Abstract
Propolis is a sticky substance that is collected from plants by honeybees. We previously demonstrated that propolins A, B, C, D, E and F, isolated from Taiwanese propolis (TP), could effectively induce human melanoma cell apoptosis and were strong antioxidant agents. In this study, we evaluated TP for free radical scavenging activity by DPPH (1,2-diphenyl-2-picrylhydrazyl). The phenolic concentrations were quantified by the Folin–Ciocalteu method. The apoptosis trigger activity in human melanoma cells was evaluated. TP contained a higher level of phenolic compounds and showed strong capability to scavenge free radicals. Additionally, TP1g, TP3, TP4 and TP7 exhibited a cytotoxic effect on human melanoma cells, with an IC50 of ∼2.3, 2.0, 3.3 and 3.3 μg/ml, respectively. Flow cytometric analysis for DNA fragmentation indicated that TP1g, TP2, TP3 and TP7 could induce apoptosis in human melanoma cells and there is a marked loss of cells from the G2/M phase of the cell cycle. To address the mechanism of the apoptosis effect of TP, we evaluated its effects on induction of apoptosis-related proteins in human melanoma cells. The levels of procaspase-3 and PARP [poly(ADP-ribose) polymerase] were markedly decreased. Furthermore, propolins A, B, C, D, E and F in TP were determined using HPLC. The results indicate that TP is a rich source of these compounds. The findings suggest that TP induces apoptosis in human melanoma cells due to its high level of propolins.
Keywords: Taiwanese propolis, propolins, radical scavenging activity, antioxidant activity, apoptosis, anti-tumor
Introduction
Propolis is a resinous hive product collected by honeybees from many plant sources. It can be yellow, green or brown depending on its source and collected season (1). Propolis is a traditional medicine used as early as 300 BC and has been reported to exert a broad spectrum of biological functions, including anticancer, anti-inflammatory, antibiotic, antioxidant and antifungal activities (2–6). It has recently gained popularity as a health food in various parts of the world, including Taiwan, Japan, Brazil, the USA and Europe, where it is claimed to promote health and prevent diseases such as cancer, inflammation, heart disease and diabetes. We are interested in the composition and biological properties of Taiwanese propolis (TP).
Propolis usually contains a variety of compositions such as terpenoids, steroids, flavonoids, phenolic acids and their esters. The composition of propolis depends on local flora, phenology of the plants and the vegetation at the site of collection. Due to the geographical difference, propolis samples from Asia, Europe and North and South America contain different chemical substances (3). The major components of propolis in Europe and China were flavonoids and phenolic acid esters (7). However, Brazilian propolis (BP) has, as its basis, terpenoids and prenylated derivatives of coumaric acids (8,9).
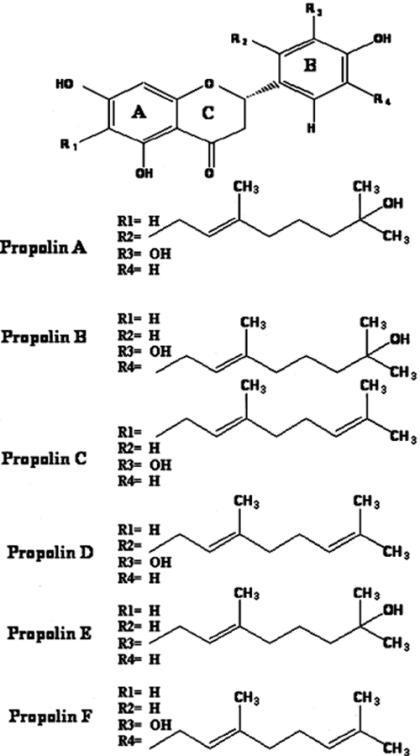
We previously identified six prenylflavanones from TP, flavonoid compounds with or without hydrated geranyl side chains, namely propolins A, B, C, D, E and F (10–12). However, these compounds included three novel ones: propolins A, B and E. Two well-known compounds, propolins C and D, were identical to the reported prenylflavanones compounds nymphaeol-A and nymphaeol-B (13) and were isolated for the first time from propolis. Propolin F was identical to the reported prenylated flavonoid compound isonymphaeol-B isolated from Japanese propolis (14). Propolins A, B and E have hydrated geranyl side chains, but propolins C, D and F have unhydrated geranyl side chains. In a previous study we demonstrated that six prenylflavanones induced apoptosis in human melanoma cells, significantly inhibited xanthine oxidase and had a strong capability to scavenge free radicals (10–12).
In this study, TP was collected from the Taipei, Taichung, Nantou and Tainan zones to compare free radical scavenging activity, phenolic levels and to evaluate cytotoxic effects and apoptosis in human melanoma cells. The apoptosis induction activity of TP in human melanoma cells was compared. The results indicated that TP was effective in inducing apoptosis in human melanoma cells, and also had a strong capacity to scavenge free radicals.
Materials and Methods
Extraction
TP collected from hives located in the areas of Taipei (TP1g, green propolis collected in Summer, and TP1b, black propolis collected in Winter), Taichung (TP4, TP5 and TP6), Nantou (TP2 and TP3) and Tainan (TP7). Propolis samples (1 g) were extracted by 95% ethyl alcohol (50 ml), sonicated for 1 h and left to stand for 23 h at 25°C. The filtered ethanol extract was evaporated to dryness under reduced pressure to yield a brown powder, which was kept at −20°C until used.
