Abstract
We previously reported the crucial role displayed by loop 3 of defensin isolated from the Mediterranean mussel, Mytilus galloprovincialis, in antibacterial and antifungal activities. We now investigated antiprotozoan and antiviral activities of some previously reported fragments B, D, E, P and Q. Two fragments (D and P) efficiently killed Trypanosoma brucei (ID50 4–12 μM) and Leishmania major (ID50 12–45 μM) in a time/dose-dependent manner. Killing of T. brucei started as early as 1 h after initiation of contact with fragment D and reached 55% mortality after 6 h. Killing was temperature dependent and a temperature of 4°C efficiently impaired the ability to kill T. brucei. Fragments bound to the entire external epithelium of T. brucei. Prevention of HIV-1 infestation was obtained only with fragments P and Q at 20 μM. Even if fragment P was active on both targets, the specificity of fragments D and Q suggest that antiprotozoan and antiviral activities are mediated by different mechanisms. Truncated sequences of mussel defensin, including amino acid replacement to maintain 3D structure and increased positive net charge, also possess antiprotozoan and antiviral capabilities. New alternative and/or complementary antibiotics can be derived from the vast reservoir of natural antimicrobial peptides (AMPs) contained in marine invertebrates.
Keywords: Trypanosoma, Leishmania, HIV, antibiotics, protozoa, virus, defensin, mussel
Introduction
One of the characteristics of animal life is mobility, offering multiple advantages among which are escape, food gathering and partner encounter for reproduction. Most marine animal life is fixed to a substratum. As a consequence, animals must adapt to this unusual situation. Obviously they cannot escape, they must protect themselves against deadly radiation, especially UVA, they compete for available space, they must avoid predation and they are exposed to colonization. According to phyla, different strategies evolved leading to various visible morphologies and behaviors. Millions of invertebrate species exist in contrast to only 45 000 vertebrate species. Among such an enormous variety of animal species one can expect to find original systems/ mechanisms with putative benefits for human health.
It is the immune system that plays a major role in determining host fitness in the wild, i.e. under the constraints imposed by ecology and life history. But immune responses are costly and theory predicts when a host should switch between constitutive (e.g. pre-existing phenoloxidase cascade) and inducible (e.g. antimicrobial peptides or AMPs) defenses, or when they should combine those defenses (1). In addition, genetic variations for resistance to pathogens are high and immune genes appear to evolve relatively rapidly (2). Most of our knowledge on the functionality of invertebrate immune systems has been obtained through laboratory-based observations, most of the time in the absence of true pathogens. The link with natural forces that drive evolution of those genes in natural populations is still missing and it constitutes the rapidly expanding field of ecological immunology (3).
Permanent conflict interactions with the environment are the natural situation for a living creature. To partially resolve this, the immune system evolved and is characterized by an enormous variety of mechanisms and effectors, including the AMPs, although their specificity is poor. In fact, AMPs are universal and extremely successful in dealing with a huge range of pathogens, including bacteria, fungi, protozoa and viruses (4). However, AMPs differ widely in amino acid sequence and 3D structures. Classically, they are arranged in three to five classes (5,6). Activity of AMPs is generally restricted to one type of target, i.e. insect defensins, diptericin, drosocin and attacin (see 7 for review), drosomycin (8), mollusc mytimycin (9) and myticin (10). Some of them are multipotent, i.e. ceratotoxins (11), mytilins (12), metchnikowin (13) and cecropins (14). All the previous peptides possess a positive surface charge, i.e. they are cationic. Some others are anionic, i.e. lung lavage fluid (15) and ovine pulmonary surfactant (16) with still unknown modes of action. Some others are released from the cleavage of macromolecules, i.e. lactoferrin (17), hen ovotransferrin (18) and crustacean hemocyanin (19).
Whatever their commercial names, all the antibiotics we used have been derived from a restricted number of proteins with only slight differences introduced into the frameworks. Systematic use and abuse of antibiotics have selected for multi-drug resistant bacteria. The number and danger of such adapted bacteria are already a major public health problem. We rapidly need new antibiotic molecules possessing original modes of action that pathogens cannot adapt to. It is the purpose of this report to present one solution derived from marine invertebrate AMPs. We previously reported antibacterial and antifungi activities (20) of fragments derived from mussel defensin structure (21). It is the purpose of this report to extend such activities to protozoa and virus, In addition, cytotoxicity towards mammal cells was evaluated.
