Abstract
DNA vaccination with the M3 gene, encoding an immune evasion molecule expressed during both the acute lytic and persistent phases of murid gammaherpesvirus 68 infection, yielded a significantly lower titer of virus in the lung than controls. The protection seen was dependent on T cells, and we mapped an epitope recognized by CD8 T cells. The immune response to this epitope follows the same kinetics as lytic cycle antigens, despite the fact that this gene is expressed in both lytic and persistent stages of infection. This has important implications for our understanding of T-cell responses to putative latency-associated gammaherpesvirus proteins and how vaccination may improve control of these viruses.
Members of the gammaherpesvirus subfamily are important human pathogens and are associated with significant diseases, such as infectious mononucleosis, nasopharyngeal carcinoma, and lymphoid malignancies. The isolation of a gammaherpesvirus that infects mice, murid gammaherpesvirus 68 (MHV-68, γHV-68; International Committee on Taxonomy of Viruses name, murid herpesvirus 4), provides an experimental model for the study of disease caused by human and animal gammaherpesviruses (3, 12, 16, 29). This virus is related to the human viruses Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) as well as animal viruses, such as ovine herpesvirus 2 and herpesvirus saimiri. As such, it is a useful model with which to test hypotheses concerning the immunological control of these viruses. Most vaccination strategies have concentrated on structural proteins or those antigens expressed during lytic replication (8, 11, 19, 30), but less is known about the efficacy of targeting latency-associated antigens. In the MHV-68 system, we and others have shown that vaccination with certain lytic-phase antigens induces protection from virus replication in the lungs and reduces the initial level of latently infected cells (9, 17). In contrast, vaccination with a latency-specific protein, M2, greatly reduced the initial level of latently infected cells but had no effect on lytic replication in the lungs (24, 25). Neither vaccination strategy reduced the level of latent virus in the mice long term. However, protection from the establishment of long-term latency by vaccination is theoretically possible, since infection with MHV-68 mutants which are unable to reactivate from or establish latency prevents residual latency after challenge with wild-type virus (4, 21). Little is known concerning the effect of priming T-cell responses that target both lytic and persistent phases of the virus life cycle. In this regard, the M3 protein of MHV-68 is an attractive target, since it is expressed during lytic virus replication as well as during persistence and is a candidate latency-associated antigen (14, 24, 26, 28). Unlike M2, which is expressed for only a few days around 14 days postinfection, mRNA for M3 is detectable for at least 1 month postinfection and probably much longer (24). What makes this protein of particular interest is its function as an immune evasion protein. The M3 protein can block the function of CC and CXC chemokines and may block sufficient CD8-T-cell recruitment into the site of infection and/or lymphoid tissues (1, 7, 27). In this study we wished to determine whether the M3 protein elicited an antiviral T-cell response and test its effect on the control of different stages of the infection.
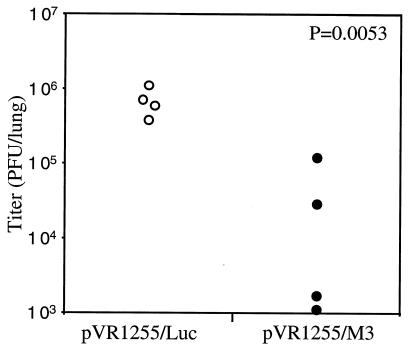
We vaccinated mice by using a technique that we have used successfully before, specifically, DNA vaccination with a gene gun, using protocols similar to those described previously (25). The complete M3 open reading frame with three copies of an influenza hemagglutinin epitope tag sequence at the 3′ end was amplified from MHV-68 DNA by PCR using the primers 5′GCGCGGCCGCGCGTCAGCCATGGC and 5′GCGGAATTCTTAAGCGTAGTCTGGAACGTCGTATGGGTAAGCGTAGTCTGGAACGTCGTATGGGTAAGCGTAGTCTGGAACGTCGTATGGGTAATGATCCCCAAAATACTCC under conditions described previously (10). The amplified product was cut with the restriction enzymes NotI and BamHI and inserted by molecular cloning between the NotI and BamHI sites of the DNA vaccine vector pVR1255 (5) to generate pVR1255/M3. Expression of M3 protein by this vector was confirmed by calcium phosphate-mediated transfection into HEK293T cells and detection of [35S]Met-labeled M3 protein in the supernatant by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography