Abstract
In most bacteria, cell division relies on the functions of an essential protein, FtsZ. FtsZ polymerizes at the future division site to form a ring-like structure, termed the Z-ring, that serves as a scaffold to recruit all other division proteins, and possibly generates force to constrict the cell. The scaffolding function of the Z-ring is well established, but the force generating function has recently been called into question. Additionally, new findings have demonstrated that the Z-ring is more directly linked to cell wall metabolism than simply recruiting enzymes to the division site. Here we review these advances and suggest that rather than generating a rate-limiting constrictive force, the Z-ring’s function may be redefined as an orchestrator of septum synthesis.
Introduction
The final step in cellular replication is the physical constriction and ultimate separation of the mother cell into two daughters. In all organisms, these dramatic morphological changes require synthesis and delivery of new material, and the generation of force for envelope ingression. For animal cells, the contractile ring generates the bulk of the force required for cytokinesis, with myosin motors burning ATP as they walk on antiparallel actin filaments to constrict the membrane. However, in most other organisms, in particular walled cells, it is difficult to deconvolve the contributions of cytoskeletal elements and cell wall metabolic enzymes to force generation. In this review, we will discuss advances made over the last several years in understanding the source of the constrictive force in bacterial division, with emphasis on the relative roles and contributions of the polymerizing GTPase, FtsZ, and the peptidoglycan (PG) cell wall synthesis machinery.
Bacterial cell division: the challenge and the machinery
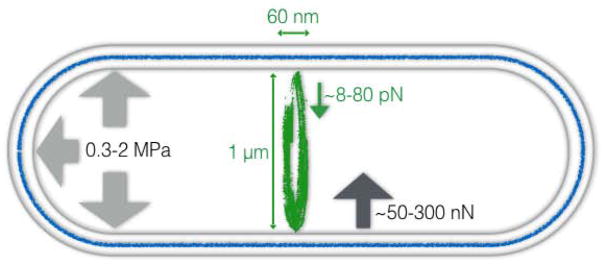
Bacterial cell division requires invagination and separation of a multi-layered cell envelope, including constriction and fission of the membrane(s), and synthesis, remodeling, and splitting of the PG cell wall at the division site. Bacterial cells possess high turgor pressures, ranging from ~ 0.3 MPa for Gram-negative Escherichia coli [1] to ~2 MPa in Gram-positive Bacillus subtilis [2]. This pressure, acting on a constriction zone of ~60 nm in axial width and ~3μm in circumference (approximately the size of the septum), would require a total force of ~50 to 300 nN to counterbalance (Fig. 1). The minimal amount of work needed to constrict the membrane to the final fission would then be at least on the order of ~10−14 Joules. This is a considerable force, as individual motor protein molecules usually generate a force on the order of a few pN [3]. Thus, the constrictive force is most likely generated through the collective effort of a large number of molecular components and/or reactions. Note here that we have not yet considered the cell wall, the rigidity of which would represent another substantial resistance for the constrictive force to overcome [4].
Figure 1.
Forces acting within the cell relevant to cell division. The Z-ring, with the approximate dimensions of the septum in E. coli labeled, is shown in green. Turgor pressure (light gray arrows) applies an outward force that must be overcome for constriction. The estimated maximum force the Z-ring might exert (green) is significantly less than the estimated required to overcome turgor pressure (dark gray). Note that these estimates do not include the force required to overcome the rigidity of the cell wall (blue).
Where does the constrictive force come from? It is almost certain that the force originates from the divisome, the essential division apparatus operating at the edge of and within the invaginating membrane. In nearly all bacteria, the structural core of the divisome is a polymerizing GTPase and homolog of eukaryotic tubulin called FtsZ [5,6]. Accessory factors facilitate assembly of FtsZ into the cytokinetic “Z-ring”, a dynamic collection of protofilaments [7]. The Z-ring is targeted to the inner membrane through FtsZ’s membrane-anchoring proteins, including the conserved actin family protein, FtsA [8,9]. About a dozen other essential cell division proteins are subsequently recruited to the division site through a network of protein-protein and protein-envelope interactions [10]. Many of these proteins are involved in cell wall synthesis and remodeling, including the division-specific transpeptidase FtsI (Penicillin Binding Protein (PBP) 3) and hydrolytic enzymes that split the peptidoglycan for daughter cell separation [11]. It is not entirely clear which components of the divisome generate a constrictive force, but the Z-ring and the septal PG metabolic machinery have each been proposed as the likely sources.
