Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia and lymphoma, an aggressive clonal malignancy of human CD4-bearing T lymphocytes. HTLV-2, although highly related to HTLV-1 at the molecular level, has not been conclusively linked to development of lymphoproliferative disorders. Differences between the biological activities of the respective tax gene products (Tax1 and Tax2) may be one factor which accounts for the differential pathogenicities associated with infection. To develop an in vitro model to investigate and compare the effects of constitutive expression of Tax1 and Tax2, Jurkat T-cell lines were infected with lentivirus vectors encoding Tax1 and Tax2 in conjunction with green fluorescent protein, and stably transduced clonal cell lines were generated by serial dilution in the absence of drug selection. Jurkat cells that constitutively express Tax1 and Tax2 (Tax1/Jurkat and Tax2/Jurkat, respectively) showed notably reduced kinetics of cellular replication, and Tax1 inhibited cellular replication to a higher degree in comparison to Tax2. Tax1 markedly activated transcription from the cdk inhibitor p21cip1/waf1 promoter in comparison to Tax2, suggesting that upregulation of p21cip1/waf1 may account for the differential inhibition of cellular replication kinetics displayed by Tax1/Jurkat and Tax2/Jurkat cells. The presence of binucleated and multinucleated cells, reminiscent of large lymphocytes with cleaved or cerebriform nuclei often seen in HTLV-1- and -2-seropositive patients, was noted in cultures expressing Tax1 and Tax2. Although Tax1 and Tax2 expression mediated elevated resistance to apoptosis in Jurkat cells after serum deprivation, Tax1 was unique in protection from apoptosis after exposure to camptothecin and etoposide, inhibitors of topoisomerase I and II, respectively. Characterization of the unique phenotypes displayed by Tax1 and Tax2 in vitro will provide information as to the relative roles of these oncoproteins and their contribution to HTLV-1 and -2 pathogenesis in vivo.
Human T-cell leukemia/lymphoma virus type 1 (HTLV-1) is the etiologic agent of a severe and fatal lymphoproliferative disorder of helper T cells termed adult T-cell leukemia/lymphoma (ATL/ATLL). Although HTLV-1 and HTLV-2 share ca. 70% sequence homology, infection with HTLV-2 is less pathogenic in comparison to infection with HTLV-1 and has not been definitively linked to the development of lymphoproliferative disorders. The HTLV-1 and -2 Tax proteins, p40tax (Tax1) and p37tax (Tax2), share many characteristic properties of viral oncoproteins, most notably the ability to immortalize lymphocytes for growth in vitro. The HTLV-1 tax gene is invariably maintained in ATL cells, suggesting that Tax1 plays a pivotal role in the initiation of leukemogenesis. There is a lack of understanding as to the inherent differences between HTLV-1 and HTLV-2, with respect to the ability to induce leukemia, although it is speculated that the respective functions of Tax1 and Tax2 play an important role in the early processes of disease.
Tax1 can alter the expression of a number of cellular genes involved in cell cycle progression and cell growth. Progression through the cell cycle is controlled by the activity of specific cellular regulators termed cyclin-dependent kinases (cdk's), which associate with cyclins. Oncogenic viruses, including HTLV-1, commonly target cdk complexes and tumor suppressor proteins to perturb progression through the cell cycle and potentiate viral replication. Tax1 has been shown to target key regulators of the cell cycle, including p21cip1/waf1, p53, p16INK4a, and cdk complexes/cyclins D1, D2, and D3 (2, 14, 19, 24, 25, 37, 44, 52, 56, 57, 64, 67, 68). p21cip1/waf1 (p21) is a cdk inhibitor which forms part of the cyclin D1/cdk2/PCNA complex and has been shown to be transcriptionally upregulated by Tax1 (14). Expression of p21 has been shown to be upregulated in ATL cells and in HTLV-1 transformed cell lines (1, 6, 11, 14, 22). Overexpression of p21 inhibits two critical checkpoints in G1 and G2, arresting cell cycle by p53-independent and -dependent pathways (34). Tax1 has been shown to suppress G1 progression by upregulating p21 expression (66). Paradoxically, Tax1 has also been shown to compel cells to egress from G0/G1 into S phase (33, 40, 54). Expression of Tax1 in Jurkat cells has also been shown to result in the accumulation of cells in G2/M (20). The pleiotropic effects of Tax1 to inactivate cellular checkpoint processes and in the inhibition of DNA repair mechanisms contributes to dysregulation of cell cycle progression.
A limited number of assays have been generated to distinguish between Tax1 and Tax2 functions. Tax1 and Tax2 are similar with respect to the ability to immortalize lymphocytes for growth in culture and in the activation of CREB/ATF and NF-κB (29, 43, 48, 49). However, Tax1 transforms rat fibroblasts more efficiently and has a higher capacity to inactivate p53 activity, in comparison to Tax2 (16, 35). Tax1 induces micronuclei formation in Cos cells in contrast to Tax2 which does not display this activity (55). Tax1 and Tax2 also have a differential ability to transactivate the intracellular adhesion molecule-1 (ICAM-1) promoter (60). Our laboratory has recently demonstrated that Tax1 suppresses hematopoiesis of CD34+ hematopoietic progenitor cells in vitro, in contrast to Tax2 which does not adversely affect hematopoietic differentiation (62). Identifying and characterizing the unique functions of Tax1 and Tax2 in hematopoietic progenitor cells and in lymphoid cell lines will provide critical insight regarding the elevated pathogenesis associated with HTLV-1 infection.
Numerous studies have shown that persistent Tax1 expression modulates apoptosis in lymphoid and in nonlymphoid cells (9, 10, 32, 41, 42, 46, 66). Tax1 has been shown to interact directly with the mitotic checkpoint protein hsMAD1 (24), which has been proposed to cause spindle checkpoint defects, aneuploidy, and nuclear abnormalities, including multinucleated cells (30). It has also been recently reported that Tax1 directly interacts and binds to DNA topoisomerase I (Top1), inhibiting the catalytic activity and interfering with DNA repair (58, 69). The loss of Top1 activity has previously been shown to affect DNA repair, DNA replication, transcription, and chromosome condensation (26, 69). In contrast, there have been very few reports investigating the effects of Tax2 on cell cycle progression and apoptosis. To more clearly understand and define the differential effects of Tax1 and Tax2 on cellular metabolism, we have generated clonally derived Jurkat cell lines that constitutively express Tax1 and Tax2 by using lentivirus vector (LV)-mediated transduction. We found that constitutive Tax1 and Tax2 expression inhibits cellular replication kinetics of T-lymphoid cell lines. Tax1 and Tax2 displayed a differential ability to activate transcription from the p21 promoter, suggesting that the inhibition of kinetics of cellular replication may correlate with the respective ability of these viral oncoproteins to inhibit cell cycle progression. Expression of Tax1 and Tax2 prevented apoptosis in cell lines after serum deprivation, although Tax1 was unique in inhibiting apoptosis after exposure to drugs which target Top1 or Top2 activity. Characterization of the unique phenotypes of Tax1 and Tax2 in lymphoid cells may allow elucidation of the molecular mechanisms responsible for the marked differences in pathogenesis as a result of infection with HTLV-1 and HTLV-2.
MATERIALS AND METHODS
Cell lines.
293T cells were cultured in Dulbeccos modified Eagle medium (DMEM; Gibco-BRL, Grand Island, N.Y.) with 10% heat-inactivated fetal bovine serum (FBS; Gemini, Calabasas, Calif.), 2 mM l-glutamine (Gibco-BRL), and 100 U of penicillin and 100 μg of streptomycin/ml (pen/strep; Gemini). Jurkat cell lines were cultured in Iscove modified Dulbecco medium (IMDM; Gibco-BRL), supplemented with 10% FBS, 2 mM l-glutamine, and pen/strep at 37°C in a humidified incubator with 5% CO2.
Generation of VSV-G-pseudotyped LVs.