as described previously (data not shown) (20). A pVR155 construct containing the luciferase (Luc) gene was used as a negative control. BALB/c mice were immunized three times at 2-week intervals with 0.6 to 1.8 μg of DNA by gene gun and then challenged with 400 PFU of MHV-68 by the intranasal (i.n.) route 2 to 3 weeks after the last boost. Lungs were removed 7 days after infection to assess the degree of protection from acute infection (Fig. 1). Mice vaccinated with pVR1255/M3 had a significantly lower titer of virus in the lungs than those vaccinated with pVR1255/Luc. The titers in both groups continued to decline at day 10 postinfection (data not shown), indicating there was not just a delay in viral replication in the M3-vaccinated group. To measure whether this protection extended to the persistent/latent phase of the infection, we infected vaccinated mice with MHV-68 and then removed the spleens at 14 days postinfection. The latent virus burden at this time point was measured using quantitative fluorescent PCR, as previously described (25). We observed a variable decrease in virus burden between M3-vaccinated and vector-alone-vaccinated groups, which was statistically significant for some experiments but not others (data not shown). We did not observe any difference in the latent virus burden between the two groups at 28 days postinfection. At both 14 and 28 days postinfection, we detected no free virus in the spleen. Therefore, we concluded that DNA vaccination with M3 conferred partial protection from the acute phase of infection in the lungs but did not have a reproducible effect on the persistent/latent stages of the infection. The lack of effect on latent infection may imply either that M3 is expressed only in a subset of latently infected cells or that it is expressed only during the lytic cycle and not in latency at all. In either case, this would not render the majority of latently infected cells susceptible to immune recognition. In support of the latter hypothesis, we have previously shown, using a latently infected B-cell line (S11), that unlike M2, M3 was not expressed in latently infected cells (6). Currently, little is known concerning heterogeneity in gene expression during MHV-68 latency; however, other gammaherpesviruses have several different latent programs of gene expression (15). It is also possible that a vaccination regime that induces a stronger T immune response against M3 will reduce the titer of latent virus to a larger extent than was seen with DNA vaccination.
FIG. 1.
DNA vaccination with M3 induces protection from acute MHV-68 infection in the lung. BALB/c mice were DNA vaccinated, using a gene gun, three times with pVR1255 encoding either the M3 gene (closed circles) or luciferase (luc) as a negative control (open circles). Two to three weeks after the final immunization, mice were infected i.n. with 400 PFU of MHV-68, and then the lungs were removed 7 days later and the titer of virus was measured by plaque assay. Each data point represents the titer from an individual mouse. P values were calculated using Student's t test. The data are representative of four experiments.
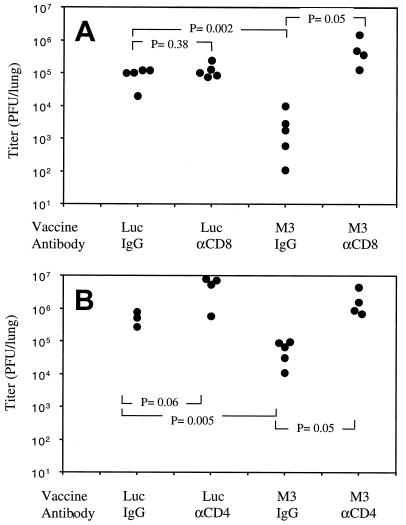
Previous studies have reported a critical role for T cells in the control of MHV-68 infection (2, 18, 22), so we wished to test whether the protection we observed was due to T cells. To test this, we vaccinated mice as described above, and then 2 to 3 weeks after the last boost we depleted T-cell subsets prior to infection. One group was depleted of CD4 T cells by intraperitoneal injection of anti-CD4 antibody (GK1.5; 300 μg on days −1, +1, and +4 relative to infection), another group received anti-CD8 antibody (TIB210), and control groups received the same dose of rat immunoglobulin G. Mice were then infected with 400 PFU of MHV-68 i.n., and 7 days later, the lungs were removed for virus titration. The protection afforded by M3 vaccination was eliminated by depletion of either CD8 (Fig. 2A) or CD4 T cells (Fig. 2B). This demonstrated that vaccination with M3 induced a T-cell response that mediated the observed protection. It also ruled out the possibility that antibodies against the M3 protein were blocking its immune evasion function and attenuating the infection. In addition, it shows that synthesis of the M3 protein itself, a secreted protein shown to bind chemokines (14, 26, 28), was not directly mediating protection from infection.
FIG. 2.
