Abstract
Introduction
Mutations in the proteinase BMP1 (bone morphogenetic 1) were recently identified in patients with osteogenesis imperfecta (OI), which can be associated with type one DGI (Dentinogenesis imperfecta). BMP1 is co-expressed in various tissues and has overlapping activities with the closely related proteinase TLL1 (mammalian tolloid-like 1). In this study we investigated whether removing the overlapping activities of BMP1 and TLL1 affects the mineralization of tooth root dentin.
Methods
Floxed alleles of the BMP1 and TLL1 genes were excised via ubiquitously expressed Cre induced by tamoxifen treatment beginning at 3 days of age (harvested at 3 weeks of age) or beginning at 4 weeks of age (harvested at 8 weeks of age). Multiple techniques, including x-ray analysis, double-labeling with calcein and alizarin-red stains for measurement of dentin formation rate, and histological and immunostaining assays were used to analyze the dentin phenotype.
Results
BMP1/TLL1 doubly knocked out mice displayed short and thin root dentin, defects in dentin mineralization, and delayed tooth eruption. Molecular mechanism studies revealed accumulation of collagens in dentin, and a sharp reduction in non-collagenous proteins such as DMP1 and DSPP. Furthermore, we found a strong reduction in TRAP, which is likely caused by defects in bone cells.
Conclusions
BMP1/TLL1 appear to play crucial roles in maintaining extracellular matrix homeostasis essential to root formation and dentin mineralization.
Keywords: Bmp1, dentin, odontoblast, Tll1, tooth root
INTRODUCTION
Dentinogenesis imperfecta (DGI) is a rare genetic disorder of tooth development characterized by discolored teeth with weak dentine structures. The condition in most patients is inherited with an autosomal dominant pattern. By Shields classification, there are three types of DGI (1). Type I is directly linked with osteogenesis imperfecta (OI), whereas the other two types of DGI have no link to OI. It has for a long time been known that OI is caused by mutations in the genes encoding collagen type I in most cases of the more frequently occurring autosomal dominant forms (2). Among the most recently described molecular defects reported for cases of recessively inherited OI have been mutations in the gene for BMP1 (bone morphogenetic protein-1), resulting in mild to severe forms the disease, depending upon the nature of the mutation (3, 4). Two of four patients with BMP1 mutations displayed typical DGI symptoms, including translucent and fragile teeth with brown discoloration and opalescent teeth, supporting the notion that BMP1 plays crucial roles in dentin formation (3).
BMP1 and TLL1 (mammalian tolloid-like 1) are encoded by two different genes but belong to a small family of extracellular metalloproteinases, which share a similar structure and play functional roles that overlap(5). For example, these proteinases enhance extracellular matrix (ECM) assembly, and accelerate collagen fibril formation by removing the C-propeptides for procollagen precursors (6).
In research of special relevance to hard tissue development, BMP1 and related proteases have been shown to process dentin matrix protein-1 (DMP1) and dentin sialophosphoprotein (DSPP), two well documented non-collagenous matrix proteins that are essential for dentin formation (7–9).
Ubiquitous constitutive knock out in mice of the BMP1 gene Bmp1 (10) or the TLL1 gene Tll1 (11) leads to embryonic death, preventing further studies of the role of these two genes during postnatal development. Thus, we generated a compound conditional knock out mouse line in which both Bmp1 and Tll1 are simultaneously deleted postnatally via tamoxifen-induced ubiquitous Cre expression in tissues (12). These null mice displayed an OI-like phenotype, including poorly formed bone, reduced processing of procollagen and DMP1, remarkably high bone turnover and defective osteocyte maturation.
In this study we attempted to study the roles of BMP1 and TLL1 in postnatal tooth development using multiple techniques. Our data revealed a more severe root defect (expanded predentin, thin dentin and short root) than that in molar crown. Furthermore, these null mice displayed delayed molar eruption. Thus, we conclude that BMP1/TLL1 activities are essential for root dentin mineralization.
