Abstract
The assembly of human cytomegalovirus (HCMV) virions is a complex process and involves the incorporation of viral transcripts. These RNAs are delivered to the newly infected cells and have the potential to be translated in the absence of HCMV gene expression. We have quantified the relative amount of RNAs in HCMV virions and infected cells with real-time reverse transcription-PCR and observed that viral and cellular RNAs are packaged in proportion to the amount of RNA within the cell at the time of assembly. To determine whether cis elements influenced RNA packaging, we constructed a recombinant HCMV mutant virus that expressed the yellow fluorescence protein (YFP) gene fused to the virion RNA UL21.5. We also constructed a mutant virus in which the UL21.5 transcription unit was replaced with the YFP gene. YFP RNA was incorporated into both viruses, indicating that RNA is incorporated in the absence of a virus-specific signal motif. Furthermore, with in situ hybridization, packaged transcripts were observed throughout the cytoplasm of the infected cells, including the site of virus assembly. Several proteins that nonspecifically interact with RNA, including the tegument protein pp28, were found within HCMV virions. These studies demonstrate that both viral and cellular RNAs are nonspecifically incorporated into HCMV, potentially through interactions with several virion proteins.
Human cytomegalovirus (HCMV) is the prototype member of the betaherpesvirus family and a ubiquitous human pathogen (20). HCMV establishes a lifelong infection after the initial exposure. Infection is usually asymptomatic in healthy individuals but may cause life-threatening disease in immunologically immature or compromised individuals, including neonates, AIDS patients, and transplant patients. The HCMV viral particle is structurally similar to that of all herpesviruses, consisting of a capsid containing a double-stranded DNA genome surrounded by a protein layer termed the tegument and a lipid bilayer studded with virally encoded glycoproteins. The tegument domain in HCMV consists of approximately 30 proteins (1) which play essential roles in both the initial stages of infection following virus entry and late stages during virion assembly. In addition, recent studies have demonstrated that HCMV particles contain viral transcripts (6, 11).
HCMV assembly and egress from infected cells involve a complex series of events that appear to be similar among all herpesviruses (reviewed in references 10, 19, and 34). Virus particle assembly initiates in the nucleus, where the genome is packaged into capsids. The capsids may associate with several tegument proteins, such as UL82-encoded pp71 (13) and UL69-encoded ppUL69 (28), proteins known to be localized to the nucleus at late times during virus replication. The mechanism used by capsids to translocate from the nucleus to the cytoplasm is likely to involve budding through the inner nuclear membrane and fusion with the outer nuclear membrane to be released into the cytoplasm (19, 35). The final tegumentation and envelopment occur within the cytoplasm. HCMV tegument proteins found within the cytoplasm late after infection include UL32-encoded pp150 (12, 29), UL99-encoded pp28 (16), UL83-encoded pp65 (12, 29), and UL25-encoded pUL25 (2). Increasing evidence suggests that the assembly of the tegument onto the maturing nucleocapsid involves a complex network of protein-protein interactions (19).
Studies in HCMV have demonstrated that the basic phosphoprotein pp150 can bind to capsids in vitro, with additional viral proteins observed binding the capsid (3, 8, 39). Immunoprecipitation experiments have suggested that interactions exist between the UL47-encoded tegument protein and several other proteins found within viral particles, including the tegument protein encoded by the UL48 gene (4). Studies in pseudorabies virus, an alphaherpesvirus, demonstrated a physical interaction between the UL37- and UL36-encoded proteins, which are the homologues of HCMV UL47 and UL48, respectively (7, 15). The final envelopment of tegument-coated particles likely takes place in cytoplasmic vacuoles (19). In HCMV, tegument proteins pp28, pp150, and pp65 are colocalized with membrane-bound viral glycoproteins within the cytoplasm in a juxtanuclear compartment (29, 34) that partially overlaps the trans-Golgi network (30). Recent studies have shown that gB is colocalized with several protein markers of Golgi-derived vacuoles that are destined for the plasma membrane (14).
HCMV virion assembly also involves the incorporation of RNA into infectious particles (6, 11, 22). Similar observations have been made in herpes simplex virus type 1 (33). In HCMV, gene array studies identified a subset of polyadenylated viral RNAs ranging in size from 0.4 to 5 kb that include UL21.5, UL106 to UL109, T/IRL 2 to T/IRL 5, T/IRL 7, and T/IRL 13 (6). These transcripts are expressed to high levels late in the replication cycle during virion assembly. With RNA-specific nucleic acid amplification, two other viral RNAs, UL65 and UL123, and two cell RNAs, those for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and U1A, were found in virions (11). The virion RNAs are delivered to newly infected cells upon virus entry and allow viral gene expression in the absence of transcription from the viral genome (6, 33). For example, the virion-associated UL21.5 mRNA is translated into a secreted glycoprotein and functions as a viral chemokine decoy receptor specifically interacting with the RANTES chemokine (D. Wang, W. Bresnahan, and T. Shenk, submitted for publication).
Two additional RNA species have been identified within HCMV virions and are found as stable RNA-DNA hybrids within the origin of replication of the HCMV genome (22). Studies in herpes simplex virus type 1 have identified a larger subset of polyadenylated viral RNAs packaged within virions (33). The incorporation of these RNAs into viral particles is mediated, at least in part, through interactions with tegument proteins encoded by the herpes simplex virus type 1 US11, UL47, and UL49 genes (32). A large number of cellular transcripts were also identified in herpes simplex virus type 1 viral particles (33).
The studies presented here examine the mechanism of RNA packaging into HCMV particles. We confirmed that cellular transcripts in addition to viral transcripts are packaged into HCMV particles, and we determined that each RNA is packaged in proportion to its level within the infected cell. We also demonstrated that incorporation of RNA occurs independently of a specific cis-acting packaging element, and we provide evidence that packaging is probably mediated through nonspecific interactions with proteins found within HCMV particles.
MATERIALS AND METHODS
Cell culture and viruses.
Primary human foreskin fibroblasts at passages 7 to 15 were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum in a 5% CO2 atmosphere at 37°C. HCMV strain AD169 was used as the wild-type virus. Wild-type AD169 and recombinant viruses were propagated on fibroblasts, and titers were determined by the 50% tissue culture infectious dose method (24).
Recombinant viruses were constructed by allelic exchange with pAD/Cre, which contains the full-length genome of HCMV strain AD169 maintained within a bacterial artificial chromosome (42). Allelic exchange employed derivatives of the delivery plasmid pGS284 (36): pGS284-sub21.5, pGS284-21.5YFP, and pGS284-YFPsub21.5. pGS284-sub21.5 contains the kanamycin resistance gene (kan) and lacZ gene cloned between UL21.5-specific flanking sequences. The 5′-flanking sequence was obtained by PCR amplification with an upstream primer at nucleotide 26034 (5′-ATTAGATCTATCTGTTACTATCGCTGTGGC-3′; AD169 accession number X17403) (7) containing a BglII site. The downstream primer at nucleotide 27045 (5′-ATTGCGGCCGCTCCATGGGGGTGACGACATCCCTAGGTCATGTGGC-3′) contains both NotI and NcoI sites.
