. The significance of the result was assessed by using Student's t test (two-sample unequal variance; two-tailed).
OptiPrep gradient purification.
OptiPrep velocity gradient ultracentrifugation was used to separate virion particles from soluble viral proteins, cell membrane-derived lipid microvesicles, and other cell debris that may interfere with subsequent analysis. OptiPrep (60% [wt/vol] iodixanol; Sigma) gradients were prepared in PBS in 1.2% increments of 6 to 18% iodixanol as previously described (9, 12). The peak virus-containing fractions were pooled, diluted in PBS, and repelleted through a 20% sucrose cushion for 1 h at 4°C at 93,000 × g to reconcentrate the viral particles.
Lipid analysis of virus replenished with sterol analogues.
The introduction of exogenous cholesterol and cholesterol analogues into CD-treated HIV-1 also was assessed by qualitative lipid analysis using high-performance liquid chromatography (HPLC). Virus purified on OptiPrep gradients was gently resuspended in 1 ml of PBS containing antioxidants (33.3 μM butylated hydroxytoluene and 33.3 mM EDTA). Sterols were extracted with 2.5 ml of methanol and 5 ml of hexane (16, 17). The solvent for the hexane phase was evaporated, and the sample was added to 200 μl of the mobile phase (44% acetonitrile, 54% 2-propanol, 2% water). For HPLC analysis of cholesterol and epicholesterol or 4-cholestenone, we used the mobile phase containing 97.5% n-hexane and 2.5% 2-propanol, while cholesterol and 7-dehydrocholesterol were better separated when 0.5% acetonitrile was added. These compounds were detected at 210 nm. Cholesterol and coprostanol or dihydrocholesterol were also separated with a mobile phase of 97.5% n-hexane and 2.5% 2-propanol, but an evaporative light scattering detector was used for detection. Peak area was used for quantification and was compared to known quantities of cholesterol or cholesterol analogues.
Flotation experiments.
WT HIV-1, CD-treated HIV-1, and HIV-1 particles replenished with 7-dehydrocholesterol, dihydrocholesterol, coprostanol, or 4-cholestenone were purified on an OptiPrep gradient and gently resuspended in 1 ml of cold raft lysis buffer (containing either 0.5% Triton [for WT HIV-1 and 293T cells only] or 0.5% Brij 98 and 1 mM EDTA, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin/ml, 150 mM sodium chloride, and 25 mM MES [4-morpholineethanesulfonic acid; pH 6.5]). The Triton viral lysates were incubated on ice for 30 min, whereas the Brij 98 viral lysates were incubated at 37°C for 5 min. Simultaneously, 107 uninfected 293T cells were washed twice in PBS and resuspended in 1 ml of raft lysis buffer. 293T cells were subjected to flotation analysis to ensure that our experimental conditions were capable of producing detergent-resistant lipid rafts from normal cell membranes. The virion lysate and the control cell lysate were then subjected to 10 strokes in a Dounce homogenizer. The homogenates were adjusted to 45% sucrose by the addition of 1 ml of 90% sucrose in MES-buffered saline (25 mM MES, 150 mM NaCl) and placed at the bottom of a 12-ml SW41 ultracentrifuge tube. A gradient was formed above the lysate by layering 2 ml (each) of 35, 30, 25, and 5% sucrose in MES-buffered saline. After ultracentrifugation at 150,000 × g for 16 h at 4°C (SW41 rotor; L-90 ultracentrifuge; Beckman), 10 1-ml fractions were collected from the top of the gradient. Proteins in the gradient fractions were precipitated with 10% trichloroacetic acid and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting for gp41 Env in the virion gradient fractions and for caveolin protein (an integral raft marker) (15, 31) in the WT virion gradient fractions and the 293T cell gradient fractions.
Immunoblotting.
Equal volumes of WT HIV-1 flotation gradient fraction samples were subjected to immunoblotting for gp41 Env to detect flotation of lipid raft-like membranes to regions of lower buoyancy in the gradient and for p24 CA to monitor the fractionation of nonraft proteins as previously described (9). To determine if gp120 Env protein levels were unchanged by lipid modification of the viral particle, virion lysates were standardized for p24 CA content and then analyzed for gp120 Env content as previously described (9). Membranes of the 293T cell control flotation gradient samples and WT HIV-1 Brij 98 raft gradient fraction samples were probed overnight at 4°C with anti-caveolin polyclonal antibodies (raised in rabbit; Transduction Laboratories), followed by secondary antibody conjugated to horseradish peroxidase. Specific proteins were visualized by enhanced chemiluminescence according to manufacturer's instructions (Amersham Pharmacia Biotech; Little Chalfont, Buckinghamshire, England).
RESULTS
Triton rafts have been implicated in the assembly and budding processes of various enveloped viruses, including HIV-1 (33, 39, 41, 44, 48, 60, 63). However, the presence of a raft within the infectious HIV-1 viral membrane has not been demonstrated. With Env-deficient HIV-1 VLPs, Holm et al. have detected Brij 98 rafts, but not Triton rafts (23), suggesting that a Brij 98 raft-like structure may be found in infectious HIV-1.
WT HIV-1 particles contain Brij 98 lipid rafts.
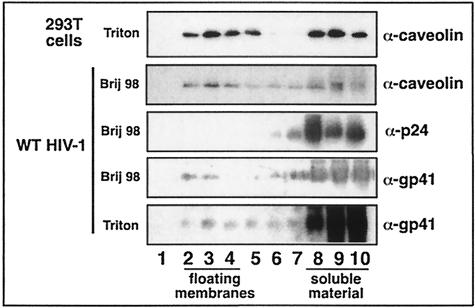
WT HIV-1 particles purified via an OptiPrep gradient were subjected to lipid raft isolation (see Materials and Methods). OptiPrep gradient purification removes contaminating microvesicles (lipid micelles derived from the virus-producing cell membrane) that may interfere with the interpretation of results (12). To ensure that the lipid raft isolation gradient was floating raft membranes, Triton-treated 293T cells were subjected to the flotation analysis and probed for caveolin (a resident raft protein) in the gradient fractions (15, 31). Fractions 3 and 4 have most of the caveolin-containing floating material (Fig. 1, lanes 3 and 4), indicating the lipid raft region of the gradient being used. Purified HIV-1 particles were treated with the detergent Brij 98 or Triton, and flotation gradient analysis was performed. We found that WT HIV-1 particles package raft-associated caveolin, and that ∼44% of the total virion-associated caveolin floated to the raft region of the gradient following treatment of WT HIV-1 with 0.5% Brij 98 (Fig. 1, lanes 2 to 4). As the HIV-1 transmembrane protein gp41 Env is anchored to the lipid bilayer of the virion, we used gp41 Env as the virion membrane marker. We found that ∼15% of the total virion gp41 Env floated to the raft region of the gradient (Fig. 1, lanes 2 to 4) when purified HIV-1 was treated with 0.5% Brij 98. In contrast, less than 6% of the total virion gp41 Env can be detected in the raft fractions when purified HIV-1 was treated with 0.5% Triton (Fig. 1, lanes 2 to 4). These data demonstrate the presence of a detergent-resistant structure in the HIV-1 membrane, which is more resistant to Brij 98 than Triton. The exclusive presence of p24 CA in the soluble fractions indicates that the virion particle was fully solubilized by the Brij 98 detergent.
