Abstract
Given the dual role of CD4 T cells as both immune effectors and targets for HIV infection, the balance of CD4 versus CD8 T cell-mediated responses induced by candidate AIDS vaccines may be critical in determining postvaccination infection outcomes. An attenuated recombinant varicella-zoster virus vaccine expressing the simian immunodeficiency virus (SIV) envelope (Env) elicited nonneutralizing Env-binding antibodies and little if any cytotoxic T lymphocyte responses in rhesus macaques (Macaca mulatta). After challenge with SIV, Env vaccinees manifested increased levels of SIV replication, more rapid CD4 depletion, and accelerated progression to AIDS compared with controls. Enhanced SIV replication correlated with increased CD4 T cell proliferation soon after SIV challenge, apparently the result of an anamnestic response to SIV antigens. Thus activation of virus-specific CD4 T cells at the time of exposure to a CD4 T cell-tropic lentivirus, in the absence of an effective CD8 response, may enhance virus replication and disease. These data suggest suggest that candidate AIDS vaccines may not simply be either efficacious or neutral; they may also have the potential to be harmful.
HIV vaccine studies strive to induce virus-specific CD4 and CD8 responses, but there have been no systematic attempts to manipulate the relative strengths of these responses to determine what might be the best balance to enable the most effective viral control and protection from disease progression. Rather, the nature of vaccine-elicited immune responses has simply been a function of the inherent characteristics of the vaccine used. An appropriate balance of CD4 and CD8 responses may be particularly important for HIV, because it preferentially replicates in activated HIV-specific CD4 T cells (1, ††), which are also key effectors required for effective immune control of HIV replication.
This study investigated the immunogenicity and protective efficacy of a replication-competent recombinant AIDS vaccine (rVZV-SIVenv) based on attenuated varicella-zoster virus (VZV) vaccine VZV-Oka, the only vaccine approved by the U.S. Food and Drug Administration that establishes persistent infection and may periodically boost immunity by intermittent virus reactivation, providing long-term protection (2). The broadly reactive cellular and humoral immune responses induced by VZV-Oka, together with an impressive safety record in immunocompromised people, make it an attractive vector for AIDS vaccine development. After challenge with the pathogenic simian immunodeficiency virus (SIV) strain SIVsmE660, rVZV-SIVenv vaccinees manifested an early expansion of CD4 T cells in conjunction with significantly enhanced levels of SIV replication. This finding may reflect a form of vaccine enhancement of disease that is unique to viruses that preferentially target activated memory CD4+ T cells for infection, the pathognomonic feature of CD4+ T cell-tropic lentiviruses such as HIV.
Materials and Methods
Construction and in Vitro Characterization of rVZV-SIVenv. A cassette containing the gp160, env, and rev genes from SIV strain smH4 driven by the human cytomegalovirus immediate early promoter and followed by a poly(A) sequence (a gift from Philip Johnson, Ohio State University, Columbus) was cut with PvuI and HindIII and inserted into the AvrII site of VZV cosmid MstIIA (3). The resulting MstIIA-SIVgp160 cosmid contains the SIV env and rev genes inserted between VZV ORFs 65 and 66. Melanoma cells were transfected with VZV cosmids encompassing the entire VZV genome to generate recombinant VZV (rVZV) or VZV expressing SIV gp160 (rVZV-SIVenv) as described (3). Lysates from melanoma cells infected with parental VZV ROka, rVZV-SIVenv, or uninfected cells were immunoblotted with rabbit antibody to HIV-2 Env [HIV-2ST gp120, a gift from Ray Sweet, GlaxoSmithKline, Upper Providence, PA (4)].
Animals, Vaccines, and SIV Challenge. Animal care and research were performed in conformity with the Guide for the Care and Use of Laboratory Animals (5), according to a protocol approved by the Emory Institutional Animal Care and Use Committee. Group 1 rhesus macaques (RMs; Macaca mulatta) were 2 years old and weighed 3.1-4.3 kg at the beginning of the study. Because VZV is cell-associated, vaccines were in the form of frozen-thawed infected human cells. The vaccine was administered at a virus dose of 107 plaque-forming units by each of three routes: intranasal, intratracheal, and intramuscular. All vaccines were safe and well tolerated, with no signs of clinical disease. Monkeys were challenged with 100 pig-tailed macaque infectious doses of SIVsmE660 (kindly provided by Vanessa Hirsch, National Institutes of Health, Bethesda, MD); in RMs this dose likely represents a <100 animal infectious dose. Group 2 RMs consisted of one RM that received its fourth parental VZV vaccine 3 years after the third immunization, and 4 naive RMs; the RMs were 2.5-6.5 years old when SIV challenged.
Immunology Assays. VZV ELISA. VZV antibodies were detected with the varicella-zoster IgG ELISA test system (Zeus Scientific, Raritan, NJ).
Immunoblotting. Plasma samples were analyzed with Western blot strips prepared from SIVsmE660 antigens (6).
Radioimmunoprecipitation to detect anti-SIV Env antibodies. HeLa cells infected overnight with vaccinia virus expressing the SIV strain smH4 Env, Gag, and Pol proteins [Wyeth-SIV (7)] were labeled with [35S]methionine; lysates were prepared, precleared with normal serum and protein A-Sepharose, and incubated with 5 μl of serum from the vaccinated monkeys, followed by protein A-Sepharose. Boiled immune complexes were analyzed by gel electrophoresis and autoradiography.