Protection is eliminated by T-cell depletion. BALB/c mice were DNA vaccinated with either pVR1255/M3 or pVR1255/Luc as described, and then groups were treated with either anti-CD8 (A) or anti-CD4 (B) antibody to deplete T-cell subsets, and the other groups were treated with rat immunoglobulin G (IgG) as a control. Depletion was initiated just prior to infection with MHV-68 i.n., and lungs were removed 7 days later. Titers of virus in the lungs are shown. Each point represents an individual mouse. P values were calculated using Student's t test. Data are representative of two experiments.
To determine the fine specificity of the T-cell response induced against the M3 protein, we synthesized overlapping 15-mer peptides covering the complete sequence of the protein (overlapping by 10 amino acids). We then used a previously described gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assay (23) to screen which peptides elicited a response from either CD8 or CD4 T cells. Spleen cells from mice vaccinated with M3 and infected for 7 days as described were the responder population in this assay. We resynthesized the peptides that scored positive in the screen and mapped an epitope, M3150-158 (AYVELQTEL), that consistently elicited IFN-γ secretion from CD8 T cells. This peptide conforms to the Kd consensus binding motif (x[Y/]xxxxxx[I/L/V]) and also binds to the Kd molecule, as determined by using an RMA-S stabilization assay (data not shown). We did not identify any additional peptides that elicited responses from purified CD4 T cells. We used this information to measure how much our DNA vaccination regime enhanced the response to M3 relative to that with mock-vaccinated mice. After vaccination and exposure to virus for 7 days, M3-vaccinated mice had 185 ± 28 spot-forming cells/106 spleen cells compared with 14 ± 10 spot-forming cells/106 spleen cells in Luc-vaccinated controls. Therefore, vaccination with M3 enhanced the CD8-T-cell response to this epitope by approximately 13-fold. Given that we could not detect a CD4 epitope within the M3 protein, it was unexpected that CD4 depletion abrogated protection elicited by DNA vaccination (Fig. 2). It has been shown that CD4 T cells play a role in the clearance of the acute infection (18), and perhaps with the enhanced virus replication observed after CD4 depletion the protective effect of vaccination is masked. Alternatively, it is formally possible that there are CD4-T-cell epitopes within M3 that were not detected using our screening regime.
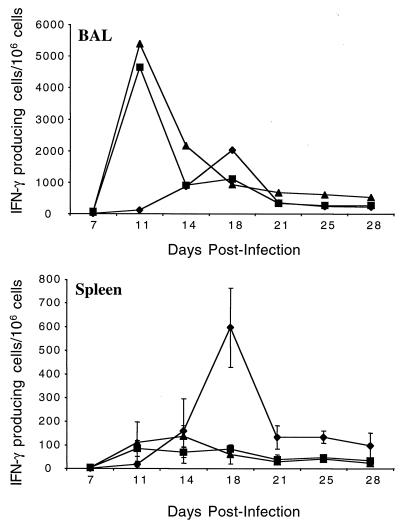
We wished to test whether the CD8-T-cell epitope we identified was recognized in the context of infection without vaccination and to establish the kinetics of this response. Therefore, we infected BALB/c mice with MHV-68, and at various times postinfection we removed the spleens and bronchoalveolar lavage (BAL) and used an IFN-γ ELISPOT assay to measure the frequency of cells responding to this epitope. For purposes of comparison, we included a known lytic cycle epitope (ORF65131-140) (23) and a known latent cycle epitope (M291-99) (6, 24). As can be seen in Fig. 3, the response to the M3 epitope was maximal in the BAL at 11 days postinfection and subsequently declined. In the spleen, the response was elevated between 11 and 14 days postinfection before declining to a steady level that was maintained until the end of the experiment. Reponses in the draining mediastinal lymph node were similar to those in the spleen (data not shown). Overall, the kinetics were strikingly similar to those for the lytic cycle ORF65131-140 epitope. As previously reported, the response to the M291-99 epitope was induced with slower kinetics (13) and was more pronounced in lymphoid tissue than in the lungs. The induction of the M3-specific T-cell response with lytic cycle kinetics is not surprising, since it is made during acute viral replication in the lungs (24). However, it was unexpected that the response declined with the same kinetics as for lytic cycle antigens, since M3 is expressed for several weeks after the establishment of viral latency. The studies showing prolonged M3 expression measured mRNA levels, so it is possible that the amount of M3 protein produced in latency is much lower than during lytic replication, which may provide less stimulus to maintain the M3-specific T-cell response.
FIG. 3.