MATERIALS AND METHODS
Mice breeding, BrdU injection, and double labeling
Mice doubly homozygous for Bmp1flox/flox and Tll1flox/flox alleles (12) were crossed to mice with a Cre-ERT2 transgene under control of the human ubiquitin C promoter (13) to obtain mice in which tamoxifen treatment induced ubiquitous BMP1/TLL1 double knock out (dKO) mice. Tamoxifen-treated Bmp1flox/flox; Tll1flox/flox mice lacking the Cre transgene were used as controls. Tamoxifen was administered to both experimental and control mice via intraperitoneal (IP) injection (100 mg/kg body weight) in two age-groups: starting with 3-day-old mice (injections 3 times per week until sacrifice) with harvest of 3-week-old mice (for study of early developmental impacts of BMP1/TLL1 ablation); and starting with 4-week-old mice (injections every day for 5 days in the 4th and 5th weeks, and then twice a week after that until sacrifice) and harvest of 8-week-old mice (for study of impacts of BMP1/TLL1 ablation on late dentin mineralization). All animal protocols were approved by the Animal Care and Use Committees at Texas A&M College of Dentistry and at the University of Wisconsin School of Medicine and Public Health.
BrdU (Invitrogen, USA) was administrated to mice at a dosage of 1 ml per 100 g bodyweight by IP injection. To measure dentin deposition rate, double-fluorescence labeling was performed as previously described (14). In brief, calcein green (Sigma-Aldrich, USA) was injected intraperitoneally 7 days before sacrifice, followed by alizarin red (Sigma-Aldrich) injections 2 days before sacrifice.
Radiography
Mandibles were analyzed using X-ray radiography (Faxitron X-Ray LLC, Lincolnshire, IL, USA). Root lengths of first molars were measured by ImageJ (NIH, Bethesda, MD, USA).
Sample preparation and histochemistry
Right mandibles were fixed in 70% ethanol and used for radiographs, and embedded in methylmethacrylate (MMA; Buehler, Lake Bluff, IL, USA) (15) for dentin formation rate measurement. Left mandibles were fixed in buffered 4% paraformaldehyde, decalcified in EDTA, and embedded in paraffin using standard histological procedures as previously described (16). Four-μm-thick serial sections were used for H&E, TRAP (tartrate-resistant acid phosphatase), Sirius red and immunohistochemistry stains. Antibodies for DMP1 and DSP (17) were generously donated by Dr. Chunlin Qin (Baylor College of Dentistry). ImageJ was used to measure the thickness of predentin and dentin and to calculate the number of osteoclasts per bone perimeter (N. Oc/B. Pm).
FITC stain and confocal imaging
The rationale for using FITC (fluorescein isothiocyanate) is that this small molecular dye fills in the non-mineralized dentin tubules but does not enter the mineral matrix (18). Thus, the content of the dye under the confocal microscope provides a visual representation of the size of dentin tubule diameters (i.e., larger diameters are equated with poor mineralization). Samples were prepared as previously described (19).
Statistical analysis