Purification and Identification of Propolins A, B, C, D, E and F
We found that an ethanol (95%) extract of TP induced cytotoxic effects in human melanoma cells. Ethanol extract was dissolved in methanol and applied to a Sephadex LH-20 column (Amersham Pharmacia Biotech AB, Uppsala, Sweden) using 95% ethanol as eluting solvent. All eluates, including fractions from the follow-up chromatographs, were assayed for human melanoma cytotoxic effects, and the active fractions were again chromatographed on Sephadex LH-20 column using 95% ethanol to elute. The active fractions were then subjected to silica gel column chromatography (Kiesel gel 60, E. Merck, Darmstadt 1, Germany) using a solvent system of n-hexane–EtOAc. Purification of the most active fraction (n-hexane:EtOAc, 30:70) was carried out on a reversed-phase preparative HPLC. Fractions of retention times at 16.5 and 22.0 min for propolins A and B, respectively, were collected. However, propolins C, D, E and F were purified from the active fractions, then subjected to silica gel column chromatography (Kiesel gel 60, E. Merck, Darmstadt 1, Germany) using a solvent system of n-hexane–EtOAc. Purification of the most active fraction (n-hexane:EtOAc, 40:60) was carried out on a reversed-phase preparative HPLC. Fractions of retention times at 9.5, 14.5, 18.5 and 25.0 min for propolins E, D, F and C, respectively, were collected. Conditions were as follows: column, Luna Phenomenex (C18, 250 × 10 mm, USA); solvent system, methanol:water (8:2); flow rate, 3 ml/min; detection, UV 280 nm. We identified the compounds as propolins A, B, C, D, E and F (Fig. 1). 1H- and 13C-NMR data are given in Tables 1 and 2.
Figure 1.
Chemical structures of propolins.
Table 1.
1H NMR assignments of propolins A, B, C, D, E and F
| Position | Propolin A δH | Position | Position B δH (J, Hz) | Position | Propolin C δH (J, Hz) | |||
|---|---|---|---|---|---|---|---|---|
| 2β | CH | 5.47 (dd,13.3, 2.7) | 2β | CH | 5.23 (dd,13.0, 3.0) | 2β | CH | 5.12 (dd, 12.8, 2.8) |
| 3β | CH2 | 2.60 (dd, 17.2, 2.7) | 3β | CH2 | 2.68 (dd, 17.2, 3.0) | 3β | CH2 | 2.60 (dd, 17.1, 2.8) |
| 3α | 3.10 (dd,17.2,13.3) | 3α | 3.03 (dd, 17.2,13.0) | 3α | 3.10 (dd, 17.1,12.8) | |||
| 6 | CH | 5.878 (d, 2.0) | 6 | CH | 5.86 (d,1.9) | 8 | CH | 5.85 (s) |
| 8 | CH | 5.882 (d, 2.0) | 8 | CH | 5.88 (d,1.9) | 2′ | CH | 6.81 (br s) |
| 5′ | CH | 6.72 (d, 8.3) | 2′ | CH | 6.76 (d,1.5) | 5′ | CH | 6.73 (d, 8.0) |
| 6′ | CH | 6.87 (d, 8.3) | 6′ | CH | 6.68 (d,1.5) | 6′ | CH | 6.68 (dd, 8.0, 1.4) |
| 1″ | CH2 | 3.46 (d, 6.3) | 1″ | CH2 | 3.33 (d,6.6) | 1″ | CH2 | 3.15 (d, 6.9) |
| 2″ | CH | 5.12 (t, 6.4) | 2″ | CH | 5.33 (t,7.0) | 2″ | CH | 5.12 (t, 6.9) |
| 4″ | CH3 | 1.64 (s) | 4″ | CH3 | 1.70 (s) | 4″ | CH3 | 1.66 (s) |
| 5″ | CH2 | 1.95 (t, 7.3) | 5″ | CH2 | 2.03 (t,6.8) | 5″ | CH2 | 1.85 (t, 6.9) |
| 6″ | CH2 | 1.42 (m) | 6″ | CH2 | 1.50 (m) | 6″ | CH2 | 1.95 (m) |
| 7″ | CH2 | 1.35 (t, 7.3) | 7″ | CH2 | 1.41 (t, 6.8) | 7″ | CH | 4.96 (t,6.9) |
| 9″ | CH3 | 1.12 (s) | 9″ | CH3 | 1.36 (s) | 9″ | CH3 | 1.46 (s) |
| 10″ | CH3 | 1.12 (s) | 10″ | CH3 | 1.36 (s) | 10″ | CH3 | 1.53 (s) |
| Position | Propolin D δH (J, Hz) | Position | Propolin E δH (J, Hz) | Position | Propolin F δH (J, Hz) | |||
|---|---|---|---|---|---|---|---|---|
| 2β | CH | 5.46 (dd, 13.4, 2.6) | 2β | CH | 5.20 (dd, 11.6, 3.0) | 2β | CH | 5.23 (dd, 12.4, 3.1) |
| 3β | CH2 | 2.60 (dd, 17.2, 2.6) | 3β | CH2 | 2.60 (dd, 17.1, 3.0) | 3β | CH2 | 2.60 (dd, 17.2, 3.1) |
| 3α | 3.10 (dd, 17.2, 13.4) | 3α | 3.10 (dd, 17.1, 11.6) | 3α | 3.10 (dd, 17.2,12.4) | |||
| 6 | CH | 5.88 (s) | 6 | CH | 5.86 (d, 2.0) | 6 | CH | 5.87 (d, 2.0) |
| 8 | CH | 5.88 (s) | 8 | CH | 5.88 (d, 2.0) | 8 | CH | 5.88 (d, 2.0) |
| 5′ | CH | 6.71 (d, 8.3) | 2′ | CH | 7.16 (d, 2.1) | 2′ | CH | 6.78 (d,1.8) |
| 5′ | CH | 6.77 (d, 8.2) | ||||||
| 6′ | CH | 6.87 (d, 8.3) | 6′ | CH | 7.10 (dd, 8.2, 2.1) | 6′ | CH | 6.67 (d, 1.8) |
| 1″ | CH2 | 3.46 (d, 6.6) | 1″ | CH2 | 3.32 (d, 6.6) | 1″ | CH2 | 3.34 (d, 7.0) |
| 2″ | CH | 5.10 (t, 6.6) | 2″ | CH | 5.30 | 2″ | CH | 5.31 (t, 7.0) |
| 4″ | CH3 | 1.64 (s) | 4″ | CH3 | 1.69 (s) | 4″ | CH3 | 1.69 (s) |
| 5″ | CH2 | 1.95 (t, 7.3) | 5″ | CH2 | 2.03 (t, 6.8) | 5″ | CH2 | 2.02 (t, 7.3) |
| 6″ | CH2 | 2.01 (m) | 6″ | CH2 | 1.50 (m) | 6″ | CH2 | 2.09 (m) |
| 7″ | CH | 5.02 (t, 7.3) | 7″ | CH2 | 1.40 (t, 6.8) | 7″ | CH | 5.09 (t, 7.3) |
| 9″ | CH3 | 1.54 (s) | 9″ | CH3 | 1.13 (s) | 9″ | CH3 | 1.55 (s) |
| 10″ | CH3 | 1.59 (s) | 10″ | CH3 | 1.13 (s) | 10″ | CH3 | 1.61 (s) |
Table 2.