Vesicular stomatitis virus protein G (VSV-G)-pseudotyped lentivirus vector virus stocks were generated as previously described (4, 62, 65). Briefly, a three-plasmid transfection system was used: transfer plasmids [pHR′CMV-GFP, pHR′CMV-Tax1/GFP, pHR′CMV-Tax2/GFP, or HR′CMV-Tax1(−)/GFP]; the packaging vector (pCMVΔR8.2ΔVPR) containing the Gag/Pol proteins of human immunodeficiency virus type 1 (HIV-1) and lacking the ψ signal (3); and a vector encoding the VSV-G envelope protein (pHCMV-G [5]). Plasmids were cotransfected into 2 × 107 293T cells by calcium phosphate precipitation. The bicistronic transfer vectors were derived from HIV-1, but they do not encode for any HIV-1 gene products. The integrated provirus encodes for a single mRNA with an internal ribosome entry site sequence driven by an immediate-early cytomegalovirus (CMV) promoter. 293T cells were incubated in DMEM supplemented with 100 μM chloroquine for 24 h; the medium was then removed and replaced with 25 ml of DMEM plus 10% FBS. Supernatants were harvested at 3 and 5 days posttransfection, filtered through a 0.45-μm-pore-size filter, pooled, and subjected to ultracentrifugation (50,000 × g for 2 h) in a SW27 rotor (Beckman, Palo Alto, Calif.). The pellet was resuspended in 1/100 initial volume in serum-free DMEM overnight at 4°C and then pooled and frozen at −80°C. Titers of virus stocks were determined by infecting HeLa cells (3 × 105) with virus stocks that were serially diluted (1:10, 1:100, and 1:500) in serum-free DMEM. Cells were analyzed for GFP at 72 h postinfection by flow cytometry. Virus titers generally ranged from 106 to 107 transducing units per ml.
Generation of stably transduced Jurkat cell lines.
Jurkat cells (106) were infected at a multiplicity of infection (MOI) of 3 with LVs in a final volume of 3.0 ml of IMDM without serum containing 8 μg of Polybrene (Sigma, St. Louis, Mo.)/ml. Cells were infected by centrifugation at 2,500 rpm (555 × g) in a Beckman-Coulter GPR centrifuge at room temperature as previously described (62). Cells were resuspended in 3.0 ml of IMDM supplemented with 10% FBS, 2 mM l-glutamine, and 100 μg of pen/strep per ml. Cells were counted, serially diluted, and plated at one cell per well in 96-well plates. After clonal expansion, cells were randomly chosen and analyzed for GFP expression by using a LSR II flow cytometer (Becton Dickinson, Mountain View, Calif.). The data were analyzed by using WinMDI 2.8 software (J. Trotter, The Scripps Research Institute, San Diego, Calif.).
Cell staining.
Hematoxylin and eosin staining of Jurkat clonal cell lines was performed as follows. Chamber slides were treated with 500 μl of 0.01% poly-l-lysine (Sigma) and then drained and air dried. Cells (105) were suspended in 500 μl of a 1:1 solution of methanol-acetone and fixed to treated chamber slides at −20°C for 10 min. Cells were dehydrated in 100% ethanol for 1 min, stained with Mayer's hematoxylin solution (1 g/liter; Sigma) for 2 min, washed with distilled water, and stained with Eosin Y solution (Sigma; 5% in water) for 1 min. Cells were rinsed with distilled water and mounted with coverslips, as described below. DAPI (4′,6′-diamidino-2-phenylindole) staining of Jurkat clonal cell lines was performed as follows. Cells (105) were fixed to poly-l-lysine-pretreated slides with 1:1 methanol-acetone as described above, and cells were permeabilized for 15 min in 0.2% Triton X-100 in phosphate-buffered saline (PBS) at room temperature. Slides were incubated in 1 ml of DAPI (1 μg/ml) in PBS for 10 min at room temperature and washed with 1× PBS. Cells were mounted by using fluorescent mounting medium (Vector Laboratories) and viewed at a ×20, ×40, and ×80 magnifications with a Nikon Eclipse microscope (Nikon Instruments, Inc., Melville, N.Y.). Photos of cells were taken on a SPOT digital microscope camera and software (Diagnostic Instruments, Inc.).
Luciferase assay.
pHTLV-1-LTR-Luc (a gift from Kuan-Teh Jeang, National Institute of Allergy and Infectious Disease, Bethesda, Md.) was transfected into Jurkat cell lines by using Lipofectamine 2000 (Invitrogen/Life Technologies, Carlsbad, Calif.) according to the manufacturer's protocol. Briefly, cells (107) were plated in 15 ml of IMDM with 10% FBS, 2 mM l-glutamine, and 100 μg of pen/strep per ml in a T-75 cell culture flask and transfected with 24 μg of pHTLV-1-LTR-Luc. Cell extracts were prepared and assayed for luciferase activity using reagents (luciferase assay system; Promega, Madison, Wis.) according to the manufacturer's protocol. The luciferase activity was assayed by using a plate reading luminometer (Luminoskan RS; Labsystems, Needham Heights, Mass.).
Apoptosis analysis.
Tax1/Jurkat, Tax2/Jurkat, and Tax1(−)/Jurkat cells (3 × 105) were incubated in 3 ml of IMDM containing 0.1 μM camptothecin (CPT), 1.0 μM etoposide (ETP), or 5 ng of taxol (TXL)/ml. After 24 and 36 h, cells were incubated with phycoerythrin-conjugated Annexin V stain (BioVision, Mountain View, Calif.) and 7-amino actinomycin D (7-AAD; Calbiochem, La Jolla, Calif.) according to previously published protocols (53, 62). Briefly, cells were washed twice with PBS and resuspended in 500 μl of binding buffer and 5 μl of Annexin V stain. Cells were incubated at room temperature for 5 min and then washed with PBS. The cell pellet was resuspended in 1 ml of PBS containing 1 μl of 7-AAD (1.0 mg/ml) and incubated for 10 min at room temperature. Cells were pelleted by centrifugation and resuspended in PBS for flow cytometric analysis by using an LSR II flow cytometer (Becton Dickinson), and data were analyzed by using WinMDI 2.8 software.
Serum starvation and cell cycle analysis.
Jurkat cells (106) were serum starved in 3 ml of IMDM with 2 mM l-glutamine, and 100 U of pen/strep per ml. After 48 h of serum starvation, cells were pelleted by centrifugation at 1,500 rpm (200 × g) in a Beckman GPR centrifuge for 5 min and resuspended in 3 ml of IMDM supplemented with l-glutamine, pen/strep, and 10% FBS. Cell cycle analysis of cells by propidium iodide staining was performed as previously described (59). Briefly, an aliquot of cells (5 × 105) was washed in ice-cold PBS and fixed in ice-cold 70% ethanol in PBS for 30 min. After being washed in PBS, cells were treated with 500 U of RNase A (Sigma)/ml at 37°C for 30 min. Cells were washed with PBS and stained in PBS with 40 μg of propidium iodide (Sigma)/ml to stain DNA. Cell cycle data were acquired on a LSR II flow cytometer and analyzed with WinMDI 2.8 software and Microsoft Excel 2000. Statistical significance was analyzed by using the Student t test (P < 0.05).
RESULTS
Generation and characterization of Tax1 and Tax2 stably transduced Jurkat T-lymphoid cell lines.