Statistical significance was determined by an independent-sample t-test using SPSS 19.0. A P value of < 0.05 was considered statistically significant.
RESULTS
DKO mice displayed poor dentine mineralization with shortened roots in the early double knock out (dKO) group
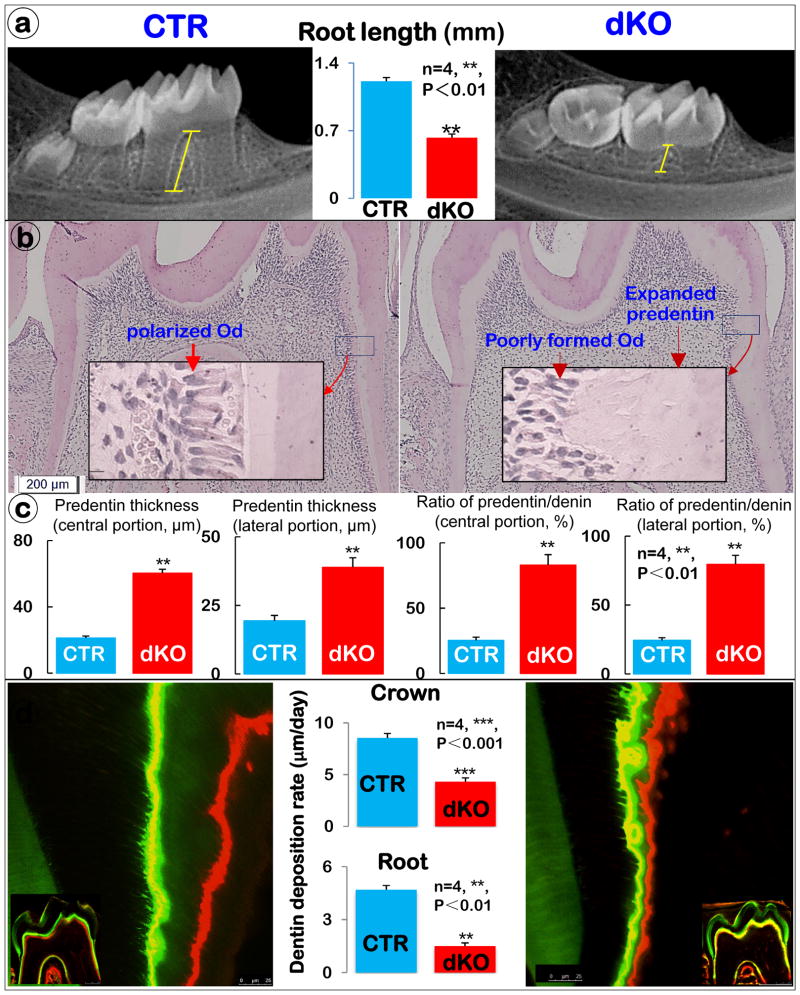
Previously we reported an OI-like long bone phenotype in Bmp1/Tll1 dKO mice, indicating a critical role of these extracellular metalloproteinases in bone mineralization (12). Here we demonstrated ablation of BMP1 in the dKO odontoblasts from both crown and root (Supplementary Fig. 1). In the dKO mice we found short roots in the 3-week-old dKO mandibles with a modest change in molar crowns by radiographs (Fig. 1a and Supplementary Fig. 2a). Quantitative data, based on the X-ray images using the technique described in our previous publications (14, 20), displayed a 50% reduction in root length of the first molars (Fig. 1a; n=4; **, P< 0.01). The histological analysis revealed an expanded predentin layer (indicating a defect in dentin mineralization), and a defect in odontoblast differentiation, as reflected by a cell arrangement and polarization (Fig. 1b). The detail analyses of dentin and predentin thickness in different areas of roots displayed a 2-fold increase in predentin thickness both in central and lateral portions in dKO mice. The ratio of predentin to dentin, indicating a non-mineralized portion of dentin, revealed nearly a 2-fold increase in the central portion and a 4-fold in lateral portion (Fig. 1c).
Figure 1. BMP1/TLL1 dKO mice display short roots and a dentinogenesis imperfecta-like phenotype (right panels).
a) X-ray examination of dKO mice revealed short molar root length. (n=4, **, P<0.01) b) H&E staining displayed some typical dentinogenesis imperfecta-like phenotype, including enlarged pulp with less pulp cells, expanded predentin, and a lack of odontoblast polarization. c) Quantitative analysis of the predentin thickness and predentin/dentin ratio in different root areas. d) Double fluorescence labeling of mandible from 3-week-old control (CTR) and dKO mice. The first injection (calcein) gave rise to a green label, whereas the second injection (Alizarin Red) produced a red line. The distance between the green and red labeling indicated the mineral deposition rate in the period between the two injections (5 days). The quantitative measurement of the distance between the two injections revealed a significantly lower mineral deposition rate both in the crown (moderate) and root dentin (severe) of dKO mice compared with the CTR group. (n=4, ***, P< 0.001, **, P< 0.01,).
To test the impact of removing both genes on dentin matrix deposition and/or mineralization, we compared the dentin deposition rate using prelabeled fluorochrome specimens. The double-labeled confocal images revealed a sharp reduction in the dentin formation rate in the dKO group with few dentin tubules labeled. This change is statistically significant between both groups, although the reduction degree of the mineralization rate in roots is more severe than that in the crown dentin (Fig. 1d; n=4, ***, P< 0.001, **, P< 0.01).