13C NMR data of propolins A, B, C, D, E and F
| Carbon No. | Propolin A δC | Propolin D δC | Propolin B δC | Propolin F δC | Propolin C δC | Propolin E δC |
|---|---|---|---|---|---|---|
| 2 | 77.8 | 77.8 | 80.7 | 80.7 | 79.9 | 80.6 |
| 3 | 43.7 | 43.7 | 44.1 | 44.0 | 43.6 | 44.2 |
| 4 | 198.2 | 198.2 | 197.7 | 197.8 | 197.3 | 197.8 |
| 5 | 165.2 | 165.1 | 164.8 | 164.8 | 162.2 | 164.8 |
| 6 | 97.1 | 97.1 | 97.0 | 97.1 | 109.0 | 97.0 |
| 7 | 168.3 | 168.4 | 168.5 | 168.5 | 165.0 | 168.4 |
| 8 | 96.2 | 96.2 | 96.2 | 96.3 | 95.3 | 96.2 |
| 9 | 165.5 | 165.5 | 165.4 | 165.4 | 161.9 | 165.2 |
| 10 | 103.2 | 103.2 | 103.4 | 103.3 | 103.0 | 103.4 |
| 1′ | 129.7 | 129.7 | 130.9 | 130.8 | 131.6 | 130.9 |
| 2′ | 128.3 | 128.3 | 111.9 | 112.0 | 114.6 | 128.9 |
| 3′ | 144.5 | 144.4 | 146.1 | 146.1 | 145.1 | 129.5 |
| 4′ | 146.5 | 146.5 | 144.6 | 144.6 | 146.4 | 156.7 |
| 5′ | 113.6 | 113.6 | 129.7 | 129.7 | 115.9 | 119.5 |
| 6′ | 118.7 | 118.7 | 119.8 | 119.7 | 119.1 | 126.3 |
| 1″ | 25.4 | 25.4 | 29.2 | 29.1 | 21.5 | 29.1 |
| 2″ | 124.7 | 124.6 | 123.9 | 123.8 | 123.5 | 123.7 |
| 3″ | 135.8 | 135.7 | 137.0 | 136.9 | 134.9 | 137.2 |
| 4″ | 16.2 | 16.4 | 16.1 | 16.2 | 16.2 | 16.1 |
| 5″ | 41.2 | 40.7 | 41.3 | 40.8 | 40.5 | 41.2 |
| 6″ | 23.7 | 27.7 | 23.7 | 27.6 | 27.4 | 23.7 |
| 7″ | 44.3 | 125.3 | 44.3 | 125.3 | 125.1 | 44.3 |
| 8″ | 71.5 | 132.3 | 71.4 | 132.3 | 131.6 | 71.5 |
| 9″ | 29.1 | 17.7 | 29.1 | 17.8 | 17.7 | 29.2 |
| 10″ | 29.2 | 25.8 | 29.2 | 25.8 | 25.8 | 29.1 |
Assignments were based on the HMQC and HMBC NMR data.
Preparation of Samples and Standards
TP extracts were prepared as 2% (w/v) extract solutions. The ethyl alcohol extracts of TP were made by sonicating them for 1 h, and letting them stand for 23 h at 25°C. The extracts were then filtered through MILLEX-GS 0.22 μm filter (Millipore, Malsheim, France) to remove impurities. All TP extracts contained propolins A, B, C, D, E and F (the chemical structures of which are depicted in Fig. 1). All standards were dissolved in ethyl alcohol and prepared at 1 mg/ml. Propolins were dissolved in standard solutions. Propolin concentrations were determined using linear calibration curves based on the peak height for each propolin. Each calibration curve contained six different concentrations of propolins: 1000, 500, 250, 125, 62.5 and 31.25 μg/ml.
Analysis Conditions
The composition of propolins in TP extracts was analyzed with a reversed-phase preparative HPLC. The conditions were: column, Luna Phenomenex (C18, 250 × 4.6 mm, USA); solvent system, methanol:water (82:18); flow rate, 1.5 ml/min; detection, UV 280 nm.
Determination of Total Phenolics
The concentration of total phenolic compounds in the fractions was determined spectrophotometrically using Folin–Ciocalteu reagent (15). The TP extract (0.1 ml) was diluted with deionized water (7.9 ml). Folin–Ciocalteu reagent (0.5 ml) was added, and the contents were mixed thoroughly. After 1 min, 0.2 ml of 7.5% (w/v) sodium carbonate solution was added, and the mixture was mixed thoroughly. The absorbance of the blue color produced was measured with a spectrophotometer at 765 nm. Phenolic content was expressed in milligrams per gram of dry weight (TP) based on a standard curve of catechin (C), which was expressed as milligrams per gram of catechin equivalent (CE).
Stable Free Radical Scavenging Capacity
The free radical scavenging capacity (10) of TP extract was measured with DPPH (1,2-diphenyl-2-picrylhydrazyl). The DPPH radical has a deep violet color due to its unpaired electron and radical scavenging capability can be followed spectrophotometrically by absorbance loss at 517 nm when the pale yellow non-radical form is produced. DPPH (0.5 ml, 0.15 mM) was added to the TP extract (30 μg/ml). The contents were mixed in a cuvette, and the optical density change at 517 nm was followed for 1 min in a UV/VIS U-3210 spectrophotometer. The absorbance of the control (DPPH radical without sample) was measured daily. The percent of DPPH decoloration of the sample was calculated according to the formula: % decoloration = (1 − Abs sample/Abs control) × 100.
Cell Culture and Cytotoxicity Assay
Human melanoma cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco), containing 10% fetal bovine serum (Gibco) and 1% penicillin–streptomycin, and maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells (1 × 105)/well were cultured in a 12-well plate and at various concentrations of TP for 24 h. Treated cells were counted, and cell viability was determined by a trypan blue exclusion assay.