An evolving hypothesis: FtsZ-mediated constrictive force
FtsZ has long been proposed as the key constrictive force generator [5]. Two main properties of FtsZ make it possible to fulfill this role. First, FtsZ is a GTPase [12,13]; intuitively, it can harvest the chemical energy released by GTP hydrolysis for mechanical work, as other motor proteins do. Based on the free energy of GTP hydrolysis (ΔG0 = ~30 kJ/mol [14]) and cellular FtsZ concentrations (~5μM in E. coli [15]), we estimate that the total amount of energy that could be released by FtsZ’s GTP hydrolysis during the constriction period is on the order of ~10−14 Joules. This amount of work, if completely harvested, is comparable to what is minimally required to counter balance the turgor pressure as discussed above. Second, FtsZ polymerizes [16–18]. Even without nucleotide hydrolysis, a biological polymer can generate force based on its mechanical properties such as its stiffness and curvature [19], and/or by dynamic polymerization and depolymerization [20]
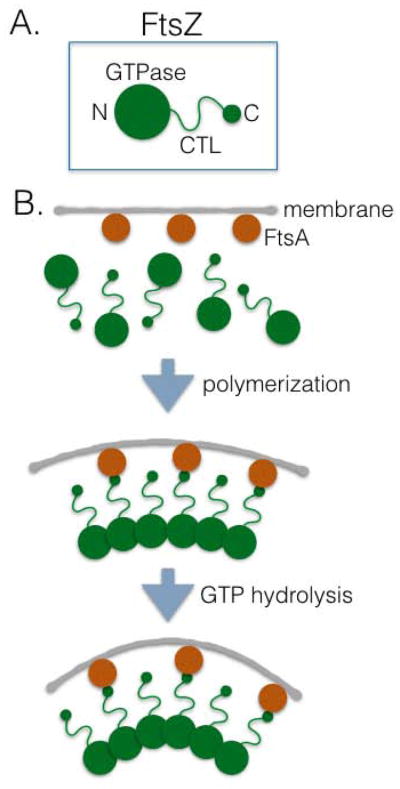
A number of FtsZ-mediated force generation mechanisms based on these two main properties have been proposed in the past decade. These include sliding [9,21], pinching [22], condensing [23], bending [24–26], assembling/disassembling [25,27] and lateral bonding [23,28] of FtsZ filaments, and are estimated to generate force in the range of ~ 8–80 pN [5,23,29]. Among the proposed mechanisms, FtsZ filament bending has accumulated the most experimental support. Electron microscopy (EM) and atomic force microscopy (AFM) studies showed that FtsZ filaments formed in the presence of GDP were highly curved, whereas those formed with GTP were straight [30] or moderately curved [31–34]. The C-terminus of FtsZ, which binds to its membrane anchoring proteins such as FtsA and ZipA in E. coli, resides on the outside of the curved filament and the N-terminus faces the inside [29,35,36] (Fig. 2A). In this configuration, if FtsZ filaments curve away from the membrane to which they are attached at the C-terminus, this would generate a pulling force (Fig. 2B).
Figure 2.
FtsZ and the generation of constrictive force. (A) Domain organization of FtsZ (green). CTL - C-terminal linker. Relative locations of the amino (N) and carboxy (C) termini, and the GTPase domain of FtsZ are labeled. (B) Proposed mechanism for generation of force through bending of FtsZ filaments. Straight or gently curved filaments can slightly deform membranes upon polymerization, but deep constriction in vitro requires GTP hydrolysis and/or FtsA-induced turnover.