FIG. 1.
WT HIV-1 particles were analyzed for lipid raft-like domains. WT HIV-1 particles purified on OptiPrep gradients were treated with 0.5% Brij 98 or Triton detergent and subjected to sucrose flotation gradient analysis (see Materials and Methods). Fractions 1 to 10 (lanes 1 to 10) were taken from the top of the gradient. 293T cells were used as the positive control for successful Triton raft isolation, with caveolin-containing raft membranes floating to fractions 3 and 4 (n = 5). Viral proteins were precipitated from each fraction and analyzed by SDS-PAGE and immunoblotting for raft-resident caveolin protein and p24 (in the Brij 98 raft gradient) and for viral gp41 envelope proteins (in both the Brij 98 and Triton raft gradients). Data are representative of the results of two experiments.
Since the use of detergents as a method for the characterization of lipid raft formation is indirect and may be subject to artifacts (53) and also does not reveal the functional requirement of Brij 98 rafts in virion particles, we pursued a complementary approach by using our cholesterol replacement system to assess whether sterols that disrupt or promote lipid raft formation contribute to HIV-1 replication. Using a model membrane system, Xu and London have observed that 4-cholestenone and coprostanol inhibit the formation of lipid raft-like structures (62), while cholesterol, 7-dehydrocholesterol, dihydrocholesterol, and, to a lesser extent, epicholesterol support the formation of these structures (61, 62). It was previously proposed that the incorporation of lipid raft-disrupting sterol analogues into the HIV-1 particle decreases viral infectivity, while lipid raft-promoting analogues support viral infectivity (9). The replacement of virion cholesterol in CD-treated HIV-1 with exogenous cholesterol restores viral infectivity and buoyant particle density to that of WT HIV-1 (9). For this study, we used buoyant density (1.149 g/ml) as an indirect marker to monitor the restoration of WT HIV-1 structure after sterol analogue replacement.
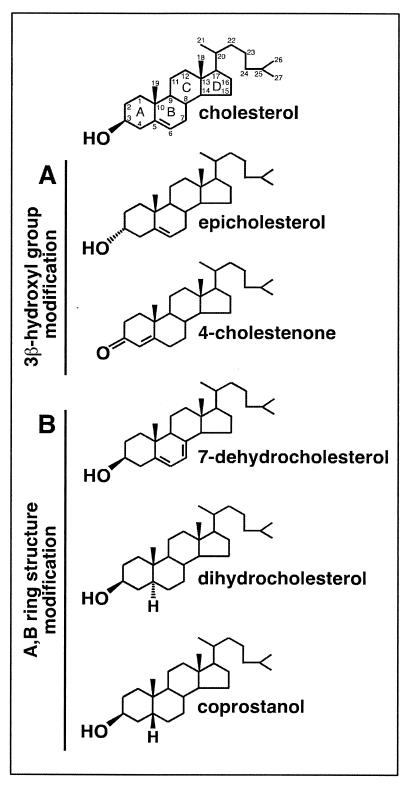
Cholesterol analogues with modifications in the fused A,B rings are readily incorporated into CD-modified HIV-1.
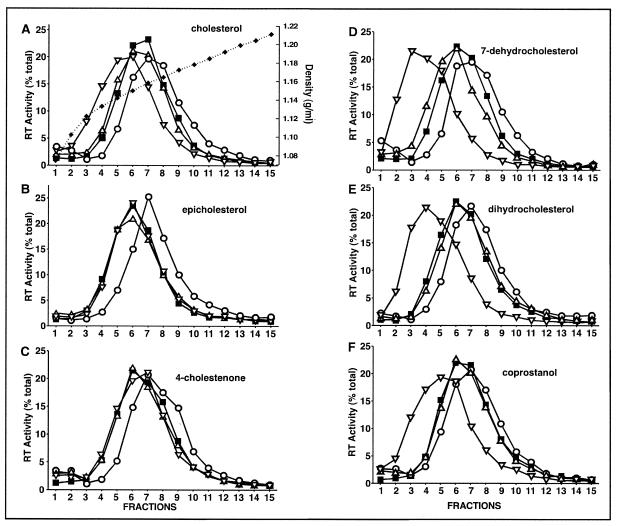
The density gradient sedimentation profiles of CD-treated HIV-1 containing various cholesterol analogues were compared. The sterol analogues used in this study have modifications in either the 3β-hydroxyl group or the fused A,B ring structure (Fig. 2). At least four different concentrations (12, 25, 50, and 100 μM) of exogenous sterol analogue were used during the replenishment of CD-treated virions. With a 100 μM concentration of exogenous analogue supplied to CD-treated HIV-1, the buoyant density of the CD-treated HIV-1 was either restored to or fell below the WT level (Fig. 3). The 3β-hydroxyl group of analogue-replenished particles supplied with a 100 μM concentration of exogenous analogue shifted the buoyant density of the CD-treated HIV-1 to within the WT level (Fig. 3B and C), whereas the fused A,B ring analogues supplied with exogenous analogue at the same concentration shifted the buoyant densities of the replenished CD-treated particles to well below that of WT HIV-1 (Fig. 3D to F). It was previously shown that the buoyant densities of lipid-modified HIV-1 shift below that of the WT when excess exogenous cholesterol was supplied to CD-treated HIV-1 (Fig. 3A) (9). These data suggest that the assorted cholesterol analogues used in this study (Fig. 2) exhibit different affinities toward the CD-treated virion particle.
FIG. 2.
