Abstract
The role of prostanoids in modulating respiratory syncytial virus (RSV) infection is unknown. We found that RSV infection in mice increases production of prostaglandin I2 (PGI2). Mice that overexpress PGI2 synthase selectively in bronchial epithelium are protected against RSV-induced weight loss and have decreased peak viral replication and gamma interferon levels in the lung compared to nontransgenic littermates. In contrast, mice deficient in the PGI2 receptor IP have exacerbated RSV-induced weight loss with delayed viral clearance and increased levels of gamma interferon in the lung compared to wild-type mice. These results suggest that signaling through IP has antiviral effects while protecting against RSV-induced illness and that PGI2 is a potential therapeutic target in the treatment of RSV.
Respiratory syncytial virus (RSV) is the leading cause of respiratory failure in young children and a significant cause of morbidity and mortality in the elderly and patients receiving bone marrow and solid organ transplants (14). Currently, there is no effective vaccine for RSV, and pharmacologic treatment is far from optimal (23). Passive antibody is available to protect selected high-risk infants from severe disease but is limited by its expense (1). The development of new therapeutic agents and vaccine approaches holds the promise of reducing morbidity and mortality from this important pathogen (14).
The role of prostanoids in modulating RSV infection in vivo is unknown. Recent reports suggest that prostaglandin I2 (PGI2) alters the host immune response in murine models of pulmonary allergic inflammation (15, 17, 24). PGI2 is the most abundant arachidonic acid metabolite in vascular tissues, and endothelial cells are the main producers of this prostanoid (9). The principal PGI2 receptor is IP, a member of a family of eight prostanoid receptors that have conserved homology in mammals, including mice and humans (3). IP is a G protein-coupled rhodopsin-type receptor that has seven transmembrane domains. Binding of PGI2 to its receptor activates adenylate cyclase via Gs in a dose-dependent manner, increasing the production of cyclic AMP (4). Northern blot analysis reveals that IP mRNA is expressed to the greatest degree in the thymus, while high levels of IP mRNA expression are also found in the spleen, heart, lung, and neurons in the dorsal root ganglia (18).
We found that FVB background mice that are heterozygous for overexpressing PGI2 synthase in the lung (PGI2 synthase OE+) were protected against RSV-induced illness as defined by weight loss and also had decreased lung peak viral replication and gamma interferon (IFN-γ) production compared to littermate controls (PGI2 synthase OE−). In contrast, IP-deficient mice (IP−/−) of the C57BL/6 background had exacerbated illness with prolonged viral replication. These results reveal that PGI2 signaling through IP modulates RSV-induced illness.
MATERIALS AND METHODS
Mice.
Pathogen-free 10- to 14-week-old mice were used in all experiments. The PGI2 synthase OE+ transgenic mice were developed with a construct consisting of a human SP-C promoter and full-length rat PGI synthase cDNA as described previously (10). The SP-C promoter allows targeted expression to alveolar and airway epithelial cells. Transgenic mice were genotyped by performing PCR on genomic DNA isolated from tails as described previously (10). Each line was propagated as heterozygotes. PGI2 synthase OE+ mice were always bred with wild-type FVB/N (Jackson Laboratory, Bar Harbor, Maine) mice to produce the experimental PGI2 synthase OE+ mice as well as the PGI2 synthase OE− littermates, which were used as controls in all of the experiments. For all of the experiments, F1 mice were used.
In the experiments measuring the PGI2 urinary metabolite, and female BALB/c mice were purchased from Charles River Laboratories (Wilmington, Mass.). The IP−/− mice were generated by homologous recombination in embryonic stem (ES) cells and were backcrossed 10 generations to the C57BL/6 genetic background (5). The C57BL/6 control wild-type mice were purchased from Jackson Laboratories. Cages, bedding, food, and water were sterilized prior to use. Room temperature was maintained at 27°C, and a 12-h-on, 12-h-off light cycle was provided. In caring for animals, the investigators adhered to the Guide for the Care and Use of Laboratory Animals prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (National Institutes of Health Publication No. 86-23, revised 1985).
Cells and virus.
HEp-2 cells were maintained in Eagle's minimal essential medium supplemented with glutamine, amphotericin, gentamicin, penicillin G, and 10% fetal bovine serum. The A2 strain of RSV was provided by Robert Chanock, National Institutes of Health. Master stocks and working stocks of RSV were prepared as previously described (12).
Mouse infection.
On day 0, mice were infected with RSV or given mock-infected culture medium intranasally as previously described (12). Briefly, the mice were anesthetized with intramuscular ketamine at 40 μg/g and xylazine at 6 μg/g. When held upright with the neck fully extended, the mice readily inhaled a 100-μl inoculum placed over their nostrils with a micropipette. The murine response to RSV infection varies with the strain of mouse. To achieve similar illness as defined by weight loss in our experiments, we administered 1.2 × 107 PFU to mice of the FVB background, 1.9 × 107 PFU in the experiments with mice of the C57BL/6 background, and 0.7 × 107 PFU in the experiments with mice of the BALB/c background. In our experiments, RSV infection with this procedure causes bronchiolitis (12). Each mouse was weighed daily as a measure of RSV-induced illness. Viral replication was ascertained by plaque assay in HEp-2 cells as previously described (12).