The most convincing evidence for force generation through FtsZ filament bending comes from reconstitution of Z-rings inside of liposomes using purified FtsZ protein. FtsZ fused to yellow fluorescent protein (YFP) and/or a membrane targeting sequence (MTS) formed slight, static indentations on associated liposomes in the presence of the slowly-hydrolyzed GTP analog GMPCPP, and constricted to smaller diameters when GTP was present and gradually depleted [37]. When assembled on the outside of liposomes, the geometry of the FtsZ-induced membrane deformation correlated with the direction of filament curvature [35]. These experiments clearly demonstrated that curved FtsZ filaments are rigid enough to bend the associated membrane, a property also common to protein crowding on membranes [38]. They further suggested that under these in vitro conditions, dynamic subunit turnover and/or an increase of FtsZ filament curvature likely mediate the observed membrane constriction.
While FtsZ-MTS promotes only moderate liposome constriction, complete fission of liposomes in the presence of GTP hydrolysis was observed, albeit at a low frequency, when FtsZ’s cognate membrane tethering protein FtsA was used to target FtsZ to liposome membranes [39]. This observation suggests that an additional contribution from FtsA, perhaps through its ability to promote FtsZ turnover from the membrane [40] is required for complete liposome fission.
Now we arrive at a picture in which FtsZ filaments alone can indeed deform membranes in vitro, but complete liposome constriction and fission requires GTP hydrolysis and the presence of FtsA. The question is then: is this FtsZ-derived force the driving force for the constriction process in cells? Here we define the driving force as the one that dictates the rate of constriction (i.e. reduction of septum diameter per unit time), which should be proportional to the amount of exerted force. In this definition, if FtsZ-generated force were the driving force, altering FtsZ properties that are proposed to generate the constrictive force should also alter the constriction rate.
A recent study examined this hypothesis, and showed that changing FtsZ assembly dynamics, GTPase activity, or amount of FtsZ in the Z-ring did not alter the constriction rate in E. coli cells; thus, the force generated by FtsZ may not be rate-limiting for constriction [41]. Whether these results hold true for other bacterial species remains to be examined, but they are consistent with previous genetic studies wherein FtsZ mutants with drastically reduced GTPase activities formed static or aberrant Z-ring structures, yet were viable at permissive temperatures [42–44]. Recent fluorescence microscopy studies also showed that FtsZ departs the midcell prior to cell wall remodeling factors at the end of cell division in E. coli [45,46], indicating that, at least at late constriction stages, the presence of FtsZ is not required. Interestingly, early temperature shift experiments of FtsZ mutants showed ~ 50% of constricting cells were able to finish constriction after being shifted to a restrictive temperature [47]. Collectively, these studies raise the possibility that FtsZ, while essential, is not the rate-limiting constriction force generator.
Another source of force: peptidoglycan synthesis
If FtsZ is not the rate-limiting constrictive force generator, what is? The most likely candidate is the PG metabolic machinery itself (Fig. 3A). It has long been appreciated that cell wall synthesis is required for cell division, as filamentation is observed when cells are treated with intermediate concentrations of penicillin or when FtsI function is inhibited [48–50]. Moreover, bacterial L forms that are induced to propagate without a cell wall do so by protrusion and resolution of large vesicles - not through an FtsZ-mediated constriction and fission mechanism [51]. This is despite the fact that FtsZ and the rest of the divisome are still produced in L forms. Conversely, chlamydial pathogens require PG metabolism to divide, but lack FtsZ and instead use MreB and its associated proteins to direct septal PG synthesis [52–56].
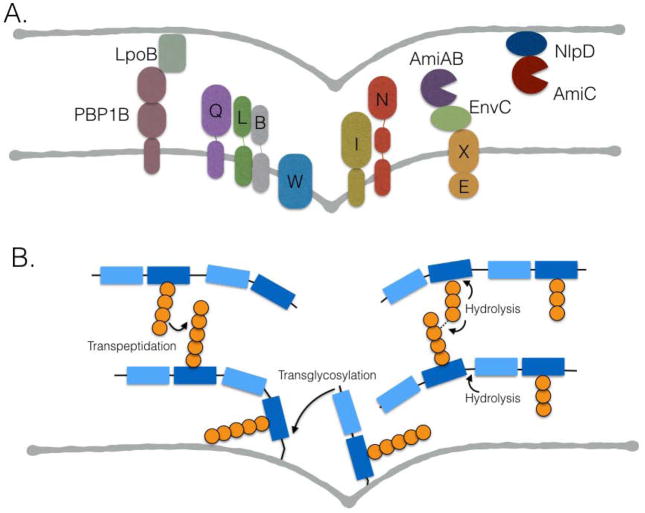
Figure 3.
