Summary
Background
Leuco-methylthioninium bis(hydromethanesulfonate; LMTM), a stable reduced form of the methylthioninium moiety, acts as a selective inhibitor of tau protein aggregation both in vitro and in transgenic mouse models. Methylthioninium chloride has previously shown potential efficacy as monotherapy in patients with Alzheimer’s disease. We aimed to determine whether LMTM was safe and effective in modifying disease progression in patients with mild to moderate Alzheimer’s disease.
Methods
We did a 15-month, randomised, controlled double-blind, parallel-group trial at 115 academic centres and private research clinics in 16 countries in Europe, North America, Asia, and Russia with patients younger than 90 years with mild to moderate Alzheimer’s disease. Patients concomitantly using other medicines for Alzheimer’s disease were permitted to be included because we considered it infeasible not to allow their inclusion; however, patients using medicines carrying warnings of methaemoglobinaemia were excluded because the oxidised form of methylthioninium in high doses has been shown to induce this condition. We randomly assigned participants (3:3:4) to 75 mg LMTM twice a day, 125 mg LMTM twice a day, or control (4 mg LMTM twice a day to maintain blinding with respect to urine or faecal discolouration) administered as oral tablets. We did the randomisation with an interactive web response system using 600 blocks of length ten, and stratified patients by severity of disease, global region, whether they were concomitantly using Alzheimer’s disease-labelled medications, and site PET capability. Participants, their study partners (generally carers), and all assessors were masked to treatment assignment throughout the study. The coprimary outcomes were progression on the Alzheimer’s Disease Assessment Scale–Cognitive Subscale (ADAS-Cog) and the Alzheimer’s Disease Co-operative Study–Activities of Daily Living Inventory (ADCS-ADL) scales from baseline assessed at week 65 in the modified intention-to-treat population. This trial is registered with Clinicaltrials.gov (NCT01689246) and the European Union Clinical Trials Registry (2012-002866-11).
Findings
Between Jan 29, 2013, and June 26, 2014, we recruited and randomly assigned 891 participants to treatment (357 to control, 268 to 75 mg LMTM twice a day, and 266 to 125 mg LMTM twice a day). The prespecified primary analyses did not show any treatment benefit at either of the doses tested for the coprimary outcomes (change in ADAS-Cog score compared with control [n=354, 6·32, 95% CI 5·31–7·34]: 75 mg LMTM twice a day [n=257] –0·02, –1·60 to 1·56, p=0·9834, 125 mg LMTM twice a day [n=250] –0·43, –2·06 to 1·20, p=0·9323; change in ADCS-ADL score compared with control [–8·22, 95% CI –9·63 to –6·82]: 75 mg LMTM twice a day –0·93, –3·12 to 1·26, p=0·8659; 125 mg LMTM twice a day –0·34, –2·61 to 1·93, p=0·9479). Gastrointestinal and urinary effects were the most common adverse events with both high doses of LMTM, and the most common causes for discontinuation. Non-clinically significant dose-dependent reductions in haemoglobin concentrations were the most common laboratory abnormality. Amyloid-related imaging abnormalities were noted in less than 1% (8/885) of participants.
Interpretation
The primary analysis for this study was negative, and the results do not suggest benefit of LMTM as an add-on treatment for patients with mild to moderate Alzheimer’s disease. Findings from a recently completed 18-month trial of patients with mild Alzheimer’s disease will be reported soon.
Funding
TauRx Therapeutics.
Introduction
Approved treatments for Alzheimer’s disease, including acetylcholinesterase inhibitors and the N-methyl-d-aspartate receptor antagonist memantine, offer only symptomatic benefit without affecting the underlying disease pathology. Despite the urgent clinical need,1,2 disease-modifying therapies have been elusive so far, with candidates that target the amyloid aspect of Alzheimer’s disease pathology proving unsuccessful across late-stage clinical trials.3
Neurofibrillary tangles, the pathology of the disease discovered by Alois Alzheimer, are made up of paired helical filaments, composed predominantly of a 12-kDa repeat-domain fragment of the microtubule-associated protein tau.4–6 Findings from several studies support a quantitative link for the spread of aggregated tau pathology to both the extent of clinical dementia and functional molecular imaging deficits noted in Alzheimer’s disease.7–9 Because the process begins at least 20 years before any clinical manifestations of Alzheimer’s disease,10 the targeting of tau aggregation offers a rational approach to both its treatment and prevention.9 The use of methylthioninium, a diaminophenothiazine, is one such approach, inhibiting tau aggregation in vitro,12,13 dissolving paired helical filaments isolated from human Alzheimer’s disease brain tissue in vitro,13 and reducing tau pathology and associated behavioural deficits in transgenic mouse tau models at brain concentrations consistent with human oral dosing.14,15
Methylthioninium chloride (commonly known as methylene blue, the chloride salt of the oxidised form of methylthioninium), has been tested clinically as monotherapy in a phase 2 study.16 The minimum safe and effective dose was identified as 138 mg/day, but dose-dependent absorption limitations restricted its use at a higher dose of 218 mg/day. We have developed a stable reduced form of the methylthioninium moiety (leuco-methylthioninium bis[hydromethanesulfonate]; LMTM) that retains tau-aggregation inhibitor activity in vitro and in vivo,13,15 has superior pharmaceutical properties in terms of solubility and pKa compared with methylthioninium chloride, and is better absorbed than the oxidised form.14
Therefore, the objective of our study was to determine whether treatment with LMTM at doses of 75 mg and 125 mg given twice a day was safe and effective in modifying disease progression in patients with mild to moderate Alzheimer’s disease.
Methods
Study design and participants
We did a 15-month phase 3, randomised, controlled, double-blind, parallel-group study at 115 academic centres and private research clinics across 16 countries in Europe, North America, Asia, and Russia. A protocol amendment was made in August, 2013, that increased the study duration from 12 to 15 months in light of the placebo decline rates reported in external studies,17,18 which were lower than our initial estimates. Approval of the study protocol (appendix) and all related documents was obtained from the appropriate Independent Ethics Committees and Institutional Review Boards for all study sites.
Eligible patients had to be younger than 90 years with a diagnosis of mild to moderate probable Alzheimer’s disease according to criteria from the National Institute of Aging and the Alzheimer’s Association, a Mini-Mental State Examination (MMSE) score of 14–26 inclusive, and a Clinical Dementia Rating (CDR) total score of 1 or 2. The target recruitment was adjusted in August, 2013, so that two-thirds of the study sample included patients with moderate Alzheimer’s disease to better reflect the expected distribution of tau pathology9 across two studies being done in Alzheimer’s disease (the present study and a study of mild Alzheimer’s disease [NCT01689233, EudraCT 21012-002847-28]). Concomitant use of acetylcholinesterase inhibitors or memantine (or both) at a stable dose for at least 18 weeks before randomisation was permitted. Patients could enter the trial whether or not they were taking currently approved Alzheimer’s disease medications, because we considered it infeasible for these drugs to be restricted, given their extensive use. Additionally, concomitant use of serotonergic antidepressant, antipsychotic (except clozapine or olanzapine) and sedative medications was permitted at stable doses where clinically feasible. However, patients taking medications with warnings or cautions about methaemoglobinaemia were excluded, because the oxidised form of methylthioninium in high doses can induce methaemoglobinaemia. Each patient had one or more study partners (generally carers) participate with them in this trial. Patients were excluded from the study if they had a substantial CNS cause for dementia other than Alzheimer’s disease. A detailed list of inclusion and exclusion criteria is provided in the protocol (appendix).
All patients provided written informed consent before enrolling in the study. Legal representatives provided consent on behalf of patients with reduced decision-making capacity. Study partners also provided consent for involvement.