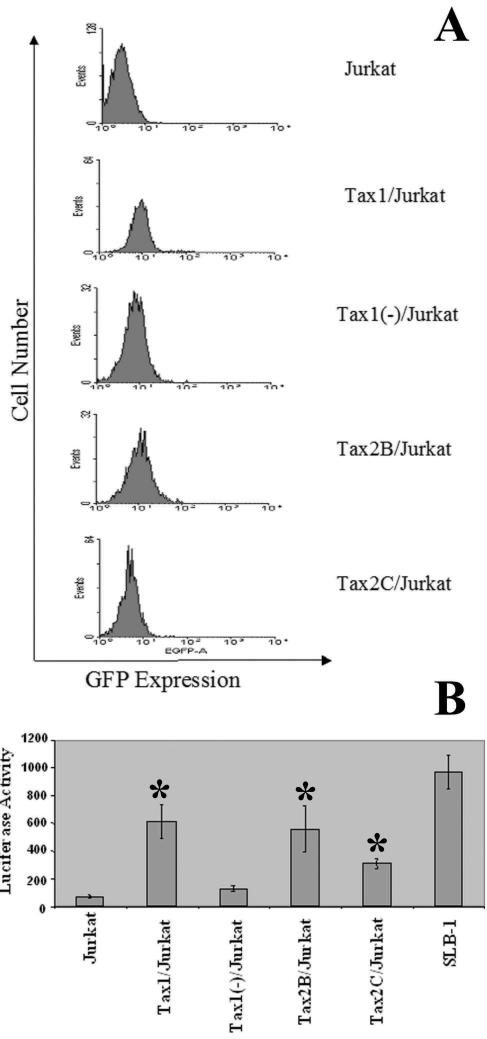
We recently described the construction of bicistronic LVs capable of coexpressing Tax1 and Tax2 in conjunction with the GFP marker gene and demonstrated that ectopic expression of Tax1 in Jurkat and CEM T-lymphoid cell lines failed to induce apoptosis (62). Jurkat cells were infected with LVs encoding Tax1, Tax2, or an antisense Tax1 construct [HR′CMV-Tax1/GFP, HR′CMV-Tax2/GFP, or HR′CMV-Tax1(−)/GFP] (MOI = 3), and cells were serially diluted and plated. Clones were expanded, randomly selected, and analyzed for GFP expression by flow cytometry. Cell lines which displayed relatively high levels of GFP expression were selected for further analysis and GFP expression was monitored after 1, 6, and 12 weeks in culture (Fig. 1A). All cell lines maintained detectable levels of GFP at each time point analyzed, although the mean fluorescence intensity of GFP decreased over time with cultivation for all clonal cell lines (Table 1). To confirm the expression of functional Tax protein, HTLV-1-LTR-Luc was transfected into Jurkat clonal cell lines. We previously demonstrated that Tax1 and Tax2 are capable of transactivating transcription from the HTLV-1 LTR (62). Transfection of HTLV-1-LTR-Luc into either Tax1 or Tax2 stably transduced Jurkat cell lines (Tax1/Jurkat, Tax2B/Jurkat, and Tax2C/Jurkat) resulted in significant induction of luciferase activity in contrast to transfection of Jurkat or Tax1(−)/Jurkat cells (Fig. 1B). Of note, Tax2C/Jurkat is a Tax2-transduced clonal Jurkat cell line that displays relatively low GFP expression and luciferase activity, suggesting that this cell line expresses relatively lower levels of Tax2 in comparison to Tax2B/Jurkat cells. Tax2 activity in Tax2C/Jurkat cells was significantly higher in comparison to Tax1(−)/Jurkat and Jurkat cells. In addition, although luciferase activity was detected in Tax1/Jurkat and Tax2B/Jurkat clones, this activity was lower than the luciferase activity detected after transfection of SLB-1 cells, an HTLV-1 transformed T-cell line that has been established in culture for many years and contains multiple proviral integrations (27). Transfection of HTLV-1-LTR-CAT into Tax1/Jurkat or Tax2B/Jurkat cell lines also showed significantly higher CAT activity in comparison to transfection of Jurkat cells or Tax1(−)/Jurkat cells (data not shown). These experiments demonstrate that LV-transduced Jurkat cells constitutively maintain functional Tax1 and Tax2 expression and that GFP expression is a valid surrogate marker of Tax1 or Tax2 expression in these clonal cell lines.
FIG. 1.
Characterization of Jurkat cell lines constitutively expressing Tax1 and Tax2. (A) Flow cytometric analysis of GFP expression. Jurkat cells were infected with LVs (MOI = 3), as previously described (62). Cells were serially diluted and clonally plated in 96-well plates at 48 h postinfection. After expansion, clones were randomly selected and analyzed for GFP expression by flow cytometry. GFP expression was assayed at 1, 6, and 12 weeks after clonal expansion, and GFP expression measured at 12 weeks is shown. The data were analyzed by using WinMDI 2.8 software. The clonal cell lines and LVs used for infection are as follows: Tax1/Jurkat, HR′CMV-Tax1/GFP; Tax1(−)/Jurkat, HR′CMV-Tax1(−)/GFP; and Tax2B/Jurkat and Tax2C/Jurkat, HR′CMV-Tax2/GFP. (B) Luciferase activity resulting from transfection of Jurkat clones with HTLV-1-LTR-Luc. Jurkat cell lines (107) were transfected with HTLV-1-LTR-Luc (24 μg; a gift from Kuan-Teh Jeang, National Institutes of Health, Bethesda Md.) by using Lipofectamine 2000 (Invitrogen). Cells were lysed in cell culture lysis reagent (Promega), and the luciferase activity was normalized for the amount of protein in each extract, as determined by a Bradford assay. SLB-1 is an HTLV-1-transformed T-cell line and contains multiple proviral integrations of HTLV-1. The experiment was performed three times, and the luciferase assays were carried out in triplicate. Error bars represent one standard deviation, and statistical analysis was performed by using the Student t test (P < 0.05), with Tax1(−)/Jurkat cells as a control. The transfection efficiencies ranged from 80 to 95% in SLB-1 cells as determined by GFP expression after cotransfection of a GFP reporter gene construct and HTLV-1-LTR-Luc.
TABLE 1.
Mean fluorescence intensity of GFP expression in Jurkat clonal cell linesa
| Cell line | MFI after cultureb for:
|
||
|---|---|---|---|
| 1 wk | 6 wk | 12 wk | |
| Jurkat | 4.61 | 3.74 | 2.78 |
| Tax1/Jurkat | 13.46 | 10.09 | 9.01 |
| Tax1(−)/Jurkat | 10.18 | 8.43 | 7.77 |
| Tax2B/Jurkat | 14.74 | 8.46 | 10.24 |
| Tax2C/Jurkat | 7.42 | 5.87 | 5.13 |
GFP expression was determined at 1, 6, and 12 weeks after clonal expansion of Jurkat cells transduced with LVs. The geometric means of the fluorescent peaks were recorded as described in Fig. 1A.
That is, the time in weeks after clonal expansion. MFI, mean fluorescence intensity.
HTLV-1 and -2 Tax inhibit proliferation of Jurkat cells.
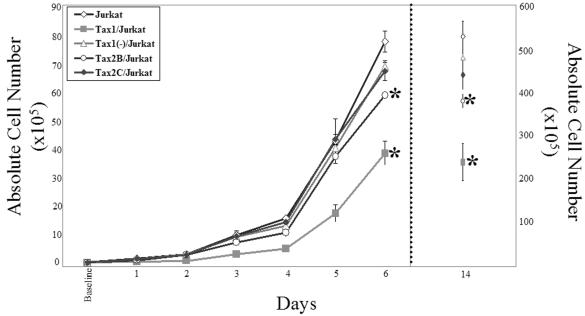
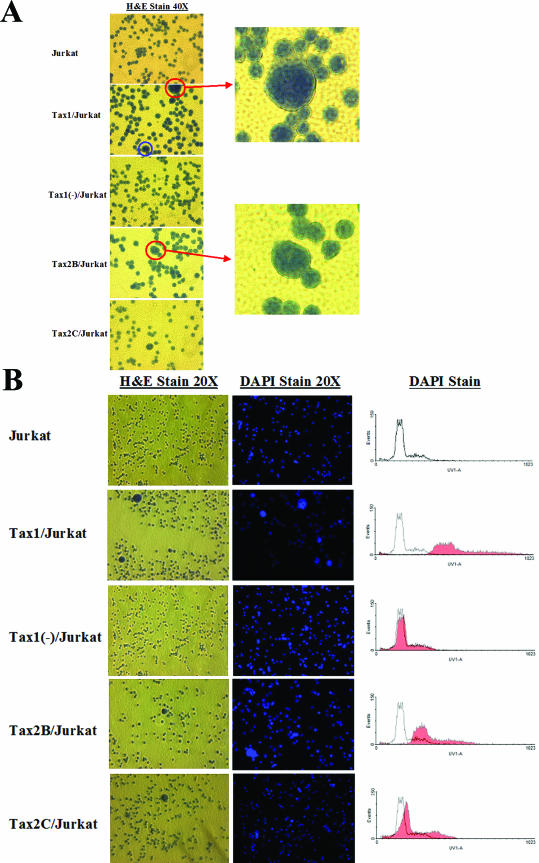
Tax1 expression has previously been shown to perturb progression through the cell cycle (28, 33, 54). To compare the kinetics of cellular replication of Tax-expressing Jurkat cell lines, cells were plated into culture and enumerated at 24-h intervals for up to 14 days (Fig. 2). Tax1/Jurkat cells grew with significantly reduced kinetics compared to Tax1(−)/Jurkat or Tax2B/Jurkat cells. These data are in concordance with previous results showing that expression of Tax1 confers antiproliferative activity in cell lines (30). Notably, Tax2B/Jurkat cells displayed slower growth rates in culture in comparison to Tax2C/Jurkat, Tax1(−)/Jurkat, or Jurkat cells, but with faster kinetics in comparison to Tax1/Jurkat cells. Since Tax2B/Jurkat demonstrates higher levels of Tax2 activity in comparison to Tax2C/Jurkat (Fig. 1), the differences in replication kinetics between Tax2B/Jurkat and Tax2C/Jurkat suggests that elevated expression of Tax2 may account for the antiproliferative effects. There was no significant difference in the growth kinetics displayed by Tax1(−)/Jurkat and Tax2C/Jurkat cell lines. Microscopic analysis of individual Jurkat clones revealed the presence of large multinucleated cells in Tax1/Jurkat, Tax2B/Jurkat, and Tax2C/Jurkat cultures, which were not detected in Tax1(−)/Jurkat or Jurkat cell cultures (Fig. 3A). The prevalence of binucleated and multinucleated Jurkat cells is reminiscent of previous reports of aneuploidy induction in adherent hamster (BHK-21), mouse fibroblasts (NIH 3T3), and human (WI-38) cell lines after ectopic expression of Tax1 with a retroviral vector (30). DAPI staining demonstrated significantly higher levels of fluorescence in Tax1/Jurkat and Tax2B/Jurkat cells in comparison to Jurkat and Tax1(−)/Jurkat cells, suggesting that these cells have higher DNA content and are multinucleated. Notably, Tax2C/Jurkat cells also demonstrated higher levels of DAPI staining in comparison to Jurkat and Tax1(−)/Jurkat cells (Fig. 3B). These results indicate that constitutive expression of Tax1 and Tax2 inhibits cellular proliferation kinetics and induces the discernible formation of binucleated and multinucleated lymphoid cells.