Two substitutions were made within the HCMV sequence to create a unique AvrII site. The 5′-flanking sequence was inserted into pGS284 with the BglII and NotI sites. The 3′-flanking sequence was obtained with an upstream primer at nucleotide 27623 (5′-AATGCGGCCGCACACGGTTTCTTCCCATAGCC-3′) containing a NotI site and a downstream primer at nucleotide 28615 (5′-ATTGCTAGCCCCAAAGACCGCTGCCTCG-3′) containing an NheI site. The 3′-flanking sequence was cloned into the NotI and NheI sites of the above pGS284 derivative. The kan/lacZ sequence was obtained from YDC54 (42) and cloned into the NcoI and NotI sites located between the HCMV flanking sequences.
pGS284-UL21.5YFP was constructed through PCR amplification of the UL21.5 sequence from pUL21.5-YFP (6), which contains the yellow fluorescence protein (YFP) gene inserted into UL21.5 at nucleotides 27500 to 27502. The sequence was amplified with an upstream primer at nucleotide 27022 (5′-CATGACCTAGGGATGTCGTCACCC-3′) containing an AvrII site and a downstream primer at nucleotide 27857 (5′-CGTCTCCCACGGACCGTGTGC-3′) containing an RsrII site. The UL21.5YFP sequence was inserted into the AvrII and RsrII sites of pGS284-subUL21.5. Plasmid pGS284-YFPsubUL21.5 was constructed by amplification of the YFP and simian virus 40 polyadenylation sequence from pEYFP-N1 (Clontech) with an upstream primer containing an AvrII site and a downstream primer containing an RsrII site and inserted into pGS284-subUL21.5. DNA sequence analysis was completed on all HCMV PCR amplification products to confirm their integrity.
Allelic exchange was performed through homologous recombination with Escherichia coli strain GS500 as previously described (37, 42). pAD/Cre subUL21.5 was made by recombination of pAD/Cre with pGS284-subUL21.5 followed by selection for kanamycin resistance and LacZ expression. pAD/Cre UL21.5YFP and pAD/Cre YFPsubUL21.5 were made through recombination of pAD/Cre subUL21.5 with pGS284-UL21.5YFP and pGS284-YFPsubUL21.5, respectively, followed by selection for the loss of kanamycin resistance and LacZ expression. The bacterial artificial chromosome constructs were analyzed by EcoRI digestion, and sequences altered through PCR were confirmed by DNA sequence analysis. The generation of virus from bacterial artificial chromosome DNA has been described previously (42). pAD/Cre subUL21.5, pAD/Cre UL21.5YFP, and pAD/Cre YFPsubUL21.5 were used to generate viruses BADsubUL21.5, BADinUL21.5YFP, and BADsubUL21.5YFP, respectively.
Quantitative real-time RT-PCR.
To isolate RNA in each experiment, fibroblasts were grown to confluency in 18 culture dishes (15-cm diameter) and infected at a multiplicity of 2 PFU/cell. At 72 h postinfection, total cellular RNA was isolated from two dishes with Trizol reagent according to the manufacturer's instructions (Invitrogen). At 96 h postinfection, medium containing cell-free virus was collected from the remaining dishes for virion RNA isolation. The medium was cleared of any cell debris by centrifugation at 3,600 × g for 15 min, and virus was pelleted onto a cushion of 20% sorbitol in 50 mM Tris (pH 7.2) and 1.0 mM MgCl2 by centrifugation at 55,000 × g for 90 min (38). The virus pellet was resuspended in 400 μl of phosphate-buffered saline (PBS) and treated with 120 U of RNaseOne (Promega) for 1.5 h at 37°C to remove any contaminating RNA from outside of the viral particles, followed by the addition of 40 μg of proteinase K for 30 min at 37°C.
To isolate noninfectious envelope particles, infectious virus, and dense bodies, pelleted virus was sedimented in a glycerol-tartrate gradient (1). Prior to RNase treatment, 400 ng of yeast DNA-free RNA was added as a control. RNA was isolated with Trizol reagent. Virion RNA was resuspended in 50 μl of diethylpyrocarbonate-treated H2O. To control for the removal of any contaminating RNA bound to the outside of the virus, reverse transcription (RT)-PCR was performed with the Titan One Tube RT-PCR system according to the manufacturer's instructions (Roche) with primers specific for yeast β-actin RNA with the upstream primer 5′-GAAGGTAGTCAAAGAAGCCAAGATAGAAC-3′ and the downstream primer 5′-TCCCAGGATTTGCCGAAAGAATGC-3′. The removal of yeast RNA from virion RNA samples was determined by the inability to amplify yeast β-actin RNA by RT-PCR. To remove contaminating DNA, samples were treated with DNase I with the DNA-free kit according to the manufacturer's instructions (Ambion). Samples were monitored for the loss of DNA by PCR with Taq DNA polymerase (Roche) and primers to the HCMV gene UL21.5 up to 40 cycles of amplification. Removal of DNA was confirmed with real-time PCR as described below by the lack of a detectable signal above background amplification seen in the no-template control reaction.
Relative quantitation was accomplished through two-step real-time RT-PCR. cDNA was synthesized with TaqMan reverse transcription reagents and random hexamers according to the manufacturer's instructions (Applied Biosystems). For each experiment, cDNA was synthesized with 19 μl of virion RNA and 1.0 μg of RNA isolated from HCMV-infected cells in 50-μl reaction volumes and incubated at 25°C, 10 min; 48°C, 30 min; and 95°C, 5 min. Real-time PCR was completed with SYBR Green PCR Master Mix and run in the 7900HT sequence detection system (SDS) with SDS software version 2.1 according to the manufacturer's instructions (Applied Biosystems). Reactions received 1.0 μl of cDNA and 0.1 μM each primer in a 25-μl reaction volume.
Real-time PCR was carried out with a single thermocycling protocol of 50°C, 2 min; 95°C, 10 min; and 40 cycles of 95°C, 15 s, followed by 60°C, 1 min. The primer pairs against both viral and cellular transcripts are listed in Table 1 and were synthesized by Integrated DNA Technologies. For each primer pair, amplification efficiencies were determined by creating a standard curve with 10-fold serial dilutions of cDNA from infected cells; the log of the relative target quantity was plotted versus the CT (cycle threshold) value. The standard curves demonstrated various amplification efficiencies between primer pairs with slopes ranging from −3.6 to −3.3 representing amplification efficiencies between 90 and 100%, respectively. A dissociation curve was generated for each primer pair and demonstrated the amplification of a single product. The sizes of the amplified products were confirmed by agarose gel electrophoresis. Reactions were completed in duplicate, and no-template controls were included for each primer pair.
TABLE 1.