Randomisation and masking
We randomly assigned patients at baseline to LMTM 75 mg twice a day or 125 mg twice a day (expressed as methylthioninium base equivalent) or control LMTM 4 mg twice a day (3:3:4) using an interactive web response system (Trident) managed by BioClinica (Audubon, PA, USA). Because LMTM is associated with both urinary19 and faecal discolouration, the low dose of 4 mg twice a day was selected as the control, on the basis of repeat dose phase 1 studies where it was the minimum dose that would allow masking to be maintained and less than the 69 mg/day dose of methylthioninium chloride that was previously reported to not have clinical efficacy.16
The randomisation was stratified according to geographical region (three levels: North America, Europe, and the rest of world), use of Alzheimer’s disease-labelled comedications (two levels: using or not using), severity of Alzheimer’s disease (two levels: mild MMSE 20–26 and moderate MMSE 14–19 inclusive) and site PET capability (two levels: yes or no). Trident generated the randomisation sequence with 600 blocks of length ten (3:3:4 treatment allocations), by use of a Java 1.6 api class random number generator that uses a 48-bit seed based on the time the randomisation list is generated. The patient randomisation file consisted of the trial randomisation number, treatment group code or description, and block number. This file was provided to the manufacturer of the investigational medicinal product and a drug kit number list was generated and subsequently uploaded into the interactive web response system (managed by BioClinica). The randomisation file and investigational medicinal product kit list were unavailable to personnel involved in doing the study and analysing the data, but were available to the unmasked statistician providing analyses exclusively for the independent Data Safety Monitoring Board who were established for oversight of accruing safety information. Clinical trial site staff were responsible for enrolment of patients by use of the interactive web response system for which all end-user roles at sites were masked. Study participants, their informant, and all assessors remained masked to treatment assignment throughout the study, and safety assessors were not permitted to be involved in the primary efficacy assessments. The study drugs were identical in appearance for all three treatment groups.
Procedures
The interventions were LMTM 4 mg, 75 mg, or 125 mg twice a day provided in blister packages as oral tablets. Patients were required to take the requisite dose twice daily for up to 65 weeks. The number of tablets taken was to be recorded in a diary, reviewed at each clinic visit. We assessed scores on the 11-item Alzheimer’s Disease Assessment Scale–cognitive subscale (ADAS-Cog) and the Alzheimer’s Disease Cooperative Study–Activities of Daily Living (ADCS-ADL) scale at baseline and every 13 weeks thereafter, with the final on-treatment visit at week 65. These assessments were repeated at the final off-treatment safety visit at week 69. We also measured scores on the Alzheimer’s Disease Cooperative Study– Clinical Global Impression of Change scale (ADCS-CGIC), administered by an independent rater at the same visits as ADAS-Cog and ADCS-ADL, and MMSE (administered at screening and weeks 26, 52, 65, and 69). We did cranial MRI scans at baseline or screening and at weeks 13, 26, 39, 62, and 65 (or at early termination), plus or minus 14 days for each timepoint, using a standardised protocol at prequalified sites. MRI data were obtained centrally by an imaging core lab (Bioclinica) and reviewed centrally by RadMD (Buckingam, PA, USA) for eligibility and safety (monitoring amyloid related imaging abnormalities). Volumetric data were used to measure changes in lateral ventricular volume, temporoparietal volume, whole brain volume, and hippocampal volume (estimated as the mean of left and right). 18F-fluorodeoxyglucose PET (18F-FDG-PET) imaging was done at screening and weeks 39 and 65 in a subset of patients at sites with this imaging capability. Changes in cerebrospinal fluid (CSF) concentrations of total tau, phospho-tau, and amyloid-β1–42 between baseline (any time during screening before first dose of study drug) and week 65 (or early termination) were measured in a subsample of patients who consented to a lumbar puncture.
Outcomes
The coprimary efficacy outcomes were differences in ADAS-Cog and ADCS-ADL scores compared with changes in the scores in the control group from baseline. Assessments were done at each site and change from baseline was computed centrally by SynteractHCR (Carlsbad, CA, USA). A protocol amendment in June, 2015, changed one of the coprimary endpoints from scores on the ADCS-CGIC to those on the ADCS-ADL in view of data from external studies,17,18 which made possible determination of relevant placebo decline estimates. The change also conformed with recommendations received from the European Medicines Agency.
We selected MRI volumetry (lateral ventricular volume) as the key secondary outcome to assess a potential therapeutic effect on the rate of brain atrophy. Lateral ventricular volume was included as the key secondary outcome rather than whole brain volume, and temporoparietal volume was added as an exploratory endpoint, on the basis of advice from the Scientific Advisory Board (SAB) before finalisation of the statistical analysis plan, but these were not reflected in a protocol amendment. Other secondary efficacy measures were ADCS-CGIC scores and MMSE scores, the extent of vascular pathology burden as indicated by Fazekas score at baseline,24 baseline bilirubin and creatinine clearance, which might suggest differences in metabolism or excretion of LMTM, and APOE ε4 allele frequency. Exploratory endpoints were whole brain volume, hippocampal volume, 18F-FDG-PET, total tau, phosphor-tau, and amyloid-β1–42; Resource Utilization in Dementia (RUD)-lite instrument scores and collection of CSF markers were added to the protocol as exploratory endpoints in August, 2013.
We monitored patients throughout the study for adverse events and concomitant medication use, and did clinical laboratory tests, including measurement of methaemoglobin by pulse CO-oximetry and measurement of vital signs; physical and neurological examinations; and 12-lead electrocardiographs at all clinic visits (screening, baseline, and weeks 2, 6, 13, 26, 39, 52, 65, and 69). By protocol, amyloid related imaging abnormalities, serotonin toxicity, and suicidality were reported as serious adverse events. We also assessed patients at all visits for suicidal ideation and intent using the Columbia-Suicide Severity Rating Scale,20 and systematically monitored them for potential serotonin syndrome using a rating scale derived from four published diagnostic criteria,21 because of a theoretical potential for serotonin syndrome.22
Statistical analysis
We aimed to enrol 833 patients to obtain data for approximately 500 patients completing the study, assuming 30–40% of participants drop out. This sample size was estimated to provide at least 90% power for detecting a treatment difference of 2·40 units on the ADAS-Cog scale and 3·80 units on the ADCS-ADL scale at a two-sided α value of 0·05 after correction for multiple comparisons. This estimation was under the assumption that both doses have an effect size corresponding to a 50% reduction in the mean expected rate of decline assumed to be 4·76 units (SD 8·85) and –7·52 units (14·06), respectively for each dose over 15 months.
The last version of the statistical analysis plan (appendix) was finalised on Feb 9, 2016, before database lock on Feb 10, and unblinding on Feb 11. The primary efficacy analyses of change from baseline in ADAS-Cog and ADCS-ADL scores to week 65 (week 52 if the withdrawal rate exceeded 40%) were done in the modified intent-to-treat (mITT) population (ie, all randomly assigned patients who took at least one dose of study treatment and had both a baseline and at least one post-baseline efficacy assessment). The primary analysis was specified as a mixed model repeated-measures analysis with an unstructured covariance matrix and no imputation for missing data. The model included visit (five levels corresponding to assessments at weeks 13, 26, 39, 52, and 65), treatment (three levels corresponding to control, 75 mg twice a day, and 125 mg twice a day), treatment-by-visit interaction, the stratification variables as additive terms, and baseline ADAS-Cog or ADCS-ADL scores as a covariate. The primary analysis model included assessment of the statistical significance of each of the stratification covariates as additive terms in the model. An exploratory post-hoc analysis was specified in the statistical analysis plan with the covariate for taking or not taking Alzheimer’s disease-labelled medications as an interaction term with treatment and an interaction term with visit in the model comparing monotherapy and addon treatment with the control population as randomly assigned. We used the same method for all secondary analyses. We used Westfall’s method for multiple comparison correction in each step (including analyses of covariates) to ensure control of the familywise error with α set at 0·05.23 In unprespecified post-hoc analyses, we compared patients taking 4 mg twice a day as monotherapy or as add-on therapy. We also compared patients taking 75 mg twice a day and 125 mg twice a day as monotherapy with those taking 4 mg twice a day as monotherapy, and patients taking 75 mg twice a day and 125 mg twice a day as add-on therapy with patients taking 4 mg twice a day as add-on therapy. Data analyses were done by SynteractHCR with SAS 9.4 (Enterprise Guide v 7.1); results were verified with R version 3.3.0 (2016-05-03). All amendments to the protocol (including those stated earlier) and others entailing mainly clarifications arising from site or monitor queries, or both, are listed in the appendix. This trial is registered at www.clinicaltrials.gov (NCT01689246) and the European Union Clinical Trials Registry (2012-002866-11).