Peptidoglycan metabolism and the generation of constrictive force. (A) E. coli divisome components that may contribute to generation of force through septum synthesis and remodeling, including the transglycosylase and transpeptidase PBP1B, the transpeptidase FtsI, and the amidases AmiA, B, and C. LpoB regulates PBP1B, FtsEX and EnvC regulate AmiA and AmiB, NlpD regulates AmiC, and FtsQ, L, B, W, and N are implicated in the initiation of and/or rate of constriction. (B) Peptidoglycan metabolic reactions that contribute to septum synthesis and remodeling. Hydrolysis reactions include those mediated by amidases, lytic transglycosylases, and endopeptidases.
Coltharp and colleagues explored the effects of altered PG synthesis rates on cell constriction by using wild-type (WT) E. coli cells with different growth rates and a temperature sensitive allele of FtsI [41]. They found that the cell constriction rate was proportional to the cell elongation rate, which is governed by PG synthesis rate in balanced growth, similar to what was observed in Caulobacter crescentus [57]. Most importantly, the constriction rate was significantly slowed in the FtsI mutant strain [41]. Substantially longer constriction periods of E. coli cells have also been previously observed in mutants of the late divisome proteins FtsI and FtsQ [47,58], and upon overexpression of FtsN [59]. In addition, mutations in FtsL and FtsB in E. coli [60,61] promote premature initiation of constriction, and mutations in FtsW and FtsI in C. crescentus [62] hyperactivate constriction. These data suggest that the late divisome, including its associated PG metabolic enzymes, instead of FtsZ, plays a central role in driving constriction.
The next question to consider is then whether the chemical energy from septal PG synthesis is sufficient to generate a force large enough to actively push the cytoplasmic membrane inward against the turgor pressure (Fig. 3B). Directly estimating septal PG synthesis-associated chemical energy in bacterial cells is not possible due to the lack of current knowledge in the orientation and density of glycan strands in the septum, and the corresponding free energy released during PG remodeling reactions such as transglycosylation, transpeptidation, and PG hydrolysis. However, as the cell wall maintains cell shape, it is certainly conceivable that its mechanical strength would be sufficient to deform the inner membrane against turgor pressure. Furthermore, theoretical studies have suggested that constriction in walled bacteria cells can be entirely driven by the chemical potential energy released from cell wall synthesis at the septum [63], and that in the absence of active cell wall remodeling, the force provided by FtsZ alone is insignificant and could not impact the constriction rate [4].
Similar to the considerations described above for bacteria, in fission yeast, it has been shown that cell constriction does not require the actomyosin contractile ring. Moreover, it was estimated that the polymerization pressure of a single β-glucan fibril on its membrane-imbedded synthase, at ~2.2 MPa, would significantly exceed the ~1 MPa turgor pressure in Schizosaccharomyces pombe, providing a means for cell wall synthesis to drive constriction [64]. In bacterial cells, peptidoglycan polymerization could potentially generate a similar magnitude of force, although clearly more work is required to obtain a quantitative, molecular understanding of how peptidoglycan synthesis might power constriction.
A role for the Z-ring in regulating peptidoglycan metabolism
As outlined above, the Z-ring appears to provide limited force for cell division, and PG synthesis is, in our view, the likely primary source of constriction force. What, then, is the role of FtsZ in promoting constriction? Certainly FtsZ acts as a scaffold, marking the site of division and recruiting the requisite machinery to promote cell wall synthesis there. Evidence exists, however, to suggest that FtsZ is more directly linked to the regulation of PG metabolism than mediating simple recruitment of enzymes to the division site.