To further address the role of BMP1/TLL1 on late dentin formation/mineralization, we deleted both genes in 4-week-old mice (when 1st-molars are largely formed) and harvested the mice at 8-week-old. The 8-week-old dKO mice showed a minor DGI-like phenotype, including an enlarged pulp-root-canal plus expanded predentin (Supplementary Fig. 2b and Fig 3a), and extended non-mineralized areas surrounding each dentin tubule (as reflected by more FITC dye filling the dentin tubules, Supplementary Fig. 3b). Quantitative data showed statistically significant differences between the dKO and control groups (Supplementary Fig. 3c). Together, the sequential studies at two different age groups support a critical role of BMP1-TLL1 in dentin (especially root dentin) formation.
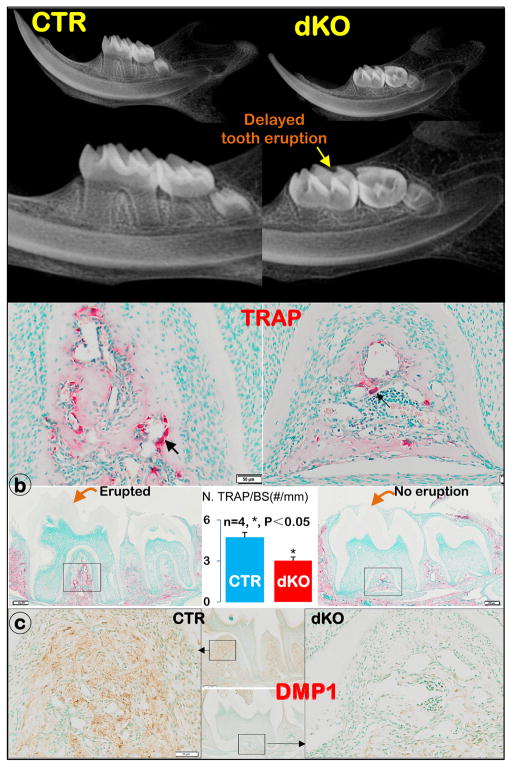
Figure 3. The dKO mice developed delayed molar eruption (right panels).
a) X-ray images showed a lack of molar eruption in the dKO mice compared to the age-matched controls, in which both 1st and 2nd molars are fully erupted; b) TRAP stain images showed more positive osteoclasts (black arrow) in CTR mice than in dKO mice, with a statistical difference between these two groups (n=4, *; P<0.05); and c) DMP1 immunostain images displayed a major defect in alveolar bone formation in the dKO mice.
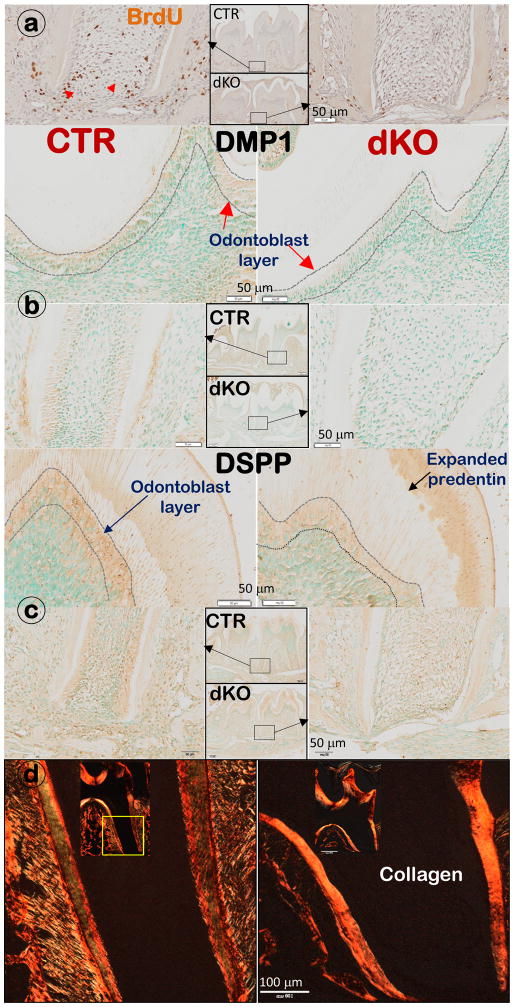
The dKO mice displayed a sharp reduction in cell proliferation and expression of DMP1 and DSPP in odontoblasts, as well as an accumulation of collagens in predentin and dentin
To understand the cellular and molecular changes in dKO molars, we conducted BrdU assays to evaluate the change in cell proliferation, which showed a sharp decrease in the BrdU positive cells in the dKO apical region (Fig. 2a). To test the impact of removing BMP1/TLL1 on cell differentiation, we measured levels of DMP1 (Fig. 2b) and DSPP (Fig. 2c), two important matrix proteins for dentin formation. Levels for both proteins were greatly reduced in the dKO odontoblast and ECM.