Target genes and primers used for quantitation of viral and cellular RNAs by real-time RT-PCR
| Gene (accession no.) | Forward primer (5′ location, nt) | Reverse primer (5′ location, nt) |
|---|---|---|
| T/IRL7 | 5′GAACGAACCAGCGAATAACCA3′ (6770) | 5′CCTAAAAGTTCGCAAAAACGATTG3′ (6670) |
| UL108 | 5′TCTGGCTCGACACAATGATCAC3′ (156077) | 5′GCTAATTGGACTTTGCCCATGT3′ (55973) |
| UL110 | 5′AAAAGAACGCCTAGCCATTGG3′ (158369) | 5′TTCAAAGGTCAGCCTGTGTATTGT3′ (158269) |
| UL83 (1) | 5′TGAGCATCTCAGGTAACCTGTTG3′ (120100) | 5′CAGCCACGGGATCGTACTG3′ (19999) |
| UL83(2) | 5′CCATGGTGGCTACGGTTCAG3′ (119545) | 5′TGCCATACGCCTTCCAATTC3′ (19443) |
| UL21.5 | 5′GCTTTGGCGGCACCTTCTCA3′ (27159) | 5′TTCGCTGCCATCTCCGTCTGTA3′ (27430) |
| UL122 | 5′ATGGTTTTGCAGGCTTTGATG3′ (169965) | 5′ACCTGCCCTTCACGATTCC3′ (169899) |
| UL123 | 5′GCCTTCCCTAAGACCACCAAT3′ (71824) | 5′ATTTTCTGGGCATAAGCCATAATC3′ (171724) |
| GAPDHa (J04038) | 5′ACCCACTCCTCCACCTTTGAC3′ (4548) | 5′CTGTTGCTGTAGCCAAATTCGT3′ (4752) |
| β-Actin (M10277) | 5′CATTGCCGACGGATGCA3′ (2684) | 5′GCCGATCCACACGGAGTACT3′ (2898) |
| Cyclin G1 (X77794) | 5′TTCATCAAATAAGTGTTCCAAACCA3′ (738) | 5′GCTTCAATTGCCGTGCAGTA3′ (817) |
| YFP | 5′GTCCAGGAGCGCACCATCT3′ (958) | 5′CGATGCCCTTCAGCTCGAT3′ (1066) |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Northern blot analysis.
Northern blot assays were completed as previously described (27) with RNA isolated from HCMV-infected cells and cell-free virus. Briefly, 10 μl of virion RNA and 2.0 μg of cell RNA were separated on a 1.0% agarose denaturing gel and blotted onto a nylon membrane with TurboBlotter following the manufacturer's instructions (Schleicher and Schuell). Antisense RNA probes for UL21.5, UL83, and UL107 (6) were synthesized with the Riboprobe System (Promega) in the presence of [32P]UTP. Quantitation was performed with a PhosphorImager (Molecular Dynamics).
RNA in situ hybridization and immunofluorescence.
Fibroblasts were grown on sterile glass coverslips in six-well plates to confluence. Cells were then infected with HCMV strain AD169 at 0.01 PFU/cell. After 72 h cells were washed in PBS, fixed for 15 min in 2% paraformaldehyde in PBS, washed with PBS, and permeabilized for 15 min in 0.1% Triton X-100 in PBS. After washing with PBS containing 0.2% Tween 20, the cells were incubated for 30 min in PBS-blocking buffer containing 2% bovine serum albumin and 0.2% Tween 20 and incubated with mouse monoclonal antibodies against pp28 (clone 10B4-28) (34) diluted 1:10 in PBS blocking buffer for 1 h at room temperature. After further washing with PBS containing 0.2% Tween 20, slides were incubated for 30 min at room temperature with goat anti-mouse immunoglobulin -lexa 546 (Molecular Probes).
Cells were refixed in 4% paraformaldehyde in PBS for 15 min to cross-link bound antibodies (18), washed with PBS, and permeabilized for 15 min in 0.2% Triton X-100 in PBS. After equilibration in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), cells were dehydrated in an ethanol series (70, 80, 90, and 100% ethanol for 5 min each), air dried, and incubated overnight at 37°C with the hybridization mixture. Digoxigenin-labeled probes corresponding to UL21.5 and UL83 were generated by PCR labeling with the PCR-digoxigenin labeling mix according to the manufacturer's protocol (Roche). The actin probe was obtained as a digoxigenin-labeled RNA probe (Roche). Digoxigenin-labeled probe (≈50 ng), 15 μg of salmon sperm DNA, and 15 μg of yeast tRNA were ethanol precipitated, resuspended in 20 μl of hybridization buffer containing 50% formamide in 2× SSC-10% dextran sulfate-0.1% sodium dodecyl sulfate and heated at 94°C for 4 min to denature the probe DNA (31). After hybridization, specimens were washed at 37°C with 55% formamide in 2× SSC, pH 7.0 (four times for 5 min each), 2× SSC (two times for 5 min each), and 0.2× SSC (two times for 5 min each) (5). Hybridized probes were detected with the fluorescent antibody enhancer set for digoxigenin detection (Roche) according to the manufacturer's protocol. The third antibody was supplemented with 1 ng of 4′,6′-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes) per ml to counterstain the chromosomal DNA. After the last wash cells were dehydrated in an ethanol series (70, 80, 90, and 100% ethanol for 5 min each), air dried, and mounted with Slow Fade (Molecular Probes). A Zeiss LSM510 was used for laser scanning microscopy.
Northwestern and Western blot analyses.
Virus extract was prepared by pelleting cell-free HCMV particles through a 20% sorbitol cushion. The pellet was resuspended in radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1.0% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, and 50 mM Tris-HCl, pH 7.8) containing EDTA-free complete protease inhibitor cocktail (Roche). Virus extract (30 μg) was separated on a sodium dodecyl sulfate-containing 10% polyacrylamide gel. Proteins were transferred to nitrocellulose for either Northwestern or Western blot analysis. Northwestern blots were completed as previously described (41). Briefly, membranes were washed sequentially in 6 M, 3 M, 1.5 M, 0.75 M, 0.375 M, and 0.187 M guanidine hydrochloride in renaturation buffer (20 mM HEPES-KOH, pH 7.5, 25 mM NaCl, 1 mM dithiothreitol), blocked for 1 h at 37°C in blocking buffer (10 mM Tris-HCl, pH 7.8, 150 mM NaCl, 2.5 mg of yeast tRNA per ml), and washed for 5 min in 10 mM Tris-HCl, pH 7.8. Membranes were incubated in NWB buffer (10 mM Tris-HCl, pH 7.8, 1 mM EDTA, 50 mM NaCl, 0.02% Ficoll, 0.02% polyvinylpyrrolidone, 0.02% bovine serum albumin) containing a radiolabeled RNA probe at 5.0 × 105 cpm/ml for 1 h at room temperature. Sense-strand RNA probes for UL21.5 and UL83 (6) were synthesized with Riboprobe Systems in the presence of [32P]UTP (Promega). Membranes were washed three times with NWB buffer for 30 min each. Proteins bound to the radiolabeled RNA were detected by autoradiography.
For Western blot analysis, membranes were blocked in PBS containing 5% milk and 0.5% Tween 20 for 1 h at room temperature and then incubated with a mouse monoclonal antibodies against pp28 (34) diluted 1:10 in PBS containing 1.0% milk and 0.5% Tween 20 for 1 h at room temperature. Membranes were washed three times with PBS containing 0.5% Tween 20. Proteins were visualized by ECL detection (Amersham) according to the manufacturer's instructions.
Affinity purification.