Measurement of 2,3-dinor-6-keto-PGF1α in urine.
Mice were placed in metabolic cages, and urine was collected daily. Three mice were placed in each cage, and each data point represents the urinary concentration of 2,3-dinor-6-keto-PGF1α for the urine collected from three mice. Nine mice were infected with RSV, and nine mice were mock infected. A gas chromatographic-mass spectrometric assay was used to measure 2,3-dinor-6-keto-PGF1α as previously described (7).
Quantitation of IFN-γ in lung tissues.
The concentration of IFN-γ in lung tissue was measured with a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, Minn.) according to the manufacturer's protocols. On day 6 after infection, each mouse was sacrificed and the lungs were harvested. The left lung from each mouse was ground with a mortar and pestle and ground glass. The solution of the ground lung and the ground glass was then centrifuged at 1,000 rpm for 10 min. The supernatant was then either frozen for later use or added to precoated wells and incubated for 2 h. Dilutions of recombinant cytokine were included for generation of a standard curve. Peroxidase-labeled anticytokine antibody was added to detect bound cytokine, and the plates were developed by the addition of tetramethylbenzidene substrate. Concentrations of IFN-γ in the lung supernatant were calculated from the standard curve produced. The cytokine level from each lung was measured in duplicate.
RSV-specific antibody titers.
Blood was drawn 30 days after infection, and after centrifugation, the serum was retained for measurement of RSV-specific antibodies. For measurement of anti-RSV-specific F antibody titers, purified F protein (the kind gift of Wyeth-Lederle, West Henrietta, N.Y.) was diluted in bicarbonate buffer (pH 9.8), added to 96-well plates (Immulon II; Nunc, Roskilde, Denmark) at 10 μg/well, and incubated overnight at 4°C. The same procedure was used for measurement of anti-RSV-specific G antibody titers. The remainder of the protocol is identical for the measurement of both anti-RSV-specific F and G antibody titers. The next morning, the plate was emptied and blocked with 200 μl of 1% bovine serum albumin (Sigma, St. Louis, Mo.). The plate was then washed four times with phosphate-buffered saline-0.5% Tween 20. The serum was then diluted to either 1:1,000 for the IP−/− and wild-type mice, both of the C57BL/6 background, or 1:4,000 for the PGI2 synthase OE+ and nontransgenic PGI2 synthase OE− littermate mice, both of the FVB background. One hundred microliters of the diluted serum was added to duplicate coated wells, and the plates were incubated overnight at 4°C. The next morning, the plates were washed five times with phosphate-buffered saline-0.5% Tween 20. After washing, 100 μl of biotinylated anti-mouse immunoglobulin G1 (IgG1) (Zymed Laboratories, South San Francisco, Calif.) was added to each well at a dilution of 1:5,000 and incubated 3 h at 37°C. The plates were washed four times with phosphate-buffered saline-0.5% Tween 20. After washing, 100 μl of streptavidin-peroxidase (Sigma) was added to each well at a dilution of 1:2,000 and incubated 1 h at 37°C. The plates were washed four times with phosphate-buffered saline without Tween 20. One hundred microliters of substrate buffer (azinobis-3-ethylbenzthiazolinesulfonic acid in citrate phosphate buffer) was added to each well. Optical density was measured with a 414-nm filter, and values were calculated from a standard curve.
IFN-α/β functional assay.
The mouse fibroblast cell line F114 was plated in 96-well plates. The next day, the ground lung supernatant samples to be assayed for IFN were diluted in medium and added to the wells in a twofold dilution series, alongside medium containing known concentrations of commercially obtained mouse IFN-α/β (Access Biomedical). After 24 h, vesicular stomatitis virus was added to the well (multiplicity of infection, ≈2). Two days after infection, cells were stained with crystal violet, and the IFN-α/β concentration was calculated by comparing the dilution giving 50% protection from cell killing with the standard interferon concentration giving similar protection.
SP-A and SP-D content.
The surfactant protein A (SP-A) and SP-D in alveolar lavage fluid were analyzed by Western blot in six mice from each genotype. Samples containing 1 μg of saturated phosphatidylcholine were used for analyses of SP-A. Proteins were separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) in the presence of β-mercaptoethanol. After electrophoresis, SP-A was transferred to nitrocellulose paper (Schleicher and Schnell, Keene, N.H.), and immunoblot analysis was carried out with dilution of 1:5,000 guinea pig anti-mouse SP-A and 1:5,000 rabbit anti-mouse SP-D. Appropriate peroxidase-conjugated secondary antibodies were used at 1:5,000 dilutions. Immunoreactive bands were detected with enhanced chemiluminescence reagents (Amersham, Chicago, Ill.). Protein bands were quantitated by densitometric analyses with Alpha Imager 2000 documentation and analysis software (Alpha Innotech, San Leandro, Calif.). The linearity of the assay was confirmed for the range of 10 to 200 ng of mouse SP-A (R2 = 0.95).