Subjects and Methods
Truncated and Variant Fragments Defensin
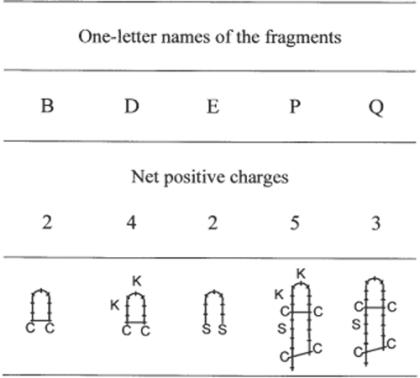
Among the 19 fragments previously reported by Romestand et al. (20) for their antibacterial and antifungi activities, we selected three fragments of nine amino acids (B, D and E) and two fragments of 19 amino acids (P and Q). Fragments D and P were the counterparts of fragments B and Q including two more positive charges each (Fig. 1). Fragment E was an open fragment B.
Figure 1.
Structure of fragments and variant analogues with special mention of disulfide bond(s) and replacements by lysines. Amino acids are expressed with one-letter codes: C, cysteine; S, serine; K, lysine. Drawings are from the sequences reported by Romestand et al. (20).
Cytotoxic Assay
Human Hela MAGIC-5 cells were incubated with fragments at 100 μM. Toxicity was evaluated after 48 h at 37°C by measuring the optical density of the culture at 570/690 nm using the In Vitro Toxicology Assay Kit (Sigma), based on reduction of the yellow tetrazolium salt MTT into purple formazan crystals by mitochondria of active cells, as reported by Hansen et al. (23).
Antiprotozoan Assay
Frozen stabilates of Trypanosoma brucei Antat1.1 were expanded in Balb/c mice (IFFA Credo). Four to five days post-infection, animals were exanguinated on heparin, and parasites were purified by DEAE–cellulose (DE52, Whatman) chromatography in PSG buffer (8.45 g/l Na2HPO4, 0.43 g/l NaH2PO4, 2.13 g/l NaCl, 8 g/l glucose, pH 8.0). Trypanosoma brucei samples (2 × 105 in 100 μl PSG) were dispensed, in triplicate, in 96-well plates containing 100 μl of serial dilutions of mussel peptides and incubated at 29°C. Living parasites were counted microscopically under contrast phase at indicated times.
Frozen stabilates of Leishmania major were injected in the footpad of Balb/c mice. Four days later, popliteal lymph nodes were collected, gently scratched and put in culture in RPMI 1640 containing 10% fetal calf serum. Trypomastigotes were allowed to grow in vitro at 29°C, regularly refreshing the medium. Leishmania major were resuspended in RPMI–10% fetal calf serum, and the killing assay was performed as described for T. brucei, with the exception that the experiments were performed in RPMI–10% fetal calf serum.
Antiviral Assay
We used a simple phenotypic assay for drug susceptibility of human immunodeficiency virus type-1 (HIV-1) using a CCR5 and CXCR4-expressing HeLa/CD4+ cell clone, named MAGIC-5B (24). One day before the assay, 50 000 MAGIC-5B cells in 880 μl Dulbecco's Modified Eagles' Medium (DMEM, Gibco) containing 10% fetal calf serum, were added to each well of a 24-well microtest plate and incubated at 37°C under 95% air–5% CO2. For the assay, 100 μl of a 10× stock solution of the fragment in DMEM were added to the cells and incubated for 15 min at 37°C. After gentle washing to remove excess of fragment, 20 μl aliquots of HIV-1 solution, corresponding to 80 000 c.p.m., were added. Alternatively, fragment solutions were incubated for 15 min with HIV-1 before addition to the cells, without washing of fragment excess. β-Galactosidase enzyme assay with Reporter lysis buffer (Promega, France) was used according to the manufacturer's instructions. Briefly, MAGIC-5B cells were incubated for 72 h at 37°C, lysed by addition of 1 ml assay 2× buffer which contained the substrate ONPG (o-nitrophenyl-β-d-galactopyranoside). Samples were incubated for 30 min to allow β-galactosidase to hydrolyze the colorless substrate to yellow o-nitrophenyl. Reaction was stopped by addition of 50 μl of 1 M Na2CO3. Absorbance at 420 nm was measured in an enzyme-linked immunosorbent assay reader (Multiskan Labsystems Bichromatic). Incubations with untreated HIV-1 (maximum infestation) in the presence of 10 μM AZT (used as inhibitor of infection) were used to verify the functionality of the assay. Except when indicated, combinations were carried out in triplicate and entire experiments were performed twice.
Statistics
Results are expressed as arithmetical mean ± SEM of at least triplicates from one experiment (see Fig. 3) or from between two and five independent experiments. Statistical significance of differences was established by Student's t-test with P < 0.015.
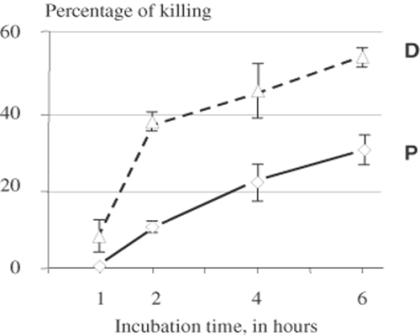
Figure 3.
