Abstract
The role of β-endorphin in the plasma glucose-lowering action of tetrandrine in streptozotocin-induced diabetic rats (STZ-diabetic rats) was investigated. The plasma glucose concentration was assessed by the glucose oxidase method. The enzyme-linked immunosorbent assay was used to determine the plasma level of β-endorphin-like immunoreactivity (BER). The mRNA levels of glucose transporter subtype 4 (GLUT4) in soleus muscle and phosphoenolpyruvate carboxykinase (PEPCK) in the liver of STZ-diabetic rats were detected by Northern blotting analysis. The expressed protein of GLUT4 or PEPCK was characterized by Western blotting analysis. Tetrandrine dose-dependently increased plasma BER in a manner parallel to the decrease of plasma glucose in STZ-diabetic rats. Moreover, the plasma glucose-lowering effect of tetrandrine was inhibited by naloxone and naloxonazine at doses sufficient to block opioid μ-receptors. Further, tetrandrine failed to produce plasma glucose-lowering action in opioid μ-receptor knockout diabetic mice. Bilateral adrenalectomy eliminated the plasma glucose-lowering effect and plasma BER-elevating effect of tetrandrine in STZ-diabetic rats. Both effects were abolished by treatment with hexamethonium or pentolinium at doses sufficient to block nicotinic receptors. Tetrandrine enhanced BER release directly from the isolated adrenal medulla of STZ-diabetic rats and this action was abolished by the blockade of nicotinic receptors. Repeated intravenous administration of tetrandrine (1.0 mg/kg) to STZ-diabetic rats for 3 days resulted in an increase in the mRNA and protein levels of the GLUT4 in soleus muscle, in addition to the lowering of plasma glucose. Similar treatment with tetrandrine reversed the elevated mRNA and protein levels of PEPCK in the liver of STZ-diabetic rats. The obtained results suggest that tetrandrine may induce the activation of nicotinic receptors in adrenal medulla to enhance the secretion of β-endorphin, which could stimulate opioid μ-receptors to increase glucose utilization or/and reduce hepatic gluconeogenesis to lower plasma glucose levels in STZ-diabetic rats.
Keywords: tetrandrine, opioid μ-receptors, β-endorphin, nicotinic receptors, glucose transporters subtype 4, phosphoenolpyruvate carboxykinase
Introduction
Both type 1 and type 2 diabetes are common and serious disorders across the world, resulting in considerable morbidity and mortality (1). Treatment of diabetes by insulin and/or oral drugs fails to prevent these complications in clinical practice, indicating the need for additional medication for diabetic patients.
Tetrandrine (6,6′,7,12-tetramethoxy-2,2′-dimethylberbam) is a bisbenzyl tetrahydroisoquinoline alkaloid extracted from the Chinese medicinal herb Radix Stephania tetrandrae, dry roots of Stephaniae tetrandrine S. Moore (Menispermaceae), which has been used traditionally as an analgesic and anti-hypertensive drug in Oriental countries (2). The principal chemical constituents in Radix Stephania tetrandrae are tetrandrine and fangchinoline (2). Tetrandrine has been introduced as a calcium channel blocker (3) and has a wide spectrum of pharmacological activities, including the modulation of cardiovascular disorders (4), anti-tumor (5) and anti-inflammatory effects (6). Fangchinoline is reported to be less potent than tetrandrine as a vasodilator or a calcium channel blocker (7). Tetrandrine has also been reported to have an active oxygen radical scavenging effect and antioxidant property (8). Moreover, tetrandrine has been shown to prevent the development of spontaneous diabetes in BioBreeding rats (9) and protect pancreatic islet beta cells from the injuries caused by alloxan (10). In addition, we have observed that tetrandrine has the ability to improve glucose utilization in streptozotocin-induced diabetic rats (STZ-diabetic rats), the type 1 diabetes-like animal model (11). These results indicate that tetrandrine seems helpful in the prevention and/or management of diabetes. However, the mechanism(s) of the effect of tetrandrine on glucose metabolism remains obscure.
It has been mentioned that exogenous β-endorphin induces an increase in circulating insulin in humans with or without diabetes mellitus (12). In fact, the effect of opioid on glucose homeostasis does not seem to be solely dependent on insulin. In a previous study (13), we found that β-endorphin may increase the glucose utilization via opioid μ-receptor activation resulting in the lowering of plasma glucose in STZ-diabetic rats. It seems that β-endorphin is involved in the regulation of glucose homeostasis. Therefore, the aim of this study is to clarify the role of β-endorphin in the plasma glucose-lowering action of tetrandrine in diabetic rats lacking insulin.
Materials and Methods
Rats and Mice
Male Wistar rats, weighing 200–250 g, were obtained from the Animal Center of National Cheng Kung University Medical College. Male BDF1 mice (as wild-type) and opioid μ-receptor knockout BDF1 mice (14), bred in the same animal center and aged 8–10 weeks were obtained from Professor H.H. Loh. Streptozotocin-induced diabetic rats (STZ-diabetic rats) were prepared by intravenously (i.v.) injecting STZ (60.0 mg/kg) (Sigma-Aldrich, Inc., Saint Louis, Missouri, USA) into male Wistar rats, 8–10 weeks of age. Mice with or without opioid μ-receptors also received an intraperitoneal (i.p.) injection of STZ at 50.0 mg/kg to induce diabetes according to the previous method (15). Animals were considered to be diabetic if they had plasma glucose concentrations of 20 mmol/l or greater in addition to polyuria and other diabetic features. Plasma insulin levels in STZ-diabetic rats were reduced to 1.29 ± 0.7 pmol/l (n = 8) following STZ injection, a level markedly lower than that of the normal rats (160.8 ± 3.7 pmol/l; n = 8), indicating insulin-dependent diabetes mellitus (IDDM). All the studies were carried out 2 weeks after the injection of STZ. All animal procedures were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health as well as the guidelines of the Animal Welfare Act.
Tetrandrine and Plasma Level of Glucose or β-endorphin
After fasting overnight, STZ-diabetic rats received an i.v. injection of tetrandrine (Sigma-Aldrich, Inc.) at the determined doses using tetrandrine dissolved in 70% alcohol as stock solution. In fact, the vehicle (70% alcohol:saline = 1:19) used to prepare tetrandrine solution did not modify the plasma glucose in the preliminary experiments; the basal plasma glucose was 24.2 ± 1.7 mmol/l and changed to 24.8 ± 2.4 mmol/l in the vehicle-treated group (n = 8). Thus, the effect of the vehicle on plasma glucose of STZ-diabetic rats could be ruled out. Animals were anesthetized with sodium pentobarbital (30.0 mg/kg, i.p.) and blood samples (0.1 ml) were collected from the tail vein for the measurement of plasma glucose concentrations or plasma β-endorphin-like immunoreactivity (BER). In the preliminary experiments, tetrandrine at 1.0 mg/kg was found to produce the maximal plasma glucose-lowering effect in STZ-diabetic rats 30 min after a single i.v. injection. Thus, the effects of tetrandrine on plasma glucose and plasma BER were determined using blood samples collected 30 min after the injection. STZ-diabetic rats that received a similar injection of the same volume of vehicle (distilled water containing 0.9% [w/v] NaCl) as that used to dissolve tetrandrine were used as controls. Further experiments were performed with pharmacological inhibitors (Research Biochemical Inc., Natick, MA) such as blockers of nicotinic receptors (hexamethonium or pentolinium) and opioid μ-receptor antagonists (naloxone or naloxonazine). These inhibitors were injected intravenously into fasted rats 30 min before the injection of tetrandrine.