Neutralizing antibody (NAb) assays. SIVsmH4 and SIVsmE660 neutralization was measured by a cell-killing assay (8, 9). Cell-free virus stocks were produced in H9 cells (SIVsmH-4) and CEMx174 cells (SIVsmE660).
Measures of complement-mediated antibody-dependent enhancement (C′-ADE) of SIVsmE660 infection. C′-ADE was assessed in MT-2 and CEMx174 cells as described (10) with minor modification. Cell-free virus (500 tissue culture ID50 units) was incubated for 1 h at 37°C with dilutions of serum samples in the presence of 1:20-diluted normal human serum as a source of complement (Sigma). Cells (50,000 per well) were added and incubated until early syncytia formation was visible in control wells (without serum). Cells were stained to determine viability (8); CEMx174 cells were stained after 3 days of incubation. No infection was seen in MT-2 cells during 12 days of incubation in the presence or absence of test samples. C′-ADE in CEMx174 cells was considered positive if cell viability was reduced by 35% relative to the number of viable cells in virus control wells.
SIV ELISA. SIV-specific antibodies were assessed by serial ELISA measures of serum antibody reactivity to lysed SIVsmE660 grown on SupT1-CCR5 cells (6). Endpoint titers were determined as the reciprocal of the highest serum dilution that gave an optical absorbance value above the values obtained with negative control sera.
Cellular immune response assays. Cytotoxic T lymphocyte (CTL) assays were 5-h 51Cr-release assays as described (11), using effectors that had been stimulated for 2 weeks with autologous Epstein-Barr virus-transformed B lymphoblastoid cell lines (B-LCLs) infected with vvSIVsmmH4env or vvVZVgE. Flow-cytometry CTL assays were performed as described (12), using B-LCLs infected with MVAsmmH4env, MVA control, vvVZVgE, or vvkip'sβgal control. ELISpot assays for IFN-γ release from antigen-specific peripheral blood mononuclear cells were performed as described (13); cells were stimulated with SIV Env peptide pools. Details of CTL and ELISpot assays are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Flow cytometry for cell surface markers. Peripheral blood mononuclear cells in whole blood were analyzed by four-color fluorescent antibody staining to determine the percentage and absolute number of specific cell subpopulations as described (14); details of the analyses are described in Supporting Materials and Methods.
Viral Load Measures. SIVsmE660 RNA was quantified with a two-step assay (15). RNA copy number was determined by comparison with an external standard curve consisting of in vitro transcripts representing bases 216-2106 of the SIVmac239 genome [HIV Sequence Database at http://hiv-web.lanl.gov/seq-db.html].
Statistical Analyses. Comparisons between rVZV-SIVenv vaccinated and control groups (rVZV vaccinees and naive RMs in groups 1 and 2) with respect to baseline percentages of Ki67+ CD4 T cells or SIV RNA levels were performed by using two-sample t tests; log-transformed viral load values were used. Spearman correlation analysis was used to estimate correlations between ELISA titers or CD4 T cell proliferation and log-transformed viral loads, and between ELISA titers and CD4 T cell proliferation.
Results
rVZV Vaccination Elicits Humoral Immune Responses. RMs are not naturally infected with VZV, although VZV-Oka and clinical VZV isolates induce antibody titers in RMs that parallel those observed in humans (16). Early studies such as ref. 16 did not ascertain whether VZV-Oka replicated in RMs or whether it elicited cellular immune responses. To test the potential of VZV recombinants to serve as vectors for HIV vaccines, we inoculated RMs with the parental rVZV or rVZV-SIVenv expressing SIVsmH4 env (Fig. 5, which is published as supporting information on the PNAS web site). Three immunizations were given 6 weeks apart; the RMs were then monitored to assess the persistence of vaccine-specific responses as a surrogate for the potential of VZV to persist and reactivate (2). After 2.5 years, a fourth immunization was given.
The vaccines induced VZV antibody titers similar to those observed in human vaccinees (Fig. 1A). Anti-SIVsmH4 Env antibodies were detected by immunoprecipitation analysis in the rVZV-SIVenv vaccinees only (Fig. 1B). An SIVsmE660-based ELISA demonstrated log10 reciprocal endpoint titers of 2.5-2.8 in the rVZV-SIVenv vaccinees and lower, but detectable, titers in the rVZV vaccinees (log10 reciprocal endpoint titers of 2.0-2.1 versus 1.3 in naive RMs), likely because of cross-reactive responses to cellular antigens shared between the cell-associated VZV vaccine preparation and the cellular antigens contained in the virus lysate-based SIV ELISA. Unlike the antibody responses in VZV-vaccinated humans (17), the responses in RMs waned over time (data not shown), suggesting that the human-derived rVZV had limited capacity to infect and/or replicate in RMs.
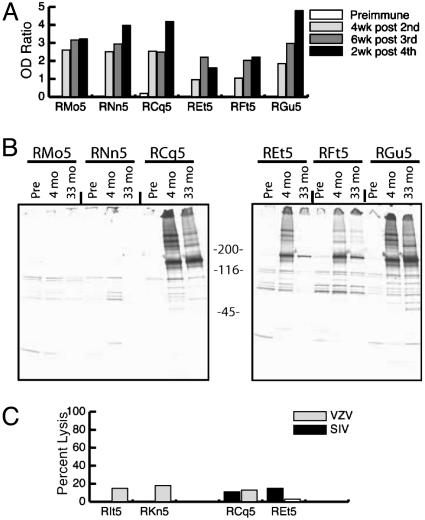
Fig. 1.