Kinetics of in vitro killing of T. brucei at 29°C in the presence of 3.1 μM of defensin fragment D and P. Values are arithmetic means of at least three replicates ± SEM (bars). Difference between D and P values are statistically extremely significant (P < 0.0001) after 2 h and significant (P = 0.016) after 4 and 6 h.
Fluorescence Detection of Fragments B and D
Biotin tagged fragments B and D were from Romestand et al. (20). Purified T. brucei were maintained in Eppendorf tubes in an ice bath throughout the procedure. Parasites (106 in 40 μl) were incubated for 30 min in an ice bath in PSG containing 10% normal mouse serum prior to adding biotinylated fragment B or D (10 μM final, in 40 μl). After 40 min, the parasites were washed twice in large volumes of PSG containing 10% normal mice serum (1400 r.p.m., 3 min) and fixed for 1 h in PBS containing 3% formaldehyde, 0.5% glutaraldehyde. After two washes in PSG containing 1% bovine serum albumin (BSA), streptavidin-FITC (7.5 μM final, Sigma) was added for 1 h. Observations were done after three washes in PSG containing 1% BSA with a Nikon ECLIPSE E600 fluorescence microscope equipped with phase contrast.
Results
Antiprotozoan activity
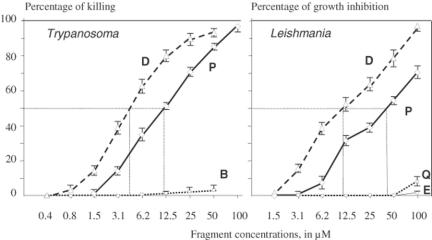
Among the tested defensin fragments, only fragments D and P were able to kill in vitro T. brucei (Fig. 2). As little as 1.5 μM of fragment D killed 17.1 ± 0.9% of the parasites after 2 h of contact at 29°C. Furthermore, 89.3 ± 1.9% of the parasites were killed by 25 μM of fragment D. Fragment P was also toxic, but to a lesser extent, as only 1.6 ± 0.6% of T. brucei were killed by 1.5 μM (P < 0.0001) and 70.3 ± 1.4% by 25 μM (P < 0.0001). Such differences in the killing capacity was statistically highly significant at all concentrations, except the lowest values for which killing was non-existent (0.4 μM) or marginal (0.8 μM). The killing capacity was reflected by the respective ID50 values: 4 μM for fragment D compared to 12 μM for fragment P. All the other tested fragments were not toxic for T. brucei, with the exception of fragment B at the highest concentration tested: 3.3 ± 1.6% killed at 50 μM.
Figure 2.
Antiprotozoan activity of truncated defensins. Trypanosoma brucei were incubated in vitro for 2 h at 29°C in survival medium in the presence of various fragment concentrations. Leishmania major were incubated for 24 h at 29°C in growing medium containing various fragment concentrations. Percentages of killing or percentages of growth inhibition were determined upon microscopic examination. Values are arithmetic means of three replicates of at least three independent experiments ± SEM (bars). For T. brucei, the difference between D and P values are statistically extremely significant (P < 0.0001) except for 0.4 and 0.8 μM. For L. major, the difference between D and P values are at least significant (P = 0.01 for 50 μM) except for 1.5 μM (P = 0.37). Dotted lines are for ID50.
When tested against L. major in vitro, again fragment D appeared as the most active. After 24 h of contact with parasites at 29°C, 6.2 μM of fragment D induced 39.0 ± 2.3% of killing whereas 100 μM induced 97.6 ± 1.4% of killing (ID50 12 μM). Concomitantly, 8.6 ± 2.9% (P = 0.0012) of killing was observed with 6.2 μM of peptide P and 70.0 ± 4.5% (P = 0.0045) with 100 μM (ID50 45 μM) (Fig. 2). No activity was noted with fragment B, even at 100 μM. Only marginal mortality was observed with 100 μM of fragments E (3.6 ± 0.6%) and Q (9.0 ± 4.5%).
Kinetics of T. brucei Killing
The kinetics of in vitro killing of T. brucei were investigated in the presence of several molarities of fragments D and P. At all concentrations, the difference in killing activity of fragments D and P could not be overcome by increasing incubation times up to 6 h. This is illustrated in Fig. 3 which shows killing activity in the presence of 3.1 μM of fragments D and P. As early as 1 h after contact with fragment D, killing was 5.5 ± 2.5%, reaching 39.5 ± 1.5% after only 2 h. At that time, fragment P induced 14.1 ± 1.6% of mortality, a highly significant difference (P < 0.0001). Between 2 and 6 h, the slopes of the two killing curves appeared parallel, reaching 53.0 ± 2.0% for fragment D at 6 h and 31.0 ± 2.0% for fragment P (P = 0.016).