FIG. 2.
Effects of Tax1 and Tax2 on Jurkat cell proliferation. Unsynchronized Jurkat cell lines (105) were plated in 3 ml of complete IMDM medium in six-well plates. Cells were stained with 20 μl of trypan blue (Gibco-BRL), and viable cells were quantified by microscopy every 24 h. The absolute number of cells in the culture on days 1 through 6 is represented on the left y axis. In one experiment, cell replication kinetics were quantified out to 14 days after plating, and these data are represented on the right y axis. These experiments were repeated three times. Error bars represent one standard deviation. Single-tailed analysis of variance (ANOVA) and Student t tests analysis was performed. ✽, P < 0.05, with Tax1(−)/Jurkat cells as a control.
FIG.3.
Multinucleation in Tax1/Jurkat and Tax2/Jurkat cell cultures. LV transduced Jurkat cells (105) were plated onto poly-l-lysine-coated chamber slides, incubated in 1:1 methanol-acetone, and stained with hematoxylin for 2 min and eosin for 1 min (H&E). Cell lines were also stained separately with DAPI, a DNA-specific stain, for 10 min at room temperature. Photographs were taken with a SPOT digital microscope camera (Diagnostic Instruments, Inc., Sterling Heights, Mich.) on a Nikon Eclipse microscope. (A) The presence of multinucleated cells was detected by H&E staining (magnification, ×40). The blue circle represents a cell undergoing mitosis for reference. The red circles represent large, multilobulated cells representing ∼1% of total cells in the Tax1/Jurkat, Tax2B/Jurkat, and Tax2C/Jurkat cell cultures. Each enlarged field is photographed at ×80. (B) Hematoxylin-and-eosin (H&E)- and DAPI-stained Jurkat cell lines. Aliquots of cells (106) were stained with H&E (left panels) or DAPI (middle panels) and observed by microscopy (magnification, ×20). DAPI-stained cells were also analyzed by flow cytometry (Becton Dickinson). The shaded overlay represents LV-transduced Jurkat cells stained with DAPI compared to the parental Jurkat cell line stained with DAPI (right panels).
Tax1 and Tax2 transcriptional transactivation of the human p21cip1/waf1 promoter.
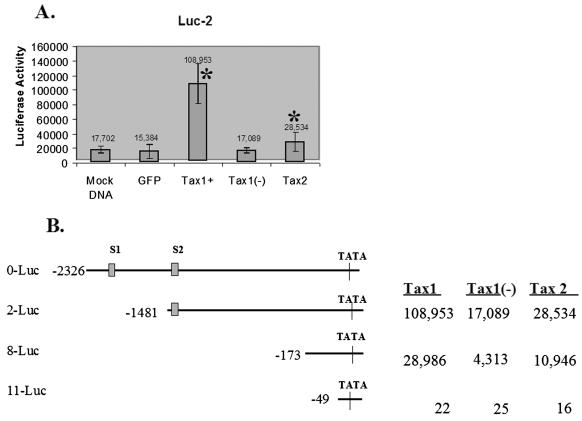
Activation of the cdk inhibitor p21cip1/waf1 (p21) inhibits cell cycle progression and cellular replication. It has previously been reported that HTLV-1 transformed lymphoid cell lines and ATL cells display high levels of endogenous p21 and that Tax1 can upregulate transcription of p21 independent of p53 binding (1, 11, 13, 15). To compare the ability of Tax1 and Tax2 to activate p21 expression, p21 promoter constructs initiating luciferase gene expression were cotransfected with LVs encoding Tax1, Tax2, Tax1(−), or GFP alone. Cotransfection of Tax1 and 2-Luc demonstrated a significantly higher level of induction of luciferase activity above basal levels compared to transfection with LVs encoding Tax2 (6.4- and 1.7-fold induction, respectively) (Fig. 4A). Tax1 and Tax2 were also functionally able to transactivate transcription from a construct encoding 173 nucleotides of the p21 promoter and lacking both p53 binding sites (8-Luc). Tax1 retained the ability to induce luciferase activity more effectively from this truncated p21 promoter construct in comparison to Tax2 (6.7- and 2.5-fold, respectively). Tax1 and Tax2 did not transactivate transcription from a minimal p21 promoter construct (49 nucleotides; 11-Luc) (Fig. 4B). This demonstrates that Tax1 is significantly more effective at transcriptional transactivation of p21 expression in comparison to Tax2. These data also confirm previous reports showing that Tax1 transactivation of the p21 promoter can occur independent of p53 binding, as previously reported (14). Moreover, the enhanced ability of Tax1 to transactivate p21 expression may account for the differences in the kinetics of replication displayed by Tax1/Jurkat cells compared to Tax2B/Jurkat and Tax2C/Jurkat cells.
FIG. 4.
Tax1 and Tax2 transactivation of the p21cip1/waf1 promoter. LV constructs encoding GFP, Tax1, Tax2, or the HTLV-1 tax gene in the antisense orientation [Tax1(−)] were cotransfected into 293T cells with plasmids encoding the p21cip1/waf1 promoter initiating expression of luciferase (a gift from Fatah Kashanchi, George Washington University, Washington, D.C.). Transfection efficiencies ranged from between 90 to 95%, as determined by fluorescence microscopic analysis of GFP expression. Luciferase activity was measured at 48 h posttransfection. (A) Luciferase activity resulting from cotransfection of LVs encoding GFP, Tax1, Tax1(−), or Tax2 and 2-Luc, a construct encoding 1,481 nucleotides of the p21 promoter (14). The number above each column represents the average luciferase activity. “Mock DNA” is a cotransfection of the p21 promoter construct (2-Luc) with pUC19 carrier DNA and represents the basal level of luciferase activity. (B) Tax1 and Tax2 transactivation of p21cip1/waf1 promoter deletion constructs. Luciferase activity resulting from cotransfection of LVs and p21 promoter constructs which retain either 173 nucleotides (8-Luc) or 49 nucleotides (11-Luc) of the 5′ p21 promoter sequences. A full-length cloned p21 promoter (0-Luc) and the locations of the p53 binding sites within the promoter (S1 and S2) are provided as a reference. Transfections were repeated three times, and luciferase reactions were carried out in triplicate. Error bars represent one standard deviation. Statistical analysis was performed by using the Student t test. ✽, P < 0.05, with Tax1(−)/Jurkat cells as a control.
Tax1 and Tax2 prevent apoptosis and compel cells into cell cycle after serum starvation.