Vaccine-induced immune responses. (A) VZV antibody titers in six RMs preimmunization and postimmunization. (B) Anti-SIV Env antibodies detected by immunoprecipitation in rVZV-SIVenv vaccinees (RCq5, REt5, RFt5, and RGu5) but not in VZV vaccinees (RMo5 and RNn5) after three immunizations (4 mo) and after a fourth immunization 2.5 years later (33 mo). (C) Weak CTL responses to SIV and VZV antigens in chromium-release assays performed 3 weeks after the third immunization at effector:target ratios of 30:1 and 20:1, respectively.
Attempts to detect VZV- or SIV-specific CD8 cellular immune responses by using methods successfully applied to previous vaccine and SIV infection studies (11, 13), demonstrated weak or absent lytic activity against VZV and SIV antigens in chromium-release assays (Fig. 1C shows a representative result). A more sensitive flow-cytometry CTL assay measuring CTL-induced caspase activation in target cells (12, 18) also demonstrated weak or absent SIV-specific CD8 T cell responses (data not shown). For all vaccinees, ELISpot assays gave high backgrounds in control wells containing cells in medium and serum only, likely reflecting immune responses to residual serum proteins that were present in the vaccine preparations. These backgrounds precluded direct ex vivo measures of antigen-specific T cell responses. However, detection of VZV- and SIV-specific antibodies indicates the in vivo priming of a CD4 T helper (Th)-dependent immune response.
SIV Replication and Disease Are Significantly Enhanced in rVZV-SIVenv Vaccinees. One month after the last boost, four RMs vaccinated with rVZV-SIVenv, two RMs vaccinated with rVZV, and two unvaccinated naive RMs were inoculated intravenously with SIVsmE660, a naturally occurring quasispecies that is genetically related to, yet distinct from, the cloned SIVsmH4 Env immunogen. Unlike commonly used challenge viruses such as SHIV-89.6P and SIVmac 239, SIVsmE660 recapitulates the variation in viral load setpoints seen in different HIV-infected humans. Although the determinants of the variable SIVsmE660 replication dynamics are not fully understood (19), known animal-to-animal differences in the extent of CD4 T cell activation at the time of virus challenge could potentially influence SIV replication in the newly infected host (20). On the day of SIV challenge, all eight RMs in this vaccine study had levels of circulating proliferating CD4 T cells (1.2-2.4% Ki67+ CD4 T cells) that were lower than those typically observed in RMs at the Yerkes Primate Research Center and elsewhere (21, 22). We speculated that this less activated immunophenotype (which resembles that of healthy human subjects in the US; data not shown) related to prolonged housing of the RMs from an early age in the clean biocontainment facility environment, a point discussed later.
After challenge, the two rVZV vaccinees and the two naive control RMs manifested an SIV replication pattern that recapitulated the low end of values previously reported for this virus (7) (Fig. 2A). In contrast, all four rVZV-SIVenv vaccinees experienced more rapid and substantially increased levels of peak viremia relative to the controls (Fig. 2A). Thereafter, the Env-vaccinated and Env-naive RMs exhibited significantly different patterns of postacute viremia [P ≤ 0.01 by day 35 postinfection (p.i.)] and the Env vaccinees suffered more rapid CD4 declines and disease progression (Fig. 2B), with CD4 counts dropping to ≤300 by the time of killing or by day 245 p.i. rVZV-SIVenv vaccinees were killed with symptoms of AIDS at weeks 26, 34, 35, and 77 p.i. The rVZV vaccinees and the naive controls maintained CD4 counts >900 through day 245 p.i. One rVZV vaccinee was killed with a non-AIDS-related condition 85 weeks p.i., whereas the remaining three RMs have survived with preserved CD4 counts for more than 2.5 years (P = 0.04). Thus prior immunization with rVZV-SIVenv had enhanced SIV replication in this cohort of RMs.
Fig. 2.
Enhanced SIV replication (A) and CD4 cell decline (B) in rVZV-SIVenv vaccinees (dashed lines) compared to controls (solid lines).
Because SIVsmE660 replication in the four control RMs was at the low end of values previously reported for this virus (7), we addressed whether the SIV inoculum used in these studies had diminished in vivo infectivity by inoculating an additional 4 naive RMs and one rVZV vaccinee with a second aliquot of the SIVsmE660 stock identical to that used for the first challenge. This second group of RMs had been housed in an outdoor field colony for 1-6 years (depending on age of the RM), with the four naive RMs having been transferred to biocontainment housing shortly before SIV challenge. The levels of circulating proliferating CD4 T cells in these RMs (2.8-5.8% Ki67+ CD4 T cells) were typical of those observed in most RMs at Yerkes and reported by others (G.S., unpublished results; refs. 21 and 22) and were higher than the levels observed in the first group of eight RMs on the day of SIV challenge (P = 0.003). In this second group of control RMs, viral peaks of 106 to 108 SIV RNA copies per ml occurred at day 14 p.i., and by 8 weeks p.i., setpoint viremia levels of 102 to 105 SIV RNA copies per ml had been established (Fig. 6, which is published as supporting information on the PNAS web site), resembling the reported range of plasma SIVsmE660 RNA levels. Nonetheless, as of 10 weeks p.i., viremia in this second group of controls was still lower than that observed in the rVZV-SIVenv vaccinees from the first group (P < 0.05). CD4 counts in these RMs remained stable for >1 year p.i.