Tetrandrine and Plasma Glucose in Opioid μ-receptor
Fasted STZ-diabetic mice with or without opioid μ-receptors were given an i.v. injection of tetrandrine at a dose of 1.0 mg/kg. After 30 min, blood samples (0.1 ml) were collected from the lower eye lid of the mice under anesthesia with pentobarbital (30.0 mg/kg, i.p.) using a chilled syringe containing 10 IU heparin.
Isolation and Incubation of Adrenal Medulla
Adrenal glands were quickly removed from the sacrificed STZ-diabetic rats and medullae were immediately dissected after removal of the cortex as described previously (16). The tissues were cut into approximately 1 mm thick slices and transferred to a glass tube fitted with a nylon mesh at the bottom to permit free interchange with the medium. The tissues were incubated for 15 min at 37°C, pH 7.4 and bubbled with a 95% O2 and 5% CO2 mixture under continuous shaking with 2 ml of modified Krebs solution (MKS) ([mmol/l]: NaCl 118, KCl 4.7, MgCl2 1.2, NaH2PO4 1.0, CaCl2 2.5, EDTA-Na 0.004, dextrose 11.1, NaHCO3 25.0 and ascorbic acid 0.11). Tissues were then transferred to fresh incubation tubes with or without nicotinic receptor antagonists at the indicated concentrations for 15 min at 37°C. They were then incubated with tetrandrine at the indicated concentrations with continuous shaking at 40 cycles/min at 37°C for 30 min. Incubation was terminated by placing the tubes on ice. The medium from each incubated sample was collected and frozen at −70°C until the β-endorphin assay was performed.
Adrenalectomized Rats
Bilateral adrenalectomy was performed in Wistar rats using the dorsal approach under pentobarbital anesthesia (30.0 mg/kg, i.p.) as described previously (17). The bilaterally adrenalectomized Wistar rats were also fed standard rat chow and 0.9% NaCl in their drinking water ad libitum. The sham-operated Wistar rats served as controls and were fed standard rat chow and water ad libitum. Animals were allowed to recover for 2 weeks after the operation. The animals appeared alert and in good health. Following recovery, diabetes was induced by an injection of STZ as described above. The effect of tetrandrine at 1.0 mg/kg was determined using blood samples collected 30 min after a single injection as described above.
Laboratory Determinations
The concentration of plasma glucose was measured by the glucose oxidase method using an analyzer (Quik-Lab, Ames, Miles Inc., Elkhart, Indiana, USA). The enzyme-linked immunosorbent assay (ELISA) for the determination of BER in plasma or in the medium incubating adrenal medulla was carried out using the commercially available kit (Peninsula Lab. Inc., CA, USA).
Determination of Gene Expression
STZ-diabetic rats were given injections of the vehicle or tetrandrine (1.0 mg/kg) every 8 h, three times a day, in the tail vein. In the preliminary experiments, tetrandrine was found to significantly modify mRNA and protein levels of glucose transporters subtype 4 (GLUT4) and hepatic phosphoenolpyruvate carboxykinase (PEPCK) in STZ-diabetic rats after a 3-day treatment. Thus, animals were sacrificed after 3 days of treatment. Normal rats received a similar treatment of the vehicle and were used as controls. After the final treatment, animals were sacrificed without fasting. Liver and soleus muscle were immediately removed, frozen in liquid nitrogen and stored at −70°C for Northern and Western blot analysis. Blood samples were also collected from the tail vein of these rats before sacrificing to evaluate the change in plasma glucose concentrations.
Northern Blot Analysis
Total RNA was extracted from soleus muscle or liver of the experimental animals using the UltraspecTM-II RNA extraction system (Bioteck, Houston, TE, USA). For Northern blot analysis, RNA (20 μg) was denatured in a solution containing 2.2 mmol/l formaldehyde and 50% formamide (v/v) by heating at 55°C for 15 min. Aliquots of total RNA were size-fractionated in a 1.2% agarose/formaldehyde gel. Gels were stained with ethidium bromide to identify the position of the 18S and 28S rRNA subunits and to confirm that equivalent amounts of undegraded RNA had been loaded. The RNA was transferred to a Hybond-N membrane (Amersham, Bucks, UK). GLUT4 and PEPCK mRNA levels were detected using full-length cDNA probes, radioactively labeled by the random primer method and hybridized under stringent conditions. Intensity of the mRNA bands on the blot was quantified by scanning densitometry (Hoefer, San Francisco, CA, USA). The response of β-actin was used as an internal standard.
Western Blot Analysis
After homogenization of the liver and soleus muscle using a glass/Teflon homogenizer, the homogenates (50 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blot analysis was performed using either anti-rat GLUT4 antibody (1:1000) (Genzyme Diagnostics, Cambridge, USA) in soleus muscle or anti-rat PEPCK antibody (1:1000) in liver. The blots were incubated with mouse monoclonal β-tubulin antibody (1:500) (Zymed Laboratories, Inc., San Francisco, California, USA) as a control to ensure that equal amounts of protein were loaded into each lane of the gel. Blots were incubated with the appropriate peroxidase-conjugated secondary antibodies. Following removal of the secondary antibody, blots were washed as described earlier and developed by autoradiography using the ECL-Western blotting system. Densities of the obtained immunoblots at 45 KDa for GLUT4, 69.5 KDa for PEPCK and 50 KDa for β-tubulin were quantified using a laser densitometer.
Statistical Analysis
The plasma glucose-lowering activity was obtained from the animals that received tetrandrine. Results of plasma glucose-lowering activity were calculated as percentage decrease of the initial value according to the formula: (Gi-Gt)/Gi × 100 where Gi was the initial glucose concentration and Gt was the plasma glucose concentration after treatment with tetrandrine.
Data are expressed as mean ± SE for the number (n) of animals in the group as indicated in the tables and figures. Repeated measures analysis of variance (ANOVA) was used to analyze the changes in plasma glucose and other parameters. The Dunnett range post-hoc comparisons were used to determine the source of significant differences where appropriate. The concentration that produced 50% of the maximum effect (EC50) was obtained from non-linear regression analysis. A P-value <0.05 was considered statistically significant.