Structures of sterols used in the present report. Variation in the 3β-hydroxyl group (A) or A,B ring structure (B) of cholesterol is shown. Conventional numerical labeling of the carbon atoms and alphabetical labeling of the four rings are indicated for cholesterol.
FIG. 3.
Density gradient profiles of lipid-modified HIV-1 where exogenous cholesterol or analogues with modification in the 3β-hydroxyl group (A to C) or with alterations in the A,B ring structure (D to F) were used. WT HIV-1 was mock-treated, CD-treated (0.5 mM), or CD-treated and incubated with exogenous cholesterol, epicholesterol, or 4-cholestenone. RT activity was detected in density gradients (A to C) of untreated (□), CD-treated (○), and CD-treated HIV-1 that had subsequently been replenished with a low concentration (▵) or a 100 μM concentration (▿) of exogenous sterol. CD-treated HIV-1 was replenished with low concentrations of exogenous sterols as follows: 25 μM cholesterol (A), 25 μM epicholesterol (B), 50 μM 4-cholestenone (C), 25 μM 7-dehydrocholesterol (D), 12 μM dihydrocholesterol (E), and 12 μM coprostanol (F). The duplicate percentage of total RT activity data points varied by <3% (the SE). Data are representative of three experiments. Corresponding densities (broken line) of each fraction (A) were determined by refractivity index measurements, where duplicate measurements varied by 0.001 g/ml (the SE).
CD-treated virion particles containing exogenous cholesterol analogues and displaying WT virion buoyant density were chosen to directly compare the impact of different features of cholesterol on HIV-1 function. Epicholesterol (25 μM), 4-cholestenone (50 μM), 7-dehydrocholesterol (25 μM), dihydrocholesterol (12 μM), and coprostanol (12 μM) were used to restore the buoyant density of CD-treated HIV-1 to the WT level, and these are denoted as “low ” sterol concentrations as shown in Fig. 3. The incorporation of exogenous cholesterol analogues into CD-treated HIV-1 particles was independently confirmed by HPLC analysis of the sterol contents of the particles (Table 1). We found that all CD-treated HIV-1 that was incubated with the low concentrations of sterols contained exogenously supplied analogue, which represents roughly 50% of the total virion sterol content.
TABLE 1.
Proportions of cholesterol to sterol analogue in lipid-modified HIV-1a
| Treatment group | Concn (μg/mg of p24) of:
|
Sterol levels vs control (%) | Cholesterol: cholesterol analogue | No. individual expts | Mean buoyant density (g/ml) | ||
|---|---|---|---|---|---|---|---|
| Cholesterol | Cholesterol analogue | Total sterol | |||||
| Control | 2.14 (0.17) | NA | 2.14 (0.17) | 100 | NA | 4 | 1.149 (0.001) |
| CD (0.5 mM) | 1.23 (0.22) | NA | 1.23 (0.22) | 57 | NA | 4 | 1.157 (0.001) |
| CD + replenishment with: | |||||||
| Cholesterol | 3.45 (0.45) | NA | 3.45 (0.45) | 161 | NA | 4 | 1.145 (0.004) |
| Epicholesterol | 1.49 (0.15) | 1.00 (0.20) | 2.49 (0.34) | 116 | 60:40 | 3 | 1.149 (0.001) |
| 4-Cholestenone | 0.91 (0.40) | 1.54 (0.89) | 2.45 (0.52) | 115 | 37:63 | 3 | 1.149 (0.001) |
| 7-Dehydrocholesterol | 0.99 (0.28) | 1.13 (0.13) | 2.12 (0.37) | 99 | 47:53 | 3 | 1.149 (0.001) |
| Dihydrocholesterol | 1.18 (0.14) | 1.22 (0.34) | 2.40 (0.45) | 112 | 49:51 | 4 | 1.151 (0.005) |
| Coprostanol | 1.91 (0.33) | 1.29 (0.28) | 3.20 (0.37) | 150 | 60:40 | 4 | 1.151 (0.004) |
Standard deviations of the means are given in parentheses. NA, not applicable.
Brij 98 rafts are present in CD-treated HIV-1 replenished with raft-promoting and -inhibiting sterol analogues.
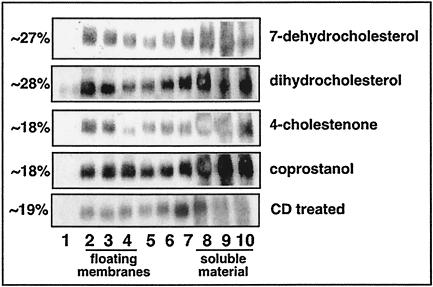
To determine the effect of replacing HIV-1 virion cholesterol with ∼50% sterol analogue (Table 1) on the presence of detergent-insoluble membrane structures in the viral envelope, we subjected lipid-modified particles purified on OptiPrep gradients to treatment with 0.5% Brij 98 followed by lipid raft isolation (see Materials and Methods). We found that Brij 98 rafts, as indicated by the proportion of gp41 Env detected in the raft region as a percentage of the total gp41 Env loaded on the gradient, were present in each of the sterol replenishment conditions tested and in CD-treated HIV-1 (Fig. 4), indicating that cholesterol-depleted virions maintain Brij 98 resistance. In comparison to the untreated WT HIV-1, in which ∼15% of the total gp41 Env forms Brij 98 raft membranes (Fig. 1), the replacement of ∼50% of virion-associated cholesterol with raft-promoting sterol analogues 7-dehydrocholesterol and dihydrocholesterol results in Brij 98 raft formation in ∼27% and ∼26% of the total gp41 Env, respectively. This finding suggests that these raft-promoting analogues have a stronger association with Brij 98 rafts in the virion lipid membrane than native cholesterol molecules, consistent with the higher affinity of these analogues toward CD-treated HIV-1. In comparison, replacement of ∼50% of virion cholesterol with the raft-disrupting sterol analogues 4-cholestenone and coprostanol produced Brij 98 rafts in ∼18% of the total gp41 Env in both cases. Compared to the ∼26 to 27% level of Brij 98 rafts produced by the raft-promoting analogues, the ∼18% level of Brij 98 rafts present within the particles replenished with raft-disrupting analogues is in agreement with the respective raft-promoting and -disrupting properties of these analogues in model membranes.
FIG. 4.