SIV Challenge Elicits Nonneutralizing Anamnestic Antibody Responses. In contrast to the detection of anti-SIVsmH4 Env antibodies by immunoprecipitation, anti-SIVsmE660 Env antibodies were not readily detected before SIV challenge by SIVsmE660 whole virus lysate-based Western blot analysis (Fig. 3A), suggesting that the immunoblots did not provide as sensitive a method of antibody detection, that the antibodies recognized conformational determinants not present in denatured Env, or that sequence differences in the two strains affected detection. Anti-Env antibodies became readily detectable by immunoblot in three of four rVZV-SIVenv vaccinees at 2 weeks p.i., and in all four RMs by 6 weeks p.i. RM RFt5 did not show detectable anti-SIVsmE660 Env gp120/160 antibodies by 6 weeks p.i., but did exhibit accelerated seroconversion to gp40, relative to controls. In control RMs, anti-SIV Env antibodies were barely detectable by 6 weeks p.i., although individual RMs began to seroconvert to other SIV antigens at this time. Thus, all four rVZV-SIVenv vaccinees demonstrated an anamnestic response to SIV Env.
Fig. 3.
Anamnestic antibody responses after SIV challenge of rVZV-SIVenv vaccinees. (A) Western blot analysis of SIV-specific antibodies in rVZV-SIVenv vaccinees (RGu5, REt5, RFt5, and RCq5), in rVZV vaccinees (RMo5 and RNn5), and in naive unvaccinated controls (RTh5; REe5 is not shown). For each RM, from left to right, four immunoblot strips corresponding to preimmune, day of challenge, and 2 and 4 weeks after challenge are shown. (B) SIVsmH4 NAb responses pre and post challenge.
An anamnestic NAb response against SIVsmH4 was induced in the three vaccinees that demonstrated the strongest anamnestic responses to gp120/160 (Fig. 3). NAbs against SIVsmH4 were not detected in vaccinee RFt5 or in the naive unvaccinated RMs, and were detected only at low levels in one of the rVZV vaccinees at 6 weeks p.i. NAbs against SIVsmE660 were undetectable in any of the RMs during the first 6 weeks of infection. Thus an anamnestic NAb response was generated against the vaccine-encoded SIVsmH4 Env, but not to the SIVsmE660 challenge virus.
Enhanced SIV Replication Correlates Directly with Early CD4 T Cell Expansion in rVZV-SIVenv Vaccinees. Anti-SIV ELISA titers on the day of SIV challenge correlated directly with both the early and setpoint levels of virus replication (Fig. 7 a and b, which is published as supporting information on the PNAS web site; P = 0.02 and P = 0.03, respectively). To explore whether enhanced SIV replication in the rVZV-SIVenv vaccinees was due to enhancing antibodies (23, 24), C′-ADE of SIVsmE660 infection was assessed by using sera from the day of challenge and through the first 6 weeks of infection. In CEMx174 cells, only very low C′-ADE could be detected in two rVZV-SIVenv vaccinees (RGu5, days 0 and 14 p.i., and REt5, 6 weeks p.i.) and one rVZV vaccinee (RMo5, 6 weeks p.i.). Thus, the rapid higher-level replication of SIVsmE660 in the rVZV-SIVenv vaccinees was not explained by C′-ADE.
Another potential mechanism of enhanced challenge virus replication was vaccine priming of SIV-specific memory CD4+ Th cells, which would be mobilized to proliferate upon virus infection, creating highly susceptible targets for SIV infection (1). In another study, we observed that CD4 T cell proliferation during acute SIV infection correlated with concomitant peak levels of SIV plasma viremia, whereas CD4 T cell proliferation that extended into chronic infection correlated with lower SIV viral load, suggesting that increased CD4 T cell proliferation during SIV infections reflects antigen-driven antiviral responses (14). We looked for evidence of CD4 T cell expansion by analyzing acute changes in proliferating CD4 T cells by flow cytometric analysis of the Ki67 nuclear antigen. We reasoned that the earliest CD4 T cell expansion upon initial SIV exposure should reflect an antigen-specific expansion of vaccine-induced memory cells. If this reasoning is correct, the magnitude of this early expansion should correlate with the levels of preexisting vaccine-induced antibodies, as these reflect a CD4 Th-dependent response. Three days after SIV challenge, rapid increases in Ki67+ CD4 T cells were observed in all rVZV-SIVenv vaccinees, increasing from a prechallenge baseline of 1.2-1.8% Ki67+ CD4 T cells in the peripheral blood to 3.7-4.6% Ki67+ CD4 T cells (Fig. 4 a-c). Thus, rVZV-SIVenv vaccinees effectively gained an additional 2.4-2.8% Ki67+ CD4 T cells during the first 3 days of SIV challenge. Naive RMs displayed virtually no increase in Ki67+ CD4 T cells during this period, whereas the rVZV vaccinees manifested only minimal increases (Fig. 4c). Likewise, SIV challenge of the second group of five control RMs also did not result in the rapid spike in CD4 T cell proliferation observed in the rVZV-SIVenv vaccinees (Fig. 8, which is published as supporting information on the PNAS web site). Thus, after SIV challenge, significant early increases in CD4 T cell proliferation were observed in all four RMs primed with SIV Env, but not in any of the nine control RMs (naive RMs or rVZV vaccinees).