Results
Tetrandrine and Plasma Glucose Concentration and Plasma BER Levels
Thirty minutes after treatment, the plasma glucose-lowering activity in STZ-diabetic rats was 7.5 ± 1.1%, 15.1 ± 1.4% and 18.4 ± 1.5% in STZ-diabetic rats receiving i.v. injections of tetrandrine at 0.05 mg/kg, 0.1 mg/kg and 0.5 mg/kg, respectively. The maximal effect (23.8 ± 1.6%) was achieved using 1.0 mg/kg of tetrandrine. Increasing the dose of tetrandrine to 1.5 mg/kg had no further effect. The basal plasma BER level in STZ-diabetic rats was 43.2 ± 5.5 pg/ml. The time course of the effect of tetrandrine on the plasma glucose and the secretion of the plasma BER from STZ-diabetic rats was preliminarily determined. Similar to the lowering of plasma glucose, plasma BER level was raised approximately 10 min later in STZ-diabetic rats receiving an i.v. injection of tetrandrine at a dose of 1.0 mg/kg. The action of tetrandrine achieved maximal levels at 30 min, which was used as the optimal time in the following experiments. A dose-dependent elevation of plasma BER level was observed in the same group of the STZ-diabetic rats receiving tetrandrine (Fig. 1) and this action occurred in parallel with the lowering of plasma glucose. Tetrandrine at a dose of 1.0 mg/kg increased the plasma BER level to 95.7 ± 6.2 pg/ml in STZ-diabetic rats and no further increase in plasma BER was observed at higher tetrandrine concentrations. Thus, 1.0 mg/kg of tetrandrine was employed in subsequent experiments.
Figure 1.
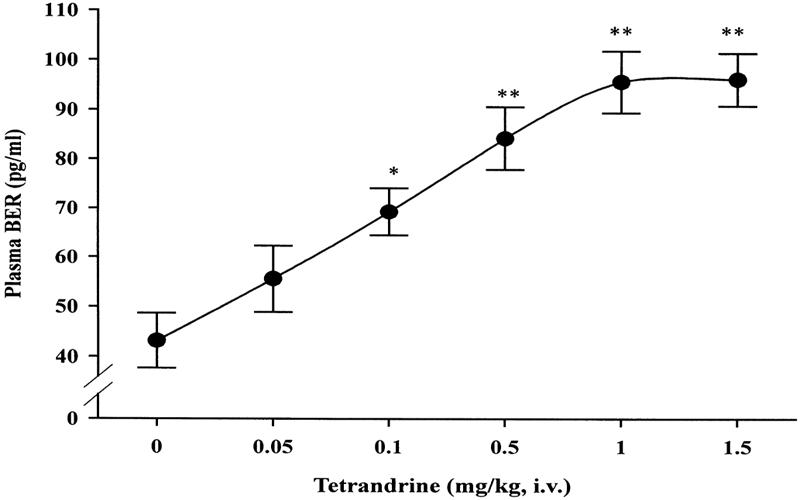
The effect of tetrandrine on plasma BER level in STZ-diabetic rats. Values (means ± SE) were obtained from each group of 8 animals receiving an intravenous (i.v.) injection of tetrandrine at the indicated dose. *P < 0.05 and **P < 0.01 vs data from animals treated with the vehicle (0) administered in the same volume.
Plasma glucose concentrations of fasting STZ-diabetic rats were also significantly (P < 0.01) decreased to 18.5 ± 1.4 mmol/l after repeated treatment with tetrandrine (1.0 mg/kg) for 3 days as compared with concentrations in vehicle-treated STZ-diabetic rats (24.8 ± 1.1 mmol/l). The plasma glucose-lowering activity was 25.4 ± 2.1% in STZ-diabetic rats after repeated treatments with tetrandrine at the effective dose. Moreover, the 3-day treatment with tetrandrine (1.0 mg/kg) did not influence the feeding behavior and/or body weight of STZ-diabetic rats.
Opioid μ-receptor Antagonists Affects Tetrandrine-induced Plasma Glucose-lowering Activity
Table 1 shows the dose-dependent action of naloxone and naloxonazine to inhibit the plasma glucose-lowering activity of tetrandrine in STZ-diabetic rats. In the presence of 10.0 μg/kg naloxone, the plasma glucose concentration in STZ-diabetic rats treated with 1.0 mg/kg tetrandrine was 23.8 ± 2.8 mmol/l, which was not statistically different from the basal level (24.2 ± 1.7 mmol/l). As with naloxone, pretreatment with naloxonazine (10.0 μg/kg) prevented the ability of tetrandrine (1.0 mg/kg) to lower the elevated plasma glucose concentrations in STZ-diabetic rats. The plasma glucose level in the naloxonazine (10.0 μg/kg)-pretreated group was 24.0 ± 2.5 mmol/l, which was near the basal level. Moreover, both naloxone and naloxonazine at the highest doses did not affect the basal plasma glucose concentration in STZ-diabetic rats.
Table 1.
Effects of opioid μ-receptor antagonists on tetrandrine-induced reduction in plasma glucose levels in STZ-diabetic rats
| Plasma glucose (mmol/l) | |
|---|---|
| n | 7 |
| Basal | 24.2 ± 1.7 |
| Tetrandrine (1.0 mg/kg, i.v.) | |
| + Vehicle | 18.0 ± 2.1** |
| + Naloxone (μg/kg, i.v.) | |
| 1.0 | 18.9 ± 1.9** |
| 5.0 | 20.5 ± 2.5 |
| 10.0 | 23.8 ± 2.8 |
| + Naloxonazine (μg/kg, i.v.) | |
| 1.0 | 19.3 ± 2.2* |
| 5.0 | 22.6 ± 1.5 |
| 10.0 | 24.0 ± 2.5 |
| Naloxone (10.0 μg/kg, i.v.) | 23.2 ± 2.6 |
| Naloxonazine (10.0 μg/kg, i.v.) | 23.7 ± 2.3 |
Data are means ± SE. Basal level shows the value from fasted animals treated with vehicle.
*P < 0.05 and
**P < 0.01 compared with the basal value, respectively.
Change in Tetrandrine-induced Plasma Glucose-Lowering Activity in Opioid μ-receptors
As shown in Fig. 2, the plasma glucose concentration of opioid μ-receptor knockout diabetic mice was not modified by tetrandrine (1.0 mg/kg) treatment (from 25.2 ± 1.8 mmol/l to 24.0 ± 2.2 mmol/l; P > 0.05). However, similar treatment with tetrandrine (1.0 mg/kg) significantly (P < 0.05) lowered the plasma glucose concentration from 23.9 ± 2.2 mmol/l to 18.2 ± 1.9 mmol/l in diabetic mice with opioid μ-receptors. The plasma glucose-lowering activity of tetrandrine in these wild-type diabetic mice was approximately 23.8 ± 2.1%, similar to the activity in STZ-diabetic rats.
Figure 2.