Brij 98 raft analysis of CD-treated HIV-1 replenished with raft-promoting and raft-disrupting sterol analogues. Concentrated HIV-1 particles were treated with CD and replenished with exogenous sterol analogues in the following concentrations: 25 μM 7-dehydrocholesterol, 12 μM dihydrocholesterol, 50 μM 4-cholestenone, and 12 μM coprostanol. Modified HIV-1 particles were purified by OptiPrep gradients, treated with 0.5% Brij 98 detergent, and subjected to sucrose flotation gradient analysis (see Materials and Methods). Fractions 1 to 10 (lanes 1 to 10) were taken from the top of the gradient. CD-treated HIV-1 was included as a control for Brij 98 detergent resistance. Viral proteins were precipitated from each fraction and analyzed by SDS-PAGE and immunoblotting for viral gp41 envelope proteins (n = 2). Quantification of protein bands was performed by using Image Gauge version 3.3 software and is represented in fractions 2 to 4 (the floating membrane region) as a percentage of the total protein loaded on the gradient (n = 2; SE, 6%).
It is important to note that the percentage of gp41 Env protein in the Brij 98 rafts of WT HIV-1 virions (∼15%) (Fig. 1) is not significantly different from the percentage of Brij 98 rafts in HIV-1 modified with raft-disrupting analogues (∼18%) (Fig. 4). This discrepancy may result in part from the notions that (i) a Brij 98 raft-like structure may still exist in the virion, as only 50% of virion-associated cholesterol was replaced with exogenous sterol analogue, and (ii) various sterol analogues have different affinities toward lipid-modified HIV-1 that can potentially influence the levels of detectable virion Brij 98 rafts after detergent solubilization. By definition, the insolubility of proteins after detergent solubilization is an in vitro phenomenon, which may adversely alter the interpretation of the results (19). Furthermore, this manner of Brij 98 raft isolation does not provide specific information on the arrangement of lipid on the virion surface or the effects of these sterols on viral infectivity.
The 3β-hydroxyl group of virion-associated cholesterol is critical for viral infectivity.
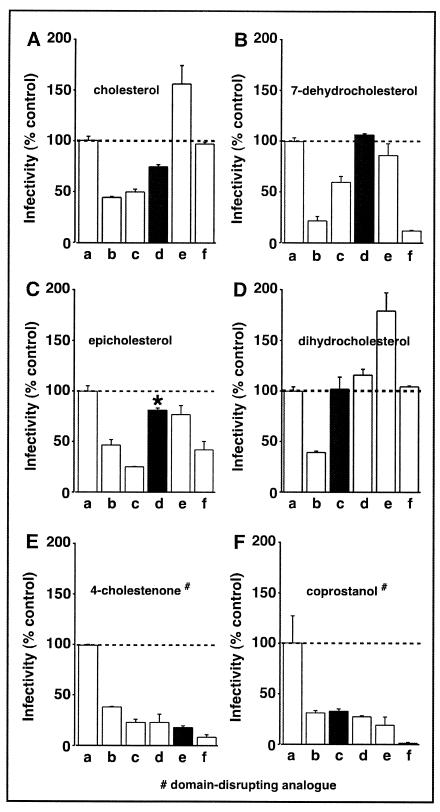
Previously, we used our virion replacement system to integrate an oxidized form of cholesterol, 5-cholestenone, into HIV-1 and observed a reduction in viral infectivity, suggesting that the 3β-hydroxyl group of cholesterol is critical for HIV-1 infectivity (9). In order to further evaluate the importance of the 3β-hydroxyl group of cholesterol in HIV-1 replication, we have replaced virion-associated cholesterol with 4-cholestenone and epicholesterol (Fig. 2). We show that the supplementation of CD-treated HIV-1 with 4-cholestenone (a natural product of cholesterol oxidase) at concentrations of between 12 and 100 μM inhibits viral infectivity (Fig. 5E), in agreement with the previous finding that the oxidative state of the 3β-hydroxyl group of cholesterol contributes to HIV-1 infectivity. When 50% of HIV-1 virion cholesterol was replaced with 4-cholestenone (exogenously supplied at a concentration of 50 μM), the lipid-modified HIV-1 displays a significant decrease in viral infectivity (20% of the WT level).
FIG. 5.
Viral infectivity of lipid-modified HIV-1. HIV-1 containing the luciferase reporter gene was mock treated, CD treated (0.5 mM), or CD treated and incubated with exogenous cholesterol (A), 7-dehydrocholesterol (B), epicholesterol (C), dihydrocholesterol (D), 4-cholestenone (E), or coprostanol (F). Lipid envelope-modified and untreated HIV-1 were normalized for p24 capsid protein content and used to infect MT2 cells. The levels of luciferase activity were used as the indicator of infectivity levels, which are reported as percentages (means ± SE of duplicate samples) of the untreated control. Infectivity levels of untreated (a), CD-treated (b), and CD-treated HIV-1 that has subsequently been incubated with a 12 (c), 25 (d), 50 (e), or 100 (f) μM concentration of exogenous sterol are shown. Filled bars represent replenished virions that exhibited WT particle density profiles (same as the low replenishment described in legends for Fig. 2 and 3). P values were 0.01, 0.2, 0.1, <0.01, and 0.01, respectively, for filled bars in panels B, C, D, E, and F; n = 4. Data are representative of three experiments. *, epicholesterol-replenished particles of WT density exhibited an infectivity range of 45 to 80%.
To assess the importance of the configuration of cholesterol at C3 and its impact on viral function, we replaced 50% of virion-associated cholesterol with exogenous epicholesterol (Fig. 2) by using 0.5 mM CD and 25 μM epicholesterol. Epicholesterol differs from cholesterol in that its 3-OH group has a different chirality than cholesterol. In contrast to the level of infectivity restored by exogenous cholesterol (Fig. 5A), the supplementation of CD-treated HIV-1 with epicholesterol partially restored viral infectivity in a variable range of 45 to 80% (Fig. 5C), but the result was not significantly different from that with the cholesterol-replenished CD-treated HIV-1 particles with WT density. Of all the sterol analogues used to replace the virion-associated cholesterol of CD-treated HIV-1, epicholesterol displayed significant variation in its capacity to restore viral infectivity, with its 80% infectivity being the best restoration that we have detected. It is worth noting that the raft-promoting capacity of epicholesterol in a model membrane system is also found between raft-promoting and raft-inhibiting analogues, such as dihydrocholesterol and coprostanol, respectively, at the physiological temperature of 37°C (62).
The fused A,B rings in cholesterol are an important determinant of viral infectivity.