Figure 2. Removing BMP1/TLL1 leads to sharp reductions in cell proliferation and differentiation in odontoblast layers, with accumulated collagen contents (right panels).
a) BrdU staining images showed a great reduction in BrdU positive cells (black arrow) in apical region in the dKO root; b) Immunostainings revealed a reduction of DMP1 in both crown and apical regions; c) Reduced expression of DSPP was observed in the dKO odontoblast layers in both crown and root areas; and d) Sirius red stain images by polarized microscopy show accumulated collagen content in dKO mice compared to age-matched controls.
Furthermore, the collagen content in the dKO root dentin, as reflected by polarized images, was much higher than in the age-matched control dentin (Fig. 2d).
In summary, the above molecular studies indicate that BMP1/TLL1 are required for maintaining stable collagen levels in dentin matrix and the accumulated, perhaps aberrantly processed, collagen may interfere with the homeostasis of non-collagenous ECM levels (such as DMP1 and DSPP).
The dKO mice exhibited delayed tooth eruption
Unexpectedly, dKO mice develop a delayed tooth eruption in molars, as detected in X-ray images (Fig. 3a, Supplement Fig. 2a). Because the osteoclast is responsible for bone resorption, we then examined TRAP positive cell numbers in alveolar bones. Both qualitative and quantitative data showed a significant reduction in TRAP positive cells in the dKO alveolar bone compared to the age-matched control group (Fig. 3b; n=4, *, P<0.05). However, there appears no apparent change in tooth eruption in the 8-week-old mice (Supplement Fig. 2b), suggesting that this defect is temporary. To explore the potential cause for the reduction in osteoclast numbers, we analyzed the DMP1 levels in alveolar bone, which showed a sharp reduction (Fig. 3c). This finding suggests a secondary effect of the defective bone cells on osteoclast function. In sum, dKO mice displayed a delayed tooth eruption due to a temporary reduction in osteoclast number, which is likely caused by a poorly formed alveolar bone.
DISCUSSION
Type one DGI, a rare disorder that is directly associated with OI, is mainly caused by mutations in the genes encoding collagen type I. Recent studies have identified mutations in BMP1, an extracellular proteinase involved in formation of the ECM, in cases of OI (3, 4). To understand the mechanism by which BMP1 contributes to mineralization, we studied the effects of deletion of the two related proteinases with overlapping functions, BMP1 and TLL1, postnatally. These null mice displayed both OI (such as poorly formed alveolar bone) and DGI (including an expanded predentin and enlarged pulp/root canal), as well as short tooth roots. The cellular and molecular studies revealed decreased pulp cell proliferation, and reduced odontoblast differentiation (as reflected in sharp reductions in DMP1 and DSPP). Data support the essential roles of BMP1/TLL1 in controlling dentin ECM homeostasis and mineralization, and raise the interesting point of whether the pathological buildup of collagens may involve aberrant collagen forms with retained C-propeptides.
Interestingly, the dKO mice also displayed delayed tooth eruption in molars. As evidence of a potential mechanism, we found a sharp reduction in TRAP in dKO alveolar bone. We further showed a reduction in both bone volume and DMP1 expression in the dKO mice, suggestive of malformed, aberrant bone cells. Because osteoclast formation and function depend on osteoblasts, we speculate that the observed minor phenotype in the osteoclast lineage (which is likely related to delayed molar eruption) is secondary to the dKO bone cells. However, as bone defects encompass both the molars and incisors, the question is raised of why molars and incisors are differentially affected, if differences in eruption are truly attributed to the influence of surrounding bone? Our working hypothesis is that incisor eruption occurs earlier than that of molars and the dKO gene excision event initiates at postnatal day 3, when the incisor is already erupted. Thus,, the incisor eruption might indeed be affected in dKO mice if tamoxifen treatment, and thus gene excision, occurred earlier in development. Of note, Bmp1/Tll1 are widely expressed in different cell types, and deletion of both genes could conceivably lead to an osteoclast-autonomous defect. However, our previous studies do not support this possibility, as there even more TRAP positive cells were found in dKO long bones than in controls (21).