Kinetics of the response to the CD8-T-cell epitope in M3. BALB/c mice were infected with 400 PFU of MHV-68 i.n., and then at the times shown, BAL and spleens were removed and cells were used in an IFN-γ ELISPOT assay. We measured the frequency of cells responding to the following peptides: M3150-158 (squares), ORF65131-140 (triangles), and M291-99 (diamonds). Data are representative of two experiments; error bars show one standard deviation. BAL was pooled from groups of six mice, whereas spleens were measured individually (n = 4 to 6).
In conclusion, we show that T cells recognizing the M3 protein of MHV-68 can afford partial protection from the acute phase of infection, but protection from the early latent phase of infection was not observed with consistency. This illustrates that a viral protein whose primary function is immune evasion can also be the target for antiviral T-cell attack. The response to a CD8-T-cell epitope in this protein is induced with kinetics similar to those for lytic cycle antigens during infection with MHV-68. This work furthers our understanding of the immune response to gammaherpesvirus proteins expressed during latency and may lead to better strategies to contain these important pathogens.
Acknowledgments
This work was supported by NIH grant AI51663-01 (E.J.U.), American Cancer Society institutional research grant no. IRG-82-003-18 (E.J.U.), a Biotechnology and Biological Sciences Research Council (United Kingdom) grant, 15/C12782 (J.P.S.), and a Wellcome Trust biomedical research collaboration grant, 054503 (J.P.S.). J.P.S. is supported by a Royal Society University Research Fellowship. D.C.D. and J.J.O. were supported by NIH/NIAID T32 training grant AI077363-11.
REFERENCES
- 1.Bridgeman, A., P. G. Stevenson, J. P. Simas, and S. Efstathiou. 2001. A secreted chemokine binding protein encoded by murine gammaherpesvirus-68 is necessary for the establishment of a normal latent load. J. Exp. Med. 194:301-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ehtisham, S., N. P. Sunil-Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flano, E., D. L. Woodland, and M. A. Blackman. 2002. A mouse model for infectious mononucleosis. Immunol. Res. 25:201-217. [DOI] [PubMed] [Google Scholar]
- 4.Fowler, P., and S. Efstathiou. 2004. Vaccine potential of a murine gammaherpesvirus-68 mutant deficient for ORF73. J. Gen. Virol. 85:609-613. [DOI] [PubMed] [Google Scholar]
- 5.Hartikka, J., M. Sawdey, F. Cornefert-Jensen, M. Margalith, K. Barnhart, M. Nolasco, H. L. Vahlsing, J. Meek, M. Marquet, P. Hobart, J. Norman, and M. Manthorpe. 1996. An improved plasmid DNA expression vector for direct injection into skeletal muscle. Hum. Gene Ther. 7:1205-1217. [DOI] [PubMed] [Google Scholar]
- 6.Husain, S. M., E. J. Usherwood, H. Dyson, C. Coleclough, M. A. Coppola, D. L. Woodland, M. A. Blackman, J. P. Stewart, and J. T. Sample. 1999. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8(+) T lymphocytes. Proc. Natl. Acad. Sci. USA 96:7508-7513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen, K. K., S. C. Chen, R. W. Hipkin, M. T. Wiekowski, M. A. Schwarz, C. C. Chou, J. P. Simas, A. Alcami, and S. A. Lira. 2003. Disruption of CCL21-induced chemotaxis in vitro and in vivo by M3, a chemokine-binding protein encoded by murine gammaherpesvirus 68. J. Virol. 77:624-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khanna, R., M. Sherritt, and S. R. Burrows. 1999. EBV structural antigens, gp350 and gp85, as targets for ex vivo virus-specific CTL during acute infectious mononucleosis: potential use of gp350/gp85 CTL epitopes for vaccine design. J. Immunol. 162:3063-3069. [PubMed] [Google Scholar]
- 9.Liu, L., E. J. Usherwood, M. A. Blackman, and D. L. Woodland. 1999. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 73:9849-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macrae, A. I., B. M. Dutia, S. Milligan, D. G. Brownstein, D. J. Allen, J. Mistrikova, A. J. Davison, A. A. Nash, and J. P. Stewart. 2001. Analysis of a novel strain of murine gammaherpesvirus reveals a genomic locus important for acute pathogenesis. J. Virol. 75:5315-5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss, D. J., C. Schmidt, S. Elliott, A. Suhrbier, S. Burrows, and R. Khanna. 1996. Strategies involved in developing an effective vaccine for EBV-associated diseases. Adv. Cancer Res. 69:213-245. [DOI] [PubMed] [Google Scholar]