In at least two contexts, FtsZ mutants with altered - but not completely eliminated –polymerization properties induced defects in PG metabolism leading to cell lysis. In the first scenario, E. coli mutant cells expressing the temperature sensitive ftsZ84 allele and lacking the DD-carboxypeptidases PBP5 and PBP7 or PBP4 and PBP7 lysed rapidly when shifted to the restrictive temperature, suggesting a functional interaction between FtsZ with aberrant activity and these otherwise non-essential low molecular weight PBPs [65]. In the second scenario, production of an FtsZ variant that lacks the disordered C-terminal linker that separates the GTPase domain from the C-terminal conserved peptide in C. crescentus or B. subtilis caused cells to lyse rapidly [66,67]. At least in C. crescentus, this FtsZ variant-induced lysis was coincident with altered PG chemistry, but occurred without obvious defects in divisome assembly, i.e. the scaffolding function of FtsZ [67]. These observations hint that FtsZ impacts specific PG metabolic activities through mechanisms that are in addition to its role in protein recruitment.
What might FtsZ provide beyond a platform for assembly of division proteins? One possible model is again inspired by observations in fission yeast. In that organism, when the actomyosin ring was disrupted, septum ingression continued, but occurred in a spatially disorganized, asymmetric manner that led to misshapen (non-circular) septa [64,68]. Careful observation and modeling of septum ingression demonstrated that an active actomyosin ring promotes septum synthesis in a curvature-dependent manner — regions of the septum with higher curvature constricted more quickly — and hence rectifies the non-circular septa [68]. As such, it was suggested that the force from actomyosin ring contraction (which is proportional to local membrane curvature) is used to modulate the spatial organization and/or activity of cell wall synthesis machineries to ensure even, processive constriction. In a similar manner, in bacterial cells, a small force from the Z-ring could locally promote septum synthesis (Fig. 4). It is also possible that, even without meaningful force generation, dynamic FtsZ assembly and disassembly in the Z-ring promotes even distribution of cell wall synthetic activity around the circumference of the septum. This hypothesis may help explain why FtsZ GTPase mutants are viable, but yield deformed septa in E. coli cells. Nevertheless, the question remains of how FtsZ, through force generation or other means, regulates the spatiotemporal organization of PG metabolic enzyme activity.
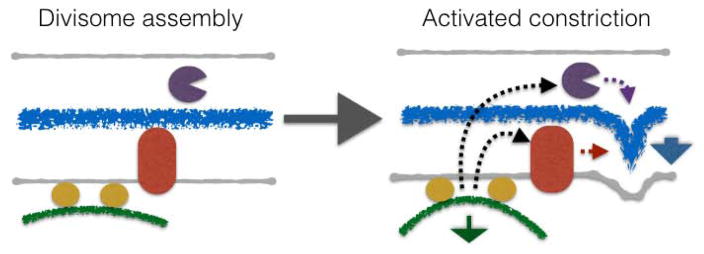
Figure 4.
Model for coordinated action of FtsZ and peptidoglycan metabolism in driving cell division. (Left) The Z-ring (green line) assembles proximal to the inner membrane through interactions with FtsA and other membrane tethering proteins (yellow). This serves to initiate assembly of the divisome, including factors required for PG synthesis (red) and hydrolysis (purple). (Right) The Z-ring provides a signal (black dashed arrows) - through generation of moderate force (green arrow) and/or other biophysical or biochemical mechanisms (black arrows)- to peptidoglycan synthetic and hydrolytic factors that provides directionality and evenness to the synthesis of an invaginating septum. The peptidoglycan synthetic (red arrow) and hydrolytic (purple arrow) activities within the divisome shape a septum (blue line) that pushes the inner membrane and provides the major force for constriction (blue arrow).
Conclusions
The discovery that FtsZ forms polymers and plays a central role in cell division early on led to the attractive hypothesis that it plays a major force-generating role in bacterial division. However, a couple of decades of careful study addressing the hypothesis that FtsZ is the primary driver of cytokinesis have failed to rigorously support that possibility. Instead, evidence points us back to the cell wall synthetic machinery as the major source of constrictive force. In moving forward, however, we are confronted with gaping holes in knowledge that prevent formulation of a quantitative physical and mechanistic model for coordinated Z-ring assembly, PG synthesis, and cell wall constriction.