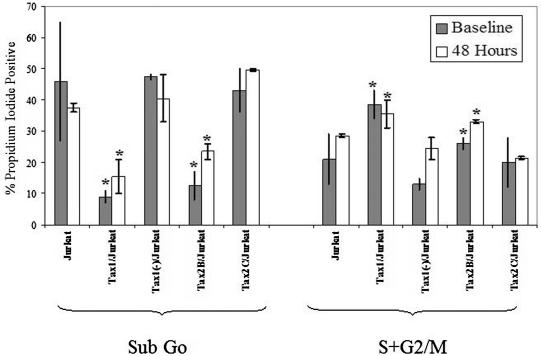
Previous studies have demonstrated that Tax1 can perturb cell cycle progression and induce cell cycle arrest in the G1 or G2/M phase (20, 21, 24, 28). In contrast, there have been no reports on the effect of Tax2 on cell cycle perturbation. To determine and compare the effect of HTLV-1 and HTLV-2 Tax on cell cycle progression in human lymphoid cells, Jurkat clonal cell lines were synchronized in G0 by serum starvation for 48 h. Cells were stained with propidium iodide and were analyzed by flow cytometry immediately after serum starvation and again after a subsequent 48 h of growth in the presence of serum (Fig. 5). The percentage of cells in sub-G0, indicative of apoptotic cells, was significantly lower in Tax1/Jurkat (9% ± 2%) and Tax2B/Jurkat (12.5% ± 4.5%) cell cultures compared to Jurkat (46% ± 19%), Tax1(−)/Jurkat (43.7% ± 1%), and Tax2C/Jurkat cells (43% ± 7%) when analyzed immediately after serum starvation. Furthermore, Tax1/Jurkat and Tax2B/Jurkat cells also showed notably higher number of cells in S+G2/M (38.5% ± 4.5% and 26% ± 2.5%, respectively) compared to Jurkat (21% ± 8%), Tax1(−)/Jurkat (13% ± 2%), and Tax2C/Jurkat cells (20% ± 8%) after serum starvation, suggesting that Tax1 and Tax2 can compel cells to progress through cell cycle in the absence of serum. Similar flow cytometric profiles were seen when cells were analyzed 48 h after growth of cells in serum. Tax1/Jurkat and Tax2B/Jurkats consistently displayed lower percentages of cells in sub-G0 and relatively higher percentages of cells in S+G2/M compared to Jurkat, Tax1(−)/Jurkat, and Tax2C/Jurkat cells. Tax2B/Jurkat cells also displayed significantly lower levels of cells in sub-G0 compared to Tax2C/Jurkats and a higher number of cells in S+G2/M when measured at 48 h, suggesting that the relative levels of Tax2 expression are important in modifying apoptosis and cell cycle arrest. These results demonstrate that elevated expression of Tax1 and Tax2 protects lymphoid cells from apoptosis and compels cells to progress through the cell cycle under conditions that normally promote cell cycle arrest. These results are in concordance with reports from other investigators (21, 23, 39, 40).
FIG. 5.
Cell cycle and apoptosis analysis of Jurkat clones after serum starvation. Clonal Jurkat cell lines (106) were serum starved by plating them in 3 ml of IMDM medium supplemented with pen/strep and glutamate in the absence of serum. After 48 h, cells were replated in 3 ml of IMDM supplemented with 10% FBS. An aliquot of cells (5 × 105) was incubated in 70% ethanol, washed with PBS, and stained with propidium iodide and analyzed by flow cytometry immediately after 48 h of serum starvation (Baseline) and 48 h after refeeding of cells in the presence serum (48 Hours). Sub-G0 (apoptotic cells) and cycling cells (S+G2/M) were quantified for each Jurkat cell line by flow cytometry, and percentages of cells in each fraction are indicated. The percentage of apoptotic cells (Sub-G0) and actively cycling cells (S+G2/M) after serum withdrawal and at 48 h after refeeding cells in the presence of serum are indicated. WinMDI 2.8 was used to quantify the cell subpopulations and analyze the data. Experiments were performed three times; the error bars represent one standard deviation. Statistical analysis was performed by using single-tailed ANOVA and Student t tests ✽, P < 0.05, with Tax1(−)/Jurkat cells as a control.
Tax1 inhibits apoptosis mediated by topoisomerase inhibitors.
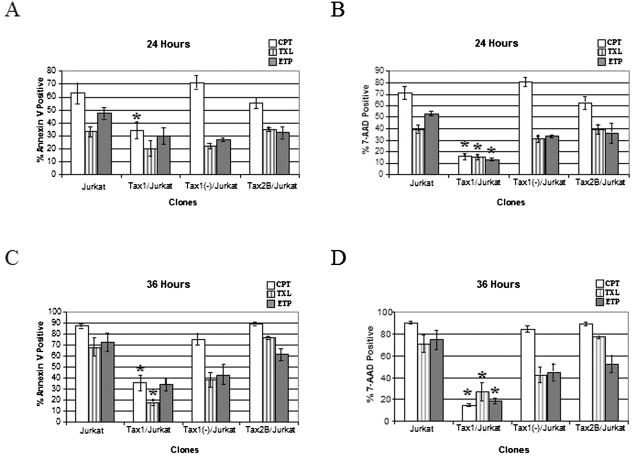
Tax1 has been reported to increase resistance of cell lines to apoptosis (12, 45, 51, 63, 70). Tax1 has recently been shown to bind and alter the activity of Top1 in vitro (58, 69). CPT and ETP are recently developed novel chemotherapeutic agents which inhibit the activity of Top1 and Top2, respectively. To ascertain whether Tax1 and Tax2 could modulate apoptosis in response to drugs that inhibit topoisomerase function, Tax1/Jurkat, Tax2B/Jurkat, Tax1(−)/Jurkat, and Jurkat cells were exposed to CPT or ETP and then analyzed by flow cytometry after staining with Annexin V and 7-AAD. Annexin V binds to phosphatidylserine on cells in early and intermediate stages of apoptosis, and 7-AAD is a dead cell exclusion dye. Tax1/Jurkat cells showed significant resistance to apoptosis induced by incubation in CPT or ETP compared to Jurkat, Tax1(−)/Jurkat, and Tax2B/Jurkat cells (Fig. 6). After 36 h in CPT, 36% of Tax1/Jurkat cells demonstrated staining with Annexin V compared to 89% of Tax2B/Jurkat cells, 88% of Jurkat cells, and 75% of Tax1(−)/Jurkat cells. Similarly, 15% of Tax1/Jurkats showed staining with 7-AAD at 36 h compared to 90, 84, and 89% of Jurkat, Tax1(−)/Jurkat, and Tax2B/Jurkat cells, respectively. Tax1/Jurkat cells also showed elevated resistance to apoptosis after incubation in ETP. Significantly fewer numbers of Tax1/Jurkat cells (34%) demonstrated staining with Annexin V at 36 h postincubation compared to Jurkat, Tax2B/Jurkat, and Tax1(−)/Jurkat cells (72, 62, and 43%, respectively). Staining with 7-AAD confirmed that Tax1/Jurkat cells were more resistant to ETP-mediated apoptosis compared to the other cell lines. Tax1/Jurkat cells also showed a modest level of protection from TXL-mediated apoptosis. These experiments demonstrate that Tax1 confers resistance to apoptosis induced by CPT and ETP, but that Tax2 does not protect cells from apoptosis mediated by topoisomerase inhibitors.
FIG. 6.
Flow cytometric analysis of Jurkat cell lines after incubation with CPT, TXL, and ETP. Jurkat cell lines were cultured in the presence of either 1.0 μM CPT, an inhibitor of Top1 (□); 1.0 μM ETP, an inhibitor of Top2 (▥); or 5 ng of TXL/ml (▪). An aliquot of the cell culture (106 cells) was analyzed by flow cytometry at 24 and 36 h after drug application, and apoptosis was assayed by staining for Annexin V (A and C) and 7-AAD (B and D). The data were compiled from three independent experiments, and statistical analysis was performed by using single-tailed ANOVA and Student t tests. ✽, P < 0.05, with Tax1(−)/Jurkat cells as a control. The error bars represent one standard deviation.
DISCUSSION
Defects in the regulation of cell cycle progression are thought to be common features of transformed cells, including cells immortalized by infection with HTLV-1. Although Tax2 shows a high degree of homology at the amino acid level compared to Tax1, distinct phenotypes for Tax2 have been reported (17, 35, 42, 70). In these studies we show that Tax1 is more effective at inhibiting cellular replication kinetics of T lymphoid cells and in transcriptionally activating p21 expression compared to Tax2. The cdk inhibitor p21 is capable of inhibiting the activity of the cell cycle kinase cdk2, a regulatory molecule important in G1/S transition. Previous studies have demonstrated that HTLV-1 infection and Tax1 expression upregulates p21 expression independent of p53 activation (1, 11, 14, 15). Although Tax1 and Tax2 exerted an antiproliferative effect on Jurkat cells in culture, we did not detect overt induction of cell cycle arrest. Moreover, we did detect the ability of Tax1 and Tax2 to compel cells to transition through cell cycle after serum arrest, in agreement with previous reports showing that Tax1 can prevent cell cycle arrest (28, 30, 43). The absence of overt G2 arrest is in contrast to reports by Haoudi and Semmes (21), describing accumulation of cells in G2/M after de novo expression of Tax1. We speculate that these differences may be attributed to the experimental cell model used since our system imposed clonal selection and constitutive expression of Tax1 during Jurkat cell expansion. The multinucleated phenotype displayed by a small percentage of Tax1/Jurkat and Tax2/Jurkat cells does, however, suggest that Tax1 and Tax2 may block the progression and completion of mitosis or cytokinesis. Our data are similar to the recent findings of Liang and Giam, who described an antiproliferative effect after retroviral transduction of Tax1 and the development of multinucleated cells in adherent cell cultures (30). It is interesting to speculate that Tax1 and Tax2 expression in Jurkat cells may reflect the induction of aneuploidy in HTLV-infected lymphoid cells, particularly since Tax1 has been shown to bind the human mitotic arrest defective (MAD1) protein (24). The morphological features displayed by Tax1/Jurkat and Tax2/Jurkat cells are reminiscent of the large, convoluted nuclei displayed by ATL tumor cells (47) and primary lymphoid cells from HTLV-1- and -2-seropositive donors (50). These nuclear abnormalities are most likely a direct consequence of Tax1 and Tax2 expression and suggest that both oncoproteins may contribute to the induction of genomic instability exhibited by HTLV-infected cells. It will be important to delineate the exact role of Tax1 and Tax2 on the perturbation of mitosis and cytokinesis in primary lymphoid cells and cell types which normally harbor HTLV infection in humans.