Role of the funding source
The funder of the study took the lead in study design, undertaking the study, data interpretation, and writing of the report. Data analyses were done independently of the funder; the funder verified results. The decision to submit for publication was taken jointly by the Scientific Advisory Board members (SG, HHF, LSS, GKW, GBF, and CMW).
Results
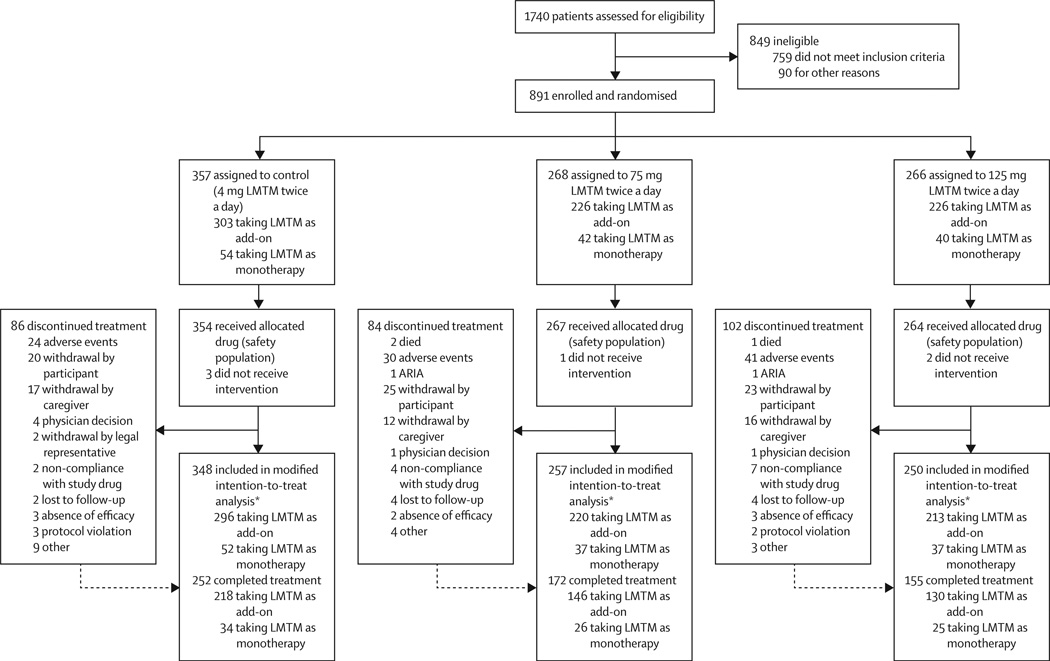
Between Jan 29, 2013, and June 26, 2014, we recruited 891 patients; the last patient visit was on Nov 30, 2015. Of 891 patients randomly assigned to treatment, 885 received at least one dose of study drug and comprised the safety population; 855 of these patients had a post-baseline efficacy assessment and comprised the mITT population (figure 1). The baseline demographic and clinical characteristics of the safety population are in table 1. Although seven patients had a CDR score of 0·5, they were not required to discontinue if they had already been assigned to treatment. 618 patients completed the study to 65 weeks (579 remained on treatment), therefore 31% of patients withdrew from the study. MRI scans from all scheduled visits were available for 880 patients before treatment, and 554 patients at 65 weeks. 18F-FDG-PET data were available from 101 patients at 65 weeks, of whom six were not taking Alzheimer’s disease-labelled treatments. Lumbar-puncture data were available from 38 patients at baseline, of whom five were not taking Alzheimer’s disease treatments.
Figure 1. Trial profile.
LMTM=leuco-methylthioninium bis(hydromethanesulfonate. ARIA= amyloid related imaging abnormalities. *Missing patients in the modified intention-to-treat analysis did not have a post-baseline efficacy assessment.
Table 1.
Baseline characteristics of the safety population
| Control 4 mg LMTM twice a day (n=354) |
75 mg LMTM twice a day (n=267) |
125 mg LMTM twice a day (n=264) |
Total (n=885) | |
|---|---|---|---|---|
| Age (years) | ||||
| Mean (SD) | 70·7 (8·5) | 71·0 (9·3) | 70·1 (9·3) | 70·6 (9·0) |
| Median (IQR) | 72·0 (65·0–77·0) | 72·0 (65·0–78·0) | 71·0 (64·0–77·0) | 72·0 (65·0–77·0) |
| Sex | ||||
| Male | 134 (38%) | 93 (35%) | 113 (43%) | 340 (38%) |
| Female | 220 (62%) | 174 (65%) | 151 (57%) | 545 (62%) |
| Ethnic origin | ||||
| Native American or Alaska Native | 2 (1%) | 3 (1%) | 2 (1%) | 7 (1%) |
| Asian | 41 (12%) | 32 (12%) | 30 (11%) | 103 (12%) |
| Black or African American | 3 (1%) | 3 (1%) | 4 (2%) | 10 (1%) |
| White | 307 (87%) | 226 (85%) | 225 (85%) | 758 (86%) |
| Other | 1 (<1%) | 0 | 2 (1%) | 3 (<1%) |
| Multiethnic | 0 | 3 (1%) | 1 (<1%) | 4 (<1%) |
| Years since diagnosis | 2·8 (2·4) | 2·9 (2·3) | 2·8 (2·2) | 2·8 (2·3) |
| Dementia severity (CDR score) | ||||
| 0·5 | 4 (1·1) | 1 (0·4) | 2 (0·8) | 7 (0·8) |
| 1 | 261 (73·7) | 209 (78·3) | 192 (72·7) | 662 (74·8) |
| 2 | 89 (25·1) | 57 (21·3) | 70 (26·5) | 216 (24·4) |
| MMSE score | ||||
| Mean (SD) | 18·6 (3·45) | 18·8 (3·44) | 18·5 (3·40) | 18·6 (3·43) |
| Median (IQR) | 18·0 (16·0–21·0) | 19·0 ((16·0–21·0) | 18·0 (15·0–21·0) | 18·0 (16·0–21·0) |
| MMSE severity | ||||
| MMSE score ≥20 | 134 (38%) | 105 (39%) | 98 (37%) | 337 (38%) |
| MMSE score <20 | 220 (62%) | 162 (61%) | 166 (63%) | 548 (62%) |
| ADAS-Cog score | ||||
| Mean (SD) | 27·2 (10·1) | 26·5 (9·4) | 26·7 (9·7) | 26·9 (9·8) |
| Median (IQR) | 26·3 (19·7–34·0) | 26·3 (18·7–32·7) | 26·3 (19·0–32·7) | 26·3 (19·3–33·0) |
| ADCS-ADL score | ||||
| Mean (SD) | 55·9 (12·7) | 58·0 (11·1) | 57·5 (12·7) | 57·0 (12·3) |
| Median (IQR) | 58·0 (48·0–65·0) | 58·5 (52·0–65·8) | 60·0 (48·0–67·9) | 59·0 (49·0–66·0) |
| Whole brain volume (cm3) | ||||
| Mean (SD) | 927 (108) | 922 (115) | 939 (101) | 929 (108) |
| Median (IQR) | 917 (847–1004) | 922 (848–993) | 934 (965–1005) | 925 (854–1002) |
| Lateral ventricular volume (cm3) | ||||
| Mean (SD) | 52 (23) | 52 (26) | 51 (23) | 52 (24) |
| Median (IQR) | 49 (35–66) | 44 (32–65) | 47 (34–62) | 47 (34–64) |
| Hippocampal volume (cm3) | ||||
| Mean (SD) | 2·3 (0·6) | 2·7 (0·6) | 2·9 (0·6) | 2·8 (0·6) |
| Median (IQR) | 2·7 (2·4–3·1) | 2·7 (2·3–3·1) | 2·8 (2·4–3·2) | 2·7 (2·4–3·1) |
| Alzheimer’s disease-approved comedications |
||||
| Acetylcholinesterase inhibitors only | 183 (52%) | 151 (57%) | 150 (57%) | 484 (55%) |
| Memantine only | 32 (9%) | 16 (6%) | 15 (6%) | 63 (7%) |
| Acetylcholinesterase inhibitors and memantine |
93 (26%) | 60 (22%) | 61 (23%) | 214 (24%) |
| Cerebrospinal fluid biomarkers (ng/L) | ||||
| Total tau | 143·9 (68·4; n=19) | 156·4 (72·5; n=15) | 113·2 (547; n=5) | 144·8 (68·2; n=39) |
| Phospho-tau | 59·2 (25·3; n=20) | 61·2 (20·3; n=15) | 58·1 (12·8; n=5) | 59·8 (21·9; n=40) |
| Aβ1–42 | 2647 (96·6; n=20) | 276·0 (85·9; n=15) | 235·8 (62·1; n=5) | 265·3 (88·0; n=40) |
| APOE genotype* | ||||
| ε4 allele present | 144/303 (48%) | 91/217 (42%) | 114/217 (53%) | 349/737 (47%) |
| ε4 allele absent | 159/303 (52%) | 126/217 (58%) | 103/217 (47%) | 388/737 (53%) |
Data are mean (SD), median (IQR), n (%), or n/N (%)· LMTM=leuco-methylthioninium bis(hydromethanesulfonate)· CDR=Clinical Dementia Rating· MMSE=Mini-Mental State Examination· ADAS-Cog=Alzheimer’s Disease Assessment Scale-Cognitive Subscale· ADCS-ADL=Alzheimer’s Disease Cooperative Study-Activities of Daily Living.