We next replaced virion-associated cholesterol with 7-dehydrocholesterol, coprostanol, or dihydrocholesterol (Fig. 3D to F; Table 1) to assess the impact of the sterol analogues on viral infectivity. 7-Dehydrocholesterol differs from cholesterol as it has an additional double bond between C7 and C8 of the B ring, giving the sterol molecule a higher oxidative state and reducing the flexibility in the B ring of the molecule (Fig. 2). In contrast, coprostanol and dihydrocholesterol are the reduced forms of cholesterol, exhibiting a lower overall oxidative state at C5 and C6. Coprostanol and dihydrocholesterol differ from each other only in their chirality at C5, which causes the sterol A ring to be positioned differently in relation to the B ring of cholesterol. The incubation of CD-treated virus with 25 μM 7-dehydrocholesterol, 12 μM dihydrocholesterol, or 12 μM coprostanol produced viral particles of WT buoyant density (Fig. 3D to F). The replacement of ∼50% of the HIV-1 virion cholesterol (Table 1) with either 7-dehydrocholesterol or dihydrocholesterol did not reduce the infectivity of HIV-1 (Fig. 5B and D). In contrast, replacing ∼40% of virion-cholesterol with coprostanol (Table 1) resulted in virion particles with 30% of WT HIV-1 infectivity (Fig. 5F). These findings suggest that the fused ring structure of virion-associated cholesterol has a role in HIV-1 infectivity.
Excess cholesterol analogues reduce infectivity.
It has been shown previously that the incubation of CD-treated HIV-1 with increasing concentrations of exogenous cholesterol will lead to the eventual suppression of HIV-1 infectivity (9). This high-dose cholesterol-suppressive activity for CD-treated HIV-1 is also detected for the sterol analogues 7-dehydrocholesterol, epicholesterol, 4-cholestenone, and coprostanol (Fig. 5B, C, E, and F), supporting the notion that only a finite amount of sterol can be accommodated by the HIV-1 virion before impairing viral infectivity (9). Similarly, for the CD-treated particles supplied with a 100 μM concentration of cholesterol or dihydrocholesterol, we detected reduced infectivity compared to viruses that were incubated with the same analogues at the lower concentration of 50 μM (Fig. 5A and D). This result indicates that an excess of raft-promoting analogues, when supplied to the CD-treated HIV-1 at a high concentration, will also suppress viral infectivity.
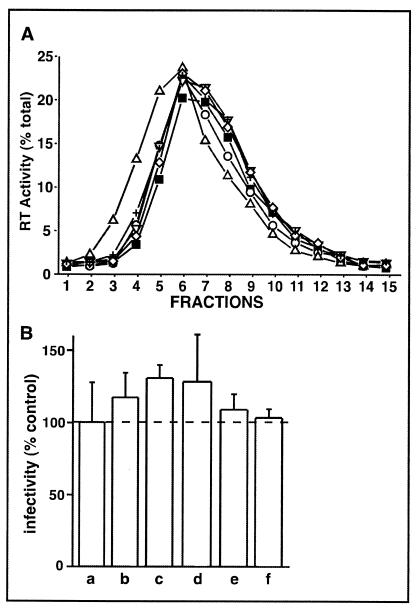
It is important to note that CD treatment of HIV-1 is a prerequisite for adjusting the buoyant particle density and viral infectivity by modifying the virion-associated cholesterol content. In the absence of CD, the sterol analogues do not alter the peak density fraction or HIV-1 infectivity from the WT control level (Fig. 6).
FIG. 6.
Density gradient profiles and infectivity levels of HIV-1 supplemented with sterol analogues in the absence of CD. WT HIV-1 was mock-treated or incubated with exogenous sterol analogues in the absence of CD (see Fig. 2). RT activity detected in density gradients (A) of untreated HIV-1 (□) or HIV-1 incubated in 100 μM concentrations of epicholesterol (○), 4-cholestenone (▵), coprostanol (▿), dihydrocholesterol (◊), or 7-dehydrocholesterol (+). Duplicate percentages for total RT activity data points varied by <3% (the SE). HIV-1 containing the luciferase reporter gene was mock treated (B, bar a) or incubated with 100 μM concentrations of epicholesterol (bar b), 4-cholestenone (bar c), coprostanol (bar d), dihydrocholesterol (bar e), or 7-dehydrocholesterol (bar f). All samples were normalized for p24 capsid protein content and used to infect MT2 cells. The levels of luciferase activity were used as indicators of infectivity levels, which are reported as percentages (means ± SE of duplicate samples) of the untreated control. Data are representative of two to three experiments.
Maintenance of gp120 envelope protein levels after replacing virion cholesterol with sterol analogues.
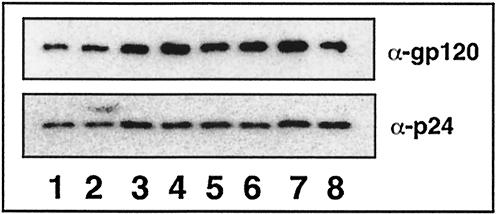
HIV-1 particles lacking viral envelope proteins are unable to infect target cells. It has previously been shown that CD-treated HIV-1 (9, 21) and CD-treated HIV-1 replenished with 5-cholestenone (9) do not affect the anchoring of HIV-1 gp120 Env protein on the virion particles. However, it remains possible that the decrease in HIV-1 infectivity observed upon replacement of virion cholesterol with the sterol analogues 4-cholestenone and coprostanol may arise in part from the interference with the anchoring of the envelope protein to the virion lipid bilayer. In order to test this theory, we analyzed the levels of gp120 envelope proteins in HIV-1 particles that have modifications in their virion lipid envelopes (Fig. 7). Similar to previous findings (9), our data show that the reduction in infectivity associated with cholesterol depletion or sterol analogue replenishment of CD-treated HIV-1 cannot be attributed to a reduction in HIV-1 envelope proteins on the virion surface.
FIG. 7.
Immunoblot analysis of HIV-1 gp120 envelope proteins and p24 CA proteins present in lipid-modified HIV-1. Concentrated HIV-1 particles were treated with CD and replenished with exogenous sterol analogues. Microvesicle contamination was removed by purification on OptiPrep gradients as described in Materials and Methods. Sample input was standardized by p24 CA content. Lane 1, untreated HIV-1 control; lane 2, CD-treated HIV-1; lane 3, CD-treated HIV-1 replenished with cholesterol; lane 4, CD-treated HIV-1 replenished with epicholesterol; lane 5, CD-treated HIV-1 replenished with 4-cholestenone; lane 6, CD-treated HIV-1 replenished with 7-dehydrocholesterol; lane 7, CD-treated HIV-1 replenished with dihydrocholesterol; lane 8, CD-treated HIV-1 replenished with coprostanol (n = 2).