Protocol for examining lung sections.
The mice were sacrificed by cervical dislocation on day 15, and the lung block was removed. The lung tissue was stored in 4% paraformaldehyde, paraffin embedded, cut in 6-μm sections, mounted, and stained with hematoxylin and eosin for routine histology and periodic acid-Schiff to assess mucus. Slides were examined by one observer in a blinded fashion. The following compartments of the lung were assessed: alveolar spaces, airways at all levels, interstitium, and vessels (both arteries and veins). Inflammatory infiltrates were evaluated for location, severity, and composition (cell types: small mononuclear cells, transformed lymphocytes, histiocytes, neutrophils, and eosinophils). The degrees of inflammation were graded as follows: 0, no infiltrate; 1+, most vessels have an infiltrate up to four cells thick; 2+, most vessels have an infiltrate five to seven cells thick; 3+, most vessels have an infiltrate greater than seven cells thick; this score also includes blood or edema fluid in the tissue space.
Statistical analysis.
Results are expressed as the mean ± standard error of the mean. Weight curves were analyzed in their entirety by t test with analysis of repeated measures. Measurements of viral replication, cytokines, and antibody titers were analyzed by t test. Differences were considered significant at P < 0.05.
RESULTS
RSV infection increases production of the stable PGI2 urinary metabolite 2,3-dinor-6-keto-PGF1α.
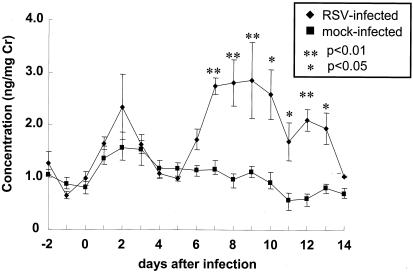
We measured the stable PGI2 metabolite 2,3-dinor-6-keto-PGF1α in the urine of wild-type BALB/c mice that were challenged intranasally with either 0.7 × 107 PFU of RSV (RSV infected) or culture medium (mock infected) to determine if PGI2 might be involved in the host response to RSV infection. RSV-infected mice had a significant increase in the production of PGI2 on days 7 to 13 compared to mice challenged intranasally with culture medium (Fig. 1). The increase in the urinary PGI2 metabolite corresponded to the peak of RSV-induced illness and early recovery phase as defined by weight loss for the FVB (Fig. 2) and C57BL/6 (see Fig. 4) wild-type mice. These results suggest that PGI2 synthesis is modulated by RSV infection.
FIG. 1.
Concentrations of the PGI2 metabolite 2,3-dinor-6-keto-PGF1α measured in RSV- and mock-infected BALB/c mice. The data shown are a combination of results from two separate experiments. **, P < 0.01 compared to the mock-infected group; *, P < 0.05 compared to the mock-infected group.
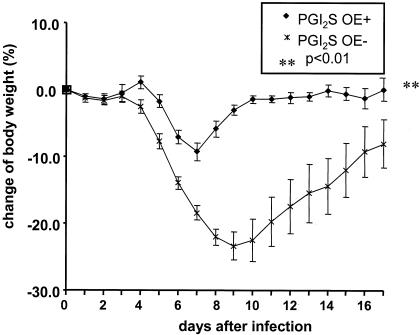
FIG. 2.
Daily weight curves of RSV-infected transgenic PGI2 synthase OE+ and nontransgenic PGI2 synthase OE− littermate control mice, shown as a percentage of the preinfection weight. The data shown are representative of three separate experiments. **, P < 0.01 compared to the PGI2 synthase OE− mice.
FIG. 4.
Daily weight curves of RSV-infected IP−/− and wild-type mice shown as a percent of the preinfection weight. The data shown is representative from two separate experiments. **P < 0.01 compared to the wild-type− mice.
Mice that overexpress PGI2 synthase are protected against RSV-induced illness.
Based on the finding that the stable PGI2 urinary metabolite was increased at the peak of infection and during recovery from illness, we hypothesized that PGI2 was protective against RSV-induced disease. We used transgenic PGI2 synthase OE+ and nontransgenic PGI2 synthase OE− littermate control mice in our model of RSV infection to test this hypothesis. Consistent high-level expression of PGI2 synthase was confirmed by increased urinary 2,3-dinor-6-keto-PGF1 in the PGI2 synthase OE+ mice compared to nontransgenic littermate mice (8.11 versus 1.30 ng/mg of creatinine). The PGI2 synthase OE+ mice were significantly protected against RSV-induced illness compared to nontransgenic littermates as defined by weight loss (P < 0.01) (Fig. 2).