Analysis of the Cell Cycle
Human melanoma cells (1 × 106) in a 100-mm dish were treated with TP extracts at a concentration of 20 μg/ml for 6 h. Cells were trypsinized and collected with ice-cold phosphate-buffered saline (PBS). The cells were resuspended in 200 μl PBS, and fixed in 800 μl of iced 100% ethanol at −20°C. After being left to stand overnight, the cell pellets were collected by centrifugation, resuspended in 1 ml of hypotonic buffer (0.5% Triton X-100 in PBS and 0.5 μg/ml RNase A), and incubated at 37°C for 30 min. Then, 1 ml of propidium iodide solution (50 μg/ml) was added, and the mixture was allowed to stand at 4°C for 30 min. Fluorescence emitted from the propidium iodide–DNA complex was quantitated after excitation of the fluorescent dye by FACScan cytometry (Becton Dickinson, San Jose, CA).
Western Blotting Assay
Human melanoma cells on 100-mm culture dishes (1.5 × 106/dish) were treated with TP at 20 μg/ml for 6 h. After treatment, cells were collected and resuspended in 100 μl Gold lysis buffer. Equal amounts of proteins (20 μg), were mixed with 5× sample buffer and resolved by 12% SDS–PAGE for β-actin, PARP and procaspase-3 detection. Proteins were electrotransferred to an immobilon membrane (PVDF; Millipore Corp, Bedford, MA), and equivalent protein loading was verified by staining the membrane with reversible dye amido black (Sigma Chemical Co.). This was followed by overnight blocking with a solution composed of 20 mM Tris–HCl (pH 7.4), 125 mM NaCl, 0.2% Tween 20, 4% non-fat dried milk and 0.1% sodium azide. Expression of β-actin, PARP and procaspase-3 was monitored by immunoblotting using specific antibodies: 1:500 of rabbit polyclonal antibodies to human PARP (Santa Cruz Biotechnology, Santa Cruz, CA), anti-procaspase-3 antibody (Pharmingen, Becton Dickinson, San Diego, CA) and anti-β-actin antibody (Cashmere Biotech, USA). These proteins were detected by chemiluminescence (ECL, Amersham).
Results
Purification and Identification of Propolins A, B, C, D, E and F
Propolins A, B, C, D, E and F (Fig. 1) were isolated through repeated chromatography of the 95% ethanol extract of the propolis glue under the guidance of A2058 cells proliferation. Final purification of the active fraction was achieved by HPLC on a reversed phase column. Previously, studies had shown that the total proportions of the active components, propolins D, E and F, in the Taiwanese propolis glue were roughly 4.8, 1.2 and 6.0%, respectively. Both 1H- and 13C-NMR (Tables 1 and 2 in CD3OD) of propolins D and F displayed absorptions characteristic of the prenylflavanone-type, very similar to those of propolins A and B, which were isolated from the same source (3 and 1%, respectively) with more polar components (10). The HRMS of a pure sample of propolins D and F indicated an M+ of 424 with the molecular composition C25H28O6. Obviously, a water molecule was lost from the geranyl side chains. Propolin D was identical to nymphaeol-B, a known compound isolated from Hernandia nymphaefolia, which has the same geranyl group on the B ring at C-2 (13). Furthermore, propolin F was identical to isonymphaeol-B, a known compound isolated from propolis collected in Okinawa, which has the same geranyl group on the B ring at C-5 (14). Moreover, the geranyl substitution of propolin E is on the B-ring at C-3, judging from the coupling patterns of the two aromatic rings (H 5.86, d, J = 2.0 Hz; 5.88, d, J = 2.0 Hz; 6.77, d, J = 8.2 Hz; 7.16, d, J = 2.1 Hz; and 7.10, dd, J = 8.2, 2.1 Hz). The HRMS of a pure sample of propolin E indicated an M+ of 426 with the molecular composition C25H30O6. Upon comparison of the 13C-NMR of propolin D in CD3OD with the reported data of nymphaeol-B, both compounds could be identified. However, propolin E was a novel compound and characterized from TP. Nymphaeol-B (propolin D) had been previously identified, but its biological function in cells has never been characterized. In Tables 1 and 2, a complete assignment of each proton and carbon of propolins A, B, C, D, E and F in d-methanol has been compiled on the basis of the 2-D NMR (HMQC, HMBC, NOESY) performed.
Total Phenolic Content
The total phenolic content of TP extracts ranged from 335 to 210 mg of CE/g of dry extract (Table 3). In general, TP (especially TP1g, TP2, TP3, TP4 and TP7) was found to have a higher phenolic content than TP1b. In all cases, the lowest amounts of phenolics were found in TP1b and TP5, ranging from 210 to 250 mg of CE/g of dry extract (Table 3).
Table 3.
Extraction of percentage, total phenolic content and radical scavenging activity of the TP extracts*
| Propolis | Extract dry weight (%)† | Total phenolic content‡ | DPPH radical scavenged (%)§ |
|---|---|---|---|
| TP1g | 84.4 ± 5.7 | 320 ± 15.0 | 49.0 ± 3.5 |
| TP1b | 62.0 ± 4.1 | 210 ± 20.0 | 33.0 ± 2.5 |
| TP2 | 80.2 ± 3.3 | 310 ± 25.0 | 41.0 ± 3.0 |
| TP3 | 75.6 ± 3.6 | 335 ± 20.0 | 40.5 ± 2.5 |
| TP4 | 65.7 ± 4.2 | 315 ± 10.0 | 30.0 ± 2.0 |
| TP5 | 54.6 ± 4.0 | 250 ± 10.0 | 13.0 ± 1.0 |
| TP6 | 68.5 ± 4.7 | 275 ± 15.0 | 43.0 ± 3.5 |
| TP7 | 77.8 ± 3.8 | 330 ± 25.0 | 43.5 ± 3.0 |
*Values are the mean of three replicates ± SE.
†Values expressed as mg of dry extract/1000 mg of propolis.
‡Values expressed as mg of CE/g of dry extract.
§Values expressed as free radical scavenging efficiency was measured with 30 μg/ml of TP extracts.
Free Radical Scavenging Activity
The free radical scavenging activities of the TP extracts (30 μg/ml) are also shown in Table 3. The values of the free radical (DPPH) scavenging activity ranged from 49.0 to 13.0% in TP extracts. The results indicate that TP extracts exhibit strong potential free radical scavenging activity.