Fig. 4.
Enhanced SIV replication correlates with early CD4 T cell proliferation. (a and b) Early changes in Ki67+ CD4 T cells and SIV RNA in a representative rVZV-SIVenv vaccinee (a) and a naive control (b). (c) Day 3 increase in Ki67+ CD4 T cells in all four rVZV-SIVenv vaccinees (dashed lines) compared with controls (solid lines). (d) Early SIV infection-induced CD4 T cell proliferation correlates with preexisting SIV antibody titers. ELISA titers are shown as the reciprocal of the log10 dilution endpoint titer. Increases in Ki67+ CD4 T cells at day 3 are shown. (e) Correlation between increased CD4 T cell proliferation at day 3 after SIV challenge and setpoint viremia.
The differences in early CD4 T cell proliferation between the rVZV-SIVenv vaccinees and control RMs correlated significantly with differences in ELISA antibody titers on the day of challenge (Fig. 4d, P = 0.009), supporting the notion that the early CD4 T cell proliferation observed in Env vaccinees represented a memory CD4 Th response to SIV Env. Furthermore, differences in early CD4 proliferation among vaccinees and controls correlated directly with the observed differences in the subsequent peak (R = 0.91, P = 0.016) and setpoint levels of SIV replication (Fig. 4e; R = 0.79, P = 0.036), suggesting that increased numbers of proliferating CD4 T cells in the rVZV-SIVenv vaccinees led to their higher levels of SIV replication, despite their higher anti-SIV antibody titers.
Environmentally Influenced Differences in Lymphocyte Activation May Explain the Variable SIVsmE660 Replication Observed in Control RMs. We hypothesized that the lower baseline levels of proliferating CD4 T cells observed in the first group of eight RMs in this vaccine study related to their reduced exposure to endemic infectious agents, such as enteric pathogens, or other immune-stimulating environmental antigens, because of prolonged housing in the biocontainment facility. (Divergent levels of immune activation in the first and second groups of RMs were not correlated with simian retrovirus, simian T lymphotropic virus type 1, or cytomegalovirus coinfection; data not shown.) To investigate the influence of housing history on basal levels of lymphocyte activation, we analyzed 25 SIV-uninfected RMs that had been housed indoors in individual cages since birth or an early age, and we compared them with 30 age-matched RMs that had been housed in an outdoor field colony since birth. Substantially increased levels of proliferating (Ki67+) T cells were observed in RMs housed outdoors (P < 0.001; Fig. 9, which is published as supporting information on the PNAS web site). Both CD25, a marker of T cell activation, and expression of the chemokine receptor CCR5 were elevated on CD4 T cells in outdoor-housed RMs (P < 0.05; Fig. 9). Increased expression of CCR5, a key SIV coreceptor, combined with elevated CD4 T cell proliferation would create more fertile targets for SIV replication and would help to explain why the second group of control animals challenged in this study exhibited more typical levels of SIVsmE660 replication (Fig. 6). However, the enhanced SIV replication observed in the rVZV-SIVenv vaccinees in this study is not explained by baseline levels of generalized CD4 T cell activation, which were low in this first group of animals; rather, enhanced SIV replication in these vaccinees occurred in concert with an early expansion of CD4 T cells that occurred upon SIV challenge (Fig. 4).
Discussion
This study provides evidence, in a relevant simian AIDS model, that certain vaccines can exacerbate CD4 T cell tropic lentivirus disease. A limited capacity of the human VZV vaccine to infect and/or replicate and persist in RMs likely precluded the rVZV-encoded antigens from undergoing the cellular processing required for effective induction of class I-restricted CD8 T cell responses; in effect, the vaccine may have resembled an inactivated immunogen that primarily elicits a Th-dependent antibody response. These observations should not be misconstrued as a condemnation of the VZV vectoring approach; rVZV may prove to be an excellent vector for the expression of heterologous antigens in hosts where it replicates well, such as humans. Rather, RMs do not appear to be a good model in which to perform preclinical tests of rVZV. Future investigation of rVZVs will require studies in host species more permissive for VZV replication, including chimpanzees and humans (25).
In RMs, expansion of virus-susceptible CD4 T target cells, in the absence of a strong CD8 T cell response, apparently led to their preferential infection by SIV, and may have resulted in compromise of the incipient SIV-specific Th responses, and consequent profound impairment of host immune control of virus replication. Several precedents exist for vaccines to shift the host-pathogen interaction in harmful ways upon subsequent exposure to live virus (26-29), including examples of vaccine-mediated enhancement of lentivirus infections (30-32). Because lentiviral vaccine-mediated enhancement does not always involve enhancing antibodies (31), a mechanism of lymphocyte activation-mediated enhancement of virus replication has been proposed (33); data consistent with such a mechanism were reported in one feline immunodeficiency virus (FIV) vaccine study (34). The discovery that CD134, a T cell activation antigen expressed primarily on CD4 T cells, is a receptor for FIV helps to explain how vaccination could prime for expansion a population of cells that would be highly susceptible to FIV infection (35). Because HIV replicates optimally in activated CCR5-expressing CD4 T cells, circumstances of memory CD4 T cell activation may lead to increased levels of virus replication. Efficient antiviral immune mechanisms, including prompt generation of virus-specific CD8 T cells, are likely to be important in containing early viremia and ameliorating the potentially harmful effects of preferential targeting of HIV-specific memory CD4 T cells activated during primary HIV infection.