Temperature Effect
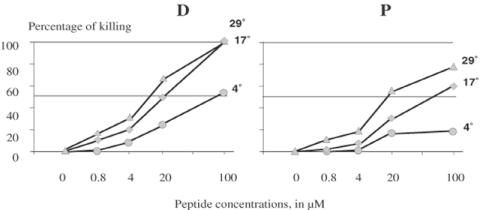
To investigate whether the defensin fragments needed to be internalized by T. brucei in order to exert their activity, killing was measured after 2 h incubations at 4, 17 and 29°C in the presence of fragments D and P at a range of concentrations. At temperature below 17°C, the membrane of trypanosomes adopts a rigid conformation that impairs phagocytic processes including endosome/lysosome fusion (25). Results indicated that incubation at 4°C efficiently impaired the ability of fragments D and P to kill the parasites (Fig. 4), suggesting that membrane fluidity is required for peptides to be active and that general cytotoxicity is highly improbable. The difference between 4 and 17 °C was statistically significant at 0.8 μM of fragment D (P < 0.0001) and 4 μM of fragment P (P = 0.0015). With the exception of 100 μM of fragment D, killing observed at 29°C was always statistically higher than at 17°C. At all tested temperatures and with all concentrations, fragment D was more toxic than fragment P, confirming the data in Figs 2 and 3.
Figure 4.
Effect of temperature on in vitro killing of T. brucei after 2 h incubation with defensin fragment D and P. Results are arithmetic means of three replicates of one experiment. SEM is not indicated as contained within marks. Differences between temperatures are at least statistically significant (P < 0.014) for fragment D, and at least statistically very significant (P < 0.0048) for fragment P. Only the killing percentages observed with 100 μM of fragment D at 17 and 29 °C and with 0.8 μM of fragment P at 4 and 17 °C were not statistically different.
Membrane Binding
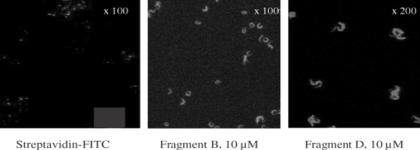
Possible interaction between defensin fragments and T. brucei membranes was visualized by fluorescence microscopy. We controlled that antiprotozoan capacity of biotinylated fragments B and D were not significantly different from untagged fragments, revealing that biotinylation did not alter such capacities. When parasites were incubated with biotinylated fragments, the entire surface of T. brucei was labeled so that peptides did not bind to a specific location, such as the flagellar pocket (Fig. 5). Surprisingly, labeling observed with both fragments B and D were similar, even though only fragment D killed the parasites. We controlled so that the FITC-labeled streptavidin used to detect the bound defensin fragments did not bind to T. brucei (Fig. 5) and had no toxic effect on viability of parasites at least over 2 h at 29°C (not shown).
Figure 5.
Binding of biotinylated defensin fragment B and D (10 μM) on T. brucei. Note that FITC-labeled streptavidin did not bind to the targets.
In Vitro Prevention of Viral Infection
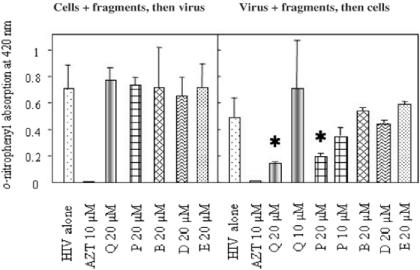
In addition to antiprotozoan activity, we were interested in putative interference of defensin fragments with virus infestation. When infected by untreated HIV-1, MAGIC-5B cells were able to metabolize the ONPG substrate, giving measurable absorption at 420 nm. Such absorption was dramatically reduced by ∼70% when viruses were pre-incubated with 20 μM of fragment Q or P (Fig. 6). No significant inhibition was observed with 10 μM of fragment Q or P. In addition, fragment B, D and E were not inhibitory, even when tested at 20 μM. Interestingly, when the MAGIC-5B cells were incubated with the same fragments, washed, then submitted to HIV-1 infection, no inhibition was observed (Fig. 6). We controlled such that 20 μM of peptide was not toxic for MAGIC-5B cells, at least up to 100 μM of fragments B, D, E, P and Q over 48 h at 37°C. In addition, the well-known inhibitor AZT used at 10 μM totally suppressed HIV-1 infection.
Figure 6.
Interference of defensin fragments Q, P, B, D and E with infection of MAGIC-5B cells by HIV-1 compared to AZT inhibitory effect. Results are from one of two independent experiments and presented as arithmetic mean values ± SEM (bars) of triplicates. Stars indicate extremely significant difference (P < 0.0001) with infection by HIV-1 alone. Note that fragment P was tested three times but only in mixture with cells and virus, without washing of excess fragment.