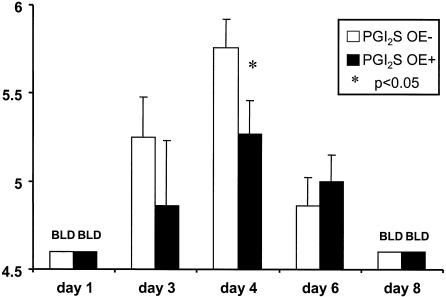
Our group has previously found that illness score correlates with weight loss (12). We found that the peak weight loss in the PGI2 synthase OE+ mice was approximately 10% of the preinfection weight, compared to 30% weight loss in the nontransgenic littermate controls. Not only were the PGI2 synthase OE+ mice protected against RSV-induced weight loss, but these mice also had decreased peak viral replication compared to the PGI2 synthase OE− mice (5.27 ± 0.19 versus 5.76 ± 0.16 log10 PFU/g of lung tissue; P < 0.05) on day 4 after infection (Fig. 3).
FIG. 3.
Viral titers in RSV-infected transgenic PGI2 synthase OE+ and nontransgenic PGI2 synthase OE− littermate control mice as measured on days 1, 3, 4, 6, and 8 after infection. The viral titers are expressed as log10 PFU per gram of lung tissue. The data shown are representative of three separate experiments. *, P < 0.05 compared to the PGI2 synthase OE− mice. BLD, below the limit of detection.
In an attempt to determine the effect of PGI2 on IFN-γ production, we measured this cytokine in the lung supernatants of the PGI2 synthase OE+ and PGI2 synthase OE− mice on day 6 after infection, the time of peak IFN-γ production in the lung. There was a significant decrease in IFN-γ levels in the lung supernatants of the PGI2 synthase OE+ compared to the PGI2 synthase OE− mice (312 ± 63 versus 532 ± 59 pg/ml; P = 0.02). These results suggest that the decreased peak viral replication that was mediated by PGI2 synthase overexpression is not mediated by production of IFN-γ.
Mice that are deficient in IP have exacerbated illness.
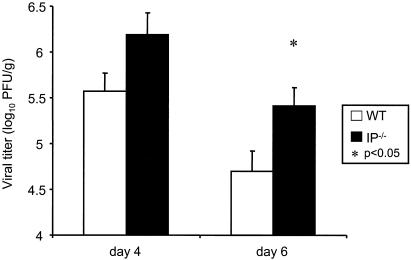
Since PGI2 synthase OE+ mice were protected against RSV-induced illness, we hypothesized that mice lacking the ability to signal through IP would have greater RSV-induced illness compared to wild-type mice that express IP. To test this hypothesis, we infected IP−/− and strain-matched (C57BL/6) wild-type mice with RSV. We found that IP−/− mice had greater weight loss than wild-type mice (Fig. 4). Although there was no difference between the IP−/− and wild-type mice in peak viral replication on day 4 after infection (data not shown), the IP−/− mice had delayed viral clearance with significantly higher lung viral replication on day 6 after infection compared to wild-type mice (5.41 ± 0.20 versus 4.70 ± 0.22 log10 PFU/g of lung tissue; P = 0.04), suggesting that the inability to signal through IP created a deficiency in antiviral immunity (Fig. 5). We also found that levels of IFN-γ in the lung supernatants were significantly greater in the IP−/− mice compared to the wild-type mice (2,260 ± 133 versus 1,799 ± 38 pg/ml; P < 0.01). These results indicate that the inability to signal through IP exacerbates RSV-induced weight loss, prolongs viral replication, and increases lung IFN-γ levels.
FIG. 5.
Viral titers in RSV-infected transgenic IP−/− and wild-type (WT) mice as measured on days 4 and 6 after infection. The viral titers are expressed as log10 PFU per gram of lung tissue. The data shown are representative of two separate experiments. **, P < 0.01 compared to the wild-type mice.
Inverse relationship between anti-RSV antibody titers and RSV-induced illness.
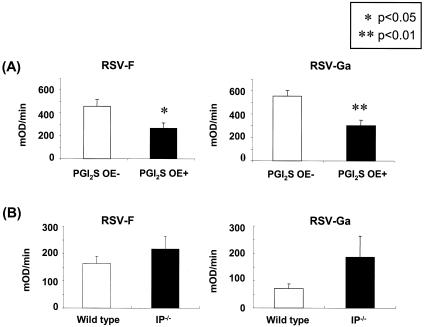
Serum was collected on day 30 after infection and IgG1 anti-RSV antibody titers to the RSV F and Ga protein were measured. PGI2 synthase OE+ mice had significantly decreased levels of IgG1 antibodies to both RSV F and RSV Ga compared to the PGI2 synthase OE− mice (Fig. 6), while there was no difference in RSV-specific IgG2a titers between the groups (data not shown). In contrast, there was no statistically significant difference between the anti-RSV IgG1 antibody titers to either RSV F or Ga in the IP−/− and wild-type mice, although there was a trend for an increase in anti-RSV antibody levels in the IP−/− mice. These results suggest that signaling through IP decreases the need for a robust humoral immune response to RSV.