DISCUSSION
The WT HIV-1 lipid envelope contains Brij 98 rafts.
Raft extraction detergents have different selectivities in their disruption of lipid-protein interactions and their enrichment of cholesterol and sphingolipids over glycerophospholipids (53). Schuck et al. (53) found that the selectivity for detergent-insoluble membranes is greater for Triton than for Brij 98, which correlates with results with these two detergents for the types of detergent-resistant membranes we isolated from the HIV-1 viral membrane. Our findings are in agreement with those of Holm et al. (23), who showed that Brij 98 rafts, but not classical Triton rafts, are found in Env-deficient HIV-1 VLPs. We then examined the role of virion Brij 98 rafts in HIV-1 replication.
The raft-promoting property of sterols is required for HIV-1 infectivity.
In this study, we have shown that the replacement of 50% of the HIV-1 cholesterol with the raft-inhibiting sterol analogues 4-cholestenone or coprostanol (62) suppresses HIV-1 infectivity, while the replacement of 50% of HIV-1 cholesterol with the raft-promoting sterol analogues 7-dehydrocholesterol and dihydrocholesterol (61, 62) did not alter viral infectivity, even though Brij 98 rafts can be isolated from both types of lipid-modified virus. It is important to note that the inhibition of infectivity did not result from a change in the amounts of Brij 98 rafts in the HIV-1 viral membrane or in the levels of virion-associated envelope protein, showing that the presence of Brij 98 rafts in HIV-1 is not sufficient to support viral replication. Suboptimal infectivity (45 to 80% of that of the WT control) was observed for virions containing epicholesterol, a sterol analogue that partially supports raft formation compared to cholesterol (62). The detection of Brij 98 detergent-insoluble rafts in virions containing raft-disrupting sterols suggests that our virion cholesterol replacement analysis can detect subtle effects on the lipid arrangement that lead to alterations in viral infectivity, but do not necessarily alter the Brij 98 insolubility of gp41 in the viral membrane, as observed in CD-treated HIV-1. Our data show a direct correlation between the capacity of the virion-associated sterol to support raft formation in a model membrane system and viral infectivity (61, 62).
Cholesterol analogues and the viral lipid envelope.
It has been hypothesized that the formation of lipid rafts in cell membranes is driven by lipid-lipid interactions such as those between cholesterol and sphingolipid (55). For example, when the 3β-hydroxyl group of cholesterol is replaced with a ketone group, as in 4-cholestenone, the hydrogen bond-accepting ability of the sterol is eliminated and hydrogen bonding to the sphingolipid polar head group is abolished. The raft-promoting sterol analogues used in this study have a molecular structure that allows them to form ordered structures with sphingolipids, whereas raft-inhibiting sterol analogues disrupt the ordered packing arrangement of phospholipids and decrease membrane rigidity. It is possible that the binding interactions that occur between coprostanol or 4-cholestenone and neighboring viral membrane molecules affect the viral membrane in a manner that disrupts fusion yet maintains a detectable Brij 98-resistant raft component. The replacement of virion cholesterol with these selected analogues will undoubtedly alter viral membrane rigidity, but in a range that is not differentiated by Brij 98 raft extraction. The correlation of infectivity levels with the incorporation of raft-promoting sterols suggests that the increased rigidity or lipid order of the viral particle, but not the presence of a physical Brij 98 resistant raft structure, plays a crucial role in viral infectivity.
Contribution of virion cholesterol to HIV-1 replication.
It was previously shown that the enriched cholesterol content of the HIV-1 particle is critical for viral infectivity and virion stability (9). Cholesterol-depleted HIV-1 virions exhibit suppressed infectivity (9, 21) through disruption of the virion lipid bilayer (9), yet display normal levels of viral protein by immunoblotting, notably of gp120 Env (9, 21), and bind to the surface CD4 receptor and coreceptor(s) of target cells (21). The cholesterol-depleted virions, however, are not internalized upon binding (21). The recent finding by Graham et al. (20) showed that cholesterol removal from the HIV-1 membrane causes virion permeabilization and further supports a role for viral cholesterol in HIV-1 replication.
Raft-promoting sterols in HIV-1.
The precise mechanistic contribution of virion-associated cholesterol in HIV-1 replication remains undefined. Virion-associated cholesterol may facilitate the HIV-1 fusion entry process through the formation and stabilization of the hemifusion stalk and the creation of the fusion pore. Following CD4 and coreceptor binding, the gp41 Env entity of the HIV-1 Env complex inserts a fusion peptide into the cell membrane (14) to draw the viral and cell membranes into close proximity. The gp41 Env then snaps back onto itself to form a helical hairpin structure that overcomes the hydration barrier between the opposing lipid bilayers, and membrane fusion is initiated via a hemifusion stalk intermediate (14, 38). The effect of cholesterol on membrane fusion may represent a general course of action for the stabilization of the fusion pore.
It is known that the lipid content of the PM can affect the arrangement of viral envelope proteins (46, 49, 50). In the presence of cholesterol, the pretransmembrane region of HIV-1 gp41 Env protein favors membrane fusion, while raft-inhibiting sterols suppress fusion (50), indicating a role for the raft-promoting capacity of cholesterol in the fusion of membranes. Furthermore, HIV-1 may contain specialized cholesterol-rich rafts that are involved in virion permeabilization and have a role in fusion (20). In another example, the envelope protein of the influenza virus requires cholesterol in the target membrane to stabilize the opening of the fusion pore. It is important to note that cholesterol analogues that support raft formation stabilized influenza virus-cell membrane pore formation, while raft-inhibiting analogues did not (46). Although Razinkov and Cohen assessed the role of cholesterol in the target membrane (46), in contrast to our study of the viral membrane, the fundamental requirement of cholesterol is its capacity to support raft formation during fusion, which is likely to be the same in viral and target membranes. A requirement for cholesterol during HIV-1 fusion may represent a general principle among other viral pathogens that require cholesterol during entry into their respective target cells (27, 34, 43, 58).
Development of a topical microbicide that adjusts the virion-associated cholesterol content.