Virus extract was prepared by pelleting cell-free virus through 20% sorbitol and resuspending in lysis buffer (10 mM Tris-HCl, pH 7.8, 50 mM NaCl, 2 mM MgCl2, 1 mM dithiothreitol, 0.1% Nonidet P-40) containing EDTA-free complete protease inhibitor cocktail (Roche) and incubated for 1 h at 4°C prior to use. Sense-strand UL21.5 RNA was synthesized in vitro with the RiboMax large scale RNA production system (Promega). Unincorporated nucleotides were removed through several precipitation steps followed by purification with a Sephadex G-50 column (Roche). UL21.5 RNA (20 μg) was linked to biotin with the 5′ EndTag nucleic acid labeling system (Vector Laboratories) and biotin maleimide (Vector Laboratories) following the manufacturer's instructions. Biotinylated UL21.5 RNA (≈10 μg) was bound to 75 μl of Dynabeads M-280 streptavidin (Dynal Biotech) according to the manufacturer's instructions. Unbound RNA was removed with a magnetic particle separator and three washes with lysis buffer. The Dynabead-RNA complex was resuspended in 75 μl of lysis buffer. Virus extract (60 μg) was incubated for 15 min at 30°C with 50 μl of the Dynabead-RNA complex and 10 μg of poly(dI-dC) (Sigma) in a final volume of 100 μl. Dynabeads alone were mixed with virus extract as a control. Unbound proteins were removed with a magnetic particle separator (Roche) and three consecutive washes with 400 μl of lysis buffer. Pelleted protein-RNA complexes were resuspended in 10 μl of sample buffer, separated on a sodium dodecyl sulfate-10% polyacrylamide gel, and analyzed by Western blotting as described above.
RESULTS
Viral and cellular RNAs are packaged proportionally to their levels within the infected cell.
Previous studies have demonstrated that both HCMV and herpes simplex virus type 1 package RNA into viral particles (6, 11, 33). To investigate the mechanism of RNA packaging in HCMV, we first quantified the relative amounts of different RNAs within viral particles compared to their levels within infected cells with real-time RT-PCR. For these experiments, fibroblasts were infected with HCMV, and total RNA was isolated from the infected cells after 72 h. Assembly of viral particles within fibroblasts begins around 48 h postinfection (20). RNA was also isolated from extracellular virions that had been treated with RNase to remove contaminating RNAs from the outside of the viral particles.
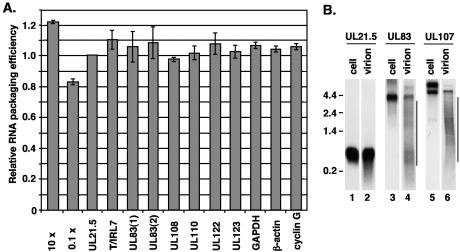
Real-time RT-PCR was performed on RNA from infected cells as well as viral particles with the primers to viral and cellular transcripts listed on Table 1. Real-time RT-PCR produces a threshold cycle (CT) value at which the fluorescence signal rises above a background level and is often used to compare changes in the levels of a single RNA target as a result of changes in the cellular environment (17). However, primer pairs against different RNAs possess various amplification efficiencies, and therefore, the results cannot be directly compared. For this reason, we measured RNA packaging into HCMV virions as a ratio of the CT value obtained from virion RNA sample to the CT value obtained from infected-cell RNA. This ratio was normalized to that of UL21.5 to define the relative amount or efficiency of RNA packaging of a given transcript. The UL21.5 transcript has been shown to be present in HCMV virions (6). We have presented the results as the inverse value, so that a greater amount of RNA packaged is represented by a larger number. With the CT values obtained from the standard curve for UL21.5 described in Materials and Methods, we estimated that a 10-fold increase in the amount of RNA packaged within the virion relative to the cell would produce a relative CT ratio of 1.22 and a 10-fold decrease would produce a CT ratio of 0.83 (Fig. 1A).
FIG. 1.
Relative quantitation of RNA packaged into HCMV particles with real-time RT-PCR. (A) Real-time RT-PCR was completed with RNA isolated as described in Materials and Methods from fibroblasts and cell-free virus following 72 h of infection. cDNA was synthesized with random hexamers, and reactions were completed with primers to numerous viral and cellular genes with SYBR green detection. The results are presented as the ratio of the CT value obtained from the virion RNA sample to the CT value obtained from RNA isolated from infected cells. The ratios were normalized to the ratio for UL21.5 and are presented as the inverse value. An estimate of the packaging efficiency is shown when 10-fold more RNA is packaged as well as 0.1-fold less RNA relative to UL21.5. The data are presented as the standard deviation from two experiments. (B) Northern blot analysis was completed with an equal volume of virion RNA and 2 μg of total RNA from infected cells. Specific transcripts were detected with 32P-labeled strand-specific probes to UL21.5, UL83, and UL107. Additional low-molecular-weight bands observed in virion RNA samples are indicated by lines. The locations of marker RNAs are indicated to the left of the gel, with sizes in kilobases.
The viral RNAs previously shown to be present in HCMV virions include transcripts encoding UL21.5, UL106 to UL110, T/IRL2 to T/IRL5, T/IRL7, and T/IRL13 (6). To confirm the packaging of these RNAs and to measure the relative amounts packaged, we performed real-time RT-PCR with random hexamers for reverse transcription and primers to UL21.5, T/IRL7, UL108, and UL110. These RNAs were incorporated into virions with efficiencies similar to that of UL21.5 (Fig. 1A). The relative CT values are summarized in Table 2. In HCMV replication, these RNAs are expressed at high levels during virus assembly. To determine if an RNA expressed at low levels during HCMV assembly could be packaged, we measured the relative levels of UL123 in virions. Interestingly, UL123 and UL21.5was packaged at similar efficiencies (Table 2, Fig. 1A).
TABLE 2.
Relative efficiency of RNA packaging within HCMV particles
| Gene | Ratio of CT values relative to that of UL21.5a
|
|||
|---|---|---|---|---|
| Total particles | NIEP | Infectious virus | DB | |
| UL21.5 | 1.0 | 1.0 | 1.0 | 1.0 |
| T/IRL7 | 1.10 ± 0.06 | NDb | ND | ND |
| UL83(1) | 1.06 ± 0.10 | ND | ND | ND |
| UL83(2) | 1.08 ± 0.10 | 1.08 ± 0.04 | 1.08 ± 0.05 | 1.04 ± 0.03 |
| UL108 | 0.98 ± 0.01 | 0.98 ± 0.02 | 0.96 ± 0.03 | 0.92 ± 0.11 |
| UL110 | 1.02 ± 0.04 | ND | ND | ND |
| UL122 | 1.08 ± 0.07 | ND | ND | ND |
| UL123 | 1.02 ± 0.04 | ND | ND | ND |
| GAPDHc | 1.07 ± 0.02 | ND | ND | ND |
| β-Actin | 1.04 ± 0.02 | 1.04 ± 0.05 | 1.03 ± 0.02 | 1.00 ± 0.02 |
| Cyclin G1 | 1.06 ± 0.02 | ND | ND | ND |
| 21.5YFP | 0.91 ± 0.05d | ND | ND | ND |
| YFP | 1.00 ± 0.04d | ND | ND | ND |
1/([CT, virion/CT, cell]/UL21.5).