What do we need to know? First, the physical orientation, density, and architecture of the glycan strands and peptide crosslinks at the septum need to be elucidated. This would allow us to better envision the spatial coordination of the divisome and cell wall synthesis, and to identify testable mechanisms for how insertion of new cell wall material might push against the inner membrane. Second, we require additional details regarding the enzymology of cell wall metabolism at the division site. Which enzymatic activities contribute to force generation and how much energy could be liberated from each reaction and used to do the work of constriction? Finally, to address directly if FtsZ-mediated force indeed guides PG synthesis, we require methods to perturb force generation without disrupting the scaffolding function of FtsZ. With these parameters and tools in hand, we can begin to approach a quantitative understanding of the forces behind bacterial cytokinesis.
Highlights.
Bacterial cell division requires generation of constrictive force
Membrane-associated FtsZ can generate force in vitro
FtsZ-generated force is limited and does not set the rate of constriction in cells
Cell wall synthesis is required for and sets the rate of constriction in cells
FtsZ-generated force likely regulates cell wall metabolism for division
Acknowledgments
We thank members of the Xiao and Goley laboratories, especially Dr. Xinxing Yang, Dr. Carla Coltharp, Elizabeth Meier, and Kousik Sundararajan, as well as Prof. Waldemar Vollmer, Dr. Sean Sun, and Dr. Ganhui Lan for thoughtful discussions during the writing of this manuscript. Work in the Goley and Xiao Laboratories on to the topic of bacterial cytokinesis is supported by the National Institutes of Health [grant numbers R01GM108640 (EDG) and 1R01GM086447 (JX)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Deng Y, Sun M, Shaevitz JW. Direct measurement of cell wall stress stiffening and turgor pressure in live bacterial cells. Phys Rev Lett. 2011;107:158101. doi: 10.1103/PhysRevLett.107.158101. [DOI] [PubMed] [Google Scholar]
- 2.Whatmore AM, Reed RH. Determination of turgor pressure in Bacillus subtilis: a possible role for K+ in turgor regulation. J Gen Microbiol. 1990;136:2521–2526. doi: 10.1099/00221287-136-12-2521. [DOI] [PubMed] [Google Scholar]
- 3.Kolomeisky AB, Fisher ME. Molecular motors: a theorist’s perspective. Annu Rev Phys Chem. 2007;58:675–695. doi: 10.1146/annurev.physchem.58.032806.104532. [DOI] [PubMed] [Google Scholar]
- 4.Lan G, Wolgemuth CW, Sun SX. Z-ring force and cell shape during division in rodlike bacteria. Proc Natl Acad Sci US A. 2007;104:16110–16115. doi: 10.1073/pnas.0702925104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erickson HP, Anderson DE, Osawa M. FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol Mol Biol Rev. 2010;74:504–528. doi: 10.1128/MMBR.00021-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meier EL, Goley ED. Form and function of the bacterial cytokinetic ring. Curr Opin Cell Biol. 2014;26:19–27. doi: 10.1016/j.ceb.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Ortiz C, Natale P, Cueto L, Vicente M. The keepers of the ring: regulators of FtsZ assembly. FEMS Microbiol Rev. 2016;40:57–67. doi: 10.1093/femsre/fuv040. [DOI] [PubMed] [Google Scholar]
- 8.Pichoff S, Lutkenhaus J. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Molecular Microbiology. 2005;55:1722–1734. doi: 10.1111/j.1365-2958.2005.04522.x. [DOI] [PubMed] [Google Scholar]
- 9**.Szwedziak P, Wang Q, Bharat TAM, Tsim M, Löwe J. Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. eLife. 2014;3:e04601. doi: 10.7554/eLife.04601. The authors reconstitute FtsZ-FtsA rings inside unilamellar vesicles and visualize them using electron cryomicroscopy and cryotomography. They propose a sliding filament mechanism of constriction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haeusser DP, Margolin W. Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat Rev Microbiol. 2016;14:305–319. doi: 10.1038/nrmicro.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egan AJF, Vollmer W. The physiology of bacterial cell division. Ann N Y Acad Sci. 2013;1277:8–28. doi: 10.1111/j.1749-6632.2012.06818.x. [DOI] [PubMed] [Google Scholar]
