Abstract
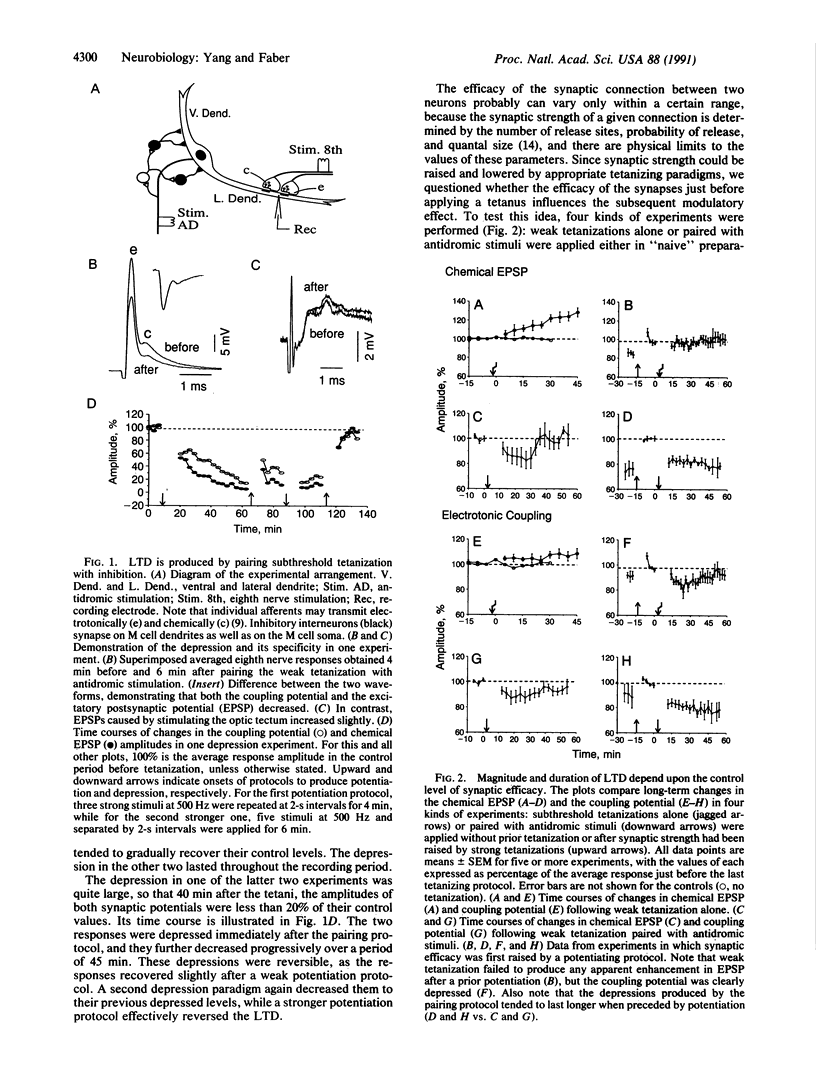
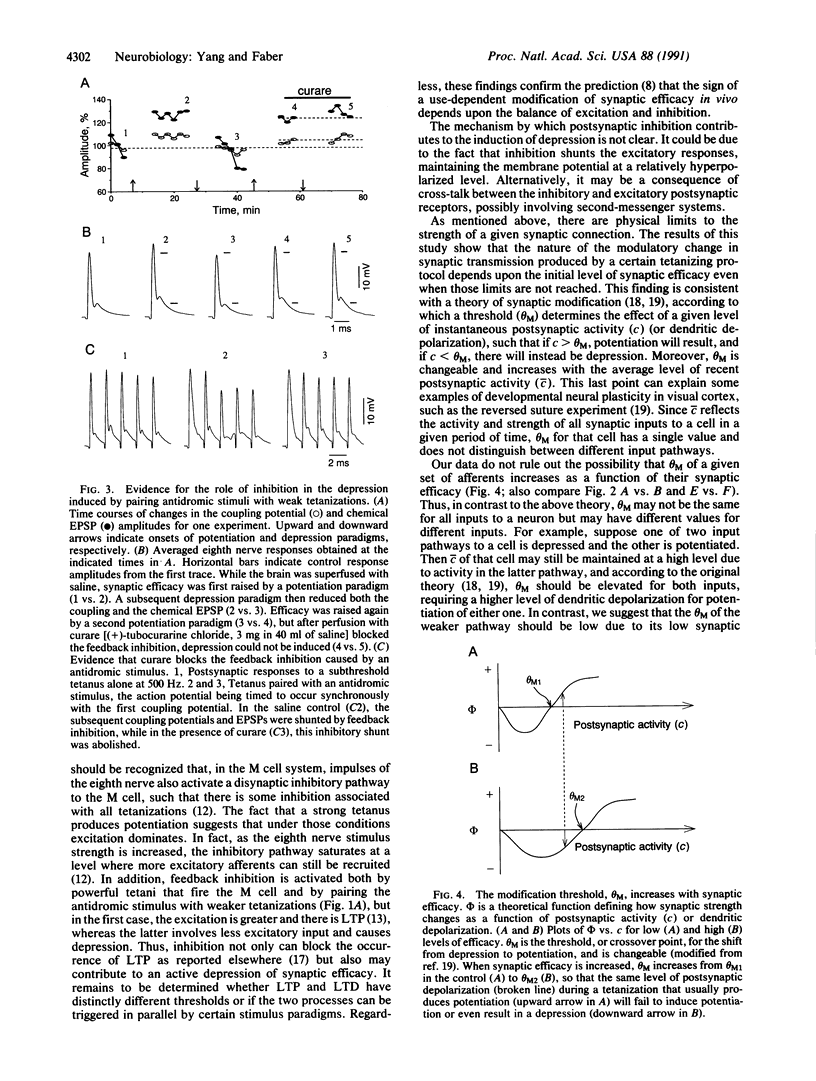
Long-term depression (LTD) of glutamatergic and electrotonic transmission can be induced at mixed synapses between eighth nerve fibers and the goldfish Mauthner (M) cell in vivo, by pairing weak presynaptic tetani with postsynaptic inhibition. This LTD can be reversed by stronger tetani that produce long-term potentiation (LTP). Moreover, the depression is more likely to occur and tends to last longer when the initial synaptic efficacy is high--that is, if the synaptic strength is first potentiated. In addition, when synaptic efficacy is initially elevated, a weak tetanization that usually results in a gradually developing potentiation instead produces no change in chemical transmission and even a depression of electrotonic coupling. Thus, the modifications in synaptic transmission caused by a certain tetanizing protocol depend upon the history of synaptic efficacy. This last concept provides an experimental basis for theoretical models concerned with pre- and postsynaptic contributions to the regulation of synaptic plasticity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artola A., Bröcher S., Singer W. Different voltage-dependent thresholds for inducing long-term depression and long-term potentiation in slices of rat visual cortex. Nature. 1990 Sep 6;347(6288):69–72. doi: 10.1038/347069a0. [DOI] [PubMed] [Google Scholar]
- Artola A., Singer W. Long-term potentiation and NMDA receptors in rat visual cortex. Nature. 1987 Dec 17;330(6149):649–652. doi: 10.1038/330649a0. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Cooper L. N., Ebner F. F. A physiological basis for a theory of synapse modification. Science. 1987 Jul 3;237(4810):42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Bienenstock E. L., Cooper L. N., Munro P. W. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci. 1982 Jan;2(1):32–48. doi: 10.1523/JNEUROSCI.02-01-00032.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURUKAWA T., FURSHPAN E. J. Two inhibitory mechanisms in the Mauthner neurons of goldfish. J Neurophysiol. 1963 Jan;26:140–176. doi: 10.1152/jn.1963.26.1.140. [DOI] [PubMed] [Google Scholar]
- Hackett J. T., Faber D. S. Mauthner axon networks mediating supraspinal components of the startle response in the goldfish. Neuroscience. 1983;8(2):317–331. doi: 10.1016/0306-4522(83)90069-6. [DOI] [PubMed] [Google Scholar]