NIEP, noninfectious enveloped particles; DB, dense bodies; ND, not determined.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Normalized to CT ratio for T/IRL7.
During late times of infection, the RNAs encoding UL83 and UL21.5 are expressed to comparable levels but were not previously detected within viral particles by Northern blot (6). To determine if UL83 can be detected in virions with real-time RT-PCR, we completed experiments with two different primer pairs to different regions of the UL83 transcript (Table 1). Both primer pairs amplified a single product of the predicted size from RNA isolated from viral particles. The ratios of the CT values indicated that UL83 RNA was packaged at a similar efficiency to UL21.5 (Table 2, Fig. 1A). Similar results were obtained with oligo(dT) to produce the cDNA for real-time PCR (data not shown).
An additional RNA expressed at high levels late during HCMV infection but was not previously observed in virions was UL122 (6). With primers specific to exon 5 of the UL122 gene, we observed this RNA to be present within virions (Table 2, Fig. 1A). Real time RT-PCR results were confirmed for UL83 by Northern blot analysis with a strand-specific probe against UL83. In this experiment, the 4-kb transcript encoding UL83 (26) was detected in virion samples (Fig. 1B, lane 4). To compare the amount of full-length RNA packaged, the levels for both UL21.5 and UL83 found in HCMV virions and infected cells were measured. Similar to the RT-PCR analysis, we compared the ratio of full-length UL83 RNA found within viral particles (Fig. 1B, lane 4) to the amount found within the infected cell (Fig. 1B, lane 3) relative to that of UL21.5 (Fig. 1B, lanes 1 and 2). We observed a lower ratio equal to 0.45 compared to 1.06 and 1.08 observed with real-time PCR. In addition to the full-length UL83 transcript, additional smaller UL83 RNA species were detected within the virion sample but not in the infected cell (Fig. 1B, lane 4). Northern blot analysis with a probe to UL107 detected the 5-kb UL106 to UL109 RNA in virions (Fig. 1B, lane 6). An upper band was also observed, representing an unspliced precursor to the 5-kb RNA (M. J. Romanowski and T. Shenk, unpublished data). As seen with UL83, a decreased level of the full-length 5-kb RNA was observed in virions, and additional smaller RNA species were detected. For both UL83 and UL107, these smaller species may represent fragments of the full-length transcript. RT-PCR, of course, does not distinguish between the full-length transcript and fragments of that RNA species.
Previous studies identified several cellular RNAs within herpes simplex virus type 1 and HCMV (11, 33). We analyzed HCMV virions for the presence of cellular RNAs by real-time RT-PCR. Incorporation of glyceraldehhyde-3-phosphate dehydrogenase, β-actin, and cyclin G1 RNAs was measured with the primers listed in Table 1. All three RNAs were detected within viral particles with real-time RT-PCR (Fig. 1A). The ratios of CT values for glyceraldehyde-3-phosphate dehydrogenase, β-actin, and cycle G1 RNAs were similar to that of UL21.5 (Table 2, Fig. 1A). Taken together, these data suggest that both viral and cellular RNAs are packaged into HCMV particles at similar efficiencies and in proportion to their abundance within the infected cell.
RNAs are packaged into all types of HCMV particles.
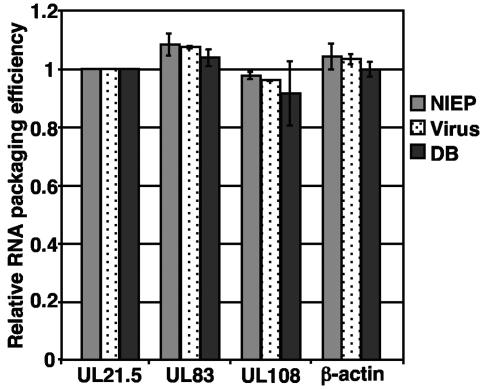
In addition to infectious virus, replication of HCMV in fibroblasts yields two types of aberrant particles known as noninfectious enveloped particles and dense bodies. Noninfectious enveloped particles are defective particles consisting of enveloped capsids lacking the viral genome, while dense bodies are enveloped particles that lack the nucleocapsid. Previous studies have demonstrated that RNA is packaged into infectious particles (6, 11). To determine if RNA is packaged into infectious particles only or into all particle types, total viral particles were pelleted through sorbitol and treated with RNase, and the three particle types were separated on a glycerol-tartrate gradient. Particle types were analyzed by real-time RT-PCR with primer pairs to a subset of RNAs which included UL21.5, UL83, UL108, and β-actin. RT-PCR identified all four RNAs within the three different particle types (Fig. 2). In comparing the CT values within each particle type, we observed that these RNAs were packaged at similar efficiencies compared to UL21.5 within noninfectious enveloped particle and dense bodies as well as infectious virus (Fig. 2), and these data are summarized in Table 2. Similar results were obtained when real-time RT-PCR was completed with oligo(dT) to synthesize the cDNA and primers to UL21.5 and UL83 (data not shown).
FIG. 2.
Relative quantitation of RNA packaged into different HCMV particles types with real-time RT-PCR. RNA was isolated from each particle type and used to quantify the relative amounts of RNA packaged. The results are presented as the ratio of CT values relative to the ratio for UL21.5 and are presented as the inverse value. The data are presented as the standard deviation from two experiments. NIEP, noninfectious enveloped particles; DB, dense bodies.
Incorporation of RNA into viral particles occurs in the absence of a specific packaging signal.
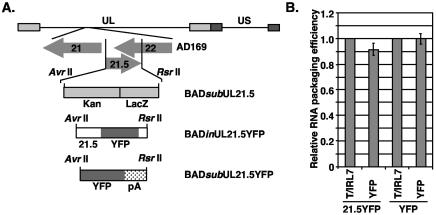
Several RNA viruses package their genomes based on specific cis-acting packaging signals recognized during assembly (9). While the packaging of cellular RNAs argues against a role for cis-acting packaging signals for HCMV, we nevertheless directly tested the possibility that HCMV RNAs contain packaging signals. Previous studies have demonstrated that an RNA containing the yellow fluorescent protein (YFP) gene fused to UL21.5 was packaged within HCMV virions (6). To determine if a sequence within the UL21.5 transcription unit could act to enhance packaging, we constructed recombinant virus BADinUL21.5YFP, which contained UL21.5 and YFP inserted into UL21.5 transcription unit (23) (Fig. 3A). A second virus, BADsubUL21.5YFP, was constructed in which the UL21.5 sequence was replaced with only the YFP gene and the simian virus 40 virus polyadenylation signal (Fig. 3A). The UL21.5 gene is nonessential for virus replication in cultured fibroblasts (D. Wang, W. Bresnahan, and T. Shenk, submitted). Both viruses were propagated in fibroblasts and replicated to similar titers.
FIG. 3.