Denominator is the available sample.
None of the treatment effects was significant for the coprimary outcome (change in ADAS-Cog score compared with control [n=354, 6·32, 95% CI 5·31 to 7·34]: 75 mg LMTM twice a day [n=257] –0·02, –1·60 to 1·56, p=0·9834, 125 mg LMTM twice a day [n=250] –0·43, –2·06 to 1·20, p=0·9323; change in ADCS-ADL score compared with control [–8·22, 95% CI –9·63 to –6·82]: 75 mg LMTM twice a day –0·93, –3·12 to 1·26, p=0·8659; 125 mg LMTM twice a day –0·34, –2·61 to 1·93, p=0·9479; table 2). Similarly, LMTM was not associated with significant treatment effects for any of the secondary outcomes (table 2, figure 2, and appendix). In the primary analysis models for coprimary and secondary clinical outcomes, patients who received LMTM as monotherapy had a lower rate of progression than did control patients or those taking LMTM as an add-on (ADAS-Cog, p<0·0001; ADCS-ADL, p=0·0174; ADCS-CGIC, p<0·0001; MMSE, p<0·0001). ADAS-Cog (p=0·0009), ADCS-ADL (p<0·0001), and ADCS-CGIC (p<0·0001) scores were likewise significantly lower in patients with mild Alzheimer’s disease, but there was no difference in MMSE score (p=0·9997). There was no effect of geographic region in any of the analyses. Treatment-emergent adverse events occurring in at least 2% of participants receiving high-dose LMTM, with the 75 mg and 125 mg dose groups greater than the control group, are shown in Table 3. Gastrointestinal and urinary related adverse events were the most common and were also the commonest reasons for discontinuing high-dose LMTM (48 of 531 patients; 9%) compared with six of 354 patients (2%) in the control group. The incidence of targeted gastrointestinal adverse events was twice as high in patients receiving LMTM as add-on therapy (241 of 761 patients; 32%) compared with those receiving LMTM alone (22 of 124 patients; 18%).
Table 2.
Efficacy analyses for primary and secondary outcomes using primary analysis with the stratification covariates as additive terms in the model
| Baseline | Control 4 mg LMTM twice a day change from baseline (n=348) |
Treatment effects | Covariate effects | |||
|---|---|---|---|---|---|---|
| 75 mg LMTM twice a day (n=257) |
125 mg LMTM twice a day (n=250) |
Severity (mild) | Taking LMTM as monotherapy |
|||
| ADAS-Cog | 27·15 (26·09–28·21) |
6·32 (5·31–7·34) |
−0·02 (−1·60 to 1·56); p=0·9834 |
−0·43 (−2·06 to1·20); p=0·9323 |
−1·03 (−1·57 to −0·49); p=0·0009 |
−2·30 (−3·35 to −1·25); p<0·0001 |
| ADCS-ADL | 55·91 (54·58–57·24) |
−8·22 (−9·63 to −6·82) |
−0·93 (−3·12 to 1·26); p=0·8659 |
−0·34 (−2·61 to 1·93); p=0·9479 |
1·62 (1·02–2·23); p<0·0001 |
2·00 (0·65–3·35); p=0·0174 |
| Lateral ventricular volume (cm3) |
52·40 (49·93–54·87) |
7·18 (6·63–7·74) |
−0·60 (−1·47 to 0·27); p=0·6049 |
−0·58 (−1·46 to 0·31); p=0·6049 |
−0·12 (−0·25 to 0·01); p=0·3490 |
−0·13 (−0·42 to −0·16); p=0·6158 |
| ADCS-CGIC | −1·03 (–1·16 to −0·90) |
−0·06 (−0·27 to 0·14); p=0·7866 |
0·01 (−0·21 to 0·22); p=0·9504 |
0·16 (0·09–0·23); p<0·0001 |
0·42 (0·27–0·57); p<0·0001 |
|
| MMSE | 18·60 (18·24–18·96) |
−3·73 (−4·23 to −3·23) |
0·06 (−0·71 to 0·84); p=0·9997 |
0·50 (− 0·29 to 1·30); p=0·6888 |
0·03 (−0·51 to 0·56); p=0·9997 |
1·95 (1·24–2·66); p<0·0001 |
Data are mean (95% CI); p value. The effect for geographic regions is not shown because it was not significant for any of the outcomes. LMTM=leuco-methylthioninium bis(hydromethanesulfonate). Treatment effects are differences with respect to control change from baseline at 65 weeks. Estimates for the covariates severity and use of Alzheimer’s disease-labelled treatments are shown. Population weights are used for all covariates in the mixed model repeated measures analysis, except for the Alzheimer’s disease treatment term where the contrast was set to taking approved Alzheimer’s disease treatments. ADAS-Cog=Alzheimer’s Disease Assessment Scale-Cognitive Subscale. ADCS-ADL=Alzheimer’s Disease Co-operative Study-Activities of Daily Living. ADCS-CGIC=Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change scale. MMSE=Mini-Mental State Examination.