Discussion
Antiprotozoan activity of defensin fragments was evaluated on the African trypanosome T. brucei which belongs to the genus that causes sleeping sickness, and on L. major that causes cutaneous leishmaniasis. Our data show that two of the five tested fragments derived from mussel defensin efficiently killed T. brucei and L. major in a time/dose-dependent manner. Differences in ID50 (4–12 μM for T. brucei versus 12–45 μM for L. major) can be due to the fact that experiments were performed in a survival medium for trypanosomes and in a growing medium for L. major. Dose–response curves of fragments D and P were parallel, with stronger killing capacity of fragment D. This stronger and faster activity of fragment D was confirmed by kinetic curves and by the effects of temperature during incubation. As revealed by observation of fluorescence, fragments bind to parasite membranes and fluidity of these membranes appears to be crucial as incubation at 4°C significantly impaired the ability to kill trypanosomes. In addition, and with the exception of the 100 μM concentration, killing was higher at 29°C compared to 17°C, suggesting that fusion endosome/lysosome is required to display killing.
Fragments D and P are the counterparts of fragments B and Q, with the addition of two positive charges located at loop 3. The presence of these extra charges appears to be fundamental for both antiprotozoan and antiviral activities as has been reported for antibacterial activity (20). Both fragments B and D bound to parasite membranes, suggests that to be positively charged is a condition sufficient to bind to the membrane. Activity appeared to be related to peptide size as fragment D (nine amino acids) is much more efficient than fragment P (19 amino acids) on protozoa. It is well known that AMPs act at the membrane level, by inserting into the phospholipid bilayer. The better activity observed with fragment D might be due to its small size, favoring penetration into the parasite membrane. Concerning antiviral activity, correlation between size and activity seems to exist as fragment Q was active in contrast to fragment B. Increasing the net positive charge (from Q to P and from B to D) did not result in better activity. In all cases, activity of fragments B and E was marginal and not sufficient to confirm the importance of folding as demonstrated with bacteria (20).
The present paper provides evidence that truncated AMPs act on structurally different targets: protozoa and viruses. Compared to numerous reports on antibacterial activity of AMPs, including truncated mussel defensin (20), there are few reports of antiviral or antiprotozoan activity. Recently, another mussel AMP, mytilin, has been reported as preventing in vivo viral infection due to interference between the virus (White Spot Syndrome Virus) and the peptide (26). Generally, antiviral activity is at the very early stages of viral multiplication as for melittin (27,28), synthetic magainin derivatives (29) and dermaseptins (30). Putative mode of action is by suppressing viral gene expression as for melittin and cecropin (31) or by binding to gp120 and CD4 as for retrocyclin (32).
Different effector structures are active on various target structures. We report here that a sequence of only nine amino acids can kill both Trypanosoma and Leishmania. Longer sequences of 18 amino acids (protegrin-1), 29 amino acids (cathelicidins) or 30–50 amino acids (α-defensins) have been reported as killing both insect and bloodstream forms of T. brucei (33). Action of cathelicidins involves disruption of parasite membrane integrity, which is in agreement with our observation of parasite labeling. Meanwhile, effects were observed with 5 μM (on procyclics) to 25 μM (on bloodstream forms) and routine experiments engaged 25–50 μM, compared to 3 μM in our experiments with truncated nine-amino-acid defensin. Concerning the antivirus activity, a longer sequence of 19 amino acids seemed necessary to prevent infection. This is in agreement with Dupuy et al. (26) who reported antiviral activity of mytilin (39 amino acids) but not of a 13 amino acid folded fragment. Minidefensins of 16–18 residues (out of 30–45) with net positive charge, β-sheet structure and intramolecular disulfide bonds have also been reported as capable of preventing in vitro HIV-1 infection (33). The question is, how can a simple structure be active on multiple target structures? Several models have been constructed to explain modes of action at the cell membrane level (35,36). But some authors have suggested that AMPs penetrate cells and also act inside the cytoplasm (37,38). Adding the antivirus activity made a unique mode of action highly improbable.
Even if short amino acid sequences were reported for potent activity towards various targets, the definition of a unique motif to be employed as universal killer is probably too optimistic. In contrast, more sophisticated peptides that combine the products of two genes have been reported (39) and one can think about chimeric peptides constructed by association of several active sites from different peptides, thus increasing the target spectrum. We must also consider the possible in vivo increase of in vitro activity by synergistic effects with other innate immune mechanisms such as phagocytosis, self antimicrobial peptides and free radicals. It is most probable that, in the near future, new antibiotic generations will be used to complement an association with classical antibiotic molecules.
Introducing a single bacteria species into Drosophila resulted in the up-regulation of more than 350 genes, most of them being unknown or having no immune-related functions (40). Constructions of expressed sequence tag (EST) libraries following challenges, released panels of immune-related genes, the vast majority with only potential immune function in shrimp (41), oysters (42,43) and mussels (44). Consequently (i) most of the mechanisms triggered by a microorganism are unknown, (ii) the immune system is not the only one responding to introduction of foreign material and (iii) complex interactions between several physiological functions must occur.