Total Phenolic Content Versus Radical Scavenging Activity
Among all the extracts analyzed, a higher level of phenolic content radical scavenging activity was found in TP extracts (Table 3). In general, extracts with a higher radical scavenging activity showed a higher phenolic content, and this was the case with TP1g, TP2, TP3, TP4 and TP7. The percentage of the extraction ranged from 84.4 to 54.6% for TP extracts. TP extracts contained a high level of phenolic content and exerted high free radical scavenging activity as shown in Table 3.
Inhibition of Cell Growth by TP Extracts
The cytotoxic effects of TP extracts were evaluated in human melanoma cells. As shown in Table 4, treatment with TP extracts at a concentration of 1.25–40 μg/ml showed a dose-dependent decrease in cell viability. The IC50 of TP extracts on human melanoma cells ranged from 2.3 to 20.0 μg/ml. The growth-inhibited activity of TP1g (collected in Summer) extracts was much stronger than that of TP1b (collected in Winter) extracts from the same zone (Taipei). The results indicated that TP composition differs depending on season, local flora and plant phenology. However, treatment of human melanoma cells with TP extracts for 24 h induced cell morphological changes. Morphological features such as condensation of chromatin of the nucleus and cell shrinkage were observed in TP extract-treated cells. It appears that the cell morphologic changes are triggered by cell apoptosis.
Table 4.
Cytotoxic activity of TP in human melanoma cells
| Propolis | Cell viability (%) at the indicated concentrations (μg/ml) | IC50(μg/ml) | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1.25 | 2.5 | 5.0 | 10.0 | 20.0 | 40.0 | ||
| TP1g | 100 | 72 ± 4.5 | 31 ± 2.5 | 4 ± 0.3 | ND | ND | ND | 2.3 |
| TP1b | 100 | 98 ± 5.5 | 99 ± 6.0 | 99 ± 5.5 | 75 ± 3.5 | 42 ± 2.5 | 2 ± 0.2 | 20.0 |
| TP2 | 100 | 98 ± 6.5 | 96 ± 5.0 | 88 ± 4.5 | 12 ± 1.5 | 2 ± 0.1 | ND | 9.5 |
| TP3 | 100 | 68 ± 3.5 | 6 ± 0.5 | 2 ± 0.2 | ND | ND | ND | 2.0 |
| TP4 | 100 | 99 ± 7.0 | 73 ± 4.3 | 5 ± 0.3 | ND | ND | ND | 3.3 |
| TP5 | 100 | 98 ± 5.5 | 90 ± 5.0 | 24 ± 2.5 | 2 ± 0.1 | ND | ND | 5.0 |
| TP6 | 100 | 97 ± 6.0 | 95 ± 5.0 | 80 ± 4.5 | 3 ± 0.2 | ND | ND | 7.0 |
| TP7 | 100 | 99 ± 7.0 | 78 ± 4.5 | 3 ± 0.5 | ND | ND | ND | 3.2 |
Cells were treated with TP crude extracts at the indicated concentrations (1.25–40 μg/ml) for 24 h. Each cell viability value is the mean ± SE of three independent experiments by a trypan blue exclusion assay. ND, not determined.
Relation Between Cell Cycle and DNA Fragmentation Caused by TP Extract Treatment
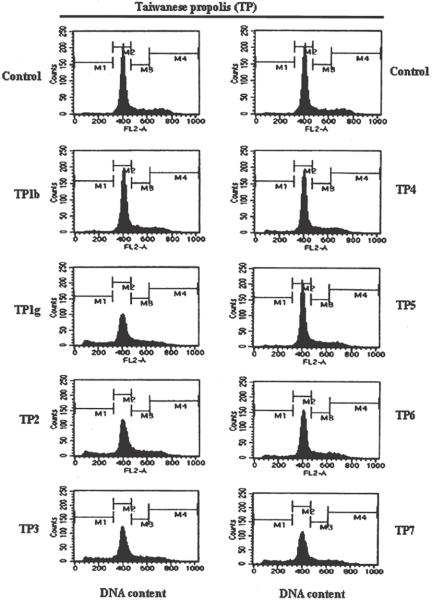
To determine the extent of cell apoptosis, DNA fragmentation represented by the sub-G1 peak in flow cytometry, human melanoma cells treated with TP extracts at a concentration of 20 μg/ml for 6 h were analyzed by flow cytometry, as shown in Fig. 2. The percentages of apoptotic human melanoma cells observed after TP1g, TP2, TP3, TP6 and TP7 extract treatment for 6 h were as follows: 16.90, 12.45, 14.93, 6.71 and 14.63%, respectively. We next questioned whether cell damage might be attributable to the cell cycle program by TP extract treatment. Table 5 shows that this is not the case. Our data allow us to conclude that TP1g, TP2, TP3 and TP7 extracts had markedly induced cytotoxic effects compared with TP1b and TP5 extracts on human melanoma cells, but these effects are mediated by apoptosis, and there is a marked loss of cells from the G2/M phase of the cell cycle.
Figure 2.
TP extracts induced apoptosis in human melanoma cells. Effect of TP extracts on the cellular sub-G1 and DNA content. The cells were treated with 20 μg/ml of TP (TP1g–TP7) extracts for 6 h and stained with PI as described in Materials and Methods. Following flow cytometric analysis, cellular DNA profiles were further analyzed by Cell Quest software. Results are from one experiment but are representative of three similar experiments.
Table 5.
Observation of the DNA content of human melanoma cells induced by TP extracts by flow cytometry
| Propolis | DNA content | |||
|---|---|---|---|---|
| M1 (sub-G1 phase) | M2 (G1 phase) | M3 (S phase) | M4 (G2/M phase) | |
| Control | 2.02 | 71.80 | 14.87 | 12.07 |
| TP1b | 2.35 | 75.63 | 14.81 | 8.04 |
| TP1g | 16.90 | 61.27 | 15.87 | 7.24 |
| TP2 | 12.45 | 62.88 | 17.19 | 8.58 |
| TP3 | 14.93 | 61.61 | 15.66 | 8.97 |
| TP4 | 4.40 | 72.33 | 15.82 | 8.33 |
| TP5 | 3.02 | 71.46 | 17.32 | 9.16 |
| TP6 | 6.71 | 68.61 | 16.56 | 9.11 |
| TP7 | 14.63 | 60.29 | 17.13 | 9.10 |
Human melanoma cells were treated with TP extracts at 20 μg/ml for 6 h. The data used are from one of three independent experiments.