It has been suggested that cholesterol-removing agents, such as CD, may potentially be used as topical microbicides against HIV-1 (21, 26, 32, 40, 45, 59). Our data have revealed important clues for the development of such a microbicide, namely: (i) CD in combination with a raft-disrupting sterol analogue further inhibits viral infectivity; (ii) CD in combination with excess sterols acts to suppress HIV-1 infection; and (iii) sterols with high affinity for the HIV particle may lower the amount of cholesterol analogue or CD required to affect the particle, thus reducing the likelihood of cytotoxic effects. One would predict that the combination of CD with a synthetic cholesterol analogue that encompasses these features would be invaluable for the formulation of a CD-based topical microbicide against HIV-1. The development of this new topical microbicide is likely to have wider applications in preventing the transmission of other sexually acquired pathogens, including the herpes simplex virus and Chlamydia.
Acknowledgments
We thank Andy Poumbourios (St. Vincent's Medical Research Institute) for purifying the HIV-1 Env antibodies (NIH AIDS Research and Reference Reagent Program) and Melissa Hill and Andy Poumbourios for critically reviewing the manuscript.
S. Campbell is the recipient of an NHMRC Ph.D. training scholarship and a scholarship from the University of Melbourne (Microbiology and Immunology Department). J. Mak is supported by a Monash Logan Fellowship and a Pharmacia-Pfizer Senior Research Fellowship. S. Crowe is supported by an NHMRC Principal Research Fellowship. This work is also supported in part by grants to J. Mak from the NHMRC, the Clive and Vera Ramaciotti Foundation, the Honda Foundation, and the Cecilia Kilkeary Foundation.
REFERENCES
- 1.Adachi, A., H. E. Gendelman, S. Koenig, T. Folks, R. Willey, A. Rabson, and M. A. Martin. 1986. Production of acquired immunodeficiency syndrome-associated retrovirus in human and nonhuman cells transfected with an infectious molecular clone. J. Virol. 59:284-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aloia, R. C., H. Tian, and F. C. Jensen. 1993. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc. Natl. Acad. Sci. USA 90:5181-5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavari, S., C. M. Bosio, E. Wiegand, G. Ruthel, A. B. Will, T. W. Geisbert, M. Hevey, C. Schmaljohn, A. Schmaljohn, and M. J. Aman. 2002. Lipid raft microdomains: a gateway for compartmentalized trafficking of Ebola and Marburg viruses. J. Exp. Med. 195:593-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley, J., and J. P. Moore. 1997. HIV-cell fusion. The viral mousetrap. Nature 387:346-348. [DOI] [PubMed] [Google Scholar]
- 5.Brown, D. A., and E. London. 1998. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 14:111-136. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. A., and E. London. 2000. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275:17221-17224. [DOI] [PubMed] [Google Scholar]
- 7.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 8.Campbell, S. M., S. M. Crowe, and J. Mak. 2001. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J. Clin. Virol. 22:217-227. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, S. M., S. M. Crowe, and J. Mak. 2002. Virion-associated cholesterol is critical for the maintenance of HIV-1 structure and infectivity. AIDS (Philadelphia) 16:2253-2261. [DOI] [PubMed] [Google Scholar]
- 10.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell. Biol. 4:309-319. [DOI] [PubMed] [Google Scholar]
- 11.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 12.Dettenhofer, M., and X. F. Yu. 1999. Highly purified human immunodeficiency virus type 1 reveals a virtual absence of Vif in virions. J. Virol. 73:1460-1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding, L., A. Derdowski, J. J. Wang, and P. Spearman. 2003. Independent segregation of human immunodeficiency virus type 1 Gag protein complexes and lipid rafts. J. Virol. 77:1916-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 15.Fra, A. M., E. Williamson, K. Simons, and R. G. Parton. 1995. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc. Natl. Acad. Sci. USA 92:8655-8659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaus, K., R. T. Dean, L. Kritharides, and W. Jessup. 2001. Inhibition of cholesterol efflux by 7-ketocholesterol: comparison between cells, plasma membrane vesicles, and liposomes as cholesterol donors. Biochemistry 40:13002-13014. [DOI] [PubMed] [Google Scholar]
- 17.Gaus, K., J. J. Gooding, R. T. Dean, L. Kritharides, and W. Jessup. 2001. A kinetic model to evaluate cholesterol efflux from THP-1 macrophages to apolipoprotein A-1. Biochemistry 40:9363-9373. [DOI] [PubMed] [Google Scholar]
- 18.Gheysen, D., E. Jacobs, F. de Foresta, C. Thiriart, M. Francotte, D. Thines, and M. De Wilde. 1989. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 59:103-112. [DOI] [PubMed] [Google Scholar]
- 19.Glebov, O. O., and B. J. Nichols. 2004. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nat. Cell Biol. [DOI] [PubMed]
- 20.Graham, D. R. M., E. Chertova, J. M. Hilburn, L. O. Arthur, and J. E. K. Hildreth. 2003. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with β-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J. Virol. 77:8237-8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyader, M., E. Kiyokawa, L. Abrami, P. Turelli, and D. Trono. 2002. Role for human immunodeficiency virus type 1 membrane cholesterol in viral internalization. J. Virol. 76:10356-10364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill, M. K., M. Shehu-Xhilaga, S. M. Crowe, and J. Mak. 2002. Proline residues within spacer peptide p1 are important for human immunodeficiency virus type 1 infectivity, protein processing, and genomic RNA dimer stability. J. Virol. 76:11245-11253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holm, K., K. Weclewicz, R. Hewson, and M. Suomalainen. 2003. Human immunodeficiency virus type 1 assembly and lipid rafts: Pr55gag associates with membrane domains that are largely resistant to Brij98 but sensitive to Triton X-100. J. Virol. 77:4805-4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karacostas, V., K. Nagashima, M. A. Gonda, and B. Moss. 1989. Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc. Natl. Acad. Sci. USA 86:8964-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller, P., and K. Simons. 1998. Cholesterol is required for surface transport of influenza virus hemagglutinin. J. Cell Biol. 140:1357-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna, K. V., K. J. Whaley, L. Zeitlin, T. R. Moench, K. Mehrazar, R. A. Cone, Z. Liao, J. E. Hildreth, T. E. Hoen, L. Shultz, and R. B. Markham. 2002. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J. Clin. Investig. 109:205-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kielian, M. C., and A. Helenius. 1984. Role of cholesterol in fusion of Semliki Forest virus with membranes. J. Virol. 52:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kilsdonk, E. P., P. G. Yancey, G. W. Stoudt, F. W. Bangerter, W. J. Johnson, M. C. Phillips, and G. H. Rothblat. 1995. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 270:17250-17256. [DOI] [PubMed] [Google Scholar]