In this study, we also observed a milder DGI-like phenotype in 8-week dKO mice than in the 3-week dKO mice. We reason that 8-week dKO mice may have a greater ability to compensate for loss of BMP1/TLL1 function. Additionally, the first 3 weeks after birth is more crucial for dentin and root formation, whereas excision of Bmp1/Tll1 sequences was initiated at 4 weeks of age (when 1st-molars are largely formed) in 8-week dKO mice.
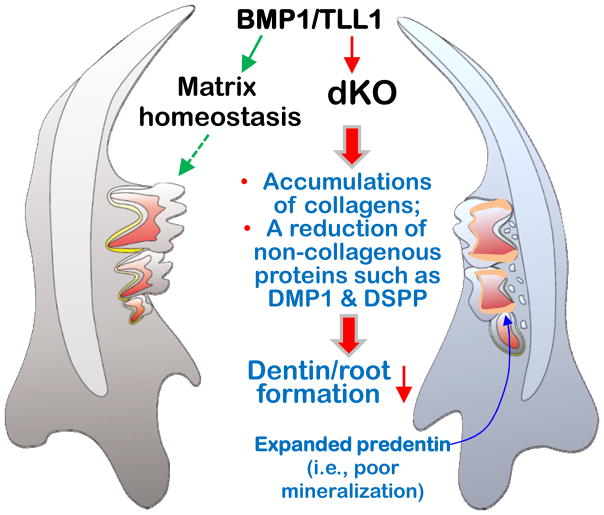
Recent studies discovered that removing NFIC (nuclear factor 1 C) (22) or osterix (23) results in defects in tooth root, but not crown, dentin formation. Studies of mechanism demonstrated a great reduction in DMP1 and DSPP levels, leading to an inhibition in odontoblast differentiation, and short thin root dentin with few dentin tubules (23). Furthermore, Dmp1-null (24) and Dspp-null mice (25) both display a similar DGI-like phenotype, including enlarged predentin and thin dentin, which supports the critical roles of these two proteins in dentin formation. In this study, we demonstrated a similar reduction in these two non-collagenous proteins in the dKO odontoblasts, which may explain in part why these null mice developed a DGI-like phenotype in both age groups. Based on these findings, we propose that BMP1 and TLL1, two extracellular proteinases maintain a homeostasis in levels of collagen and non-collagenous proteins, which is required for normal mineralization. Removing these proteinases destroys this homeostatic balance, leading to abnormal collagen accumulations, but reductions in those non-collagenous proteins (DMP1 and DSPP) important to mineralization. As a result, a DGI-like phenotype occurs (Fig. 4).
Figure 4. Depictions of a critical role for BMP1/TLL1 during postnatal tooth root formation.
Here we propose that the closely related proteinases BMP1/TLL1 are required for maintaining matrix homeostasis during postnatal tooth development. Ablation of these two proteinases led to shortened roots and severe deficits in dentin mineralization, and exhibiting of a DGI-like phenotype.
Supplementary Material
Supplementary Figure 1. Representative immunostaining images show a marked reduction in BMP1 signals in the dKO odontoblast layers (right panel) in both crown and root compared to the age-matched control (left panel).
Supplementary Figure 2. a) X-ray examinations revealed shortened roots and delayed molar eruptions in 3-week-old dKO mice. The age-matched control mandibulae are presented in the top panel and the dKO mandibulae are shown in the lower panel. b) X-ray examinations revealed shortened roots in 8-week-old dKO mice. The age-matched control mandibulae are presented in the top panel and the dKO mandibulae are shown in the lower panel.