Quantitation with real-time RT-PCR of YFP-containing transcripts from recombinant HCMV viruses. (A) The recombinant virus BADsubUL21.5 was constructed by replacing the UL21.5 transcription unit with a cassette containing the kanamycin resistance (kan) and β-galactosidase (lacZ) genes. The cassette was replaced with the UL21.5 gene fused with the YFP gene in BADinUL21.5YFP and with the YFP gene and the polyadenylation signal (pA) from simian virus 40 virus in BADsubUL21.5YFP. (B) Total RNA was isolated from fibroblasts infected for 72 h and cell-free virus for both HCMV recombinant strains BADinUL21.5YFP and BADsubUL21.5YFP. Quantitative RT-PCR was completed with primers specific to RNAs containing YFP and T/IRL 7. The results are presented as the ratio of the CT value obtained from the virion RNA sample to the CT value obtained from RNA isolated from infected cells. The ratios were normalized to the CT ratio for T/IRL 7 and are presented as the inverse value. The data are presented as the standard deviation from two experiments.
To determine whether RNAs containing the YFP sequence were packaged into virus particles, we infected fibroblasts with BADinUL21.5YFP and BADsubUL21.5YFP and isolated RNA from cells and cell-free virus particles. With real-time RT-PCR, YFP-containing RNAs were detected in virions isolated from both recombinant viruses (Fig. 3B). To determine the relative amounts of RNA packaged, we determined the CT ratios in virions and cells, and these ratios were normalized to that for the RNA encoding T/IRL7, which is packaged with an efficiency similar to that of the UL21.5 RNA (Fig. 1A). The results demonstrate that both YFP-containing RNAs were packaged at similar efficiencies to T/IRL7 (Table 2, Fig. 3B). These data indicate that RNA packaging into HCMV virions occurs independently of a specific cis-acting packaging element. In addition, these experiments confirmed the observation that transcripts are packaged in proportion to their levels within the infected cell.
Packaged RNAs are evenly distributed throughout the cytoplasm of the infected cell, including the site of virus assembly.
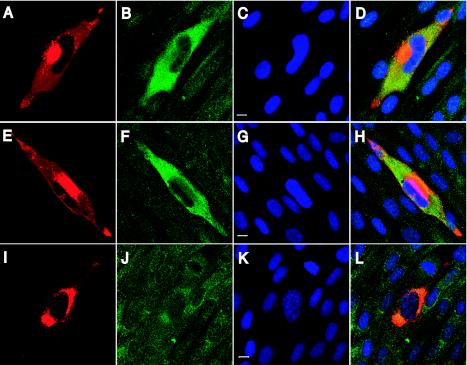
Sequence-independent packaging into viral particles may require transcripts to be localized to the site of virus assembly within the infected cell. To test this possibility, HCMV RNAs were localized in the infected cell by immunofluorescence in combination with in situ hybridization. Fibroblasts were infected for 72 h and analyzed by immunofluorescence with an antibody specific for the tegument protein pp28 and in situ hybridization with probes to UL21.5, UL83, and β-actin. The viral protein pp28 plays an essential role in virus assembly and is mainly localized to the juxtanuclear HCMV assembly site (30, 30, 34), as seen in Fig. 4. In the same cells, a probe against UL21.5 showed the transcript to be distributed throughout the cytoplasm of the infected cell (Fig. 4B), including the site of assembly as defined by pp28 (Fig. 4D). We observed little signal within the nucleus, suggesting that UL21.5 RNA is predominantly located within the cytoplasm at late times during infection. Hybridization with a probe to UL83 showed a distribution similar to that of UL21.5 (Fig. 4F) and overlapping pp28 in the infected cell (Fig. 4H). We observed β-actin RNA to be distributed throughout the cytoplasm in both uninfected and infected fibroblasts (Fig. 4J). These studies demonstrate that transcripts packaged within HCMV are localized throughout the cytoplasm, suggesting that RNA is not specifically targeted to the assembly site.
FIG. 4.
Localization of several packaged RNAs in infected fibroblasts during HCMV assembly. Fibroblasts were infected at a multiplicity of infection of 0.01 PFU/ml and processed for combined immunofluorescence and in situ hybridization at 72 h postinfection. In situ hybridization was completed with probes to UL21.5 (A to D), UL83 (E to H), and β-actin (I to L), shown in green. Immunofluorescence was completed with an antibody to the tegument protein pp28 (A, E, and I), shown in red. DNA was counterstained and appears blue (C, G, and K). Merged multicolor images (D, H, and L) and bars representing 10 μm (C, G, and K) are also shown.
HCMV particles contain several proteins that nonspecifically interact with RNA.
Our studies have demonstrated that transcripts are packaged in proportion to their levels within the infected cell independent of a cis-acting packaging element. These observations suggest that RNA is packaged without specificity into HCMV virions. We were next interested in identifying proteins within HCMV viral particles that may contribute to RNA packaging. Studies in herpes simplex virus type 1 identified several proteins within viral particles that could bind RNA, including proteins encoded by the US11, UL47, and UL49 genes (32). Analysis of the HCMV genome sequence failed to identify homologues to these proteins or HCMV sequences which contained the US11 arginine-rich RNA-binding motif (25).
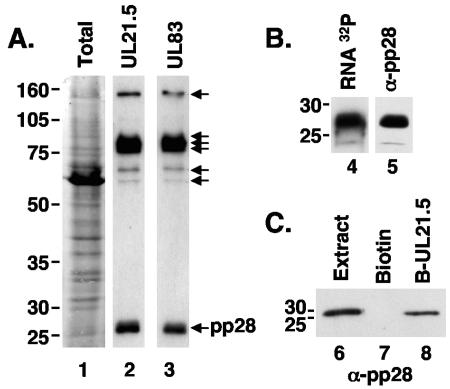
To identify proteins within HCMV virions that have the potential to bind RNA, we separated virion proteins isolated from cell-free virus by denaturing polyacrylamide gel electrophoresis and transferred them to a nitrocellulose membrane. The membrane was treated with decreasing amounts of guanidine HCl to renature proteins and then incubated with a 32P-labeled RNA generated by in vitro transcription of the full-length UL21.5 coding sequence. These experiments reproducibly identified seven bands that bound UL21.5 RNA (Fig. 5A, lane 2). We repeated these experiments with a probe containing part of the UL83 coding sequence to determine if these interactions were specific to UL21.5 RNA and observed the same set of bands (Fig. 5A, lane 3). In addition, the binding of the UL21.5 probe was efficiently inhibited by the addition of increasing amounts of yeast total RNA (data not shown). These experiments suggest that the observed RNA binding activities are not restricted to UL21.5 RNA.
FIG. 5.
Several proteins within HCMV particles interact nonspecifically with RNA. (A) Virion proteins isolated from cell-free virus were separated on a sodium dodecyl sulfate-10% polyacrylamide gel and transferred to nitrocellulose. Membranes were washed with decreasing amounts of guanidine HCl and incubated with 32P-labeled UL21.5 (lane 2) and UL83 (lane 3) sense-strand RNA probes. Seven species consistently observed to bind radiolabeled RNA are indicated by arrows. Lane 1 shows total virion proteins stained with Ponceau S. The masses of marker proteins (in kilodaltons) are indicated to the left of the gel. (B) Electrophoretically separated proteins isolated from cell-free virus were hybridized to 32P-labeled UL21.5 sense-strand RNA probes (lane 4) or an antibody to the tegument protein pp28 (lane 5). (C) Affinity purification with UL21.5 RNA bound to magnetic beads and proteins isolated from cell-free virus. In vitro-transcribed UL21.5 RNA was covalently attached to biotin and bound to streptavidin-coated magnetic beads. Proteins isolated from cell-free virus were incubated with bead-RNA complexes (lane 8) or beads alone (lane 7). Bound proteins were eluted and subjected to Western blot analysis with an antibody to pp28. Lane 6 represents crude virion extract.