Immune research focuses on polypeptide structures, relying on genetic sequences. However, recognition is also mediated by carbohydrate moieties, sometimes combined with lipids, which constitute the usual external barriers. This is particularly the case for protozoan parasites and bacteria with the crucial role of LPS. In addition, an enormous variety of molecular structures, mostly cyclic, isolated from marine environments have been reported to exert anti-infectious activity (45) and to affect the cardiovascular, immune and nervous systems (46). Some active compounds are as simple as iron (47) or free radicals (48). Consequently, the ribosomally synthesized peptides involved in immune mechanisms must not be considered separately, but combined with all the other molecular moieties employed by living creatures to ensure a more sophisticated picture.
Acknowledgments
Thanks to Benoit Stijlemans for help with microscopy. We are grateful to Claude Granier and Bernard Romestand for the design and synthesis of defensin fragments. We are indebted to Prof. Edwin L. Cooper (UCLA, David Geffen School of Medicine) for English improvement of the manuscript. This work, supported by the Bilateral Scientific Cooperation program of Flanders (BWS03/06), was performed in frame of an Interuniversity Attraction Pole Program.
References
- 1.Shudo E, Iwasa Y. Inducible defense against pathogens and parasites: optimal choice among multiple options. J Theor Biol. 2001;209:233–247. doi: 10.1006/jtbi.2000.2259. [DOI] [PubMed] [Google Scholar]
- 2.Hurst L, Smith N. Do essential genes evolve slowly? Curr Biol. 1999;9:747–750. doi: 10.1016/s0960-9822(99)80334-0. [DOI] [PubMed] [Google Scholar]
- 3.Rolff J, Siva-Jothy MT. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. [DOI] [PubMed] [Google Scholar]
- 4.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 5.Gallo RL, Huttner KM. Antimicrobial peptides: an emerging concept in cutanous biology. J Invest Dermatol. 1998;111:739–743. doi: 10.1046/j.1523-1747.1998.00361.x. [DOI] [PubMed] [Google Scholar]
- 6.Bulet P, Hetru C, Dimarcq JL, Hoffmann D. Antimicrobial peptides in insects: structure and function. Develop Comp Immunol. 1999;23:329–344. doi: 10.1016/s0145-305x(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 7.Cociancich S, Bulet P, Hétru C, Hoffmann JA. The inducible antibacterial peptides of insects. Parasitol Today. 1994;10:132–139. doi: 10.1016/0169-4758(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 8.Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hétru C, et al. Insect immunity: septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem. 1994;269:33159–33163. [PubMed] [Google Scholar]
- 9.Charlet M, Chernysh S, Philippe H, Hetru C, Hoffmann JA, Bulet P. Innate immunity: isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J Biol Chem. 1996;271:21808–21813. doi: 10.1074/jbc.271.36.21808. [DOI] [PubMed] [Google Scholar]
- 10.Mitta G, Hubert F, Noël T, Roch Ph. Myticin, a novel cysteine-rich antimicrobial peptide isolated from hemocytes and plasma of the mussel, Mytilus galloprovincialis. Eur J Biochem. 1999;265:71–78. doi: 10.1046/j.1432-1327.1999.00654.x. [DOI] [PubMed] [Google Scholar]
- 11.Rosetto M, Manetti AGO, Giordano PC, Marri L, Amons R, Baldari CT, et al. Molecular characterization of ceratotoxin C, a novel antibacterial female-specific peptide of the ceratotoxin family from the medfly Ceratitis capitata. Eur J Biochem. 1996;241:330–337. doi: 10.1111/j.1432-1033.1996.00330.x. [DOI] [PubMed] [Google Scholar]
- 12.Mitta G, Vandenbulcke F, Hubert F, Salzet M, Roch Ph. Involvement of mytilins in mussel antimicrobial defense. J Biol Chem. 2000;275:12954–12962. doi: 10.1074/jbc.275.17.12954. [DOI] [PubMed] [Google Scholar]
- 13.Levashina EA, Ohresser S, Bulet P, Reichhart JM, Hoffmann JA. Metchnikowin, a novel immune-inducible prolin-rich peptide from Drosophila with antibacterial and antifungal properties. Eur J Biochem. 1995;233:694–700. doi: 10.1111/j.1432-1033.1995.694_2.x. [DOI] [PubMed] [Google Scholar]