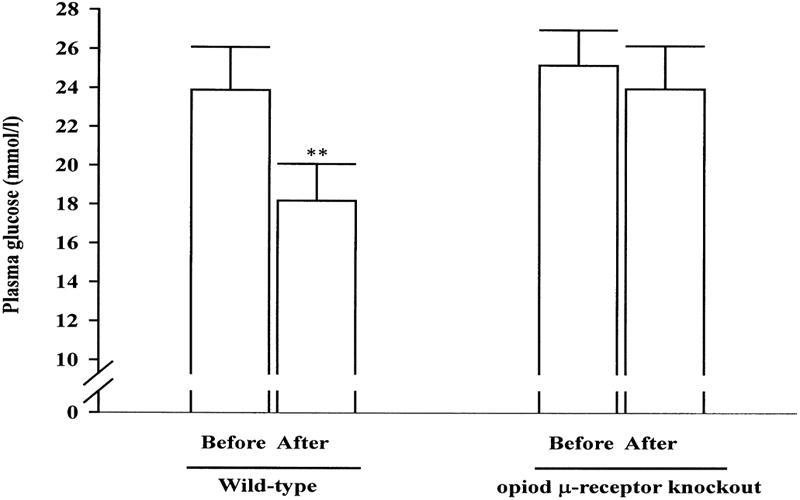
Effects of tetrandrine (1.0 mg/kg, i.v.) on plasma glucose concentrations in opioid μ-receptor knockout mice and wild-type controls. Values (means ± SE) were obtained from each group of 7 animals. **P < 0.01 vs data obtained from animals before treatment in each group.
Tetrandrine-induced Actions in STZ-diabetic Rats by Bilateral Adrenalectomy
Two weeks after bilateral adrenalectomy, there were no significant differences in the basal plasma levels of glucose between adrenalectomized STZ-diabetic rats and sham-operated control rats (Table 2). The basal plasma BER was not significantly different in STZ-diabetic rats that received adrenalectomy as compared with the sham-operated group; these results are consistent with our previous data (18). However, both the decrease in plasma glucose and the increase in plasma BER induced by tetrandrine (1.0 mg/kg) were not observed in STZ-diabetic rats with bilateral adrenalectomy, while these effects persisted in the sham-operated STZ-diabetic rats (Table 2).
Table 2.
Effect of adrenalectomy on the tetrandrine-induced changes in plasma concentrations in glucose and BER in STZ-diabetic rats
| Adrenalectomized group | Sham-operated group | |
|---|---|---|
| n | 8 | 8 |
| Plasma glucose (mmol/l) | ||
| Basal | 23.8 ± 2.1 | 24.2 ± 1.8 |
| Vehicle | 23.6 ± 2.3 | 23.9 ± 2.3 |
| Tetrandrine (1.0 mg/kg, i.v.) | 23.4 ± 1.8 | 18.4 ± 1.9** |
| Plasma BER (pg/ml) | ||
| Basal | 43.1 ± 4.7 | 39.9 ± 3.7 |
| Vehicle | 42.6 ± 4.3 | 41.8 ± 4.1 |
| Tetrandrine (1.0 mg/kg, i.v.) | 6.1 ± 3.8 | 94.2 ± 4.9** |
Data are means ± SE. Basal level shows the value from fasted animals without treatment.
**P < 0.01 vs basal value in each group.
Tetrandrine Increases the Secretion of BER from Isolated Adrenal Medulla
The spontaneous secretion of BER from the isolated adrenal medulla of STZ-diabetic rats was 95.5 ± 7.5 pg/mg protein. The amount of BER in the medium increased significantly after incubation with tetrandrine in a concentration-dependent manner from 0.001 μmol/l to 10.0 μmol/l (Fig. 3). Tetrandrine at 1.0 μmol/l increased the amount of BER in the medium to 167.4 ± 6.3 pg/mg protein and no further stimulatory effect was observed at higher tetrandrine concentrations (Fig. 3). The EC50 of tetrandrine to increase BER secretion from rat adrenal medulla was approximately 2.6 nmol/l.
Figure 3.
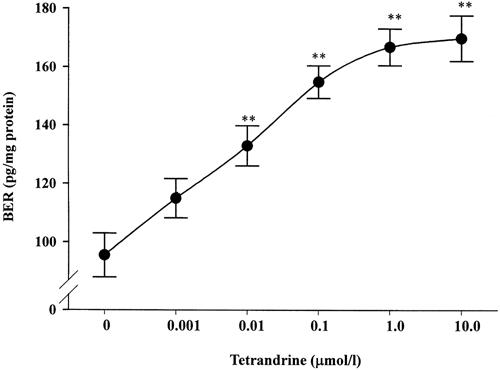
Effect of tetrandrine on BER secretion from isolated adrenal medulla of STZ-diabetic rats. Results expressed as pg/mg protein are the means ± SE of 7 determinations. *P < 0.05 and **P < 0.01 vs data from samples treated with MKS (0), respectively.
Nicotinic Receptor Antagonists on Tetrandrine-induced Plasma Glucose-lowering Activity
Table 3 shows that the increase in plasma BER by tetrandrine in STZ-diabetic rats was progressively attenuated in a dose-dependent manner by hexamethonium or pentolinium given 30 min before the injection of tetrandrine (1.0 mg/kg). Pretreatment with hexamethonium or pentolinium at 7.5 mg/kg completely abolished the activity of tetrandrine. Moreover, hexamethonium or pentolinium at the effective dose of 7.5 mg/kg failed to influence the basal plasma BER level in STZ-diabetic rats.
Table 3.
Effects of nicotinic receptor antagonists on the tetrandrine-induced changes in BER and glucose concentrations in plasma of STZ-diabetic rats
| Plasma glucose (mmol/l) | Plasma BER (pg/ml) | |
|---|---|---|
| n | 8 | 8 |
| Basal | 24.8 ± 1.5 | 39.3 ± 2.5 |
| Tetrandrine (1.0 mg/kg, i.v.) | ||
| + Vehicle | 18.7 ± 1.8** | 96.1 ± 5.8** |
| + Hexamethonium (mg/kg, i.v.) | ||
| 2.5 | 20.4 ± 2.3* | 90.3 ± 4.8** |
| 5.0 | 22.5 ± 1.9 | 70.2 ± 4.6* |
| 7.5 | 23.8 ± 1.6 | 42.0 ± 5.7 |
| + Pentolinium (mg/kg, i.v.) | ||
| 2.5 | 21.0 ± 2.0 | 85.2 ± 5.3** |
| 5.0 | 23.1 ± 1.8 | 62.5 ± 6.0* |
| 7.5 | 24.2 ± 1.7 | 39.3 ± 5.2 |
| Hexamethonium (7.5 mg/kg, i.v.) | 24.6 ± 2.4 | 38.5 ± 4.9 |
| Pentolinium (7.5 mg/kg, i.v.) | 25.1 ± 2.9 | 37.2 ± 5.1 |
Data are means ± SE. Basal level shows the value from fasted animals treated with vehicle.
*P < 0.05 and
**P < 0.01 compared with basal value, respectively.
Similar to the effect on plasma BER, the plasma glucose-lowering activity of tetrandrine at 1.0 mg/kg was decreased in a dose-dependent manner in the presence of hexamethonium. Pretreatment with hexamethonium at the highest dose (7.5 mg/kg) completely abolished the plasma glucose-lowering activity of tetrandrine. Similarly, the plasma glucose-lowering activity of tetrandrine (1.0 mg/kg) in STZ-diabetic rats was attenuated by pretreatment with pentolinium. However, no change in plasma glucose concentration was found in STZ-diabetic rats that received either antagonist alone at maximal doses (Table 3).