FIG. 6.
Measurement of IgG1 anti-RSV antibody titers to the RSV F and Ga proteins on day 30 after infection in PGI2 synthase OE+ and PGI2 synthase OE− mice as well as in RSV-infected IP−/− and wild-type (WT) mice. **, P < 0.01 compared to the PGI2 synthase OE− mice; *, P < 0.05 compared to the PGI2 synthase OE− mice. The data shown are combined from three separate experiments for the PGI2 synthase OE+ and PGI2 synthase OE− mice and two separate experiments for the IP−/− and wild-type mice.
PGI2 signaling protects against RSV-induced lung edema.
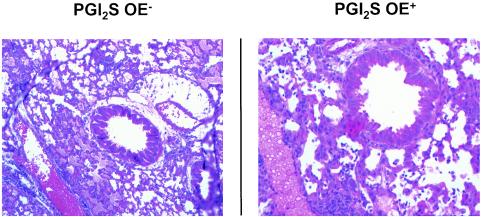
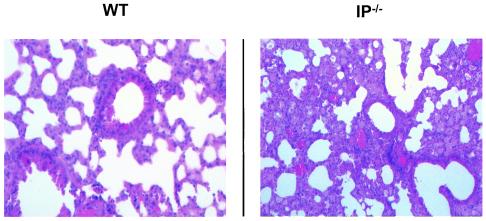
Lungs from three mice in each group were harvested on day 8 after infection to examine the inflammatory infiltrate and structural consequences of RSV infection. In both the RSV-infected PGI2 synthase OE+ and PGI2 synthase OE− groups (Fig. 7), there was 1+ (mild) inflammation consisting primarily of lymphocytosis in the bronchovascular and perivenous spaces. In the lungs of both the PGI2 synthase OE+ and PGI2 synthase OE− groups, there was focal edema; however, the edema was greater in the PGI2 synthase OE− group. There was a similar degree of lymphocytic inflammation in the bronchovascular and perivenous spaces of the lungs of the IP−/− and their wild-type control group, but there was marked widespread edema in the parenchyma of the RSV-infected IP−/− mice that was not present in the lungs of the wild-type mice (Fig. 8). Thus, neither overexpression of PGI2 synthase nor deficiency in IP had an effect on RSV-induced pulmonary parenchymal lymphocytosis. However, PGI2 synthase overexpression decreased edema in the lungs of RSV-infected mice of the FVB background, while the inability to signal through IP led to edema in the alveoli in C57BL/6 background mice.
FIG. 7.
Lung sections of PGI2 synthase OE− and PGI2 synthase OE+ mice harvested on day 8 and stained with hematoxylin and eosin. The sections are representative of three mice in each group.
FIG. 8.
Lung sections of wild-type (WT) and IP−/− mice harvested on day 8 and stained with hematoxylin and eosin. The sections are representative of three mice in each group.
PGI2 signaling does not affect IFN-α/β production.
In order to determine the effect of PGI2 signaling on RSV-induced IFN-α/β production, we measured IFN-α/β lung activity on day 1 after infection. We found that there was no difference in IFN-α/β lung activity in the PGI2 synthase OE+ and PGI2 synthase OE− groups (4,310 ± 1,596 versus 4,096 ± 1,122 U/lung, respectively; n = 5 for each group). We also found that there was no difference in IFN-α/β lung activity in the IP−/− and wild-type groups (6,554 ± 1,003 versus 4,915 ± 819 U/lung, respectively; n = 5 for each group). Therefore, neither overexpression of PGI2 synthase nor deficiency of IP had an effect on IFN-α/β production 1 day after infection.
PGI2 synthase overexpression had no effect on SP-A and SP-D production.
In order to determine if PGI2 regulates SP-A and SP-D production and if such regulation might explain the decrease in peak viral titers in the PGI2 synthase OE+ group, we performed Western blots for surfactant proteins in bronchoalveolar lavage fluid. In uninfected mice, we found no difference in the levels of SP-A and SP-D in bronchoalveolar lavage fluid between the PGI2 synthase OE+ and PGI2 synthase OE− groups (data not shown, n = 4 per group).
DISCUSSION
Our results reveal that PGI2 synthase OE+ mice are protected against RSV-induced illness and have decreased viral replication, while mice that lack IP had exacerbated weight loss and have delayed viral clearance in comparison. These results reveal that PGI2 signaling through IP protects against RSV-induced disease.