It is noteworthy that stably expressing Tax cell lines have been notoriously difficult to generate by using transient-transfection methods and drug selection strategies (10, 31, 36, 38). Tax1/Jurkat cells display relatively lower levels of Tax1 than SLB-1 cells, an HTLV-1-transformed lymphoid cell line. The antiproliferative activity displayed by cells which constitutively express Tax1 and Tax2 suggests that ectopic expression of Tax confers a selective disadvantage for cell growth in culture. We are currently evaluating whether the activation of p21 accounts for the inhibition of cellular replication kinetics in Jurkat cells and whether the abnormal cellular morphology is due to defects in cellular replication or inhibition of mitosis. Although p21 transcriptional transactivation by Tax1 has previously been described, the differential upregulation of p21 expression exhibited by Tax1 in comparison to Tax2 may be an important distinguishing feature of the elevated pathogenesis associated with HTLV-1 infection. We previously showed that Tax1 suppressed hematopoiesis in transduced human hematopoietic progenitor (CD34+) cells, in contrast to Tax2, which lacked this activity (62). It was recently demonstrated that p21 is a key intracellular mediator of CD34+ cell division and maturation (7, 8, 18, 61). The marked ability of Tax1 to suppress hematopoiesis may be directly attributable to the induction of endogenous p21 in hematopoietic progenitor cells. We are currently evaluating the status of p21 expression in LV transduced CD34+ cells to fully characterize the role of the Tax oncoproteins on maturation and differentiation of hematopoietic progenitor cells. We speculate that HTLV-1 infection of CD34+ cells and suppression of hematopoiesis may play an essential role in establishing viral latency in vivo, an event which may account for the elevated pathogenesis associated with infection of HTLV-1 in contrast to infection with HTLV-2.
Tax1 and Tax2 expression inhibited apoptosis after serum deprivation in Jurkat cells, and these results are in concordance with previous reports demonstrating that Tax1 prevents apoptosis in murine fibroblasts after serum withdrawal (51). Notably, Tax1 also conferred resistance to apoptosis after incubation with the topoisomerase inhibitors CPT or ETP, a function that Tax2 did not exhibit. Interestingly, Top1 functions by aiding in chromosome condensation via the 13S condensing protein complex in the mitotic phase of the cell cycle (26). It was reported that Tax binds to the C terminus of Top1, preventing DNA binding and altering the catalytic activity of Top1 (58). It will be of interest to determine whether the direct interaction of Tax1 with Top1 accounts for mediating resistance to drug-induced apoptosis and whether Tax2 is also able to interact with Top1, particularly since topoisomerase inhibitors are chemotherapeutic agents currently in clinical use. It is interesting that the specific function of Tax1 in mediating resistance to CPT- and ETP-induced apoptosis is consistent with previous data demonstrating that Tax1 protects cells from apoptosis due to DNA damage (20). We speculate that differences in the ability of Tax1 to uniquely protect cells from apoptosis after DNA damage may be a critical distinguishing component of the association of HTLV-1 with leukemogenesis. Characterization of the activities of Tax1 and Tax2 clearly suggests that these viral proteins have both unique and distinct features in exerting protection from apoptosis. Tax1 has a more robust ability to modulate cell cycle progression and apoptosis compared to Tax2. Further delineation of the unique functions of Tax1 and Tax2 will be important in ultimately characterizing the elevated oncogenicity associated with HTLV-1 infection. Illumination of the unique roles of these viral oncoproteins in the early phases of HTLV infection, particularly in primary hematopoietic cells, will be crucial to understanding the molecular basis of HTLV-1 and HTLV-2 pathogenesis.
Acknowledgments
This study was supported by the National Institutes of Health (1R29 CA77567 and RO1 RR14324) and the Leukemia Research Foundation.
We gratefully acknowledge and thank Fatah Kashanchi for providing p21/luciferase vectors.
REFERENCES
- 1.Akagi, T., H. Ono, and K. Shimotohno. 1996. Expression of cell-cycle regulatory genes in HTLV-1-infected T-cell lines: possible involvement of Tax1 in the altered expression of cyclin D2, p18Ink4, and p21Waf1/Cip1/Sdi1. Oncogene 12:1645-1652. [PubMed] [Google Scholar]
- 2.Ariumi, Y., A. Kaida, J. Y. Lin, M. Hirota, O. Masui, S. Yamaoka, Y. Taya, and K. Shimotohno. 2000. HTLV-1 tax oncoprotein represses the p53-mediated trans-activation function through coactivator CBP sequestration. Oncogene 19:1491-1499. [DOI] [PubMed] [Google Scholar]
- 3.Blomer, U., L. Naldini, T. Kafri, D. Trono, I. M. Verma, and F. H. Gage. 1997. Highly efficient and sustained gene transfer in adult neurons with a lentivirus vector. J. Virol. 71:6641-6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buchschacher, G. L., Jr., and F. Wong-Staal. 2000. Development of lentiviral vectors for gene therapy for human diseases. Blood 95:2499-2504. [PubMed] [Google Scholar]
- 5.Burns, J. C., T. Friedmann, W. Driever, M. Burrascano, and J. K. Yee. 1993. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors: concentration to very high titer and efficient gene transfer into mammalian and nonmammalian cells. Proc. Natl. Acad. Sci. USA 90:8033-8037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cereseto, A., F. Diella, J. C. Mulloy, A. Cara, P. Michieli, R. Grassmann, G. Franchini, and M. E. Klotman. 1996. p53 functional impairment and high p21waf1/cip1 expression in human T-cell lymphotropic/leukemia virus type I-transformed T cells. Blood 88:1551-1560. [PubMed] [Google Scholar]
- 7.Cheng, T., N. Rodrigues, H. Shen, Y. Yang, D. Dombkowski, M. Sykes, and D. T. Scadden. 2000. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287:1804-1808. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, T., H. Shen, N. Rodrigues, S. Stier, and D. T. Scadden. 2001. Transforming growth factor β1 mediates cell-cycle arrest of primitive hematopoietic cells independent of p21Cip1/Waf1 or p27Kip1. Blood 98:3643-3649. [DOI] [PubMed] [Google Scholar]
- 9.Chlichlia, K., M. Busslinger, M. E. Peter, H. Walczak, P. H. Krammer, V. Schirrmacher, and K. Khazaie. 1997. ICE-proteases mediate HTLV-1 Tax-induced apoptotic T-cell death. Oncogene 14:2265-2272. [DOI] [PubMed] [Google Scholar]
- 10.Chlichlia, K., G. Moldenhauer, P. T. Daniel, M. Busslinger, L. Gazzolo, V. Schirrmacher, and K. Khazaie. 1995. Immediate effects of reversible HTLV-1 tax function: T-cell activation and apoptosis. Oncogene 10:269-277. [PubMed] [Google Scholar]
- 11.Chowdhury, I. H., A. Farhadi, X. F. Wang, M. L. Robb, D. L. Birx, and J. H. Kim. 2003. Human T-cell leukemia virus type 1 Tax activates cyclin-dependent kinase inhibitor p21Waf1/Cip1 expression through a p53-independent mechanism: inhibition of cdk2. Int. J. Cancer 107:603-611. [DOI] [PubMed] [Google Scholar]
- 12.Copeland, K. F., A. G. Haaksma, J. Goudsmit, P. H. Krammer, and J. L. Heeney. 1994. Inhibition of apoptosis in T cells expressing human T-cell leukemia virus type I Tax. AIDS Res. Hum. Retrovir. 10:1259-1268. [DOI] [PubMed] [Google Scholar]