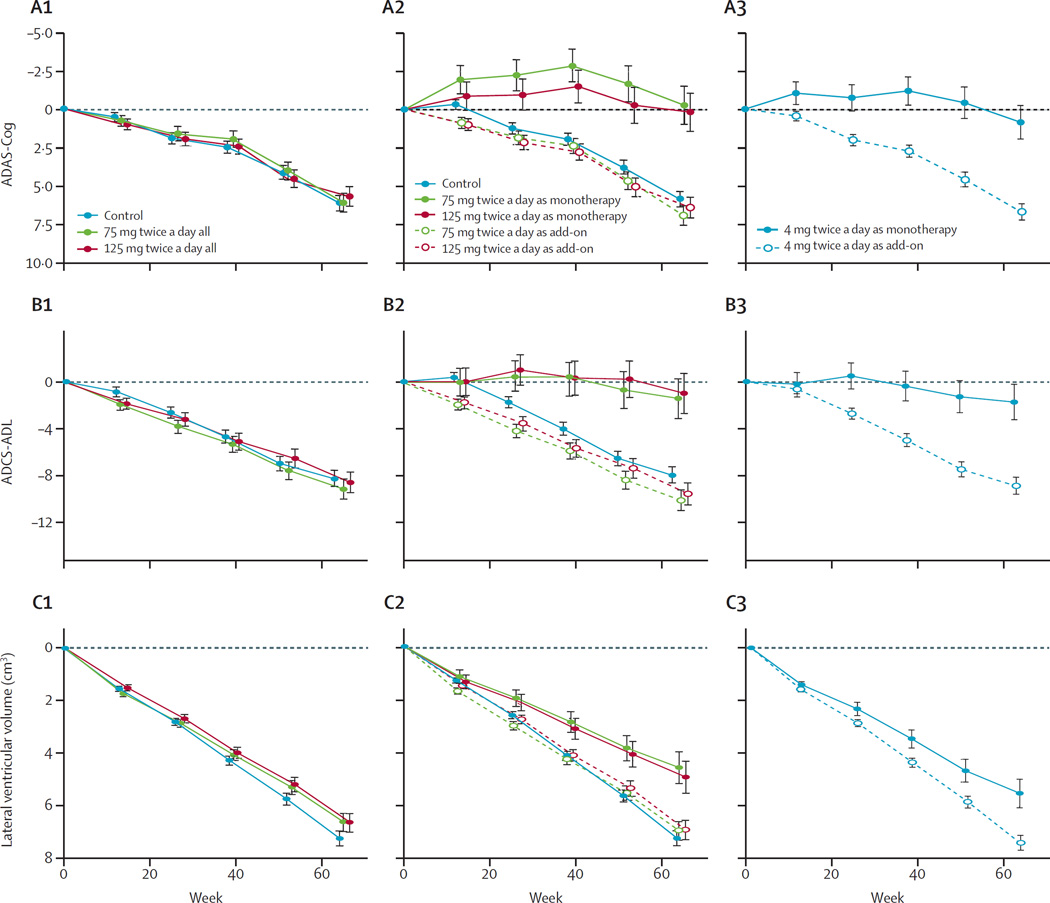
Figure 2. Least squares estimates of mean change from baseline in ADAS-Cog (A), ADCS-ADL (B), and lateral ventricular volume (C).
We did the estimates with either the primary analysis model with Alzheimer’s disease comedication status as an additive term in the model (A1, B1, C1), or a prespecified repeat of the primary analysis with Alzheimer’s disease comedication status as an interaction term in the model showing effect of leuco-methylthioninium bis(hydromethanesulfonate) treatment as either monotherapy or as add-on to existing Alzheimer’s disease treatments (A2, B2, C2). In both these analyses, the control group is as randomised. In a further non-prespecified analysis, we compared patients randomised to the control arm taking 4 mg leuco-methylthioninium bis(hydromethanesulfonate) treatment as either monotherapy or as add-on to existing Alzheimer’s disease treatments (A3, B3, C3). Numbers of participants analysed in each of the study group are shown in tables 2 and 3, and in the appendix, and numbers completing treatment with 4 mg, 75 mg, or 125 mg twice daily are shown in figure 1, according to Alzheimer’s disease comedication status. Corresponding results for ADCS-CGIC and MMSE are shown in the appendix. ADAS-Cog=Alzheimer’s Disease Assessment Scale–Cognitive Subscale. ADCS-ADL=Alzheimer’s Disease Co-operative Study–Activities of Daily Living. ADCS-CGIC=Alzheimer’s Disease Cooperative Study–Clinical Global Impression of Change scale.
Table 3.
Most common treatment-emergent adverse events occurring in at least 2% of patients receiving LMTM that were greater than in the control group
| Control 4 mg LMTM twice a day (n=354) |
75 mg LMTM twice a day (n=267) |
125 mg LMTM twice a day (n=264) |
|
|---|---|---|---|
| At least one treatment-emergent adverse event | 296 (84%) | 224 (84%) | 229 (87%) |
| Blood and lymphatic system disorders | 17 (5%) | 29 (11%) | 25 (9%) |
| Anaemia | 10 (3%) | 22 (8%) | 15 (6%) |
| Gastrointestinal disorders | 87 (25%) | 105 (39%) | 111 (42%) |
| Diarrhoea | 33 (9%) | 63 (24%) | 67 (25%) |
| Nausea | 14 (4%) | 22 (8%) | 19 (7%) |
| Vomiting | 2 (1%) | 25 (9%) | 18 (7%) |
| Abdominal pain | 11 (4%) | 9 (3%) | 9 (3%) |
| Infections and infestations | 88 (25%) | 83 (31%) | 76 (29%) |
| Urinary tract infection | 29 (8%) | 29 (11%) | 26 (10%) |
| Investigations | 80 (23%) | 87 (33%) | 80 (30%) |
| Blood folate decreased | 21 (6%) | 18 (7%) | 19 (7%) |
| Weight decreased | 6 (2%) | 8 (3%) | 10 (4%) |
| Haemoglobin decreased | 2 (1%) | 12 (4%) | 7 (3%) |
| Metabolism and nutrition disorders | 47 (13%) | 49 (18%) | 36 (13%) |
| Folate deficiency | 11 (3%) | 10 (4%) | 15 (6%) |
| Nervous system disorders | 105 (30%) | 91 (34%) | 64 (24%) |
| Cognitive disorder | 6 (2%) | 6 (2%) | 6 (2%) |
| Renal and urinary disorders | 29 (8%) | 61 (23%) | 65 (25%) |
| Dysuria | 3 (1%) | 7 (3%) | 27 (10%) |
| Micturition urgency | 4 (1%) | 7 (3%) | 6 (2%) |
| Pollakiuria | 6 (2%) | 15 (6%) | 18 (7%) |
| Urinary incontinence | 9 (3%) | 18 (7%) | 12 (5%) |
| Respiratory, thoracic, and mediastinal disorders | 28 (8%) | 32 (12%) | 22 (8%) |
| Cough | 12 (3%) | 14 (5%) | 11 (4%) |
LMTM=leuco-methylthioninium bis(hydromethanesulfonate).
Adverse events of special interest included haemolytic anaemia, serotonin syndrome, and amyloid related imaging abnormalities. No cases of clinically significant haemolytic anaemia occurred. The incidence of anaemia-related events was 115 of 531 patients (22%) receiving high-dose LMTM, compared with 58 of 354 (16%) in those receiving the control dose. Dose-related mean decreases in haemoglobin concentrations were maximal at 6 weeks (–6·6 g/L for the 75 mg group and –10·8 g/L for the 125 mg group), with no change in the control arm (0·1 g/L). Although 196 of 885 patients (22%) who entered the study were taking a selective serotonin-reuptake inhibitor (SSRI), only two had transient symptoms consistent with serotonergic excess, and the temporal course and presentation were not consistent with serotonin syndrome in either case. In total, eight of 885 patients (<1%) developed amyloid related imaging abnormalities (six H-types and two E-types) during the study, with no dose relationship. There was no indication of increase in suicidality at higher doses compared with control.
Nine participants died, three in each group; no deaths were judged by the investigator as related to treatment. The most common reasons for death were progression of Alzheimer’s disease (one assigned to LMTM 125 mg twice a day and two to control) or cancer (one in each treatment group); one patient assigned to LMTM 75 mg twice a day had a myocardial infarction and no cause was identified for the remaining two deaths. An additional 96 patients had one or more other non-fatal serious adverse events evenly distributed between the three treatment groups. The number of serious adverse events and incidence by body system most commonly affected were judged by the investigator as possibly related to treatment in only 20 of 139 cases (14%), the most common being convulsion (all four incidences occurring in the control group).