TP Extracts Induce Apoptosis via Activated Caspase-3 and PARP
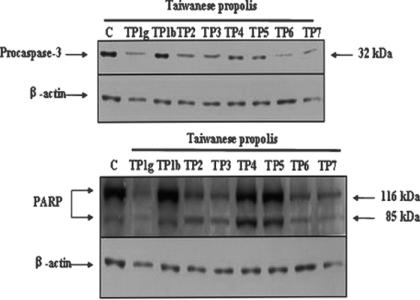
Initiator capases (including 8, 9, 10 and 12) are closely coupled to proapoptotic signals. Once activated, these caspases cleave and activate downstream effector caspases (including 3, 6 and 7), which in turn cleave cytoskeletal and nuclear proteins such as PARP and lamin A, and finally induce apoptosis. We tried to evaluate whether caspase-3 signals were involved in the apoptotic cell death induced by TP extracts in human melanoma cells. Treatment of human melanoma cells with TP extracts at a concentration of 20 μg/ml for 6 h resulted in dramatic decreases in PARP and procaspase-3 protein levels (Fig. 3). The data indicated that TP1g, TP2, TP3, TP4, TP6 and TP7 extracts were much more active than TP1b and TP5 in activating caspase-3 and PARP on human melanoma cells.
Figure 3.
Induction of apoptosis via caspase-3 and activation of PARP. Human melanoma cells were incubated with 20 μg/ml of TP extracts for 6h. Cell lysates were prepared, and subjected to SDS–PAGE. Procaspase-3 and PARP were determined by immunoblotting using specific antibodies. The results are representative of those from three separate experiments.
Separation of Authentic Standard Propolins by HPLC
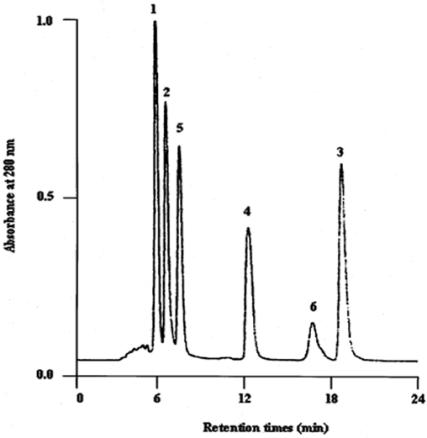
We further investigated various propolis compositions capable of inducing apoptosis on human melanoma cells. Our previous studies had demonstrated that propolins were a powerful inducer of apoptosis in human melanoma cells. A mixture of six propolins (A, B, C, D, E and F) were separated by HPLC as described in Materials and Methods, and a baseline resolution was achieved as shown in Fig. 4. The HPLC separation of these propolis constituents was accomplished in 24 min.
Figure 4.
HPLC separation of standard authentic compounds. The peaks are:1, propolin A (500 μg/ml); 2, propolin B (500 μg/ml); 3, propolin C (500 μg/ml); 4, propolin D (500 μg/ml); 5, propolin E (500 μg/ml); and 6, propolin F (500 μg/ml). The propolins were analyzed with a reversed-phase preparative HPLC. The conditions were: column-Luna Phenomenex (C18, 250 × 4.6 mm); solvent system, methanol:water (82:18); flow rate: 1.5 ml/min; detection, UV 280 nm.
HPLC Separation of Propolins in TP Extracts
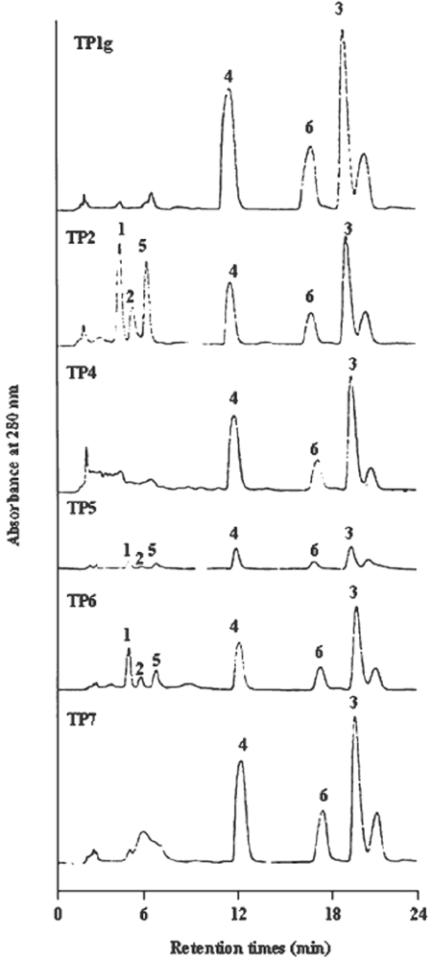
The HPLC profiles of TP extracts are shown in Fig. 5. We found that propolins C, D and F were most prominent amongst the TP extracts (especially those of TP1g, TP2, TP3, TP4 and TP7). Propolin D appeared at similar levels to propolin C. More propolin F was found in TP1g, TP2, TP3, TP4 and TP7 than in other TP extracts, while propolin E was only found in TP2. The highest amounts of both propolins A and B were contained in TP1b and TP2. These results are all shown in Table 6. The results indicate that TP contains higher levels of propolins C, D and F.
Figure 5.
Analysis of TP. The composition of propolins in TP extracts was analyzed with reversed-phase preparative HPLC. The conditions were: column-Luna Phenomenex (C18, 250 × 4.6 mm); solvent system, methanol:water (82:18); flow rate: 1.5 ml/min; detection, UV 280 nm.
Table 6.