Nicotinic Receptor Antagonists Blocks Tetrandrine-stimulated BER Secretion from Isolated Adrenal Medulla of STZ-diabetic Rats
Table 4 shows that both hexamethonium and pentolinium blocked the tetrandrine-stimulated BER secretion from isolated rat adrenal medulla in a concentration-dependent manner. Pre-incubation with hexamethonium at 1.0 μmol/l reduced BER secretion induced by 1.0 μmol/l tetrandrine from 160.7 ± 6.1 to 95.7 ± 6.2 pg/mg protein. This was not different from the basal value (90.2 ± 5.8 pg/mg protein) obtained from vehicle-treated samples. Further, pentolinium at 1.0 μmol/l reversed the increase in BER secretion stimulated by tetrandrine (1.0 μmol/l) to the basal level. However, hexamethonium or pentolinium, at a concentration (1.0 μmol/l) sufficient to completely block nicotinic receptors, did not modify the spontaneous secretion of BER from the isolated adrenal medulla of STZ-diabetic rats (Table 4).
Table 4.
Effects of nicotinic receptor antagonists on tetrandrine-induced changes in BER in isolated adrenal medulla of STZ-diabetic rats
| BER (pg/mg protein) | |
|---|---|
| n | 7 |
| Basal | 90.2 ± 5.8 |
| Tetrandrine (1.0 μmol/l) | |
| + Vehicle | 160.7 ± 6.1** |
| + Hexamethonium (μmol/l) | |
| 0.01 | 140.7 ± 9.5** |
| 0.1 | 121.2 ± 7.3* |
| 1.0 | 95.7 ± 6.2 |
| + Pentolinium (μmol/l) | |
| 0.01 | 133.5 ± 8.3** |
| 0.1 | 111.9 ± 6.7* |
| 1.0 | 91.3 ± 7.9 |
| Hexamethonium (1.0 μmol/l) | 89.8 ± 6.1 |
| Pentolinium (1.0 μmol/l) | 87.1 ± 8.2 |
Data are means ± SE.
*P < 0.05 and
**P < 0.01 compared with basal value obtained from samples only treated with vehicle (MKS), respectively.
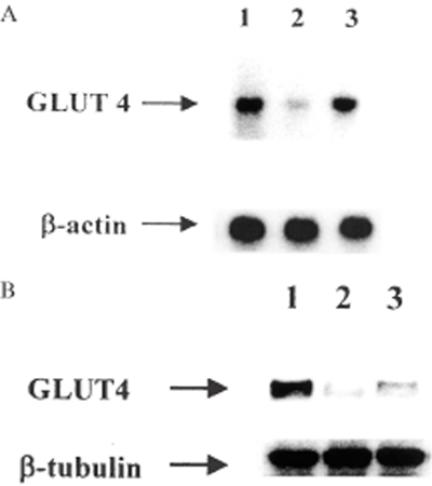
Tetrandrine Elevates the mRNA and Protein Levels of GLUT4 in Soleus Muscle
Similar to our previous study (Cheng et al., 2001b), the mRNA level of GLUT4 in isolated soleus muscle from vehicle-treated STZ-diabetic rats was approximately 45% of that of vehicle-treated normal rats (Fig. 4). Repeated treatment of STZ-diabetic rats with tetrandrine (1.0 mg/kg) for 3 days resulted in an elevation of GLUT4 mRNA level in soleus muscle to a level approximately 77% of that of vehicle-treated normal rats. However, similar treatment with tetrandrine (1.0 mg/kg) in normal rats did not (P > 0.05) modify the mRNA level of GLUT4 as compared with vehicle-treated normal rats (data not shown).
Figure 4.
A: Representative response of mRNA level for GLUT4 or β-actin in soleus muscle isolated from normal or STZ-diabetic rats receiving repeated treatment with tetrandrine (1.0 mg/kg) or the same volume of vehicle three times a day for 3 days. B: Identification of protein level of GLUT 4 or β-tubulin using immunoblot analysis. Lanes show vehicle-treated normal rats (lane 1), vehicle-treated STZ-diabetic rats (lane 2) and tetrandrine-treated STZ-diabetic rats (lane 3).
The protein level of GLUT4 in soleus muscle of vehicle-treated STZ-diabetic rats was significantly reduced to approximately 50% of that of the vehicle-treated normal rats (Fig. 4). Similar to the effect on mRNA level, repeated oral treatment with tetrandrine (1.0 mg/kg) significantly elevated the protein level of GLUT4 in soleus muscle of STZ-diabetic rats to a level approximately 82% of that of vehicle-treated normal rats. However, similar treatment with tetrandrine (1.0 mg/kg) in normal rats did not (P > 0.05) modify the mRNA and protein level of GLUT4 in isolated soleus muscle as compared with the vehicle-treated normal rats (data not shown). Quantification of the mRNA and protein level for GLUT4 are shown in Table 5.
Table 5.
Quantification of the mRNA and protein levels for GLUT4 in isolated soleus muscle and hepatic PEPCK from STZ-diabetic rats repeatedly treated with tetrandrine
| Normal rats, vehicle-treated | STZ-diabetic rats | ||
|---|---|---|---|
| Vehicle-treated | Tetrandrine-treated | ||
| n | 6 | 6 | 6 |
| GLUT4 (arbitrary units) | |||
| mRNA/β-actin | 1.63 ± 0.05 | 0.72 ± 0.06** | 1.25 ± 0.05* |
| Protein/β-tubulin | 1.49 ± 0.04 | 0.75 ± 0.05** | 1.23 ± 0.06* |
| PEPCK (arbitrary units) | |||
| mRNA/β-actin | 1.31 ± 0.06 | 2.95 ± 0.08** | 1.95 ± 0.07* |
| Protein/β-tubulin | 1.22 ± 0.04 | 2.85 ± 0.06** | 1.77 ± 0.06* |
Data are means ± SE.
*P < 0.05 and
**P < 0.01 compared with vehicle-treated normal rats.
Tetrandrine Elevates the mRNA and Protein Levels of Hepatic PEPCK
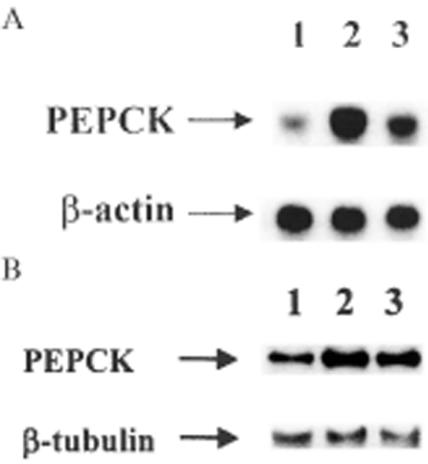
Figure 5 shows that the mean level of mRNA encoding PEPCK was elevated nearly 2.3-fold in untreated STZ-diabetic rats as compared with normal rats; this is consistent with our previous data (19). This increase in the mRNA level in the liver of STZ-diabetic rats was inhibited to about 66% of the vehicle-treated STZ-diabetic rats by repeated treatment with tetrandrine (1.0 mg/kg), although the value was still higher than that of vehicle-treated normal rats. Similar treatment with tetrandrine (1.0 mg/kg) in normal rats did not (P > 0.05) modify the mRNA level of hepatic PEPCK as compared with the vehicle-treated normal rats (data not shown).
