Abstract
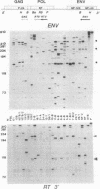
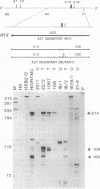
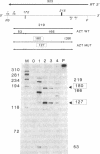
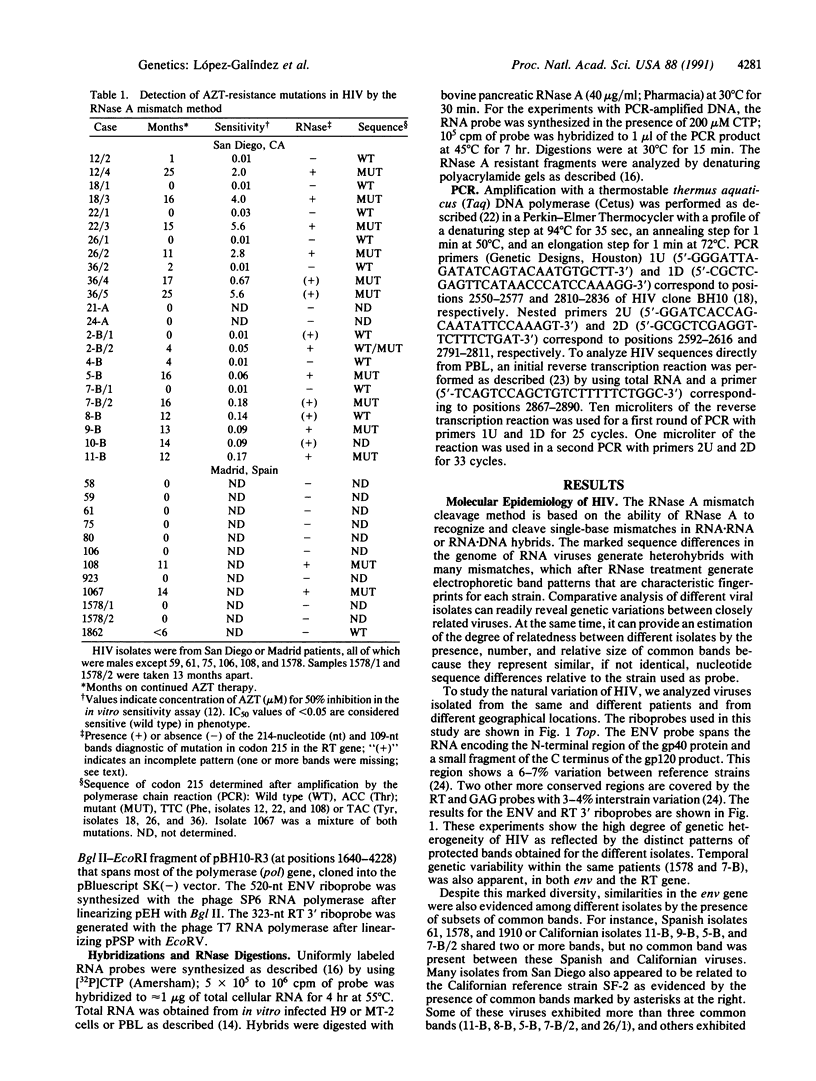
The RNase A mismatch cleavage method has been applied to the characterization of natural genetic variation of human immunodeficiency virus (HIV) from different geographical areas. The approach provides a rapid and simple assay for the analysis of differences in closely related viral isolates and allows the establishment of phylogenetic relationships between epidemiologically distinct viruses. Our results show a broad clustering of circulating viruses according to their geographical distribution. We also have analyzed the temporal appearance of mutations associated with the acquisition of resistance to 3'-azido-3'-deoxythymidine (AZT). The results show that mutations in codon 215 of the viral reverse transcriptase can be detected readily by this method in HIV isolates and also directly in peripheral blood from HIV-infected individuals after in vitro amplification of viral sequences with the polymerase chain reaction. The specific recurrence of identical double-nucleotide substitutions in epidemiologically and geographically distant viruses suggests that the restricted amino acid substitutions at this position selected by drug exposure are a critical, rate-limiting step in the acquisition of drug resistance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alizon M., Wain-Hobson S., Montagnier L., Sonigo P. Genetic variability of the AIDS virus: nucleotide sequence analysis of two isolates from African patients. Cell. 1986 Jul 4;46(1):63–74. doi: 10.1016/0092-8674(86)90860-3. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Boucher C. A., Tersmette M., Lange J. M., Kellam P., de Goede R. E., Mulder J. W., Darby G., Goudsmit J., Larder B. A. Zidovudine sensitivity of human immunodeficiency viruses from high-risk, symptom-free individuals during therapy. Lancet. 1990 Sep 8;336(8715):585–590. doi: 10.1016/0140-6736(90)93391-2. [DOI] [PubMed] [Google Scholar]
- Cristina J., López J. A., Albó C., García-Barreno B., García J., Melero J. A., Portela A. Analysis of genetic variability in human respiratory syncytial virus by the RNase A mismatch cleavage method: subtype divergence and heterogeneity. Virology. 1990 Jan;174(1):126–134. doi: 10.1016/0042-6822(90)90061-u. [DOI] [PubMed] [Google Scholar]
- Domingo E., Sabo D., Taniguchi T., Weissmann C. Nucleotide sequence heterogeneity of an RNA phage population. Cell. 1978 Apr;13(4):735–744. doi: 10.1016/0092-8674(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Eigen M. Selforganization of matter and the evolution of biological macromolecules. Naturwissenschaften. 1971 Oct;58(10):465–523. doi: 10.1007/BF00623322. [DOI] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Looney D., Rose A., Gallo R. C., Saag M. S., Shaw G. M., Hahn B. H., Wong-Staal F. Biologically diverse molecular variants within a single HIV-1 isolate. Nature. 1988 Aug 4;334(6181):444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- Goodenow M., Huet T., Saurin W., Kwok S., Sninsky J., Wain-Hobson S. HIV-1 isolates are rapidly evolving quasispecies: evidence for viral mixtures and preferred nucleotide substitutions. J Acquir Immune Defic Syndr. 1989;2(4):344–352. [PubMed] [Google Scholar]