Because taking LMTM as monotherapy showed significant benefit in the primary analysis model, a further prespecified post-hoc analysis was undertaken in the whole population, which included Alzheimer’s disease comedication status as an interaction term with LMTM treatment and as an interaction term with visit in the model. In patients taking LMTM as monotherapy, the differences with respect to all controls as randomly assigned were significant after correction for multiple comparisons on all treatment outcomes (table 4). In patients taking the same doses of LMTM as add-on to approved Alzheimer’s disease treatments, the decline was indistinguishable from controls. In a non-prespecified post-hoc analysis we compared control patients taking 4 mg twice a day either as monotherapy or add-on therapy. The differences were again significant for all treatment outcomes (appendix). None of the outcomes differed significantly between 4 mg twice a day and either of the two higher doses as monotherapy, and there were no differences in patients receiving 4 mg as add-on to existing treatments (appendix). Figure 2 shows the results for ADAS-Cog, ADCS-ADL, and lateral ventrical volume. The corresponding results for ADCS-CGIC and MMSE are in the appendix.
Table 4.
Efficacy analyses for primary and secondary outcomes using prespecified analysis with the covariate for LMTM as monotherapy or add-on as an interaction term with treatment and an interaction term with visit in the model
| Control (4 mg twice a day) change from baseline (n=348) |
Add-on LMTM therapy (baseline and treatment effect) | Monotherapy LMTM (baseline and treatment effect) | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 75 mg twice a day (n=220) |
125 mg twice a day (n=213) |
Baseline | 75 mg twice a day (n=37) | 125 mg twice a day (n=37) |
||
| ADAS-Cog | 5·98 (4·99–6·98) |
2675 (26·05–27·45) |
1·02 (−0·58 to 2·61); 0·3622 |
0·50 (−1·15 to 2·14); 0·5555 |
26·18 (24·42–27·94) | −6·25 (−8·92 to −3·59); p<0·0001 |
−579 (−8·47 to −3·11); p<0·0001 |
| ADCS-ADL | −7·92 (−9·29 to −6·55) |
5773 (56·86–58·60) |
−2·16 (−4·37 to 0·05); p=0·1027 |
−1·62 (−3·91 to 0·68); p=0·1674 |
5473 (52·39–57·07) | 6·48 (2·87–10·09); p=0·0013 |
6·93 (3·29–10·57); p=0·0007 |
| Lateral ventricular volume (cm3) |
7·19 (6·64–773) | 5275 (50·99–54·51) |
−0·27 (−1·14 to 0·60); p=0·7334 |
−0·31 (−1·19 to 0·58); p=0·7334 |
4572 (41·03–50·41) | −2·71 (−4·00 to −1·42); p=0·0002 |
−2·35 (−3·64 to –1·05); 0·0011 |
| ADCS-CGIC | −0·97 (−1·10 to −0·84) |
−0·22 (−0·43 to −0·01); p=0·0738 |
−0·10 (−0·31 to 0·12); p=0·3891 |
0·90 (0·54–1·26); p<0·0001 |
0·59 (0·23–0·95); p=0·0037 | ||
| MMSE | −347 (−3·95 to −2·98) |
18·58 (18·33–18·83) |
−0·25 (−1·04 to 0·55); p=0·7756 |
0·11 (−0·71 to 0·93); p=0·7885 |
19·30 (18·69–19·91) | 1·92 (0·46–3·39); p=0·0287 |
2·82 (1·36–4·27); p=0·0006 |
Data are mean (95% CI); p value. Baseline values are shown according to add-on treatment status. Treatment effects are shown as differences with respect to change from baseline in the control arm as randomised at 65 weeks. LMTM=leuco-methylthioninium bis(hydromethanesulfonate). ADAS-Cog=Alzheimer’s Disease Assessment Scale-Cognitive Subscale. ADCS-ADL=Alzheimer’s Disease Co-operative Study-Activities of Daily Living. ADCS-CGIC=Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change scale. MMSE=Mini-Mental State Examination.
The prespecified analyses were repeated for patients with mild and moderate Alzheimer’s disease as separate subgroups (prespecified in the statistical analysis plan). Efficacy on all outcomes was again restricted to patients taking LMTM as monotherapy, with treatment benefits being more consistent in patients with mild disease than in those with moderate disease (appendix).
Baseline characteristics of patients taking LMTM as monotherapy or add-on were compared in post-hoc analyses (appendix). We noted no differences in age or sex distribution, baseline ADAS-Cog or MMSE scores, or time between diagnosis and randomisation. Patients with mild (but not moderate) Alzheimer’s disease who were not taking these medications were marginally worse on the ADCS-ADL scale, had a slightly larger hippocampal volume and smaller lateral ventricular volume on baseline MRI, and had a lower APOE ε4 carrier frequency. There were no differences in whole brain volume, temporoparietal volume, or in the extent of vascular pathology burden in patients with mild and moderate disease.24 There was likewise no difference in the initial rate of expansion of lateral ventricular volume until after 6 months (figure 2). No differences were noted for baseline bilirubin or creatinine clearance, which might have suggested differences in metabolism or excretion of LMTM. In an analysis of sites with at least three patients, we noted that patients with mild (but not moderate) disease who had not been prescribed Alzheimer’s disease comedications were more likely to have come from geographies with more limited access to these treatments (Russia, eastern Europe [Poland and Croatia], and Malaysia). These patients represented 56% (n=70/126) of those taking LMTM as monotherapy. Patients at these sites did otherwise not differ in other baseline measures or time from diagnosis. They were also not taking other medications with higher frequency. Age at which education was completed was lower in moderate (but not mild) patients not taking Alzheimer’s disease treatments. Analyses of patients pooled for mild and moderate disease showed similar results.
For patients taking LMTM 75 mg twice a day and 125 mg twice a day doses, beneficial effects on temporoparietal volume and whole brain volume were restricted to patients taking LMTM as monotherapy and were noted in both patients with mild and moderate disease (appendix). Benefits for hippocampal atrophy were noted only in patients with mild disease at the highest dose. 18F-FDG-PET data were not analysed further, because only six patients receiving LMTM as monotherapy were in the centres with this capability. Similarly, these few patients precluded further analysis of CSF data. RUD-lite, although included as an exploratory outcome, will be analysed in conjunction with the recently completed study in patients with mild Alzheimer’s disease (NCT01689233, EudraCT 21012-002847-28).
Discussion
The primary analysis for our study was negative, and the results do not suggest benefit of LMTM as an add-on treatment for mild to moderate Alzheimer’s disease. The results did not show a treatment benefit for either of the coprimary outcomes at either the 75 mg twice a day or 125 mg twice a day doses in the prespecified analysis. Similarly, LMTM was not associated with significant treatment effects for any of the secondary outcomes. However, the primary prespecified analysis model in the whole population (which included evaluation of statistical significance of the stratification covariates corrected for multiple comparisons) showed that patients taking LMTM as monotherapy, and also those with mild disease, had significantly lower decline than control patients or those taking LMTM as an add-on to existing Alzheimer’s disease treatments. Given the substantial interaction of LMTM treatment with Alzheimer’s disease co-medication status, an analysis prespecified in the statistical analysis plan with this covariate as an interaction term in the model was undertaken in the whole population as the first supporting analysis. This finding supported a significant treatment benefit on both cognition and activities of daily living for patients taking LMTM as monotherapy at both of the doses tested compared with controls as randomly assigned, and might suggest that there was an unexplained attenuating effect of existing Alzheimer’s disease treatments on the effect of LMTM. The higher dose of 125 mg twice a day resulted in similar efficacy to that seen at the 75 mg twice a day dose. The same benefit was shown when patients taking LMTM 4 mg twice a day as monotherapy were compared with those taking this dose as an add-on treatment. We noted the same pattern of monotherapy efficacy for the secondary clinical outcomes (ADCS-CGIC and MMSE scores) and reduction in lateral ventricular volume, and all remained significant after correction for multiple comparisons. The reduction in lateral ventricular volume was confirmed by corresponding increases in temporoparietal volume and whole brain volume at all monotherapy doses tested. There is therefore concordance between reductions in clinical decline and reductions in progression of brain atrophy.