Figure 5.
A: Representative response of mRNA level for PEPCK or β-actin in liver isolated from normal or STZ-diabetic rats receiving repeated treatment with tetrandrine (1.0 mg/kg) or the same volume of vehicle three times daily for 3 days. B: Identification of protein level of PEPCK or β-tubulin using immunoblot analysis. Lanes show vehicle-treated normal rats (lane 1), vehicle-treated STZ-diabetic rats (lane 2) and tetrandrine-treated STZ-diabetic rats (lane 3).
In parallel with the data from mRNA, the protein level of PEPCK in the liver of vehicle-treated STZ-diabetic rats was approximately 2.3-fold of the vehicle-treated normal rats. Repeated treatment of STZ-diabetic rats with tetrandrine resulted in a marked reduction in the protein level of PEPCK to approximately 60% of that of vehicle-treated STZ-diabetic rats (Fig. 5). Meanwhile, the hepatic PEPCK protein levels in normal rats pretreated with tetrandrine were unchanged when compared with those in the vehicle-treated group (data not shown). Quantification of the protein level for PEPCK is shown in Table 5.
Discussion
In the present study, we found that tetrandrine can dose-dependently increase the plasma BER in STZ-diabetic rats in a manner parallel to the lowering of plasma glucose as mentioned in our previous report (11). In STZ-diabetic rats, the deficiency of insulin has been documented (20). In the present study, the plasma insulin level in STZ-diabetic rats was only approximately 1/120 of that in normal rats. Thus, mediation of endogenous insulin is negligible in this STZ-diabetic rat model. In fact, plasma glucose-lowering activity is not entirely dependent on insulin regulation; for example, physical exercise is traditionally considered beneficial in the treatment of diabetes (21). In addition, our previous reports (16,18,19,22) indicated that activation of opioid μ-receptors plays a role in the regulation of glucose metabolism during an insulin-deficient state. The physiological actions of endogenous β-endorphins are mediated in part by opioid μ-receptors (23). Therefore, an elevation of endogenous β-endorphin that can activate the opioid receptors seems related to the plasma glucose-lowering activity of tetrandrine in STZ-diabetic rats.
Although β-endorphin is released along with adrenocorticotrophic hormone from the pituitary gland (24), the adrenal gland is also a source of β-endorphin (25,26). Our previous reports have demonstrated that secretion of opioids from the adrenal gland is associated with a decrease in plasma glucose in STZ-diabetic rats (16,18,22), which is consistent with the view that pituitary gland-independent release of endogenous opioids is operative in other organs (25,26). In an attempt to identify the adrenal gland as the source of tetrandrine-induced release of β-endorphin, adrenalectomy was performed in STZ-diabetic rats. We found that the plasma glucose-lowering activity of tetrandrine was abolished following bilateral adrenalectomy in STZ-diabetic rats. Moreover, no increase in plasma BER was observed in adrenalectomized diabetic rats that received tetrandrine at the effective doses. Thus, secretion of endogenous β-endorphin from the adrenal gland is likely to be responsible for the plasma glucose-lowering action of tetrandrine in STZ-diabetic rats.
Splanchnic nerve stimulation increases the release of opioids from the adrenal gland into the adrenal vein (27,28). The release of opioids appears to be mediated by a cholinergic nicotinic receptor (27,28). In the presence of hexamethonium or pentolinium, antagonists specific for the nicotinic receptor, the plasma glucose-lowering activity of tetrandrine was blocked in a dose-dependent manner. Moreover, the increase in plasma BER by tetrandrine was sensitive to both nicotinic receptor antagonists. Therefore, mediation of the adrenal nicotinic receptor activation seems responsible for the increase in endogenous β-endorphin secretion by tetrandrine. The direct effect of tetrandrine on the secretion of β-endorphin was also characterized using isolated adrenal medulla. We found that tetrandrine enhanced BER secretion from isolated adrenal medulla of STZ-diabetic rats in a concentration-dependent manner. In addition, hexamethonium or pentolinium inhibited the tetrandrine-stimulated secretion of BER, indicating that tetrandrine may enhance β-endorphin secretion in the adrenal medulla via an activation of nicotinic receptors. These results are consistent with the finding that nicotinic receptors play a role in the regulation of adrenal β-endorphin release (27,28).
Recently, we observed that activation of opioid μ-receptors in peripheral tissues lowers plasma glucose by improving glucose utilization during the insulin deficient state (19). The response to tetrandrine in STZ-diabetic rats was inhibited by blockade of opioid μ-receptors using naloxone or naloxonazine. In addition, we employed opioid μ-receptor knockout mice receiving STZ to confirm the role of opioid μ-receptors in the action of tetrandrine. In contrast to wild-type diabetic mice having opioid μ-receptors, the plasma glucose-lowering activity of tetrandrine was depleted in opioid μ-receptor knockout diabetic mice. These data suggest that opioid μ-receptors play a role in the plasma glucose-lowering activity of tetrandrine under the insulin deficient state.
In diabetes, elevation of blood glucose is a consequence of increased hepatic glucose output together with reduced peripheral glucose utilization (29). Skeletal muscle is a major site of glucose disposal (30). Glucose transportation, which depends on insulin-stimulated translocation of glucose carriers to the cell membrane, is the rate-limiting step in carbohydrate metabolism of skeletal muscle (31). A family of glucose transporters (GLUT) mediates glucose transport across the cell membrane, and the subtype named GLUT4 is predominant in skeletal muscle (32). Reduction in insulin-mediated glucose uptake resulting from decreased expression of GLUT4 mRNA and protein in diabetes has been reported (33). In a previous study, we found that the plasma glucose-lowering activity of tetrandrine was associated with the enhancement of glucose uptake and glycogen synthesis in STZ-diabetic rats (11). Moreover, we have demonstrated that endogenous β-endorphin via the activation of the opioid μ-receptors is a positive regulator of glucose utilization and a negative modulator of hepatic gluconeogenesis in the insulin deficient state (19,22). In STZ-diabetic rats, plasma glucose levels were reduced by a 3-day repeated treatment with tetrandrine. Since long-term exposure is required for the activation of mRNA levels (34), GLUT4 gene expression was examined in STZ-diabetic rats that received a 3-day repeated treatment with tetrandrine. We found that both the mRNA and protein levels of GLUT4 were increased by tetrandrine after repeated injections for 3 days. Thus, it is reasonable to speculate that an increase in the gene expression of GLUT4 may contribute to the enhancement of glucose utilization after repeated tetrandrine treatment. It has been documented that phospholipase C (PLC) and protein kinase C (PKC) play a key role in the signals of opioid μ-receptors (23). Moreover, it has been indicated that PKC is involved in the rate-limiting step in GLUT4 mRNA expression (32). Therefore, the PLC-PKC pathway is related to the signals of opioid μ-receptors in the regulation of GLUT4 gene expression although an understanding of the detailed mechanism needs further investigations.