Several features of this study differ from other AIDS vaccine studies in ways that may explain the observed enhancement of SIV infection. First, in contrast to homologous vaccine antigens and challenge viruses used in most vaccine studies, the heterologous SIVsmE660 challenge more closely resembles the circumstances of human exposure to HIV quasispecies that will have only partial sequence similarity to the HIV vaccine immunogens previously administered to the HIV-exposed and newly infected individual. SIVsmE660 infection elicited anamnestic SIVsmH4-specific NAb responses, indicating cross-reactive memory CD4 T cells and B cells, but the antibodies did not limit SIVsmE660 replication. The SIVsmH4-vaccine-specific NAb response is consistent with previous studies demonstrating a dichotomy in the neutralization phenotypes of SIVsmH4 and SIVsmE660 and highly strain-specific NAbs during the first 6 weeks of infection (36). (Similarly, current HIV vaccine candidates also do not elicit cross-reactive NAbs against primary isolates, but likely will elicit cross-reactive CD4 Th responses.) Second, in contrast to the CXCR4-using SHIV89.6P used for many vaccine challenge studies, the CCR5-tropic SIVsmE660 would be highly tropic for activated memory CD4 T cells (37). Third, the more variable replication dynamics of SIVsmE660 suggests that this virus may be influenced by variations in the availability of activated CD4+ target T cells. In this study, SIVsmE660 replication was clearly linked to the magnitude of early CD4 T cell expansion in the rVZV-SIVenv vaccinees. This vaccine-mediated enhancement of virus replication would likely have been be masked by exceedingly virulent viruses, as the uniformly robust replication of virulent (and CXCR4-using) viruses may not be influenced by alterations in the availability of activated memory CD4 T cell targets in newly infected hosts (38, 39). Vaccine-mediated enhancement would also have been masked by higher levels of generalized immune activation at the time of SIV challenge, as increased numbers of activated (non-SIV-specific) CD4 T cells appear to lead to increased SIVsmE660 replication (Fig. 6). Vaccine-mediated enhancement of SIVsmE660 replication was not observed in a study of recombinant modified vaccinia virus Ankara expressing SIV Env (46), perhaps reflecting differences in CD4 or CD8 responses induced by the vaccine and/or differences in the baseline levels of generalized immune activation extant in the animal cohorts studied.
Rather than prevent infection, current HIV vaccines in development are anticipated to provide partial protection that would, after HIV exposure, reduce setpoint levels of HIV replication, slow disease progression, and reduce secondary virus transmission (40). In the absence of sterilizing vaccine protection, the dual role of CD4 T cells as both anti-HIV effectors and HIV-susceptible targets suggests that a suboptimal balance of vaccine-induced CD4 and CD8 responses could lead to enhanced virus replication. Newer HIV vaccine concepts target elicitation of strong CD8 T cell responses (40), as well as CD4 responses required for effective CD8 T cell function. However, earlier concepts that have progressed to phase III trials have been shown to elicit primarily CD4 proliferation and antibody responses, but limited or no CD8 CTL responses in immunized human volunteers (41, 42). Currently, a canarypox recombinant expressing several HIV antigens as a priming immunogen (ALVAC vCP1521 expresses gp120/gp41 from a clade E primary isolate and clade B-derived LAI Gag, Pro, and gp41) and a clade B- and E-derived gp120 subunit boost are being tested in a phase III trial. The stated rationale for the study is that, although this strategy is not expected to induce better CD8 T cell or NAb responses against primary HIV isolates, it may induce an augmented CD4 T-cell response (primarily to the boosted HIV Env) (43). Although it is not clear whether our results are relevant to current HIV vaccine trials, they do raise the possibility that predominantly CD4-dependent non-NAb-inducing immunogens could prime hosts for enhanced HIV replication upon subsequent HIV exposure.
Limited data on HIV “breakthrough” infections in trials of HIV Env protein-based immunogens have not revealed significant differences in HIV incidence in vaccinees versus placebo controls (44, 45). If a vaccine were to exacerbate HIV disease upon subsequent exposure, the ability to detect such vaccine-mediated disease enhancement would be determined by the levels and character of the HIV-specific CD4 T cell response elicited by the vaccine, and the timing, frequency and type of monitoring performed on breakthrough infections, as well as when such infections would be treated with antiretroviral therapy. In future HIV vaccine efficacy trials, it will be important to carefully compare levels of postvaccination/preinfection HIV-specific CD4 T cell responses with the pattern of postinfection viremia and disease.
Questions remain regarding the precise mechanism of vaccine-mediated enhancement of SIV infection observed in this study. Is the enhancement due to a type of antibody-dependent enhancement for which we do not have reliable in vitro assays, such as for Fc receptor-mediated enhancement? Is the effect due to immunization with Env in particular, or is it simply related to the elicitation of CD4 but not CD8 responses to viral antigens? Are the observations unique to the VZV vaccine vector when combined with SIV Env? Studies that compare alternative vaccine strategies that differentially elicit CD4-focused, CD8-focused, or more balanced responses for relative beneficial and detrimental outcomes should enable direct evaluation of these responses. AIDS vaccines may need to be tailored to achieve the optimal balance of CD4 and CD8 responses, wherein sufficient help for the elicitation of functional CD8 T cell responses is engendered but accelerated destruction of host antiviral immune responses and accelerated disease does not arise.