- 14.Alan AR, Earle ED. Sensitivity of bacterial and fungal plant pathogens to the lytic peptides, MSI-99, magainin II, and cecropin B. Mol Plant Microbe Interact. 2002;15:701–708. doi: 10.1094/MPMI.2002.15.7.701. [DOI] [PubMed] [Google Scholar]
- 15.Ellison RT, 3rd, Boose D, La Force FM. Isolation of an antibacterial peptide from human lung lavage fluid. J Infect Dis. 1985;151:1123–1129. doi: 10.1093/infdis/151.6.1123. [DOI] [PubMed] [Google Scholar]
- 16.Brogden KA, De Lucca AJ, Bland J, Elliott S. Isolation of an ovine pulmonary surfactant-associated anionic peptide bactericidal for Pasteurella haemolytica. Proc Natl Acad Sci USA. 1996;93:412–416. doi: 10.1073/pnas.93.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuwata H, Yip TT, Yip CL, Tomita M, Hutchens TW. Bactericidal domain of lactoferrin: detection, quantitation, and characterization of lactoferricin in serum by SELDI affinity mass spectrometry. Biochem Biophys Res Commun. 1998;245:764–773. doi: 10.1006/bbrc.1998.8466. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim HR, Sugimoto Y, Aoki T. Ovotransferrin antimicrobial peptide (OTAP-92) kills bacteria through a membrane damage mechanism. Biochim Biophys Acta. 2000;1523:196–205. doi: 10.1016/s0304-4165(00)00122-7. [DOI] [PubMed] [Google Scholar]
- 19.Destoumieux-Garzon D, Saulnier D, Garnier J, Jouffrey C, Bulet P, Bachère E. Crustacean immunity. Antifungal peptides are generated from the C-terminus of shrimp hemocyanin in response to microbial challenge. J Biol Chem. 2001;276:47070–47077. doi: 10.1074/jbc.M103817200. [DOI] [PubMed] [Google Scholar]
- 20.Romestand B, Molina F, Richard V, Roch Ph, Granier C. Key role of the loop connecting the two beta strands of mussel defensin in its antimicrobial activity. Eur J Biochem. 2003;270:1–9. doi: 10.1046/j.1432-1033.2003.03657.x. [DOI] [PubMed] [Google Scholar]
- 21.Yang YS, Mitta G, Chavanieu A, Calas B, Sanchez JF, Roch Ph, et al. Solution structure and activity of the synthetic four disulfide bond Mediterranean mussel defensin, MGD-1. Biochemistry. 2000;39:14436–14447. doi: 10.1021/bi0011835. [DOI] [PubMed] [Google Scholar]
- 22.Tam JP, Wu C, Liu W, Zhang JW. Disulfide bond formation in peptides by dimethyl sulfoxide. Scope and applications. J Am Chem Soc. 1991;113:6657–6662. [Google Scholar]
- 23.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–210. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 24.Hachiya A, Aizawa-Matsuoka S, Tanaka M, Takahashi Y, Ida S, Gatanaga H, et al. Rapid and Simple Phenotypic Assay for Drug Susceptibility of Human Immunodeficiency Virus Type 1 Using CCR5-Expressing HeLa/CD4+ Cell Clone 1–10 (MAGIC-5) Antimicrob Agents Chemother. 2001;45:495–501. doi: 10.1128/AAC.45.2.495-501.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magez S, Geuskens M, Beschin A, Del Favero H, Verschueren R, Lucas R, et al. Specific uptake of tumor necrosis factor-a is involved in growth control of Trypanosoma brucei. J Cell Biol. 1997;137:715–727. doi: 10.1083/jcb.137.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dupuy JW, Bonami JR, Roch Ph. A synthetic antibacterial peptide from Mytilus galloprovincialis reduces mortality due to white spot syndrome virus in palaemonid shrimp. J Fish Dis. 2004;27:57–64. doi: 10.1046/j.1365-2761.2003.00516.x. [DOI] [PubMed] [Google Scholar]
- 27.Marcos JF, Beachy RN, Houghten RA, Blondelle SE, Perez-Paya E. Inhibition of plant virus infection by analogs of melittin. Proc Natl Acad Sci USA. 1995;92:12466–12469. doi: 10.1073/pnas.92.26.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baghian A, Jaynes J, Enright F, Kousoulas G. An amphipathic alpha-helical synthetic peptide analogue of melittin inhibits Herpes Simplex virus-1 (HSV-1)-induced cell fusion and virus spread. Peptides. 1997;18:177–183. doi: 10.1016/s0196-9781(96)00290-2. [DOI] [PubMed] [Google Scholar]
- 29.Egal M, Conrad M, MacDonald DL, Maloy WL, Motley M, Genco CA. Antiviral effects of synthetic membrane-active peptides on Herpes Simplex Virus, Type 1. Int J Antimicrobial Agents. 1999;13:57–60. doi: 10.1016/s0924-8579(99)00094-1. [DOI] [PubMed] [Google Scholar]