- Harada S., Koyanagi Y., Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985 Aug 9;229(4713):563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- Holland J., Spindler K., Horodyski F., Grabau E., Nichol S., VandePol S. Rapid evolution of RNA genomes. Science. 1982 Mar 26;215(4540):1577–1585. doi: 10.1126/science.7041255. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Darby G., Richman D. D. HIV with reduced sensitivity to zidovudine (AZT) isolated during prolonged therapy. Science. 1989 Mar 31;243(4899):1731–1734. doi: 10.1126/science.2467383. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Lopez-Galindez C., Lopez J. A., Melero J. A., de la Fuente L., Martinez C., Ortin J., Perucho M. Analysis of genetic variability and mapping of point mutations in influenza virus by the RNase A mismatch cleavage method. Proc Natl Acad Sci U S A. 1988 May;85(10):3522–3526. doi: 10.1073/pnas.85.10.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Myers R. M., Larin Z., Maniatis T. Detection of single base substitutions by ribonuclease cleavage at mismatches in RNA:DNA duplexes. Science. 1985 Dec 13;230(4731):1242–1246. doi: 10.1126/science.4071043. [DOI] [PubMed] [Google Scholar]
- Popovic M., Sarngadharan M. G., Read E., Gallo R. C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science. 1984 May 4;224(4648):497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- Ratner L., Haseltine W., Patarca R., Livak K. J., Starcich B., Josephs S. F., Doran E. R., Rafalski J. A., Whitehorn E. A., Baumeister K. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985 Jan 24;313(6000):277–284. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanchez-Pescador R., Power M. D., Barr P. J., Steimer K. S., Stempien M. M., Brown-Shimer S. L., Gee W. W., Renard A., Randolph A., Levy J. A. Nucleotide sequence and expression of an AIDS-associated retrovirus (ARV-2). Science. 1985 Feb 1;227(4686):484–492. doi: 10.1126/science.2578227. [DOI] [PubMed] [Google Scholar]
- Starcich B. R., Hahn B. H., Shaw G. M., McNeely P. D., Modrow S., Wolf H., Parks E. S., Parks W. P., Josephs S. F., Gallo R. C. Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell. 1986 Jun 6;45(5):637–648. doi: 10.1016/0092-8674(86)90778-6. [DOI] [PubMed] [Google Scholar]
- Winter E., Yamamoto F., Almoguera C., Perucho M. A method to detect and characterize point mutations in transcribed genes: amplification and overexpression of the mutant c-Ki-ras allele in human tumor cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7575–7579. doi: 10.1073/pnas.82.22.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Shaw G. M., Hahn B. H., Salahuddin S. Z., Popovic M., Markham P., Redfield R., Gallo R. C. Genomic diversity of human T-lymphotropic virus type III (HTLV-III). Science. 1985 Aug 23;229(4715):759–762. doi: 10.1126/science.2992084. [DOI] [PubMed] [Google Scholar]