Supplementary Material
Acknowledgments
We thank V. Hirsch for providing the SIVsmE660 challenge stock; J. Lifson, J. Bess, Jr., M. Williamson, and J. Kissner for assistance with antibody studies; M. Hotic for assistance with SIV RNA assays; D. Anderson for assistance with pathology studies; and R. Ahmed for insightful discussions. This work was supported by an Elizabeth Glaser Scientist Award (to M.B.F.) and National Institutes of Health Grants PO1 AI 46007 and P30 AI 50409.
Abbreviations: C′-ADE, complement-mediated antibody-dependent enhancement; CTL, cytotoxic T lymphocyte; Env, viral envelope protein; NAb, neutralizing antibody; p.i., postinfection; RM, rhesus macaque; SIV, simian immunodeficiency virus; Th, T helper; VZV, varicella-zoster virus; VZV-Oka, attenuated VZV vaccine; rVZV, recombinant VZV.
Footnotes
Hamer, D., Keystone Symposia, HIV Vaccine Development: Progress and Prospects, April 12-18, 2004, Whistler, BC, Canada.
References
- 1.Douek, D. C., Brenchley, J. M., Betts, M. R., Ambrozak, D. R., Hill, B. J., Okamoto, Y., Casazza, J. P., Kuruppu, J., Kunstman, K., Wolinsky, S., et al. (2002) Nature 417, 95-98. [DOI] [PubMed] [Google Scholar]
- 2.Krause, P. R. & Klinman, D. M. (2000) Nat. Med. 6, 451-454. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, J. I. & Seidel, K. E. (1993) Proc. Natl. Acad. Sci. USA 90, 7376-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivey-Hoyle, M., Culp, J., Chaikin, M., Hellmig, B., Matthews, T., Sweet, R. & Rosenberg, M. (1991) Proc. Natl. Acad. Sci. USA 88, 512-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute of Laboratory Animal Resources, National Research Council (1996) Guide for the Care and Use of Laboratory Animals (Natl. Acad. Press, Washington, DC).
- 6.Lifson, J. D., Rossio, J. L., Arnaout, R., Li, L., Parks, T. L., Schneider, D. K., Kiser, R. F., Coalter, V. J., Walsh, G., Imming, R. J., et al. (2000) J. Virol. 74, 2584-2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsch, V. M., Fuerst, T. R., Sutter, G., Carroll, M. W., Yang, L. C., Goldstein, S., Piatak, M., Jr., Elkins, W. R., Alvord, W. G., Montefiori, D. C., et al. (1996) J. Virol. 70, 3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montefiori, D. C., Robinson, W. E., Jr., Schuffman, S. S. & Mitchell, W. M. (1988) J. Clin. Microbiol. 26, 231-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch, V., Adger-Johnson, D., Campbell, B., Goldstein, S., Brown, C., Elkins, W. R. & Montefiori, D. C. (1997) J. Virol. 71, 1608-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montefiori, D. C., Reimann, K. A., Letvin, N. L., Zhou, J. & Hu, S. L. (1995) AIDS Res. Hum. Retroviruses 11, 963-970. [DOI] [PubMed] [Google Scholar]
- 11.Safrit, J. T., Andrews, C. A., Zhu, T., Ho, D. D. & Koup, R. A. (1994) J. Exp. Med. 179, 463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu, L., Chahroudi, A., Silvestri, G., Wernett, M. E., Kaiser, W. J., Safrit, J. T., Komoriya, A., Altman, J. D., Packard, B. Z. & Feinberg, M. B. (2002) Nat. Med. 8, 185-189. [DOI] [PubMed] [Google Scholar]
- 13.Larsson, M., Jin, X., Ramratnam, B., Ogg, G. S., Engelmayer, J., Demoitie, M. A., McMichael, A. J., Cox, W. I., Steinman, R. M., Nixon, D. & Bhardwaj, N. (1999) AIDS 13, 767-777. [DOI] [PubMed] [Google Scholar]
- 14.Garber, D. A., Silvestri, G., Barry, A. P., Fedanov, A., Kozyr, N., McClure, H., Montefiori, D. C., Larsen, C. P., Altman, J. D., Staprans, S. I. & Feinberg, M. B. (2004) J. Clin. Invest. 113, 836-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amara, R., Villinger, F., Altman, J., Lydy, S., O'Neil, S., Staprans, S., Montefiori, D., Xu, Y., Herndon, J., Wyatt, L., et al. (2001) Science 292, 69-74. [DOI] [PubMed] [Google Scholar]
- 16.Asano, Y., Albrecht, P., Behr, D. E., Neff, B. J., Vickers, J. H. & Rastogi, S. C. (1984) J. Med. Virol. 14, 305-312. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, C. E., Stancin, T., Fattlar, D., Rome, L. P. & Kumar, M. L. (1997) Pediatrics 100, 761-766. [DOI] [PubMed] [Google Scholar]