The lowest-molecular-weight RNA-binding protein (Fig. 5B, lane 4) comigrated with the abundant UL99-encoded tegument protein pp28, which was identified by Western blot assay (Fig. 5B, lane 5). To verify the ability of pp28 to interact with RNA, we incubated protein extracts prepared from cell-free virus with UL21.5 RNA linked to magnetic beads. Several proteins bound to UL21.5 RNA, and one of the proteins isolated was pp28, which bound UL21.5 RNA-containing beads (Fig. 5C, lane 8) but not beads alone (Fig. 5C, lane 7). Taken together, these studies demonstrate that the tegument protein pp28 can nonspecifically interact with RNA and is likely to contribute to RNA packaging. The remaining RNA-binding proteins in virus preparations have not been identified.
DISCUSSION
In this study, we demonstrated that the amount of a specific RNA packaged into HCMV virions is in proportion to its level within the infected cell. This includes transcripts that are expressed at high levels late during infection, such as the RNA of the UL21.5 gene as well as RNAs expressed at much lower levels, including the immediate-early UL123 RNA (Fig. 1A). Several cellular RNAs were also observed in virions in proportion to their levels within infected cells (Fig. 1A). The extensive treatment of virions with RNase prior to RNA isolation and the discovery of packaged RNA within gradient-purified virions makes it unlikely that the results are due to RNA from contaminating fragments of cells. These results indicate that both cellular and viral transcripts are packaged into HCMV particles without specificity.
A previous study from our laboratory reported that a specific subset of viral transcripts are incorporated (6). The transcript encoded by the UL83 gene was not observed by Northern blot in HCMV virions or in newly infected cells. With quantitative RT-PCR, we demonstrated that UL83 RNA is packaged within HCMV virions and at an efficiency similar to that of UL21.5 (Fig. 1A). However, by Northern blot, we observed a substantial decrease in the amount of full-length UL83 RNA incorporated (Fig. 1B). Similar observations were made for the 5-kb RNA (Fig. 1B). These observations suggest that the differences observed between the two studies are due to instability of the UL83 transcript within HCMV particles. Our present observations are consistent with the work of Greijer et al. (11), which suggested that UL65 and UL123 RNAs were nonspecifically incorporated into virions.
Work done in herpes simplex virus type 1 identified several viral transcripts in virions that were consistently identified in multiple experiments, and a significantly higher number of transcripts were positive in at least one experiment (33). In addition, numerous cellular transcripts were identified within herpes simplex virus type 1 virions (33). The cellular transcripts were representative of the abundant RNAs within the cell, which suggests that RNAs are packaged in proportion to their levels within the infected cell. Our observations support the view that cellular RNAs are nonspecifically packaged into virions.
The packaging of full-length RNA genomes during RNA virus assembly is believed to involve interactions between cis-mediated packaging elements in genomic RNA and virus-encoded RNA-binding proteins. These interactions have been demonstrated for several viruses, including retroviruses, where the Gag polyprotein facilitates the specific packaging of two full-length genomes through interaction with the highly structured psi packaging signal (9). In our studies, we observed that YFP RNA, which did not contain any HCMV sequence, was packaged efficiently (Fig. 3B). The finding that cellular RNAs (Fig. 1A) and the artificial YFP RNA (Fig. 3B) are incorporated into virions at the same efficiency as viral RNAs argues strongly that the incorporation is not mediated by a cis-acting motif in the RNA.
Our studies also demonstrated that HCMV virions contain several proteins with nonspecific RNA-binding activity (Fig. 5). RNA-binding proteins have been identified in herpes simplex virus type 1 particles (32). One of the HCMV proteins observed to interact with RNA was the abundant tegument protein pp28. Analysis of the protein sequence failed to identify known RNA-binding consensus sites within pp28 but did reveal that pp28 was highly hydrophilic. It is possible that the nonspecific RNA-binding activity is mediated by electrostatic interactions. In general, nascent mRNAs associate with proteins to form ribonucleoprotein complexes. Nonspecific protein-protein interactions may also contribute to RNA packaging.
RNAs encoded by UL21.5 and UL83 genes are localized to the cytoplasm of HCMV-infected cells, with little signal observed within the nucleus (Fig. 4). These transcripts appeared to be distributed throughout the cytoplasm, including the cytoplasmic site of virus assembly. We also demonstrated that different particle types package RNA, including noninfectious enveloped particles and dense bodies in addition to infectious virus. Dense bodies lack a nucleocapsid and are formed within the cytoplasm of infected cells (38). These aberrant particles are predominantly composed of the tegument protein pp65 but contain additional tegument proteins, including pp28 (1). The observation that RNA is packaged into dense bodies argues that transcripts are acquired within the cytoplasm and are located within the viral tegument, consistent with previous findings in HCMV, where the majority of packaged RNA was detected within the tegument domain (11).
Why is RNA nonspecifically packaged into HCMV virions? Even though RNAs appear to be packaged through a sequence-independent mechanism, the virus could regulate the relative levels of packaged RNAs by controlling the level for each RNA during the assembly phase of the replication cycle. Studies of the UL21.5 gene revealed the importance of HCMV virion RNA during the virus replication cycle. The UL21.5 transcript is delivered to the cell (6) and translated to produce a secreted glycoprotein, which functions as a viral chemokine decoy receptor specifically interacting with the RANTES chemokine (D. Wang, W. Bresnahan, and T. Shenk, submitted). It is also possible that RNA plays a structural role in HCMV assembly. Recent studies in retroviruses suggest that the viral RNA genome as well as nonspecifically incorporated cellular RNAs are important for virus assembly and are critical for particle integrity (21, 40). It is possible that the RNA-protein interactions that we have observed help to organize the structure of the tegument domain during virion assembly.
Acknowledgments
We thank D. Yu for help with the AD169 bacterial artificial chromosome system and E. Xu for providing the yeast reagents. In addition, we thank F. Goodrum, C. Kulesza, and E. Murphy for critical reading of the manuscript.
This work was supported by a grant from the National Institutes of Health to T.S. (CA85786), and S.S.T. was supported by Postdoctoral Fellowship grant PF-02-126-01-MBC from the American Cancer Society.