The decline in the entire control group as randomly assigned is consistent with that reported in other studies with similar distributions of patients taking or not taking Alzheimer’s disease comedications.17,18 Similarly, progression of brain atrophy in the mild Alzheimer’s disease group measured by change of lateral ventricular volume was similar to data available from the Alzheimer’s Disease Neuroimaging Initiative programme.25,26 The similarity in the decline noted in the control group to findings from other studies supports the face validity of the present trial as being representative of trial populations in mild or moderate Alzheimer’s disease.
The overall safety of LMTM as monotherapy is consistent with previous findings with methylthioninium chloride.16 Adverse events affecting the gastrointestinal and urinary tracts were the most common and were also the most common reason for discontinuing high-dose LMTM. Reporting of reductions in red-cell indices was greater in patients receiving higher doses of LMTM, which is consistent with effects previously described for methylthioninium chloride.14 Although 22% of patients were taking SSRIs, only two had transient symptoms meeting any of the criteria for serotonin toxicity, although neither was taking an SSRI (or any other serotonergic drug). None of the nine deaths that occurred during the study was judged as being related to treatment. Eight patients developed amyloid related imaging abnormalities during the study and no dose relationship was evident. This frequency is consistent with the placebo rates reported in other trials.17,18
The reason for the apparent benefit in the monotherapy subgroup, but not in patients taking LMTM as add-on to existing treatments, has not yet been accounted for. An interference with tau-aggregation inhibitor activity in vitro has been ruled out13 (unpublished data), as has an effect of oral LMTM on cholinergic efficacy of donepezil in the scopolamine mouse model (unpublished data). Likewise, absorption effects have been ruled out in preliminary analyses of plasma data from a subset of patients (unpublished data). However, the absorption and distribution of the methylthioninium moiety is complex, and plasma concentrations do not reflect brain concentration.14 Avenues being explored include further blood analyses, the potential effect of cholinergic pathology on cognition and brain atrophy27 in tau transgenic mouse models, the interaction between cholinesterase and amyloid pathology,28 and whether induction of transporters by chronic administration of symptomatic treatments for Alzheimer’s disease29,30 might lower the concentration of methylthioninium at the site of action.
Although the cognitive benefit noted in our study when we compared LMTM monotherapy with add-on treatment is similar to that reported in a previous study of methylthioninium chloride monotherapy,16 the absence of an explanation for the unexpected pharmacological interaction we have documented remains an important weakness. A further limitation of our study is that it was not designed to test the efficacy of LMTM as monotherapy versus add-on to existing symptomatic treatments. The findings are therefore open to the criticism that the groups taking or not taking Alzheimer’s disease-labelled treatments in addition to LMTM might not have been comparable, because of inherently different rates of progression as a result of having less severe disease, or because the proportion of mild (but not moderate) patients with the APOE ε4 allele was low for a trial of Alzheimer’s disease. Additionally, patients who are not taking other Alzheimer’s medications might have less severe disease. However, we excluded several obvious confounding factors, including age, sex, clinical severity at baseline, time from diagnosis to randomisation, use of other medications, extent of coexisting vascular pathology, initial rate of expansion of lateral ventricles, and biological factors that could have affected metabolism or excretion of methylthioninium. The unexpected benefit noted with the 4 mg dose delivered as monotherapy compared with add-on has made simple comparisons according to Alzheimer’s disease treatment status uninformative, and there were no differences by dose within monotherapy or add-on groups. The significant benefit seen at the 4 mg monotherapy dose raises the possibility that the minimum effective dose for LMTM might be substantially less than for methylthioninium chloride.16
The few patients taking LMTM as monotherapy raises the possibility that the benefit noted in this group was a chance finding. However, the possible treatment effect occurred in 126 patients assigned to three different treatment arms. The results do not suggest benefit of LMTM as an add-on treatment for mild to moderate Alzheimer’s disease.
Research in context.
Evidence before this study
Approved treatments for Alzheimer’s disease offer symptomatic benefit without affecting the underlying disease pathology. The development of therapies aiming to reduce the rate of disease progression has focused for many years on the amyloid pathology—so far without success. Pathological aggregation of tau protein to form the neurofibrillary tangles discovered by Alois Alzheimer is highly correlated with clinical impairment in Alzheimer’s disease and begins 20 years before clinical symptoms appear. Targeting this process with an inhibitor of tau aggregation therefore provides a rational approach both to treatment and prevention. We searched PubMed on June 29, 2016, for randomised placebo-controlled studies of Alzheimer’s disease published in English since Jan 1, 1990, using the search terms “Alzheimer”, “trial”, and “tau” in any field. We identified two phase 2 studies in which progressive supranuclear palsy, a neurodegenerative disease also associated with prominent tau aggregation pathology, was treated with tideglusib (12 months, 146 participants) and davunetide (18 months, 313 participants) aiming to inhibit tau phosphorylation. However, neither drug showed significant benefit in progressive supranuclear palsy. Methylthioninium, a diaminophenothiazine that inhibits aggregation in vitro and in transgenic tau mouse models, is the only tau-aggregation inhibitor used in a clinical trial for Alzheimer’s disease. In a phase 2 trial, in which the oxidised form of methylthioninium was given as monotherapy to patients with mild or moderate Alzheimer’s disease, clinical benefit was shown with 138 mg/day methylthioninium, but not with 218 mg/day.
Added value of this study
Our phase 3 study assessed a large study population during 15 months of treatment with a novel chemical entity to provide the methylthioninium moiety in a stable reduced form, which enabled high doses to be absorbed in an efficacious form. 75 mg and 125 mg leuco-methylthioninium bis(hydromethanesulfonate) (LMTM) given twice a day did not show any treatment benefit for the coprimary outcome of progression on the Alzheimer’s Disease Assessment Scale– Cognitive Subscale (ADAS-Cog) and the Alzheimer’s Disease Co-operative Study–Activities of Daily Living Inventory (ADCS-ADL) scales, or for secondary outcomes, at either of the doses when compared with the control group as randomly assigned. In post-hoc analyses, LMTM monotherapy had significant interaction on these endpoints, including brain atrophy, when compared with the control group (comprising patients treated or not treated with drugs approved for Alzheimer’s disease), with a clinically acceptable safety profile.
Implications of all the available evidence
The primary analysis of this study was negative, and the results do not suggest benefit of LMTM as an add-on treatment for patients with mild to moderate Alzheimer’s disease. Findings from a recently completed 18-month trial of patients with mild Alzheimer’s disease will be reported soon.