- 18.Lichterfeld, M., Yu, X. G., Waring, M. T., Mui, S. K., Johnston, M., Cohen, D., Addo, M. M., Zaunders, J., Alter, G., Pae, E., et al. (2004) Blood 104, 487-494. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein, S., Brown, C. R., Dehghani, H., Lifson, J. D. & Hirsch, V. M. (2000) J. Virol. 74, 9388-9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang, Z. Q., Wietgrefe, S. W., Li, Q., Shore, M. D., Duan, L., Reilly, C., Lifson, J. D. & Haase, A. T. (2004) Proc. Natl. Acad. Sci. USA 101, 5640-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakrabarti, L. A., Lewin, S. R., Zhang, L., Gettie, A., Luckay, A., Martin, L. N., Skulsky, E., Ho, D. D., Cheng-Mayer, C. & Marx, P. A. (2000) J. Virol. 74, 1209-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaur, A., Hale, C. L., Ramanujan, S., Jain, R. K. & Johnson, R. P. (2000) J. Virol. 74, 8413-8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montefiori, D. C. (1997) Springer Semin. Immunopathol. 18, 371-390. [DOI] [PubMed] [Google Scholar]
- 24.Siebelink, K. H., Tijhaar, E., Huisman, R. C., Huisman, W., de Ronde, A., Darby, I. H., Francis, M. J., Rimmelzwaan, G. F. & Osterhaus, A. D. (1995) J. Virol. 69, 3704-3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen, J. I., Moskal, T., Shapiro, M. & Purcell, R. H. (1996) J. Med. Virol. 50, 289-292. [DOI] [PubMed] [Google Scholar]
- 26.Corapi, W. V., Darteil, R. J., Audonnet, J. C. & Chappuis, G. E. (1995) J. Virol. 69, 2858-2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham, B. S., Johnson, T. R. & Peebles, R. S. (2000) Immunopharmacology 48, 237-247. [DOI] [PubMed] [Google Scholar]
- 28.Hilleman, M. R. (2001) Vaccine 20, 651-665. [DOI] [PubMed] [Google Scholar]
- 29.Mongkolsapaya, J., Dejnirattisai, W., Xu, X., Vasanawathana, S., Tangthawornchaikul, N., Chairunsri, A., Sawasdivorn, S., Duangchinda, T., Dong, T., Rowland-Jones, S., et al. (2003) Nat. Med. 9, 921-927. [DOI] [PubMed] [Google Scholar]
- 30.Russo, P., Vitu, C., Fontaine, J. & Vignoni, M. (1993) Comp. Immunol. Microbiol. Infect. Dis. 16, 131-136. [DOI] [PubMed] [Google Scholar]
- 31.Raabe, M. L., Issel, C. J. & Montelaro, R. C. (1999) Virology 259, 416-427. [DOI] [PubMed] [Google Scholar]
- 32.Giannecchini, S., Isola, P., Sichi, O., Matteucci, D., Pistello, M., Zaccaro, L., Del Mauro, D. & Bendinelli, M. (2002) J. Virol. 76, 6882-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson, J., Moraillon, A., Baud, S., Cuisinier, A. M., Sonigo, P. & Pancino, G. (1997) J. Virol. 71, 9640-9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richardson, J., Broche, S., Baud, S., Leste-Lasserre, T., Féméniac, F., Levy, D., Moraillon, A., Pancinoe, G. & Sonigo, P. (2002) J. Gen. Virol. 83, 2515-2521. [DOI] [PubMed] [Google Scholar]
- 35.Shimojima, M., Miyazawa, T., Ikeda, Y., McMonagle, E. L., Haining, H., Akashi, H., Takeuchi, Y., Hosie, M. J. & Willett, B. J. (2004) Science 303, 1192-1195. [DOI] [PubMed] [Google Scholar]
- 36.Ourmanov, I., Bilska, M., Hirsch, V. M. & Montefiori, D. C. (2000) J. Virol. 74, 2960-2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feinberg, M. B. & Moore, J. P. (2002) Nat. Med. 8, 207-210. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz, D. H. (1994) Immunol. Today 15, 54-57. [DOI] [PubMed] [Google Scholar]
- 39.Altes, H. K., Wodarz, D. & Jansen, V. A. (2002) J. Theor. Biol. 214, 633-646. [DOI] [PubMed] [Google Scholar]
- 40.Garber, D. A., Silvestri, G. & Feinberg, M. B. (2004) Lancet Infect. Dis. 4, 397-413. [DOI] [PubMed] [Google Scholar]
- 41.Gupta, K., Hudgens, M., Corey, L., McElrath, M. J., Weinhold, K., Montefiori, D. C., Gorse, G. J., Frey, S. E., Keefer, M. C., Evans, T. G., et al. (2002) J. Acquired Immune Defic. Syndr. 29, 254-261. [DOI] [PubMed] [Google Scholar]
- 42.Cohen, J. (2002) Science 295, 1616-1617. [DOI] [PubMed] [Google Scholar]
- 43.Cohen, J. (2003) Science 302, 1309-1310. [DOI] [PubMed] [Google Scholar]
- 44.Graham, B. S., McElrath, M. J., Connor, R. I., Schwartz, D. H., Gorse, G. J., Keefer, M. C., Mulligan, M. J., Matthews, T. J., Wolinsky, S. M., Montefiori, D. C., et al. (1998) J. Infect. Dis. 177, 310-319. [DOI] [PubMed] [Google Scholar]
- 45.Connor, R. I., Korber, B. T., Graham, B. S., Hahn, B. H., Ho, D. D., Walker, B. D., Neumann, A. U., Vermund, S. H., Mestecky, J., Jackson, S., et al. (1998) J. Virol. 72, 1552-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ourmanov, I., Brown, C. R., Moss, B., Carroll, M., Wyatt, L., Pletneva, L., Goldstein, S., Venzon, D. & Hirsch, V. M. (2000) J. Virol. 74, 2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.