We found that production of the stable urinary metabolite of PGI2 is increased by RSV infection. This result is not surprising because a cyclooxygenase enzyme, COX-2, that produces PGI2 is induced as a result of inflammatory stimuli such as tumor necrosis factor alpha that can be generated in the lung as a result of RSV infection (2, 22). However, the expression of the PGI2 metabolite closely corresponds with the period of weight loss that occurs with RSV infection, suggesting that PGI2 may be an important factor in protection from RSV-induced illness. Measures of acute respiratory illness, such as tachypnea, have been noted as early as 1 day after RSV infection (25) and do not correspond to the urinary PGI2 metabolite that we assayed.
Studies of the murine model of RSV infection reveal that a vigorous adaptive immune response is a key factor in disease severity (11). For instance, when CD4+ and CD8+ lymphocyte subsets are depleted by administration of neutralizing antibody, there is no illness as defined by weight loss or decreased activity, yet RSV replication is prolonged (11). This suggests that the host immune response instead of the viral cytocidal effect is an important determinant of RSV-induced illness. However, we found in our experiments with PGI2 synthase OE+ and IP−/− mice and their respective controls that signaling through IP not only protected against weight loss but also decreased viral replication while decreasing IFN-γ production. Given the small yet statistically significant changes in viral titers that occurred in the PGI2 synthase OE+ and IP−/− groups in comparison with the respective wild-type mice, it is uncertain whether these changes in viral titers influenced the more dramatic changes seen in weight changes in the groups in response to RSV infection; however, this possibility cannot be ruled out.
Of further note, we found that RSV-specific antibody levels were significantly lower in the PGI2 synthase OE+ mice and tended to be higher in the IP−/− mice. The most likely explanation for the decreased IFN-γ levels and anti-RSV antibody titers in the PGI2 synthase OE+ mice is an inhibition in peak viral replication in these animals, as a lower antigen load resulted in a decreased need for a robust adaptive immune response to the virus. This suggests that the protective effect of PGI2 is more likely through cells of the innate immune response, such as NK cells or macrophages.
PGI2 is known to have a direct effect on NK cells. Iloprost, a PGI2 analog, increases the NK lytic activity of spleen cells in an ex vivo model of experimental neoplastic metastasis (6). Natural killer cells are activated by both IFN-α and IFN-β (13). PGI2 affects NK cell function as well as monocyte and macrophage activity. SM-10906, a stable PGI2 analog, suppressed the production of tumor necrosis factor and interleukin-1 in lipopolysaccharide-stimulated rat pleural resident monocytic cells (19). In addition, PGI2 regulates the production of tumor necrosis factor alpha in an in vitro assay of murine peritoneal macrophages stimulated with zymosan (21). Carbacyclin, a PGI2 analog, reduces tumor necrosis factor alpha production from peritoneal macrophages by 50% while increasing the production of interleukin-10 (21).
PGI2 has only recently been recognized to have immunomodulatory effects in vivo. In murine pulmonary allergen challenge models, mice lacking IP have augmented allergic inflammation as characterized by increases in plasma extravasation, leukocyte accumulation, and type 2 cytokine production in the airway (24). In addition, allergically sensitized IP−/− mice have increased serum antigen-specific IgE and total IgE levels (24). In wild-type mice, PGI2 production is induced by exposure to an allergen, and IP is expressed by CD4+ type 2 lymphocytes but not by type 1 lymphocytes (24). In vitro, PGI2 and its stable analog carbaprostacyclin augment interleukin-10 production by CD4+ type 2 cells (15). PGI2 also has antineoplastic effects. In carcinogenesis models, PGI2 synthase OE+ mice have significantly reduced numbers of lung tumors that are proportional to PGI2 synthase transgene expression (16).
There are only a few reports on PGI2 in viral infection, but some suggest that PGI2 may regulate virus-induced illness. For instance, in human immunodeficiency virus-infected patients, the plasma half-life of PGI2 is diminished to15 to 161 s, compared to 9 to 12 min in noninfected patients; human immunodeficiency virus-infected patients with central nervous system manifestations of their disease have even greater decreases (mean, 34 s) compared to those without such symptoms (mean, 84 s) (20). In lung microvascular endothelial cells from sheep with bluetongue virus infection, PGI2 is decreased compared to infected endothelial cells from cattle (8). This decrease in PGI2 production in blue tongue-infected ovine endothelial cells is interesting in that marked pulmonary edema and microvascular thrombosis occur in sheep infected with bluetongue virus but rarely if ever occurs in bluetongue virus-infected cattle (8).
Our histopathology results also suggest that signaling through IP has protective effects against lung edema caused by RSV infection. We found that PGI2 synthase overexpression decreased edema in the lungs of RSV-infected mice of the FVB background, while the inability to signal through IP led to edema in the alveoli in C57BL/6 background mice. These results suggest that the protective effect of signaling through IP might be a result not only of immunomodulation of RSV infection, but also of preventing vascular leaks leading to lung edema.