Supplementary Figure 3. DKO mice display a mild DGI-like phenotype at later stages. a) Images of x-ray analyses (top corner panels) and H&E staining (lower and center panels) of 1st molars revealed an expanded pulp-root canal in the dKO mice, in which BMP1/TLL1 were ablated starting at 4-weeks of age and harvested at 8 weeks of age. The enlarged H&E stains reveal residual predentin layer in the dKO mice, but not in the age-matched controls. b) FITC staining images show increased dye infiltration in the dKO dentin tubules at a high magnification, indicative of expanded dentin tubules (right panels). c) Quantitative data show increased root length and expanded predentin in 8-week-old dKO mice.
Significance box.
The problem with tooth regeneration in dentistry is the root problem. Here we reported a severe root dentin phenotype in Bmp1/Tll1 null mice, indicating a critical role of Bmp1/Tll1 in root formation. This finding will aid in future tooth regeneration.
Research highlights.
Deletions of Bmp1/Tll1 result in short tooth roots and severe defects in dentin mineralization similar to DGI-like phenotype
Bmp1/Tll1 null mice display an unexpected delayed tooth eruption.
Bmp1/Tll1 null mice show accumulations of collagens but a reduction in non-collagenous proteins such as DMP1 and DSPP
This study provides a novel evidence that Bmp1/Tll1 is required for maintenance of ECM homeostasis, which is the foundation for dentin mineralization.
Acknowledgments
This study was partially supported by NIH grants AR047746 to DSG and DE025014 to JQF.
Footnotes
The authors deny any conflicts of interest related to this study.
This research is original and free of conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shields ED, Bixler D, el-Kafrawy AM. A proposed classification for heritable human dentine defects with a description of a new entity. Archives of oral biology. 1973;18(4):543–553. doi: 10.1016/0003-9969(73)90075-7. [DOI] [PubMed] [Google Scholar]
- 2.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet. 2016;387(10028):1657–1671. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syx D, Guillemyn B, Symoens S, Sousa AB, Medeira A, Whiteford M, et al. Defective Proteolytic Processing of Fibrillar Procollagens and Prodecorin Due to Biallelic BMP1 Mutations Results in a Severe, Progressive Form of Osteogenesis Imperfecta. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2015;30(8):1445–1456. doi: 10.1002/jbmr.2473. [DOI] [PubMed] [Google Scholar]
- 4.Asharani PV, Keupp K, Semler O, Wang W, Li Y, Thiele H, et al. Attenuated BMP1 function compromises osteogenesis, leading to bone fragility in humans and zebrafish. Am J Hum Genet. 2012;90(4):661–674. doi: 10.1016/j.ajhg.2012.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge G, Greenspan DS. Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res C Embryo Today. 2006;78(1):47–68. doi: 10.1002/bdrc.20060. [DOI] [PubMed] [Google Scholar]
- 6.Kessler E, Takahara K, Biniaminov L, Brusel M, Greenspan DS. Bone morphogenetic protein-1: the type I procollagen C-proteinase. Science (New York, NY) 1996;271(5247):360–362. doi: 10.1126/science.271.5247.360. [DOI] [PubMed] [Google Scholar]
- 7.Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279(2):980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- 8.von Marschall Z, Fisher LW. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1) Matrix Biol. 2010;29(4):295–303. doi: 10.1016/j.matbio.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuchiya S, Simmer JP, Hu JC, Richardson AS, Yamakoshi F, Yamakoshi Y. Astacin proteases cleave dentin sialophosphoprotein (Dspp) to generate dentin phosphoprotein (Dpp) Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(1):220–228. doi: 10.1002/jbmr.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, et al. Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development. 1996;122(11):3587–3595. doi: 10.1242/dev.122.11.3587. [DOI] [PubMed] [Google Scholar]
- 11.Clark TG, Conway SJ, Scott IC, Labosky PA, Winnier G, Bundy J, et al. The mammalian Tolloid-like 1 gene, Tll1, is necessary for normal septation and positioning of the heart. Development. 1999;126(12):2631–2642. doi: 10.1242/dev.126.12.2631. [DOI] [PubMed] [Google Scholar]