Levels of propolin A, B, C, D, E and F in TP (mg/g of propolis)
| Propolis | Propolin A | Propolin B | Propolin C | Propolin D | Propolin E | Propolin F |
|---|---|---|---|---|---|---|
| TP1g | ND | ND | 106.5 ± 5.9 | 103.5 ± 8.5 | ND | 193.0 ± 14.5 |
| TP1b | 29.5 ± 3.7 | 66.5 ± 4.6 | ND | 5.0 ± 0.4 | ND | ND |
| TP2 | 25.5 ± 2.0 | 8.6 ± 0.7 | 64.2 ± 4.3 | 46.5 ± 3.5 | 30.9 ± 2.8 | 90.6 ± 7.4 |
| TP3 | ND | ND | 98.8 ± 6.8 | 45.7 ± 3.2 | ND | 188.1 ± 11.6 |
| TP4 | ND | ND | 63.4 ± 3.6 | 58.8 ± 3.5 | ND | 90.6 ± 6.3 |
| TP5 | ND | ND | 6.7 ± 0.3 | 3.4 ± 0.2 | ND | 16.9 ± 1.8 |
| TP6 | 0.31 ± 0.03 | ND | 43.6 ± 2.7 | 29.9 ± 2.0 | ND | 59.4 ± 3.1 |
| TP7 | ND | ND | 84.0 ± 4.5 | 86.9 ± 6.3 | ND | 149.4 ± 12.0 |
Samples were prepared as described in text. TP1 g and TP1b samples were purchased from Taipei, TP2 and TP3 samples were purchased from Nantou, TP4, TP5 and TP6 samples were purchased from Taichung, TP7 was purchased from Tainan. The values are averages (n = 3), and are given in 1 g of propolis on 50 ml 95% alcohol, or 2% (w/v).
Induction of Apoptosis on Human Melanoma Cells by Propolins
We further evaluated whether propolins of TP induce apoptosis on human melanoma cells. The cytotoxicity effect of six different propolins was first evaluated in human melanoma cells (Table 7). Treatment with propolins in various concentrations (1.25–40 μg/ml) caused a dose-dependent cytotoxicity. The IC50 of propolins A, B, C, D, E, F and CAPE (caffeic acid phenethyl ester, one of the components isolated and identified from propolis), on human melanoma cells were 6.0, 7.5, 3.6, 3.4, 5.0, 15.0 and 8.5 μg/ml, respectively. The growth-inhibiting activity of propolins were ranked as follows: propolin D > propolin C > propolin E > propolin A > propolin B > propolin F. Our previous studies had demonstrated that propolin C was much more active than CAPE in inhibiting the growth of cancer cells (11). Taken together, these findings suggest that propolins of TP extracts contribute to apoptosis induction in human melanoma cells.
Table 7.
Cytotoxic activity of propolins and CAPE in human melanoma cells
| Compound | IC50 (μg/ml) |
|---|---|
| Propolin A | 6.0 |
| Propolin B | 7.5 |
| Propolin C | 3.6 |
| Propolin D | 3.4 |
| Propolin E | 5.0 |
| Propolin F | 10.0 |
| CAPE | 8.5 |
Cells were treated with propolins and CAPE at concentrations ranging from 1.25 to 40 μg/ml for 24 h.
Coordinated Effect of Propolins on Apoptosis Induction in Human Melanoma Cells
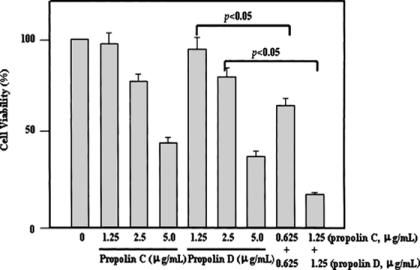
TP extracts (especially those of TP1g, TP3, TP4 and TP7) were found to contain high levels of propolin C and propolin D, and the IC50 was low for both of these propolins. We next questioned whether propolin C plus propolin D could have a synergistic effect on apoptosis induction in human melanoma cells. Our results indicated that propolin C plus propolin D (0.625 + 0.625 or 1.25 + 1.25 μg/ml) displays much stronger apoptosis induction in human melanoma cells than either propolin C and D alone at the same total dose (1.25 or 2.5 μg/ml), as shown in Fig. 6.
Figure 6.
Cooperation of the propolins to induce cytotoxic effect in human melanoma cells. The cells were treated with varying concentrations (1.25–5.0 μg/ml of propolin C and propolin D) and combinations of propolin C and propolin D at the same concentrations (0.625 + 0.625 or 1.25 + 1.25 μg/ml) for 12 h and subsequent cell viability was measured by a trypan blue exclusion assay. Results represent the mean ± SE of three independent experiments.
Discussion
Propolis, a complex mixture of plant metabolites, possesses a broad spectrum of biological activity including antibiotic, anticancer, antioxidant and anti-inflammatory activities (2–4). The chemical composition of propolis is complicated and varied. Location, season and environmental condition are important factors influencing propolin content of propolis (16–18). In our previous work, we found that the alcohol extract of TP had interesting antiproliferative activity against six different cancer cell lines, including human melanoma cells (A2058), murine melanoma cells (B16F10), human breast cancer cells (MCF-7), human hepatoblastoma cells (HepG2), human hepatocellular carcinoma cells (Hep3B) and human colon adenocarcinoma cells (HT-29) (10–12). Six prenylflavanone compounds were isolated and characterized from TP and called propolins A, B, C, D, E and F through an antiproliferative activity-guided purification. Two of these compounds, propolins C and D, were found to be identical to the reported prenylflavanone compounds nymphaeol-A and nymphaeol-B, respectively (13). However, no biological activities of these two compounds have ever been reported. The isolation and characterization of propolin C and propolin D from bee propolis are described for the first time; these two compounds are powerful inducers of apoptosis in human melanoma cells.
The aim of this study was to determine phenolic levels, free radical scavenging activity, cytotoxic effects and induction of apoptosis in human melanoma cells of TP extracts. Our data indicated that TP extracts exhibited potent antioxidative activities as shown in Table 3. TP extracts also showed relatively high levels of phenolic concentrations as compared with BP (data not shown). Taken together, we suggest that TP extracts contained higher levels of phenolic compounds and scavenged free radicals more efficiently. We further investigated the cytotoxic effects of the TP and BP extracts. Our data indicated that TP extracts (especially those of TP1g, TP3, TP4 and TP7) are more effective inducers of cytotoxic activity than BP extracts (data not shown). The IC50 values for TP1g, TP3, TP4 and TP7 were 2.3, 2.0, 3.3 and 3.2 μg/ml, respectively. TP1b (black propolis, collected in Winter) and TP1g (green propolis, collected in Summer) are TP extracts collected from Taipei. TP1b was found to possess the lowest total phenol content (210 mg/g) and contained the highest levels of propolin A and propolin B in HPLC analysis. TP1g was found to possess the highest amount of total phenol content (320 mg/g) and contained the highest levels of propolin C (106.5 mg/g) and propolin D (103.5 mg/g). Taken together, these findings suggest that plants and season are key factors influencing propolis composition.