In the only prior report investigating the effect of PGI2 on viral infection in mice, Zavagno and colleagues used a BALB/c mouse model of vaccinia virus infection to investigate the role of the different prostaglandins on illness and viral clearance (26). They found that mice treated with either aspirin or indomethacin, which are cyclooxygenase inhibitors and therefore inhibitors of prostaglandin synthesis, had a marked increase in mortality for vaccinia virus infection over nontreated mice (26). The mice treated with the nonsteroidal anti-inflammatory agents had delayed viral clearance with inhibition of the antibody response, whereas control mice had a higher survival rate with lower virus yield and normal antibody responses. These studies showed that PGD2 and PGF2α conferred little or no protection to mice against the lethal effects of vaccinia virus, while mice treated with PGE1 had a dramatic increase in mortality after infection. In contrast, the mice treated with PGI2 had greatly enhanced survival.
In conclusion, we found that mice overexpressing PGI2 synthase were protected against RSV-induced weight loss, while mice lacking the PGI2 receptor IP had exacerbated weight loss after RSV infection. In addition, the mice that overexpressed PGI2 synthase had decreased peak viral replication, lower lung IFN-γ levels, and lower serum RSV-specific antibody levels, suggesting that PGI2 overexpression decreased RSV antigen load and downregulated the adaptive immune response. In contrast, IP−/− mice had delayed viral clearance, higher IFN-γ levels in the lung, and slightly increased serum RSV-specific antibody levels, suggesting that the inability to signal through IP increased RSV antigenemia and led to an augmented adaptive immune response.
These results reveal that PGI2 signaling through its receptor IP protected against RSV-induced illness and decreased viral replication. Therefore, PGI2 may be a useful therapy in the prophylaxis of infants at risk for the severe consequences of RSV infection.
Acknowledgments
This work was supported by an American Academy of Allergy, Asthma and Immunology ERT Award, R01-AI 054660, R01-AI 045512, GM 15431, DK 48831, DK 26657, CA 77839, and Fukushima Medical University.
We thank Jeffrey A. Whitsett and William M. Hull for assistance with the measurement of surfactant protein concentrations and Edith Sanella for measurement of antibody titers.
REFERENCES
- 1.American Academy of Pediatrics Committee on Infectious Diseases and Committee of Fetus and Newborn. 1998. Prevention of respiratory syncytial virus infections: indications for the use of palivizumab and update on the use of RSV-IGIV. Pediatrics 102:1211-1216. [DOI] [PubMed] [Google Scholar]
- 2.Aung, S., J. A. Rutigliano, and B. S. Graham. 2001. Alternative mechanisms of respiratory syncytial virus clearance in perforin knockout mice lead to enhanced disease. J. Virol. 75:9918-9924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breyer, R. M., C. K. Bagdassarian, S. A. Myers, and M. D. Breyer. 2001. Prostanoid receptors: subtypes and signaling. Annu. Rev. Pharmacol. Toxicol. 41:661-690. [DOI] [PubMed] [Google Scholar]
- 4.Breyer, R. M., C. R. Kennedy, Y. Zhang, and M. D. Breyer. 2000. Structure-function analyses of eicosanoid receptors. Physiologic and therapeutic implications. Ann. N. Y. Acad. Sci. 905:221-231. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, Y., S. C. Austin, B. Rocca, B. H. Koller, T. M. Coffman, T. Grosser, J. A. Lawson, and G. A. Fitzgerald. 2002. Role of prostacyclin in the cardiovascular response to thromboxane A2. Science 296:539-541. [DOI] [PubMed] [Google Scholar]
- 6.Costantini, V., A. Giampietri, M. Allegrucci, G. Agnelli, G. G. Nenci, and M. C. Fioretti. 1991. Mechanisms of the antimetastatic activity of stable prostacyclin analogues: modulation of host immunocompetence. Adv. Prostaglandin Thromboxane Leukot. Res. 21B:917-920. [PubMed] [Google Scholar]
- 7.Daniel, V. C., T. A. Minton, N. J. Brown, J. H. Nadeau, and J. D. Morrow. 1994. Simplified assay for the quantification of 2,3-dinor-6-keto-prostaglandin F1 alpha by gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Appl. 653:117-122. [DOI] [PubMed] [Google Scholar]