- 13.de La Fuente, C., L. Deng, F. Santiago, L. Arce, L. Wang, and F. Kashanchi. 2000. Gene expression array of HTLV type 1-infected T cells: up-regulation of transcription factors and cell cycle genes. AIDS Res. Hum. Retrovir. 16:1695-1700. [DOI] [PubMed] [Google Scholar]
- 14.de La Fuente, C., F. Santiago, S. Y. Chong, L. Deng, T. Mayhood, P. Fu, D. Stein, T. Denny, F. Coffman, N. Azimi, R. Mahieux, and F. Kashanchi. 2000. Overexpression of p21waf1 in human T-cell lymphotropic virus type 1-infected cells and its association with cyclin A/cdk2. J. Virol. 74:7270-7283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de la Fuente, C., L. Wang, D. Wang, L. Deng, K. Wu, H. Li, L. D. Stein, T. Denny, F. Coffman, K. Kehn, S. Baylor, A. Maddukuri, A. Pumfery, and F. Kashanchi. 2003. Paradoxical effects of a stress signal on pro- and anti-apoptotic machinery in HTLV-1 Tax expressing cells. Mol. Cell Biochem. 245:99-113. [DOI] [PubMed] [Google Scholar]
- 16.Endo, K., A. Hirata, K. Iwai, M. Sakurai, M. Fukushi, M. Oie, M. Higuchi, W. W. Hall, F. Gejyo, and M. Fujii. 2002. Human T-cell leukemia virus type 2 (HTLV-2) Tax protein transforms a rat fibroblast cell line but less efficiently than HTLV-1 Tax. J. Virol. 76:2648-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feuer, G., S. A. Stewart, S. M. Baird, F. Lee, R. Feuer, and I. S. Chen. 1995. Potential role of natural killer cells in controlling tumorigenesis by human T-cell leukemia viruses. J. Virol. 69:1328-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Furukawa, Y. 1998. Cell cycle regulation of hematopoietic stem cells. Hum. Cell 11:81-92. [PubMed] [Google Scholar]
- 19.Haller, K., T. Ruckes, I. Schmitt, D. Saul, E. Derow, and R. Grassmann. 2000. Tax-dependent stimulation of G1 phase-specific cyclin-dependent kinases and increased expression of signal transduction genes characterize HTLV type 1-transformed T cells. AIDS Res. Hum. Retrovir. 16:1683-1688. [DOI] [PubMed] [Google Scholar]
- 20.Haoudi, A., R. C. Daniels, E. Wong, G. Kupfer, and O. J. Semmes. 2003. Human T-cell leukemia virus-I tax oncoprotein functionally targets a subnuclear complex involved in cellular DNA damage-response. J. Biol. Chem. 278:37736-37744. [DOI] [PubMed] [Google Scholar]
- 21.Haoudi, A., and O. J. Semmes. 2003. The HTLV-1 tax oncoprotein attenuates DNA damage induced G1 arrest and enhances apoptosis in p53 null cells. Virology 305:229-239. [DOI] [PubMed] [Google Scholar]
- 22.Iwanaga, R., K. Ohtani, T. Hayashi, and M. Nakamura. 2001. Molecular mechanism of cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. Oncogene 20:2055-2067. [DOI] [PubMed] [Google Scholar]
- 23.Jeang, K. T., S. G. Widen, O. J. t. Semmes, and S. H. Wilson. 1990. HTLV-1 transactivator protein, tax, is a trans-repressor of the human beta-polymerase gene. Science 247:1082-1084. [DOI] [PubMed] [Google Scholar]
- 24.Jin, D. Y., F. Spencer, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93:81-91. [DOI] [PubMed] [Google Scholar]
- 25.Kawata, S., Y. Ariumi, and K. Shimotohno. 2003. p21Waf1/Cip1/Sdi1 prevents apoptosis as well as stimulates growth in cells transformed or immortalized by human T-cell leukemia virus type 1-encoded tax. J. Virol. 77:7291-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimura, K., and T. Hirano. 1997. ATP-dependent positive supercoiling of DNA by 13S condensin: a biochemical implication for chromosome condensation. Cell 90:625-634. [DOI] [PubMed] [Google Scholar]
- 27.Koeffler, H. P., I. S. Chen, and D. W. Golde. 1984. Characterization of a novel HTLV-infected cell line. Blood 64:482-490. [PubMed] [Google Scholar]
- 28.Lemoine, F. J., and S. J. Marriott. 2001. Accelerated G1 phase progression induced by the human T-cell leukemia virus type I (HTLV-1) Tax oncoprotein. J. Biol. Chem. 276:31851-31857. [DOI] [PubMed] [Google Scholar]
- 29.Lewis, M. J., N. Sheehy, M. Salemi, A. M. VanDamme, and W. W. Hall. 2002. Comparison of CREB- and NF-κB-mediated transactivation by human T lymphotropic virus type II (HTLV-II) and type I (HTLV-I) tax proteins. Virology 295:182-189. [DOI] [PubMed] [Google Scholar]
- 30.Liang, M. H., T. Geisbert, Y. Yao, S. H. Hinrichs, and C. Z. Giam. 2002. Human T-lymphotropic virus type 1 oncoprotein tax promotes S-phase entry but blocks mitosis. J. Virol. 76:4022-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, Y., Y. Wang, M. Yamakuchi, S. Masuda, T. Tokioka, S. Yamaoka, I. Maruyama, and I. Kitajima. 2001. Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene 20:2514-2526. [DOI] [PubMed] [Google Scholar]
- 32.Los, M., K. Khazaie, K. Schulze-Osthoff, P. A. Baeuerle, V. Schirrmacher, and K. Chlichlia. 1998. Human T-cell leukemia virus-I (HTLV-I) Tax-mediated apoptosis in activated T cells requires an enhanced intracellular prooxidant state. J. Immunol. 161:3050-3055. [PubMed] [Google Scholar]
- 33.Low, K. G., L. F. Dorner, D. B. Fernando, J. Grossman, K. T. Jeang, and M. J. Comb. 1997. Human T-cell leukemia virus type 1 Tax releases cell cycle arrest induced by p16INK4a. J. Virol. 71:1956-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macleod, K. F., N. Sherry, G. Hannon, D. Beach, T. Tokino, K. Kinzler, B. Vogelstein, and T. Jacks. 1995. p53-dependent and -independent expression of p21 during cell growth, differentiation, and DNA damage. Genes Dev. 9:935-944. [DOI] [PubMed] [Google Scholar]
- 35.Mahieux, R., C. A. Pise-Masison, P. F. Lambert, C. Nicot, L. De Marchis, A. Gessain, P. Green, W. Hall, and J. N. Brady. 2000. Differences in the ability of human T-cell lymphotropic virus type 1 (HTLV-1) and HTLV-2 tax to inhibit p53 function. J. Virol. 74:6866-6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto, K., H. Shibata, J. I. Fujisawa, H. Inoue, A. Hakura, T. Tsukahara, and M. Fujii. 1997. Human T-cell leukemia virus type 1 Tax protein transforms rat fibroblasts via two distinct pathways. J. Virol. 71:4445-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mulloy, J. C., T. Kislyakova, A. Cereseto, L. Casareto, A. LoMonico, J. Fullen, M. V. Lorenzi, A. Cara, C. Nicot, C. Giam, and G. Franchini. 1998. Human T-cell lymphotropic/leukemia virus type 1 Tax abrogates p53-induced cell cycle arrest and apoptosis through its CREB/ATF functional domain. J. Virol. 72:8852-8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata, K., K. Ohtani, M. Nakamura, and K. Sugamura. 1989. Activation of endogenous c-fos proto-oncogene expression by human T-cell leukemia virus type I-encoded p40tax protein in the human T-cell line, Jurkat. J. Virol. 63:3220-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuveut, C., and K. T. Jeang. 2002. Cell cycle dysregulation by HTLV-1: role of the tax oncoprotein. Front. Biosci. 7:D157-D163. [DOI] [PubMed] [Google Scholar]
- 40.Neuveut, C., K. G. Low, F. Maldarelli, I. Schmitt, F. Majone, R. Grassmann, and K. T. Jeang. 1998. Human T-cell leukemia virus type 1 Tax and cell cycle progression: role of cyclin D-cdk and p110Rb. Mol. Cell. Biol. 18:3620-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicot, C., and R. Harrod. 2000. Distinct p300-responsive mechanisms promote caspase-dependent apoptosis by human T-cell lymphotropic virus type 1 Tax protein. Mol. Cell. Biol. 20:8580-8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nicot, C., R. Mahieux, S. Takemoto, and G. Franchini. 2000. Bcl-XL is up-regulated by HTLV-I and HTLV-II in vitro and in ex vivo ATLL samples. Blood 96:275-281. [PubMed] [Google Scholar]