Mammalian cells store glycogen in the liver for using it in the production of glucose 6-phosphate in glycolysis (35). PEPCK, which catalyzes a regulatory step in gluconeogenesis, is one of the key enzymes in hepatic carbohydrate metabolism (36). Studies on diabetic animals have shown that augmented gluconeogenesis is a major factor in the increased plasma glucose that appears in the fasting and postabsorptive states (29). In the present study, the enhanced gene expression of PEPCK resulting in increases in mRNA and protein levels in diabetic rats was suppressed by a 3-day repeated treatment with tetrandrine. Thus, the decrease in PEPCK appeared to be also related to the plasma glucose-lowering activity of tetrandrine. Since gene expression of PEPCK in liver is regulated by a number of hormones (36), further experiments are necessary to identify the hormones involved in the effect of tetrandrine on the hepatic gene expression of PEPCK. Opioid μ-receptor activation might act as a negative regulator modifying the gene expression of PEPCK in the insulin deficient state. In fact, it has been mentioned that signals for insulin to inhibit PEPCK expression did not mediate through the PKC pathways (36). Signals for opioid μ-receptors to regulate the hepatic PEPCK gene expression need to be clarified in the future.
Long-term treatment of dogs with tetrandrine at a higher oral dose of 40.0 mg/kg may induce focal necrosis of liver cells (37). However, the beneficial effect of tetrandrine at an oral dose of 10 mg/kg per day on experimental hepatic fibrosis induced by bile duct ligation and scission in rats has also been documented (38). In the present study, the dose of tetrandrine effective in lowering the plasma glucose or increasing the plasma BER in STZ-diabetic rats is less than that required to produce these effects in liver (37,38). A 3-day repeated treatment with tetrandrine did not lead to toxicity in STZ-diabetic rats. Thus, tetrandrine may be useful in the future for therapeutic control of type 1 diabetes.
In conclusion, our results suggest that activation of nicotinic receptors in the adrenal medulla by tetrandrine may enhance the secretion of endogenous β-endorphin from the adrenal gland of STZ-diabetic rats. The plasma glucose-lowering activity of tetrandrine was mediated by the release of β-endorphin to stimulate opioid μ-receptors, thereby increasing glucose utilization in peripheral tissues by an increase in GLUT4 gene expression and/or an attenuation of elevated hepatic PEPCK gene expression. Therefore, an improvement in glucose utilization in peripheral tissues and the decline of hepatic gluconeogenesis resulting in the decrease of plasma glucose by tetrandrine in STZ-diabetic rats is mainly mediated by endogenous β-endorphin.
Acknowledgments
We thank Professor HH Loh (Department of Pharmacology, University of Minnesota Medical School, Minneapolis, USA) for the kind supply of opioid μ-receptor knockout mice and the wild-type mice. Thanks are also due to Professor RW Hanson (Department of Biochemistry, School of Medicine, Case Western Reserve University, Cleveland, Ohio, USA), Professor C Makepeace (Department of Cell Biology and Physiology, School of Medicine, Washington University, St. Louis, Missouri, USA) and Professor SS Liu (Department of Microbiology and Immunology, National Cheng Kung University, Tainan City, Taiwan, ROC) for kindly providing us the plasmid containing cDNAs. Moreover, the kind donation of antibodies specific to PEPCK from Professor DK Granner (Department of Molecular Physiology and Biophysics, Vanderbilt University School of Medicine, Nashville, Tennessee, USA) is acknowledged. The present study is supported in part by a grant from the National Science Council (NSC90–2320-B006–039) of the Republic of China.
References
- 1.Lopez-Candales A. Metabolic syndrome X: a comprehensive review of the pathophysiology and recommended therapy. J Med. 2001;32:283–300. [PubMed] [Google Scholar]
- 2.Sutter MC, Wang YX. Recent cardiovascular drugs from Chinese medicinal plants. Cardiovasc Res. 1993;27:1891–1901. doi: 10.1093/cvr/27.11.1891. [DOI] [PubMed] [Google Scholar]
- 3.Felix JP, King VF, Shevell JL, Garcia ML, Kaczorowski GJ, Bick IR, et al. Bis(benzylisoquinoline) analogs of tetrandrine block L-type calcium channels: evidence for interaction at the diltiazem-binding site. Biochemistry. 1992;31:11793–11800. doi: 10.1021/bi00162a017. [DOI] [PubMed] [Google Scholar]
- 4.Kwan CY, Achike FI. Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: cardiovascular effects and mechanisms of action. Acta Pharmacol Sin. 2002;23:1057–1068. [PubMed] [Google Scholar]
- 5.Lee JH, Kang GH, Kim KC, Kim KM, Park DI, Choi BT, et al. Tetrandrine-induced cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Int J Oncol. 2002;21:1239–1244. [PubMed] [Google Scholar]
- 6.Shen YC, Chen CF, Wang SY, Sung YJ. Impediment to calcium influx and reactive oxygen production accounts for the inhibition of neutrophil Mac-1 up-regulation and adhesion by tetrandrine. Mol Pharmacol. 1999;55:186–193. doi: 10.1124/mol.55.1.186. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Zhang YH, Oh KW, Ahn HY. Vasodilating and hypotensive effects of fangchinoline and tetrandrine on the rat aorta and the stroke-prone spontaneously hypertensive rat. J Ethnopharmacol. 1997;58:117–123. doi: 10.1016/s0378-8741(97)00092-5. [DOI] [PubMed] [Google Scholar]
- 8.Cao ZF. Scavenging effect of tetrandrine of active oxygen radicals. Planta Med. 1996;62:413–414. doi: 10.1055/s-2006-957928. [DOI] [PubMed] [Google Scholar]
- 9.Lieberman I, Lentz DP, Trucco GA, Seow WK, Thong YH. Prevention by tetrandrine of spontaneous development of diabetes mellitus in BB rats. Diabetes. 1992;41:616–619. doi: 10.2337/diab.41.5.616. [DOI] [PubMed] [Google Scholar]
- 10.Sun GR, Zhang GF, Wei YJ, Yang DS, Zhang JX, Tian ZB. Protective effect of tetrandrine on pancreatic islet cells damaged by alloxan in rats. Acta Pharmacol Sin. 1994;46:161–167. [PubMed] [Google Scholar]