- 30.Belaid A, Aouni M, Khelifa R, Trabelsi A, Jemmali M, Hani K. In vitro antiviral activity of dermaseptins against Herpes Simplex virus type 1. J Med Virol. 2002;66:229–234. doi: 10.1002/jmv.2134. [DOI] [PubMed] [Google Scholar]
- 31.Wachinger M, Kleinschmidt A, Winder D, von Pechmann N, Ludvigsen A, Neumann M, et al. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J Gen Virol. 1998;79:731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J Immunol. 2003;170:4708–4716. doi: 10.4049/jimmunol.170.9.4708. [DOI] [PubMed] [Google Scholar]
- 33.McGwire BS, Olson CL, Tack BF, Engman DM. Killing of African trypanosomes by antimicrobial peptides. J Infect Dis. 2003;188:146–152. doi: 10.1086/375747. [DOI] [PubMed] [Google Scholar]
- 34.Cole AM, Lehrer RI. Minidefensins: antimicrobial peptides with activity against HIV-1. Curr Pharm Des. 2003;9:1463–1473. doi: 10.2174/1381612033454667. [DOI] [PubMed] [Google Scholar]
- 35.Matsuzaki K, Nakamura A, Murase O, Sugishita KI, Fujii N, Miyajima K. Modulation of magainin 2-lipid bilayer interactions by peptide charges. Biochemistry. 1997;36:2104–2111. doi: 10.1021/bi961870p. [DOI] [PubMed] [Google Scholar]
- 36.Huang HW. Action of antimicrobial peptides: two-stade model. Biochemistry. 2000;39:8347–8352. doi: 10.1021/bi000946l. [DOI] [PubMed] [Google Scholar]
- 37.Hancock REW, Chapple DS. Peptide antibiotics. Antimicrob Agents Chemother. 1999;43:1317–1323. doi: 10.1128/aac.43.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drin G, Cottin S, Blanc E, Rees A, Temsamani J. Studies on the internalization mechanism of cationic cell-penetrating peptides. J Biol Chem. 2003;278:31192–311201. doi: 10.1074/jbc.M303938200. [DOI] [PubMed] [Google Scholar]
- 39.Tang YQ, Yuan J, Osapay G, Osapay K, Tran D, Miller CJ, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 40.Irving P, Troxler L, Heuer TS, Belvin M, Kopczynski C, Reichhart JM, et al. A genome-wide analysis of immune responses in Drosophila. Proc Natl Acad Sci USA. 2001;98:15119–15124. doi: 10.1073/pnas.261573998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross PS, Bartlett TC, Browdy CL, Chapmann RW, Warr GW. Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific White Shrimp, Litopenaeus vannamei, and the Atlantic White Shrimp, L. setiferus. Develop Comp Immunol. 2001;25:565–577. doi: 10.1016/s0145-305x(01)00018-0. [DOI] [PubMed] [Google Scholar]
- 42.Jenny MJ, Ringwood AH, Lacy ER, Lewitus AJ, Kempton JW, Gross PS, et al. Potential indicators of stress response identified by Expressed Sequence Tag analysis of hemocytes and embryos from the American oyster, Crassostrea virginica. Mar Biotechnol. 2002;4:81–93. doi: 10.1007/s10126-001-0072-8. [DOI] [PubMed] [Google Scholar]
- 43.Gueguen Y, Cadoret JP, Flament D, Barreau-Roumiguière C, Girardot AL, Garnier J, et al. Immune gene discovery by expressed sequence tags generated from hemocytes of the bacteria-challenged oyster, Crassostrea gigas. Gene. 2003;303:139–145. doi: 10.1016/s0378-1119(02)01149-6. [DOI] [PubMed] [Google Scholar]
- 44.Venier P, Pallavicini A, De Nardi B, Lanfranchi G. Towards a catalogue of genes transcribed in multiple tissues of Mytilus galloprovincialis. Gene. 2003;314:29–40. doi: 10.1016/s0378-1119(03)00708-x. [DOI] [PubMed] [Google Scholar]
- 45.Donia M, Hamann MT. Marine natural products and their potential applications as anti-infective agents. Lancet Infect Dis. 2003;3:338–348. doi: 10.1016/S1473-3099(03)00655-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mayer AMS, Hamann MT. Marine pharmacology in 2000: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalaria, antiplatelet, antituberculosis, and antiviral activities; Affecting the cardiovascular, immune and nervous systems and other miscellaneous mechanisms of action. Mar Biotechnol. 2004;6:37–52. doi: 10.1007/s10126-003-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bullen J, Griffiths E, Rogers H, Ward G. Sepsis: the critical role of iron. Microbes Infect. 2000;2:409–415. doi: 10.1016/s1286-4579(00)00326-9. [DOI] [PubMed] [Google Scholar]
- 48.Nappi AJ, Ottaviani E. Cytotoxicity and cytotoxic molecules in invertebrates. BioEssays. 2000;22:469–480. doi: 10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]