- 12.Muir AM, Ren Y, Butz DH, Davis NA, Blank RD, Birk DE, et al. Induced ablation of Bmp1 and Tll1 produces osteogenesis imperfecta in mice. Hum Mol Genet. 2014;23(12):3085–3101. doi: 10.1093/hmg/ddu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1(1):113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Ye L, Yu S, Zhang S, Xie Y, McKee MD, et al. Rescue of odontogenesis in Dmp1-deficient mice by targeted re-expression of DMP1 reveals roles for DMP1 in early odontogenesis and dentin apposition in vivo. Developmental biology. 2007;303(1):191–201. doi: 10.1016/j.ydbio.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Lu Y, Chen L, Gao T, D’Souza R, Feng JQ, et al. DMP1 processing is essential to dentin and jaw formation. Journal of dental research. 2011;90(5):619–624. doi: 10.1177/0022034510397839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fen JQ, Zhang J, Dallas SL, Lu Y, Chen S, Tan X, et al. Dentin matrix protein 1, a target molecule for Cbfa1 in bone, is a unique bone marker gene. J Bone Miner Res. 2002;17(10):1822–1831. doi: 10.1359/jbmr.2002.17.10.1822. [DOI] [PubMed] [Google Scholar]
- 17.Baba O, Qin C, Brunn JC, Wygant JN, McIntyre BW, Butler WT. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004;23(6):371–379. doi: 10.1016/j.matbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Ciani C, Doty SB, Fritton SP. An effective histological staining process to visualize bone interstitial fluid space using confocal microscopy. Bone. 2009;44(5):1015–1017. doi: 10.1016/j.bone.2009.01.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren Y, Han X, Ho SP, Harris SE, Cao Z, Economides AN, et al. Removal of SOST or blocking its product sclerostin rescues defects in the periodontitis mouse model. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29(7):2702–2711. doi: 10.1096/fj.14-265496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Z, Zhang H, Zhou X, Han X, Ren Y, Gao T, et al. Genetic evidence for the vital function of Osterix in cementogenesis. J Bone Miner Res. 2012;27(5):1080–1092. doi: 10.1002/jbmr.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muir AM, Ren Y, Butz DH, Davis NA, Blank RD, Birk DE, et al. Induced ablation of Bmp1 and Tll1 produces osteogenesis imperfecta in mice. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DS, Park JT, Kim HM, Ko JS, Son HH, Gronostajski RM, et al. Nuclear factor I-C is essential for odontogenic cell proliferation and odontoblast differentiation during tooth root development. J Biol Chem. 2009;284(25):17293–17303. doi: 10.1074/jbc.M109.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Jiang Y, Qin C, Liu Y, Ho SP, Feng JQ. Essential Role of Osterix for Tooth Root but not Crown Dentin Formation. J Bone Miner Res. 2015;30(4):742–746. doi: 10.1002/jbmr.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ye L, MacDougall M, Zhang S, Xie Y, Zhang J, Li Z, et al. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal tooth development. The Journal of biological chemistry. 2004;279(18):19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- 25.Sreenath TL, Cho A, Thyagarajan T, Kulkarni AB. Odontoblast-specific expression of cre recombinase successfully deletes gene segments flanked by loxP sites in mouse teeth. Genesis. 2003;35(2):94–99. doi: 10.1002/gene.10170. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Representative immunostaining images show a marked reduction in BMP1 signals in the dKO odontoblast layers (right panel) in both crown and root compared to the age-matched control (left panel).
Supplementary Figure 2. a) X-ray examinations revealed shortened roots and delayed molar eruptions in 3-week-old dKO mice. The age-matched control mandibulae are presented in the top panel and the dKO mandibulae are shown in the lower panel. b) X-ray examinations revealed shortened roots in 8-week-old dKO mice. The age-matched control mandibulae are presented in the top panel and the dKO mandibulae are shown in the lower panel.
Supplementary Figure 3. DKO mice display a mild DGI-like phenotype at later stages. a) Images of x-ray analyses (top corner panels) and H&E staining (lower and center panels) of 1st molars revealed an expanded pulp-root canal in the dKO mice, in which BMP1/TLL1 were ablated starting at 4-weeks of age and harvested at 8 weeks of age. The enlarged H&E stains reveal residual predentin layer in the dKO mice, but not in the age-matched controls. b) FITC staining images show increased dye infiltration in the dKO dentin tubules at a high magnification, indicative of expanded dentin tubules (right panels). c) Quantitative data show increased root length and expanded predentin in 8-week-old dKO mice.