- 12.de Boer P, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 13.RayChaudhuri D, Park JT. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 14.Voet D, Voet JG. Biochemistry, The Expression and Transmission of Genetic Information. Wiley; 2004. [Google Scholar]
- 15.Fu G, Huang T, Buss J, Coltharp C, Hensel Z, Xiao J. In vivo structure of the E. coli FtsZ-ring revealed by photoactivated localization microscopy (PALM) PLoS ONE. 2010;5:e12682. doi: 10.1371/journal.pone.0012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bramhill D, Thompson CM. GTP-dependent polymerization of Escherichia coli FtsZ protein to form tubules. Proc Natl Acad Sci US A. 1994;91:5813–5817. doi: 10.1073/pnas.91.13.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erickson HP, Taylor DW, Taylor KA, Bramhill D. Bacterial cell division protein FtsZ assembles into protofilament sheets and minirings, structural homologs of tubulin polymers. Proc Natl Acad Sci US A. 1996;93:519–523. doi: 10.1073/pnas.93.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popp D, Iwasa M, Narita A, Erickson HP, Maéda Y. FtsZ condensates: an in vitro electron microscopy study. Biopolymers. 2009;91:340–350. doi: 10.1002/bip.21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen MH, Morris EJ, Weitz DA. Mechanics and dynamics of reconstituted cytoskeletal systems. Biochim Biophys Acta. 2015;1853:3038–3042. doi: 10.1016/j.bbamcr.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theriot JA. The polymerization motor. Traffic. 2000;1:19–28. doi: 10.1034/j.1600-0854.2000.010104.x. [DOI] [PubMed] [Google Scholar]
- 21.Hörger I, Velasco E, Mingorance J, Rivas G, Tarazona P, Vélez M. Langevin computer simulations of bacterial protein filaments and the force-generating mechanism during cell division. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;77:011902. doi: 10.1103/PhysRevE.77.011902. [DOI] [PubMed] [Google Scholar]
- 22.Li Z, Trimble MJ, Brun YV, Jensen GJ. The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J. 2007;26:4694–4708. doi: 10.1038/sj.emboj.7601895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lan G, Daniels BR, Dobrowsky TM, Wirtz D, Sun SX. Condensation of FtsZ filaments can drive bacterial cell division. Proc Natl Acad Sci US A. 2009;106:121–126. doi: 10.1073/pnas.0807963106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh B, Sain A. Origin of contractile force during cell division of bacteria. Phys Rev Lett. 2008;101:178101. doi: 10.1103/PhysRevLett.101.178101. [DOI] [PubMed] [Google Scholar]
- 25.Allard JF, Cytrynbaum EN. Force generation by a dynamic Z-ring in Escherichia coli cell division. Proc Natl Acad Sci US A. 2009;106:145–150. doi: 10.1073/pnas.0808657106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Hsin J, Zhao L, Cheng Y, Shang W, Huang KC, Wang H-W, Ye S. FtsZ protofilaments use a hinge-opening mechanism for constrictive force generation. Science. 2013;341:392–395. doi: 10.1126/science.1239248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Surovtsev IV, Morgan JJ, Lindahl PA. Kinetic modeling of the assembly, dynamic steady state, and contraction of the FtsZ ring in prokaryotic cytokinesis. PLoS Comput Biol. 2008;4:e1000102. doi: 10.1371/journal.pcbi.1000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hörger I, Campelo F, Hernández-Machado A, Tarazona P. Constricting force of filamentary protein rings evaluated from experimental results. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;81:031922. doi: 10.1103/PhysRevE.81.031922. [DOI] [PubMed] [Google Scholar]