- Ito M., Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982 Dec 13;33(3):253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- Komatsu Y., Toyama K., Maeda J., Sakaguchi H. Long-term potentiation investigated in a slice preparation of striate cortex of young kittens. Neurosci Lett. 1981 Nov 4;26(3):269–274. doi: 10.1016/0304-3940(81)90144-0. [DOI] [PubMed] [Google Scholar]
- Korn H., Faber D. S., Triller A. Probabilistic determination of synaptic strength. J Neurophysiol. 1986 Feb;55(2):402–421. doi: 10.1152/jn.1986.55.2.402. [DOI] [PubMed] [Google Scholar]
- Lee K. S. Sustained enhancement of evoked potentials following brief, high-frequency stimulation of the cerebral cortex in vitro. Brain Res. 1982 May 13;239(2):617–623. doi: 10.1016/0006-8993(82)90538-8. [DOI] [PubMed] [Google Scholar]
- Levy W. B., Steward O. Synapses as associative memory elements in the hippocampal formation. Brain Res. 1979 Oct 19;175(2):233–245. doi: 10.1016/0006-8993(79)91003-5. [DOI] [PubMed] [Google Scholar]
- Lin J. W., Faber D. S. Synaptic transmission mediated by single club endings on the goldfish Mauthner cell. I. Characteristics of electrotonic and chemical postsynaptic potentials. J Neurosci. 1988 Apr;8(4):1302–1312. doi: 10.1523/JNEUROSCI.08-04-01302.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton P. K., Sejnowski T. J. Associative long-term depression in the hippocampus induced by hebbian covariance. Nature. 1989 May 18;339(6221):215–218. doi: 10.1038/339215a0. [DOI] [PubMed] [Google Scholar]
- Steward O., Tomasulo R., Levy W. B. Blockade of inhibition in a pathway with dual excitatory and inhibitory action unmasks a capability for LTP that is otherwise not expressed. Brain Res. 1990 May 21;516(2):292–300. doi: 10.1016/0006-8993(90)90930-a. [DOI] [PubMed] [Google Scholar]
- Yang X. D., Korn H., Faber D. S. Long-term potentiation of electrotonic coupling at mixed synapses. Nature. 1990 Dec 6;348(6301):542–545. doi: 10.1038/348542a0. [DOI] [PubMed] [Google Scholar]
- Zottoli S. J., Hordes A. R., Faber D. S. Localization of optic tectal input to the ventral dendrite of the goldfish Mauthner cell. Brain Res. 1987 Jan 13;401(1):113–121. doi: 10.1016/0006-8993(87)91170-x. [DOI] [PubMed] [Google Scholar]