- 29.Kwong, P. D., R. Wyatt, J. Robinson, R. W. Sweet, J. Sodroski, and W. A. Hendrickson. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lenard, J., and R. W. Compans. 1974. The membrane structure of lipid-containing viruses. Biochim. Biophys. Acta 344:51-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li, S., K. S. Song, and M. P. Lisanti. 1996. Expression and characterization of recombinant caveolin. Purification by polyhistidine tagging and cholesterol-dependent incorporation into defined lipid membranes. J. Biol. Chem. 271:568-573. [PubMed] [Google Scholar]
- 32.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 33.Lindwasser, O. W., and M. D. Resh. 2001. Multimerization of human immunodeficiency virus type 1 Gag promotes its localization to barges, raft-like membrane microdomains. J. Virol. 75:7913-7924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu, Y. E., T. Cassese, and M. Kielian. 1999. The cholesterol requirement for Sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J. Virol. 73:4272-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu, Y. E., and M. Kielian. 2000. Semliki Forest virus budding: assay, mechanisms, and cholesterol requirement. J. Virol. 74:7708-7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luan, P., L. Yang, and M. Glaser. 1995. Formation of membrane domains created during the budding of vesicular stomatitis virus. A model for selective lipid and protein sorting in biological membranes. Biochemistry 34:9874-9883. [DOI] [PubMed] [Google Scholar]
- 37.Manie, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. 2003. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell 14:926-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen, D. H., and J. E. Hildreth. 2000. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74:3264-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen, D. H., and D. Taub. 2002. CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168:4121-4126. [DOI] [PubMed] [Google Scholar]
- 41.Ono, A., and E. O. Freed. 2001. Plasma membrane rafts play a critical role in HIV-1 assembly and release. Proc. Natl. Acad. Sci. USA 98:13925-13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ott, D. E. 2002. Potential roles of cellular proteins in HIV-1. Rev. Med. Virol. 12:359-374. [DOI] [PubMed] [Google Scholar]
- 43.Phalen, T., and M. Kielian. 1991. Cholesterol is required for infection by Semliki Forest virus. J. Cell Biol. 112:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickl, W. F., F. X. Pimentel-Muinos, and B. Seed. 2001. Lipid rafts and pseudotyping. J. Virol. 75:7175-7183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popik, W., T. M. Alce, and W.-C. Au. 2002. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4+ T cells. J. Virol. 76:4709-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Razinkov, V. I., and F. S. Cohen. 2000. Sterols and sphingolipids strongly affect the growth of fusion pores induced by the hemagglutinin of influenza virus. Biochemistry 39:13462-13468. [DOI] [PubMed] [Google Scholar]
- 47.Renkonen, O., L. Kaarainen, K. Simons, and C. G. Gahmberg. 1971. The lipid class composition of Semliki Forest virus and plasma membranes of the host cells. Virology 46:318-326. [DOI] [PubMed] [Google Scholar]
- 48.Rousso, I., M. B. Mixon, B. K. Chen, and P. S. Kim. 2000. Palmitoylation of the HIV-1 envelope glycoprotein is critical for viral infectivity. Proc. Natl. Acad. Sci. USA 97:13523-13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saez-Cirion, A., M. J. Gomara, A. Agirre, and J. L. Nieva. 2003. Pre-transmembrane sequence of Ebola glycoprotein. Interfacial hydrophobicity distribution and interaction with membranes. FEBS Lett. 533:47-53. [DOI] [PubMed] [Google Scholar]
- 50.Saez-Cirion, A., S. Nir, M. Lorizate, A. Agirre, A. Cruz, J. Perez-Gil, and J. L. Nieva. 2002. Sphingomyelin and cholesterol promote HIV-1 gp41 pretransmembrane sequence surface aggregation and membrane restructuring. J. Biol. Chem. 277:21776-21785. [DOI] [PubMed] [Google Scholar]
- 51.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 52.Scheiffele, P., M. G. Roth, and K. Simons. 1997. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 16:5501-5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuck, S., M. Honsho, K. Ekroos, A. Shevchenko, and K. Simons. 2003. Resistance of cell membranes to different detergents. Proc. Natl. Acad. Sci. USA 100:5795-5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shehu-Xhilaga, M., S. M. Crowe, and J. Mak. 2001. Maintenance of the Gag/Gag-Pol ratio is important for human immunodeficiency virus type 1 RNA dimerization and viral infectivity. J. Virol. 75:1834-1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 56.Simons, K., and D. Toomre. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 1:31-41. [DOI] [PubMed] [Google Scholar]
- 57.Slotte, J. P. 1999. Sphingomyelin-cholesterol interactions in biological and model membranes. Chem. Phys. Lipids 102:13-27. [DOI] [PubMed] [Google Scholar]
- 58.Stang, E., J. Kartenbeck, and R. G. Parton. 1997. Major histocompatibility complex class I molecules mediate association of SV40 with caveolae. Mol. Biol. Cell 8:47-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Viard, M., I. Parolini, M. Sargiacomo, K. Fecchi, C. Ramoni, S. Ablan, F. W. Ruscetti, J. M. Wang, and R. Blumenthal. 2002. Role of cholesterol in human immunodeficiency virus type 1 envelope protein-mediated fusion with host cells. J. Virol. 76:11584-11595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vincent, S., D. Gerlier, and S. N. Manie. 2000. Measles virus assembly within membrane rafts. J. Virol. 74:9911-9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, X., R. Bittman, G. Duportail, D. Heissler, C. Vilcheze, and E. London. 2001. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J. Biol. Chem. 276:33540-33546. [DOI] [PubMed] [Google Scholar]
- 62.Xu, X., and E. London. 2000. The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 39:843-849. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, J., A. Pekosz, and R. A. Lamb. 2000. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycoproteins. J. Virol. 74:4634-4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng, Y., A. Plemenitas, T. Linnemann, O. T. Fackler, and B. M. Peterlin. 2001. Nef increases infectivity of HIV via lipid rafts. Curr. Biol. 11:875-879. [DOI] [PubMed] [Google Scholar]