- 11.Chen WC, Hayakawa S, Yamamoto T, Huang LW, Liu IM, Cheng JT. The plasma glucose-lowering action of tetrandrine in streptozotocin-induced diabetic rats (submitted) [DOI] [PubMed]
- 12.Curry DL, Li CH. Stimulation of insulin secretion by beta-endorphin (1–27 and 1–31) Life Sci. 1987;40:2053–2058. doi: 10.1016/0024-3205(87)90097-x. [DOI] [PubMed] [Google Scholar]
- 13.Cheng JT, Liu IM, Tzeng TF, Tsai CC, Lai TY. Plasma glucose-lowering effect of b-endorphin in streptozotocin-induced diabetic rats. Horm Meta Res. 2002;34:570–576. doi: 10.1055/s-2002-35418. [DOI] [PubMed] [Google Scholar]
- 14.Loh HH, Liu HC, Cavalli A, Yang W, Chen YF, Wei LN. Opioid μ receptor knockout in mice: effects on ligand-induced analgesia and morphine lethality. Mol Brain Res. 1998;54:321–326. doi: 10.1016/s0169-328x(97)00353-7. [DOI] [PubMed] [Google Scholar]
- 15.Liu IM, Chi TC, Shiao GC, Lin MT, Cheng JT. Loss of plasma glucose-lowering response to cold stress in opioid mu-receptor knock-out diabetic mice. Neurosci Lett. 2001;307:81–84. doi: 10.1016/s0304-3940(01)01938-3. [DOI] [PubMed] [Google Scholar]
- 16.Cheng JT, Liu IM, Kuo DH, Lin MT. Stimulatory effect of phenylephrine on the secretion of β-endorphin from rat adrenal medulla in vitro. Auton Neurosci Basic Clinic. 2001;93:31–35. doi: 10.1016/s1566-0702(01)00321-6. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki H, Zweifach BW, Forrest MJ, Schmid-Schönbein GW. Modification of leukocyte adhesion in spontaneously hypertensive rats by adrenal corticosteroids. J Leukoc Biol. 1995;57:20–26. [PubMed] [Google Scholar]
- 18.Cheng JT, Liu IM, Chi TC, Tzeng TF. Release of β-endorphin by prostaglandin E2 to lower plasma glucose in streptozotocin-induced diabetic rats. Horm Meta Res. 2001;33:439–443. doi: 10.1055/s-2001-16236. [DOI] [PubMed] [Google Scholar]
- 19.Cheng JT, Liu IM, Chi TC, Tzeng TF, Lu FH, Chang CJ. Plasma glucose-lowering effect of tramadol in streptozotocin-induced diabetic rats. Diabetes. 2001;50:2815–2821. doi: 10.2337/diabetes.50.12.2815. [DOI] [PubMed] [Google Scholar]
- 20.Wohaieb SA, Godin DV. Alterations in free radical tissue-defense mechanisms in streptozotocin-induced diabetes in rat. Effect of insulin treatment. Diabetes. 1987;36:1014–1018. doi: 10.2337/diab.36.9.1014. [DOI] [PubMed] [Google Scholar]
- 21.Wasserman DH, Zinman B. Exercise in individuals with IDDM. Diabetes Care. 1994;17:924–937. doi: 10.2337/diacare.17.8.924. [DOI] [PubMed] [Google Scholar]
- 22.Cheng JT, Liu IM, Tzeng TF, Tsai CC, Lai TY. Plasma glucose-lowering effect of beta-endorphin in streptozotocin-induced diabetic rats. Horm Metab Res. 2002;34:570–576. doi: 10.1055/s-2002-35418. [DOI] [PubMed] [Google Scholar]
- 23.Pasternak GW. Pharmacological mechanisms of opioid analgesics. Clin Neuropharmacol. 1993;16:1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Vargo T, Rossier J, Minick S, Ling N, Rivier C, Vale W, et al. β-endorphin and adrenal corticotropin are secreted concomitantly by the pituitary gland. Science. 1977;197:1367–1369. doi: 10.1126/science.197601. [DOI] [PubMed] [Google Scholar]
- 25.Arefolov VA, Dmitriev AD, Tennov AV, Val'dman AV. Detection of the pro-opiomelanocortin peptide fragments—beta-endorphin and ACTH—in the adrenals of rats and mice by immunohistochemistry. Biull Eksp Biol Med. 1986;101:445–447. [PubMed] [Google Scholar]
- 26.Mitsuma T, Nogimori T, Sun DH, Chaya M. Thyrotropin-releasing hormone reduces the plasma levels of beta-endorphin-like immunoreactivity in rats. Exp Clin Endocrinol. 1987;89:55–60. doi: 10.1055/s-0029-1210627. [DOI] [PubMed] [Google Scholar]
- 27.Hanbauer I, Kelly GD, Saiani L, Yang HY. [Met5]-enkephalin-like peptides of the adrenal medulla: release by nerve stimulation and functional implications. Peptides. 1982;3:469–473. doi: 10.1016/0196-9781(82)90109-7. [DOI] [PubMed] [Google Scholar]
- 28.Hexum TD, Russett LR. Stimulation of cholinergic receptor mediated secretion from the bovine adrenal medulla by neuropeptide Y. Neuropeptides. 1989;13:35–41. doi: 10.1016/0143-4179(89)90019-x. [DOI] [PubMed] [Google Scholar]
- 29.Consoli A, Nurjhan N, Capani F, Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989;38:550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- 30.Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin-and insulin-mediated glucose uptake in human. Am J Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- 31.Ziel FH, Venkatesan N, Davidson MB. Glucose transport is rate limiting for skeletal muscle glucose metabolism in normal and STZ-induced diabetic rats. Diabetes. 1988;37:885–890. doi: 10.2337/diab.37.7.885. [DOI] [PubMed] [Google Scholar]
- 32.Pessin JE, Bell GI. Mammalian facilitative glucose transporter family: structure and molecular regulation. Annu Rev Physiol. 1992;54:911–930. doi: 10.1146/annurev.ph.54.030192.004403. [DOI] [PubMed] [Google Scholar]
- 33.Berger J, Biswas C, Vicario PP, Strout HV, Saperstein R, Pilch PF. Decreased expression of the insulin-responsive glucose transporter in diabetes and fasting. Nature. 1989;340:70–72. doi: 10.1038/340070a0. [DOI] [PubMed] [Google Scholar]
- 34.Henriksen EJ, Bourey RE, Rodnick KJ, Korany IL, Permutt MA, Holloszy JO. Glucose transporter protein content and glucose transport capacity in rat skeletal muscles. Am J Physiol. 1990;259:E593–E598. doi: 10.1152/ajpendo.1990.259.4.E593. [DOI] [PubMed] [Google Scholar]
- 35.Bollen M, Keppens S, Stalmans W. Specific features of glycogen metabolism in the liver. Biochem J. 1998;336:1–31. doi: 10.1042/bj3360019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu Rev Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- 37.Tainlin L, Tingyi H, Changqi Z, Peipei Y, Qiong Z. Studies of the chronic toxicity of tetrandrine in dogs: an inhibitor of silicosis. Ecotoxicol Environm Safety. 1982;6:528–534. doi: 10.1016/0147-6513(82)90034-3. [DOI] [PubMed] [Google Scholar]
- 38.Park PH, Nan JX, Park EJ, Kang HC, Kim JY, Ko G, et al. Effect of tetrandrine on experimental hepatic fibrosis induced by bile duct ligation and scission in rats. Pharm Toxicol. 2000;87:261–268. doi: 10.1034/j.1600-0773.2000.pto870604.x. [DOI] [PubMed] [Google Scholar]