Propolis from different continents was reported to have cytotoxic and antitumor effects (19–22). In this study, we found that propolins (especially propolins D, C, E, A and B) had strong antitumor effects and could be identified as unique constituents of TP in HPLC analysis as shown in Fig. 5, while BP (four samples) was undetectable by HPLC assay (data not shown). These results suggest that propolins are special components of TP that can confer cytotoxic effects. In addition, the IC50 of ethyl alcohol extracts from TP (especially those of TP1g, TP3, TP4 and TP7) was lower than that of propolin C or propolin D treatment alone, indicating that propolin C and propolin D can exert a synergistic effect on apoptosis induction in human melanoma cells as shown in Fig. 6. These data demonstrate that TP contains potent antioxidant compounds, propolins, powerful inducers of apoptosis in human melanoma cells and prenylflavanone compounds, as shown in Tables 1 and 2.
Acknowledgments
We thank Chun-Mao Lin for providing excellent technical support. The present research was supported by the National Science Council, NSC 91-2320-B-002-068.
References
- 1.Ghisalberti EL. Propolis: a review. Bee World. 1979;60:59–84. [Google Scholar]
- 2.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–571. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 3.Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 4.Burdock GA. Review of the biological properties and toxicity of bee propolis (Propolis) Food Chem Toxicol. 1998;36:347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 5.Grunberger D, Banerjee R, Eisinger K, Oltz EM, Efros L, Caldwell M, et al. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experimentia. 1988;44:230–232. doi: 10.1007/BF01941717. [DOI] [PubMed] [Google Scholar]
- 6.Dobrowolski JW, Vohora SB, Sharma K, Shah SA, Naqvi SAH, Dandiya PC. Antibacterial, antifungal, antiamoebic, anti-inflammatory and antipyretic studies on propoli bee products. J Ethnopharmacol. 1991;35:77–82. doi: 10.1016/0378-8741(91)90135-z. [DOI] [PubMed] [Google Scholar]
- 7.Bankova VS, Castro SLD, Marcucci MC. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. [Google Scholar]
- 8.Tazawa S, Warashina T, Noro T. Studies on the constituents of Brazilian propolis II. Chem Pharm Bull. 1999;47:1388–1392. [Google Scholar]
- 9.Marcucci MC, Bankova V. Chemical composition, plant origin and biological activity of Brazilian propolis. Curr Topics Phytochem. 1999;2:115–123. [Google Scholar]
- 10.Chen CN, Wu CL, Shy HS, Lin JK. Cytotoxic prenylflavanones from Taiwanese propolis. J Nat Prod. 2003;66:503–506. doi: 10.1021/np0203180. [DOI] [PubMed] [Google Scholar]
- 11.Chen CN, Wu CL, Lin JK. Propolin C from propolis induces apoptosis through activating caspases, Bid, and cytochrome c release in human melanoma cells. Biochem Pharmacol. 2004;67:53–66. doi: 10.1016/j.bcp.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Chen CN, Wu CL, Lin JK. Apotosis of human melanoma cells induced by the novel compounds propolin A and propolin B from Taiwanese propolis. J Cell Biochem. doi: 10.1016/j.canlet.2006.01.016. in press. [DOI] [PubMed] [Google Scholar]
- 13.Yakushijin K, Shibayama K, Murata H, Furukawa H. New prenylflavanones from Hernandia nymphaefolia (Presl) kubitzki. Heterocycles. 1980;14:397–401. [Google Scholar]
- 14.Kumazawa S, Goto H, Hamasaka T, Fukumoto S, Fujimoto T, Nakayama T. A new prenylated flavonoid from propolis collected in Okinawa, Japan. Biosci Biotechnol Biochem. 2004;68:260–262. doi: 10.1271/bbb.68.260. [DOI] [PubMed] [Google Scholar]
- 15.Julkunen-Tiitto R. Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J Agric Food Chem. 1985;33:213–217. [Google Scholar]
- 16.Vanhaelen M, Vanhaelen-Fastre R. Propolis-Origine, Micrographie, Composition Chimique et Activite therapeutique. J Pharma Belgique. 1979;34:253–259. [PubMed] [Google Scholar]
- 17.Cheng PC, Wong G. Honey bee propolis: prospects in medicine. Bee World. 1996;77:8–15. [Google Scholar]
- 18.Oliverira VC, Bastos EM. Morphological and anatomical aspects of the leaf of Baccharis dracunculifolia DC. (Asteraceae) as regards to the identification of the botanical origin of propolis. Acta Botanica Brasilica. 1998;12:431–439. [Google Scholar]
- 19.Banskota AH, Tezuka Y, Prasain JK, Matsushige K, Saiki I, Kadota S. Chemical constituents of Brazilian propolis and their cytotoxic activity. J Nat Prod. 1998;61:896–900. doi: 10.1021/np980028c. [DOI] [PubMed] [Google Scholar]
- 20.Song YS, Park EH, Jung KJ, Jin C. Inhibition of angiogenesis by propolis. Arch Pharm Res. 2002;25:500–504. doi: 10.1007/BF02976609. [DOI] [PubMed] [Google Scholar]
- 21.Usia T, Banskota AH, Tezuka Y, Midorikawa K, Matsushige K, Kadota S. Constituents of Chinese propolis and their antiproliferative activities. J Nat Prod. 2002;65:673–676. doi: 10.1021/np010486c. [DOI] [PubMed] [Google Scholar]
- 22.Banskota AH, Nagaoka T, Sumioka LY, Tezuka Y, Awale S, Midorikawa K, et al. Antiproliferative activity of the Netherlands propolis and its active principles in cancer cell lines. J Ethnopharmacol. 2002;80:67–73. doi: 10.1016/s0378-8741(02)00022-3. [DOI] [PubMed] [Google Scholar]