- 12.Nash, A. A., B. M. Dutia, J. P. Stewart, and A. J. Davison. 2001. Natural history of murine gamma-herpesvirus infection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obar, J. J., S. G. Crist, D. C. Gondek, and E. J. Usherwood. 2004. Different functional capacities of latent and lytic antigen-specific CD8 T cells in murine gammaherpesvirus infection. J. Immunol. 172:1213-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parry, C. M., J. P. Simas, V. P. Smith, C. A. Stewart, A. C. Minson, S. Efstathiou, and A. Alcami. 2000. A broad spectrum secreted chemokine binding protein encoded by a herpesvirus. J. Exp. Med. 191:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rickinson, A. B., and E. Kieff. 1996. Epstein-Barr virus, p. 2397-2446. In D. M. Knipe, B. N. Fields, and P. M. Howley (ed.), Fields virology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 16.Simas, J. P., and S. Efstathiou. 1998. Murine gammaherpesvirus 68: a model for the study of gammaherpesvirus pathogenesis. Trends Microbiol. 6:276-282. [DOI] [PubMed] [Google Scholar]
- 17.Stevenson, P. G., G. T. Belz, M. R. Castrucci, J. D. Altman, and P. C. Doherty. 1999. A gamma-herpesvirus sneaks through a CD8(+) T cell response primed to a lytic-phase epitope. Proc. Natl. Acad. Sci. USA 96:9281-9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson, P. G., R. D. Cardin, J. P. Christensen, and P. C. Doherty. 1999. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J. Gen. Virol. 80:477-483. [DOI] [PubMed] [Google Scholar]
- 19.Stewart, J. P., N. Micali, E. J. Usherwood, L. Bonina, and A. A. Nash. 1999. Murine gamma-herpesvirus 68 glycoprotein 150 protects against virus-induced mononucleosis: a model system for gamma-herpesvirus vaccination. Vaccine 17:152-157. [DOI] [PubMed] [Google Scholar]
- 20.Stewart, J. P., and C. M. Rooney. 1992. The interleukin-10 homolog encoded by Epstein-Barr virus enhances the reactivation of virus-specific cytotoxic T cell and HLA-unrestricted killer cell responses. Virology 191:773-782. [DOI] [PubMed] [Google Scholar]
- 21.Tibbetts, S. A., J. S. McClellan, S. Gangappa, S. H. Speck, and H. W. Virgin IV. 2003. Effective vaccination against long-term gammaherpesvirus latency. J. Virol. 77:2522-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tibbetts, S. A., L. F. van Dyk, S. H. Speck, and H. W. Virgin IV. 2002. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J. Virol. 76:7125-7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Usherwood, E. J. 2002. A new approach to epitope confirmation by sampling effector/memory T cells migrating to the lung. J. Immunol. Methods 266:135-142. [DOI] [PubMed] [Google Scholar]
- 24.Usherwood, E. J., D. J. Roy, K. Ward, S. L. Surman, B. M. Dutia, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T Cells. J. Exp. Med. 192:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usherwood, E. J., K. A. Ward, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2001. Latent antigen vaccination in a model gammaherpesvirus infection. J. Virol. 75:8283-8288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Berkel, V., J. Barrett, H. L. Tiffany, D. H. Fremont, P. M. Murphy, G. McFadden, S. H. Speck, and H. I. Virgin. 2000. Identification of a gammaherpesvirus selective chemokine binding protein that inhibits chemokine action. J. Virol. 74:6741-6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Berkel, V., B. Levine, S. B. Kapadia, J. E. Goldman, S. H. Speck, and H. W. Virgin IV. 2002. Critical role for a high-affinity chemokine-binding protein in gamma-herpesvirus-induced lethal meningitis. J. Clin. Investig. 109:905-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Berkel, V., K. Preiter, H. W. Virgin IV, and S. H. Speck. 1999. Identification and initial characterization of the murine gammaherpesvirus 68 gene M3, encoding an abundantly secreted protein. J. Virol. 73:4524-4529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Virgin, H. W., and S. H. Speck. 1999. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr. Opin. Immunol. 11:371-379. [DOI] [PubMed] [Google Scholar]
- 30.Wilson, A. D., K. Lovgren-Bengtsson, M. Villacres-Ericsson, B. Morein, and A. J. Morgan. 1999. The major Epstein-Barr virus (EBV) envelope glycoprotein gp340 when incorporated into Iscoms primes cytotoxic T-cell responses directed against EBV lymphoblastoid cell lines. Vaccine 17:1282-1290. [DOI] [PubMed] [Google Scholar]