- 8.DeMaula, C. D., M. A. Jutila, D. W. Wilson, and N. J. MacLachlan. 2001. Infection kinetics, prostacyclin release and cytokine-mediated modulation of the mechanism of cell death during bluetongue virus infection of cultured ovine and bovine pulmonary artery and lung microvascular endothelial cells. J. Gen. Virol. 82:787-794. [DOI] [PubMed] [Google Scholar]
- 9.Galie, N., A. Manes, and A. Branzi. 2001. Medical therapy of pulmonary hypertension. The prostacyclins. Clin. Chest Med. 22:529-537. [DOI] [PubMed] [Google Scholar]
- 10.Geraci, M. W., B. Gao, D. C. Shepherd, M. D. Moore, J. Y. Westcott, K. A. Fagan, L. A. Alger, R. M. Tuder, and N. F. Voelkel. 1999. Pulmonary prostacyclin synthase overexpression in transgenic mice protects against development of hypoxic pulmonary hypertension. J. Clin. Investig. 103:1509-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham, B. S., L. A. Bunton, P. F. Wright, and D. T. Karzon. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Investig. 88:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, B. S., M. D. Perkins, P. F. Wright, and D. T. Karzon. 1988. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 26:153-162. [DOI] [PubMed] [Google Scholar]
- 13.Guidotti, L. G., and F. V. Chisari. 2001. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu. Rev. Immunol. 19:65-91. [DOI] [PubMed] [Google Scholar]
- 14.Hall, C. B. 2001. Respiratory syncytial virus and parainfluenza virus. N. Engl. J. Med. 344:1917-1928. [DOI] [PubMed] [Google Scholar]
- 15.Jaffar, Z., K. S. Wan, and K. Roberts. 2002. A key role for prostaglandin I2 in limiting lung mucosal Th2, but not Th1, responses to inhaled allergen. J. Immunol. 169:5997-6004. [DOI] [PubMed] [Google Scholar]
- 16.Keith, R. L., Y. E. Miller, Y. Hoshikawa, M. D. Moore, T. L. Gesell, B. Gao, A. M. Malkinson, H. A. Golpon, R. A. Nemenoff, and M. W. Geraci. 2002. Manipulation of pulmonary prostacyclin synthase expression prevents murine lung cancer. Cancer Res. 62:734-740. [PubMed] [Google Scholar]
- 17.Nagao, K., H. Tanaka, M. Komai, T. Masuda, S. Narumiya, and H. Nagai. 2003. Role of prostaglandin I2 in airway remodeling induced by repeated allergen challenge in mice. Am. J. Respir. Cell Mol. Biol. 29:314-320. [DOI] [PubMed] [Google Scholar]
- 18.Narumiya, S., Y. Sugimoto, and F. Ushikubi. 1999. Prostanoid receptors: structures, properties, and functions. Physiol. Rev. 79:1193-1226. [DOI] [PubMed] [Google Scholar]
- 19.Oh-ishi, S., I. Utsunomiya, T. Yamamoto, Y. Komuro, and Y. Hara. 1996. Effects of prostaglandins and cyclic AMP on cytokine production in rat leukocytes. Eur. J. Pharmacol. 300:255-259. [DOI] [PubMed] [Google Scholar]
- 20.Pirich, C., Y. Efthimiou, J. O'Grady, C. Zielinski, and H. Sinzinger. 1996. Apolipoprotein A and biological half-life of prostaglandin I2 in HIV-1 infection. Thromb. Res. 81:213-218. [DOI] [PubMed] [Google Scholar]
- 21.Shinomiya, S., H. Naraba, A. Ueno, I. Utsunomiya, T. Maruyama, S. Ohuchida, F. Ushikubi, K. Yuki, S. Narumiya, Y. Sugimoto, A. Ichikawa, and S. Oh-ishi. 2001. Regulation of TNFalpha and interleukin-10 production by prostaglandins I(2) and E(2): studies with prostaglandin receptor-deficient mice and prostaglandin E-receptor subtype-selective synthetic agonists. Biochem. Pharmacol. 61:1153-1160. [DOI] [PubMed] [Google Scholar]
- 22.Smith, W. L., D. L. Dewitt, and R. M. Garavito. 2000. Cyclooxygenases: structural, cellular, and molecular biology. Annu. Rev. Biochem. 69:145-182. [DOI] [PubMed] [Google Scholar]
- 23.Staat, M. A. 2002. Respiratory syncytial virus infections in children. Semin. Respir. Infect. 17:15-20. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi, Y., S. Tokuoka, T. Masuda, Y. Hirano, M. Nagao, H. Tanaka, N. Inagaki, S. Narumiya, and H. Nagai. 2002. Augmentation of allergic inflammation in prostanoid IP receptor deficient mice. Br. J. Pharmacol. 137:315-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Schaik, S. M., N. Obot, G. Enhorning, K. Hintz, K. Gross, G. E. Hancock, A. M. Stack, and R. C. Welliver. 2000. Role of interferon gamma in the pathogenesis of primary respiratory syncytial virus infection in BALB/c mice. J. Med. Virol. 62:257-266. [DOI] [PubMed] [Google Scholar]
- 26.Zavagno, G., B. Jaffe, and M. Esteban. 1987. Role of prostaglandins and non-steroid anti-inflammatory drugs in the pathogenicity of vaccinia virus. J. Gen. Virol. 68:593-600. [DOI] [PubMed] [Google Scholar]