- 43.Ohtani, K., R. Iwanaga, M. Arai, Y. Huang, Y. Matsumura, and M. Nakamura. 2000. Cell type-specific E2F activation and cell cycle progression induced by the oncogene product Tax of human T-cell leukemia virus type I. J. Biol. Chem. 275:11154-11163. [DOI] [PubMed] [Google Scholar]
- 44.Pise-Masison, C. A., K. S. Choi, M. Radonovich, J. Dittmer, S. J. Kim, and J. N. Brady. 1998. Inhibition of p53 transactivation function by the human T-cell lymphotropic virus type 1 Tax protein. J. Virol. 72:1165-1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Portis, T., J. C. Harding, and L. Ratner. 2001. The contribution of NF-κB activity to spontaneous proliferation and resistance to apoptosis in human T-cell leukemia virus type 1 Tax-induced tumors. Blood 98:1200-1208. [DOI] [PubMed] [Google Scholar]
- 46.Rivera-Walsh, I., M. Waterfield, G. Xiao, A. Fong, and S. C. Sun. 2001. NF-κB signaling pathway governs TRAIL gene expression and human T-cell leukemia virus-I Tax-induced T-cell death. J. Biol. Chem. 276:40385-40388. [DOI] [PubMed] [Google Scholar]
- 47.Rosenblatt, J. D., I. S. Chen, and W. Wachsman. 1988. Infection with HTLV-I and HTLV-II: evolving concepts. Semin. Hematol. 25:230-246. [PubMed] [Google Scholar]
- 48.Ross, T. M., A. C. Minella, Z. Y. Fang, S. M. Pettiford, and P. L. Green. 1997. Mutational analysis of human T-cell leukemia virus type 2 Tax. J. Virol. 71:8912-8917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross, T. M., M. Narayan, Z. Y. Fang, A. C. Minella, and P. L. Green. 2000. Human T-cell leukemia virus type 2 tax mutants that selectively abrogate NFκB or CREB/ATF activation fail to transform primary human T cells. J. Virol. 74:2655-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sacher, R. A., N. L. Luban, D. I. Ameti, S. Friend, G. B. Schreiber, and E. L. Murphy. 1999. Low prevalence of flower cells in USA blood donors infected with human T-lymphotrophic virus types I and II. Br. J. Haematol. 105:758-763. [DOI] [PubMed] [Google Scholar]
- 51.Saggioro, D., S. Barp, and L. Chieco-Bianchi. 2001. Block of a mitochondrial-mediated apoptotic pathway in Tax-expressing murine fibroblasts. Exp. Cell Res. 269:245-255. [DOI] [PubMed] [Google Scholar]
- 52.Santiago, F., E. Clark, S. Chong, C. Molina, F. Mozafari, R. Mahieux, M. Fujii, N. Azimi, and F. Kashanchi. 1999. Transcriptional up-regulation of the cyclin D2 gene and acquisition of new cyclin-dependent kinase partners in human T-cell leukemia virus type 1-infected cells. J. Virol. 73:9917-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmid, I., C. H. Uittenbogaart, B. Keld, and J. V. Giorgi. 1994. A rapid method for measuring apoptosis and dual-color immunofluorescence by single laser flow cytometry. J. Immunol. Methods 170:145-157. [DOI] [PubMed] [Google Scholar]
- 54.Schmitt, I., O. Rosin, P. Rohwer, M. Gossen, and R. Grassmann. 1998. Stimulation of cyclin-dependent kinase activity and G1- to S-phase transition in human lymphocytes by the human T-cell leukemia/lymphotropic virus type 1 Tax protein. J. Virol. 72:633-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semmes, O. J., F. Majone, C. Cantemir, L. Turchetto, B. Hjelle, and K. T. Jeang. 1996. HTLV-I and HTLV-II Tax: differences in induction of micronuclei in cells and transcriptional activation of viral LTRs. Virology 217:373-379. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki, T., H. Hirai, J. Fujisawa, T. Fujita, and M. Yoshida. 1993. A transactivator Tax of human T-cell leukemia virus type 1 binds to NF-κB p50 and serum response factor (SRF) and associates with enhancer DNAs of the NF-κB site and CArG box. Oncogene 8:2391-2397. [PubMed] [Google Scholar]
- 57.Suzuki, T., S. Kitao, H. Matsushime, and M. Yoshida. 1996. HTLV-1 Tax protein interacts with cyclin-dependent kinase inhibitor p16INK4A and counteracts its inhibitory activity toward CDK4. EMBO J. 15:1607-1614. [PMC free article] [PubMed] [Google Scholar]
- 58.Suzuki, T., M. Uchida-Toita, T. Andoh, and M. Yoshida. 2000. HTLV-1 tax oncoprotein binds to DNA topoisomerase I and inhibits its catalytic activity. Virology 270:291-298. [DOI] [PubMed] [Google Scholar]
- 59.Takemoto, S., J. C. Mulloy, A. Cereseto, T. S. Migone, B. K. Patel, M. Matsuoka, K. Yamaguchi, K. Takatsuki, S. Kamihira, J. D. White, W. J. Leonard, T. Waldmann, and G. Franchini. 1997. Proliferation of adult T-cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc. Natl. Acad. Sci. USA 94:13897-13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanaka, Y., M. Hayashi, S. Takagi, and O. Yoshie. 1996. Differential transactivation of the intercellular adhesion molecule 1 gene promoter by Tax1 and Tax2 of human T-cell leukemia viruses. J. Virol. 70:8508-8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taniguchi, T., H. Endo, N. Chikatsu, K. Uchimaru, S. Asano, T. Fujita, T. Nakahata, and T. Motokura. 1999. Expression of p21Cip1/Waf1/Sdi1 and p27Kip1 cyclin-dependent kinase inhibitors during human hematopoiesis. Blood 93:4167-4178. [PubMed] [Google Scholar]
- 62.Tripp, A., Y. Liu, M. Sieburg, J. Montalbano, S. Wrzesinski, and G. Feuer. 2003. Human T-cell leukemia virus type 1 Tax oncoprotein suppression of multilineage hematopoiesis of CD34+ cells in vitro. J. Virol. 77:12152-12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukahara, T., M. Kannagi, T. Ohashi, H. Kato, M. Arai, G. Nunez, Y. Iwanaga, N. Yamamoto, K. Ohtani, M. Nakamura, and M. Fujii. 1999. Induction of Bcl-xL expression by human T-cell leukemia virus type 1 Tax through NF-κB in apoptosis-resistant T-cell transfectants with Tax. J. Virol. 73:7981-7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van, P. L., K. W. Yim, D. Y. Jin, G. Dapolito, A. Kurimasa, and K. T. Jeang. 2001. Genetic evidence of a role for ATM in functional interaction between human T-cell leukemia virus type 1 Tax and p53. J. Virol. 75:396-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wrzesinski, S., R. Seguin, Y. Liu, S. Domville, V. Planelles, P. Massa, E. Barker, J. Antel, and G. Feuer. 2000. HTLV type 1 Tax transduction in microglial cells and astrocytes by lentiviral vectors. AIDS Res. Hum. Retrovir. 16:1771-1776. [DOI] [PubMed] [Google Scholar]
- 66.Yamada, T., S. Yamaoka, T. Goto, M. Nakai, Y. Tsujimoto, and M. Hatanaka. 1994. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J. Virol. 68:3374-3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yao, J., and B. Wigdahl. 2000. Human T-cell lymphotropic virus type I genomic expression and impact on intracellular signaling pathways during neurodegenerative disease and leukemia. Front. Biosci. 5:D138-D168. [DOI] [PubMed] [Google Scholar]
- 68.Yoshida, M. 1995. HTLV-1 oncoprotein Tax deregulates transcription of cellular genes through multiple mechanisms. J. Cancer Res. Clin. Oncol. 121:521-528. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida, M., and T. Suzuki. 2000. HTLV type 1 Tax oncoprotein binds to DNA topoisomerase I and inhibits its catalytic activity. AIDS Res. Hum. Retrovir. 16:1639-1645. [DOI] [PubMed] [Google Scholar]
- 70.Zehender, G., S. Varchetta, C. De Maddalena, C. Colasante, A. Riva, L. Meroni, M. Moroni, and M. Galli. 2001. Resistance to Fas-mediated apoptosis of human T-cell lines expressing human T-lymphotropic virus type-2 (HTLV-2) Tax protein. Virology 281:43-50. [DOI] [PubMed] [Google Scholar]