REFERENCES
- 1.Baldick, C. J., Jr., and T. Shenk. 1996. Proteins associated with purified human cytomegalovirus particles. J. Virol. 70:6097-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Battista, M., G. Bergamini, M. Boccuni, F. Campanini, A. Ripalti, and M. Landini. 1999. Expression and characterization of a novel structural protein of human cytomegalovirus, pUL25. J. Virol. 73:3800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter, M. K., and W. Gibson. 2001. Cytomegalovirus basic phosphoprotein (pUL32) binds to capsids in vitro through its amino one-third. J. Virol. 75:6865-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechtel, J. T., and T. Shenk. 2002. Human cytomegalovirus UL47 tegument protein functions after entry and before immediate-early gene expression. J. Virol. 76:1043-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bell, P., L. J. Montaner, and G. G. Maul. 2001. Accumulation and intranuclear distribution of unintegrated human immunodeficiency virus type 1 DNA. J. Virol. 75:7683-7691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bresnahan, W. A., and T. Shenk. 2000. A subset of viral transcripts packaged within human cytomegalovirus particles. Science 288:2373-2376. [DOI] [PubMed] [Google Scholar]
- 7.Chee, M. S., A. T. Bankier, S. Beck, R. Bohni, C. M. Brown, R. Cerny, T. Horsnell, C. A. d. Hutchison, T. Kouzarides, J. A. Martignetti, et al. 1990. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr. Top. Microbiol. Immunol. 154:125-169. [DOI] [PubMed] [Google Scholar]
- 8.Chen, D., H. Jiang, M. Lee, F. Liu, and Z. H. Zhou. 1999. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology 260:10-16. [DOI] [PubMed] [Google Scholar]
- 9.Fields, B. N., D. M. Knipe, and P. M. Howley. 1996. Fundamental virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 10.Gibson, W. 1996. Structure and assembly of the virion. Intervirology 39:389-400. [DOI] [PubMed] [Google Scholar]
- 11.Greijer, A. E., C. A. J. Dekkers, and J. M. Middeldorp. 2000. Human cytomegalovirus virions differentially incorporate viral and host cell RNA during the assembly process. J. Virol. 74:9078-9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hensel, G., H. Meyer, S. Gartner, G. Brand, and H. F. Hern. 1995. Nuclear localization of human cytomegalovirus tegument protein pp150 (ppUL32). J. Gen. Virol. 76:1591-1601. [DOI] [PubMed] [Google Scholar]
- 13.Hensel, G. M., H. H. Meyer, I. Buchmann, D. Pommerehne, S. Schmolke, B. Plachter, K. Radsak, and H. F. Kern. 1996. Intracellular localization and expression of the human cytomegalovirus matrix phosphoprotein pp71 (ppUL82): evidence for its translocation into the nucleus. J. Gen. Virol. 77:3087-3097. [DOI] [PubMed] [Google Scholar]
- 14.Homman-Loudiyi, M., K. Hultenby, W. J. Britt, and C. Soderberg-Naucler. 2003. Envelopment of human cytomegalovirus occurs by budding into Golgi-derived vacuole compartments positive for gB, Rab 3, trans-Golgi network 46, and mannosidase II. J. Virol. 77:3191-3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 76:3065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Landini, M. P., B. Severi, G. Furlini, and D. de Giorgi. 1987. Hum. cytomegalovirus structural components: intracellular and intraviral localization of p28 and p65069 by immunoelectron microscopy. Virus Res. 8:15-23. [DOI] [PubMed] [Google Scholar]
- 17.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and 2-ΔΔCT method. Methods 25:401-408. [DOI] [PubMed] [Google Scholar]
- 18.Maul, G. G., A. M. Ishov, and R. D. Everett. 1996. Nuclear domain 10 as preexisting potential replication start sites of herpes simplex virus type-1. Virology 217:67-75. [DOI] [PubMed] [Google Scholar]
- 19.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mocarski, E. S. 1996. Cytomegaloviruses and their replication, p. 2447-2492. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 21.Muriaux, D., J. Mirro, D. Harvin, and A. Rein. 2001. RNA is a structural element in retrovirus particles. Proc. Natl. Acad. Sci. USA 98:5246-5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prichard, M. N., S. Jairath, M. E. T. Penfold, S. St. Jeor, M. C. Bohlman, and G. S. Pari. 1998. Identification of persistent RNA-DNA hybrid structures within the origin of replication of human cytomegalovirus. J. Virol. 72:6997-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawlinson, W. D., and B. G. Barrell. 1993. Spliced transcripts of human cytomegalovirus. J. Virol. 67:5502-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 25.Roller, R. J., L. L. Monk, D. Stuart, and B. Roizman. 1996. Structure and function in the herpes simplex virus 1 RNA-binding protein US11: mapping of the domain required for ribosomal and nucleolar association and RNA binding in vitro. J. Virol. 70:2842-2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruger, B., S. Klages, B. Walla, J. Albrecht, B. Fleckenstein, P. Tomlinson, and B. Barrell. 1987. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J. Virol. 61:446-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sambrook, J., and D. W. Russels. 2001. Molecular cloning: a laboratory manual, 3rd ed., vol. 1. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 28.Sanchez, V., P. C. Angeletti, J. A. Engler, and W. J. Britt. 1998. Localization of human cytomegalovirus structural proteins to the nuclear matrix of infected human fibroblasts. J. Virol. 72:3321-3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez, V., E. Sztul, and W. J. Britt. 2000. Human cytomegalovirus pp28 (UL99) localizes to a cytoplasmic compartment which overlaps the endoplasmic reticulum-Golgi-intermediate compartment. J. Virol. 74:3842-3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schroer, J., I. Holker, and W. Doerfler. 1997. Adenovirus type 12 DNA firmly associates with mammalian chromosomes early after virus infection or after DNA transfer by the addition of DNA to the cell culture medium. J. Virol. 71:7923-7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sciortino, M., B. Taddeo, A. Poon, A. Mastion, and B. Roizman. 2002. Of the three tegument proteins that package mRNA in herpes simplex virions, one (VP22) transports the mRNA to uninfected cells for expression prior to viral infection. Proc. Natl. Acad. Sci. USA 99:8318-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sciortino, M.-T., M. Suzuki, B. Taddeo, and B. Roizman. 2001. RNAs extracted from herpes simplex virus 1 virions: apparent selectivity of viral but not cellular RNAs packaged in virions. J. Virol. 75:8105-8116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva, M. C., Q. C. Yu, L. Enquist, and T. Shenk. 2003. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J. Virol. 77:10594-10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment-deenvelopment-reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith, G. A., and L. W. Enquist. 1999. Construction and transposon mutagenesis in Escherichia coli of a full-length infectious clone of pseudorabies virus, an alphaherpesvirus. J. Virol. 73:6405-6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith, G. A., and L. W. Enquist. 2000. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc. Natl. Acad. Sci. USA 97:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stinski, M. F. 1976. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J. Virol. 19:594-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trus, B., W. Gibson, N. Cheng, and A. C. Stevens. 1999. Capsid structure of simian cytomegalovirus from cryoelectron microscopy: evidence for tegument attachment sites. J. Virol. 73:2181-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, S.-W., and A. Aldovini. 2002. RNA incorporation is critical for retrovirus particle integrity after cell membrane assembly of gag complexes. J. Virol. 76:11853-11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilusz, J. 1999. Detecting RNA:protein interactions and isolating cDNA clones by Northwestern screening, p. 181-193. In S. Haynes (ed.), RNA-protein interaction protocols. Humana Press Inc., Totowa, N.J.
- 42.Yu, D., G. A. Smith, L. W. Enquist, and T. E. Shenk. 2002. Construction of a self-excisable bacterial artificial chromosome containing the full-length human cytomegalovirus genome and mutagenic analysis of the diploid genes TRL/IRL13 in cell culture. J. Virol. 76:2316-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]