Acknowledgments
Declaration of interests
SG has received clinical trial support from Lilly and Roche in DIAN-TU, TauRx Therapeutics (TauRx), and Lundbeck; has been a data safety monitoring board (DSMB) member of ADCS, ATRI, API, and Eisai; and has been a scientific adviser to Affiris, Boehringer Ingelheim, Lilly, Roche, Servier, Sanofi, Schwabe, Takeda, and TauRx. HHF has received clinical trial support from TauRx, Lilly, and Roche; has served as a DSMB member for Eisai and data monitoring committee member for Genentech/Banner Health; has served as a member of a scientific advisory board for TauRx and Tau Consortium, and has been a consultant to Arena and Merck Pharmaceuticals. LSS has received grant and research support from Baxter, Genentech, Johnson & Johnson, Eli Lilly, Lundbeck, Novartis, Pfizer, Roche, TauRx, and NIH. Within the last 3 years he has served as a consultant for, and received consulting fees from, Abbvie, AC Immune, Allon, AstraZeneca, Baxter, Biogen Idec, Biotie, Bristol-Myers Squibb, Cerespir, Chiesi, Cognition, Elan, Eli Lilly, Forum (EnVivo), GlaxoSmithKline, Johnson & Johnson, Lundbeck, MedAvante, Merck, Novartis, Piramal, Pfizer, Roche, Servier, Takeda, TauRx, Toyama (FujiFilm), and Zinfandel. GKW has been a scientific adviser to Cytox, GSK Research and Development, Nutricia, Red and Yellow Memory Services, Roche Products, Shire Pharmaceutical Development, and TauRx. GBF has served in advisory boards for Lilly, BMS, Bayer, Lundbeck, Elan, Astra Zeneca, Pfizer, TauRx, Wyeth, GE, and Baxter; is a member of the editorial board of Lancet Neurology; has received grants from Wyeth, Lilly, Lundbeck Italia, GE, Avid/Lilly, Roche, Piramal, and the Alzheimer’s Association; and has received lecture fees when speaking at the invitation of Lundbeck, Piramal, and GE. JHH, JMDS, CRH, and CMW are officers of, and hold beneficial interests in, TauRx. HJM was an officer of TauRx during design and planning of this trial; he was a full-time officer at FORUM (formerly EnVivo Pharmaceuticals). PB, KAK, DJW, BOS, CSD, RTS, LB, and KS are paid consultants to TauRx. JMDS, CRH, and CMW are inventors on patents relating to LMTM and tau-aggregation inhibitors that are owned by WisTa Laboratories, an affiliate of TauRx.
The study was sponsored by TauRx Therapeutics (Singapore).
We gratefully acknowledge study investigators and the generosity of study participants.
Footnotes
See Online for appendix
Contributors
JHH, HJM, PB, KAK, DJW, BOS, CSD, RTS, LB, KS, JMDS, CRH, and CMW were involved in study design and data interpretation. SG, HHF, LSS, GKW, GBF, JHH, PB, KAK, DJW, BOS, CSD, RTS, LB, KS, JMDS, CRH, and CMW were involved in the data analysis. All authors critically revised the report, commented on drafts of the manuscript, and approved the final report.
References
- 1.Winblad B, Amouyel P, Andrieu S, et al. Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol. 2016;15:455–532. doi: 10.1016/S1474-4422(16)00062-4. [DOI] [PubMed] [Google Scholar]
- 2.Prince MJ, Wimo A, Guerchet MM, et al. World Alzheimer report 2015—the global impact of dementia: an analysis of prevalence, incidence, cost and trends. London: Alzheimer’s Disease International; 2015. [Google Scholar]
- 3.Mullane K, Williams M. Alzheimer’s therapeutics: continued clinical failures question the validity of the amyloid hypothesis-but what lies beyond? Biochem Pharmacol. 2013;85:289–305. doi: 10.1016/j.bcp.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Wischik CM, Crowther RA, Stewart M, Roth M. Subunit structure of paired helical filaments in Alzheimer’s disease. J Cell Biol. 1985;100:1905–1912. doi: 10.1083/jcb.100.6.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wischik CM, Novak M, Edwards PC, Klug A, Tichelaar W, Crowther RA. Structural characterization of the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA. 1988;85:4884–4888. doi: 10.1073/pnas.85.13.4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wischik CM, Novak M, Thøgersen HC, et al. Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer’s disease. Proc Natl Acad Sci USA. 1988;85:4506–4510. doi: 10.1073/pnas.85.12.4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brier MR, Gordon B, Friedrichsen K, et al. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Science Transl Med. 2016;8:338ra66. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maruyama M, Shimada H, Suhara T, et al. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron. 2013;79:1094–1108. doi: 10.1016/j.neuron.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wischik CM, Harrington CR, Storey JMD. Tau-aggregation inhibitor therapy for Alzheimer’s disease. Biochem Pharmacol. 2014;88:529–539. doi: 10.1016/j.bcp.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Braak H, Del Tredici K. The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 2011;121:171–181. doi: 10.1007/s00401-010-0789-4. [DOI] [PubMed] [Google Scholar]
- 11.Lai RYK, Harrington CR, Wischik CM. Absence of a role for phosphorylation in the tau pathology of Alzheimer’s disease. Biomolecules. 2016;6:19. doi: 10.3390/biom6020019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wischik CM, Edwards PC, Lai RYK, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proc Natl Acad Sci USA. 1996;93:11213–11218. doi: 10.1073/pnas.93.20.11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrington CR, Storey JMD, Clunas S, et al. Cellular models of aggregation-dependent template-directed proteolysis to characterize tau aggregation inhibitors for treatment of Alzheimer’s disease. J Biol Chem. 2015;290:10862–10875. doi: 10.1074/jbc.M114.616029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baddeley TC, McCaffrey J, Storey JMD, et al. Complex disposition of methylthioninium redox forms determines efficacy in tau aggregation inhibitor therapy for Alzheimer’s disease. J Pharmacol Exp Ther. 2015;352:110–118. doi: 10.1124/jpet.114.219352. [DOI] [PubMed] [Google Scholar]
- 15.Melis V, Magbagbeolu M, Rickard JE, et al. effects of oxidized and reduced forms of methylthioninium in two transgenic mouse tauopathy models. Behav Pharmacol. 2015;26:353–368. doi: 10.1097/FBP.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wischik CM, Staff RT, Wischik DJ, et al. Tau aggregation inhibitor therapy: an exploratory phase 2 study in mild or moderate Alzheimer’s disease. J Alzheimer’s Dis. 2015;44:705–720. doi: 10.3233/JAD-142874. [DOI] [PubMed] [Google Scholar]
- 17.Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doody RS, Thomas RG, Farlow M, et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 19.DiSanto AR, Wagner JG. Pharmacokinetics of highly ionized drugs. II. Methylene blue-absorption, metabolism, and excretion in man and dog after oral administration. J Pharmaceut Sci. 1972;61:1086–1090. doi: 10.1002/jps.2600610710. [DOI] [PubMed] [Google Scholar]
- 20.Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alusik S, Kalatova D, Paluch Z. Serotonin syndrome. Neuroendocrinol Lett. 2014;35:265–273. [PubMed] [Google Scholar]
- 22.Ramsay RR, Dunford C, Gillman PK. Methylene blue and serotonin toxicity: inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. Br J Pharmacol. 2007;152:946–951. doi: 10.1038/sj.bjp.0707430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- 24.Westfall PH. Multiple testing of general contrasts using logical constraints and correlations. J Am Stat Assoc. 1997;92:299–306. [Google Scholar]
- 25.Frisoni GB, Fox NC, Jack CR, Scheltens P, Thompson PM. The clinical use of structural MRI in Alzheimer disease. Nat Rev Neurol. 2010;6:67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nestor SM, Rupsingh R, Borrie M, et al. Ventricular enlargement as a possible measure of Alzheimer’s disease progression validated using the Alzheimer’s disease neuroimaging initiative database. Brain. 2008;131:2443–2454. doi: 10.1093/brain/awn146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol. 2016;73:721–732. doi: 10.1001/jamaneurol.2016.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inestrosa NC, Alvarez A, Pérez CA, et al. Acetylcholinesterase accelerates assembly of amyloid-β-peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16:881–891. doi: 10.1016/s0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed LA, Keller JN, Kaddoumi A. Role of P-glycoprotein in mediating rivastigmine effect on amyloid-β brain load and related pathology in Alzheimer’s disease mouse model. Biochim Biophys Acta. 2016;1862:778–787. doi: 10.1016/j.bbadis.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed LA, Qosa H, Kaddoumi A. Age-related decline in brain and hepatic clearance of amyloid-beta is rectified by the cholinesterase inhibitors donepezil and rivastigmine in rats. ACS Chem Neurosci. 2015;6:725–736. doi: 10.1021/acschemneuro.5b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]