- 29.Hsin J, Gopinathan A, Huang KC. Nucleotide-dependent conformations of FtsZ dimers and force generation observed through molecular dynamics simulations. Proc Natl Acad Sci US A. 2012;109:9432–9437. doi: 10.1073/pnas.1120761109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu C, Reedy M, Erickson HP. Straight and curved conformations of FtsZ are regulated by GTP hydrolysis. J Bacteriol. 2000;182:164–170. doi: 10.1128/jb.182.1.164-170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.González JM, Vélez M, Jiménez M, Alfonso C, Schuck P, Mingorance J, Vicente M, Minton AP, Rivas G. Cooperative behavior of Escherichia coli cell-division protein FtsZ assembly involves the preferential cyclization of long single-stranded fibrils. Proc Natl Acad Sci US A. 2005;102:1895–1900. doi: 10.1073/pnas.0409517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamon L, Panda D, Savarin P, Joshi V, Bernhard J, Mucher E, Mechulam A, Curmi PA, Pastré D. Mica surface promotes the assembly of cytoskeletal proteins. Langmuir. 2009;25:3331–3335. doi: 10.1021/la8035743. [DOI] [PubMed] [Google Scholar]
- 33.Huecas S, Schaffner-Barbero C, García W, Yébenes H, Palacios JM, Díaz JF, Menéndez M, Andreu JM. The interactions of cell division protein FtsZ with guanine nucleotides. J Biol Chem. 2007;282:37515–37528. doi: 10.1074/jbc.M706399200. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Bjornson K, Redick SD, Erickson HP. A rapid fluorescence assay for FtsZ assembly indicates cooperative assembly with a dimer nucleus. Biophys J. 2005;88:505–514. doi: 10.1529/biophysj.104.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osawa M, Anderson DE, Erickson HP. Curved FtsZ protofilaments generate bending forces on liposome membranes. EMBO J. 2009;28:3476–3484. doi: 10.1038/emboj.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Housman M, Milam SL, Moore DA, Osawa M, Erickson HP. FtsZ Protofilament Curvature Is the Opposite of Tubulin Rings. Biochemistry. 2016;55:4085–4091. doi: 10.1021/acs.biochem.6b00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osawa M, Anderson DE, Erickson HP. Reconstitution of contractile FtsZ rings in liposomes. Science. 2008;320:792–794. doi: 10.1126/science.1154520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stachowiak JC, Schmid EM, Ryan CJ, Ann HS, Sasaki DY, Sherman MB, Geissler PL, Fletcher DA, Hayden CC. Membrane bending by protein--protein crowding. Nat Cell Biol. 2012;14:944–949. doi: 10.1038/ncb2561. [DOI] [PubMed] [Google Scholar]
- 39.Osawa M, Erickson HP. Liposome division by a simple bacterial division machinery. Proc Natl Acad Sci USA. 2013 doi: 10.1073/pnas.1222254110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40*.Loose M, Mitchison TJ. The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat Cell Biol. 2014;16:38–46. doi: 10.1038/ncb2885. In this study, the authors reconstitute dynamic FtsZ assembly on supported lipid bilayers and observe filament superstructure and turnover using total internal fluorescence microscopy. Their data indicate that FtsA promotes assembly of chiral FtsZ structures on membranes and that FtsA stimulates FtsZ turnover. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Coltharp C, Buss J, Plumer TM, Xiao J. Defining the rate-limiting processes of bacterial cytokinesis. Proc Natl Acad Sci USA. 2016;113:E1044–53. doi: 10.1073/pnas.1514296113. This study follows the rate of cell constriction in E. coli using super-resolution light microscopy and determine that peptidoglycan metabolism, not FtsZ, sets the rate of constriction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Addinall SG, Lutkenhaus J. FtsZ-spirals and -arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Molecular Microbiology. 1996;22:231–237. doi: 10.1046/j.1365-2958.1996.00100.x. [DOI] [PubMed] [Google Scholar]
- 43.Lu C, Stricker J, Erickson HP. Site-specific mutations of FtsZ--effects on GTPase and in vitro assembly. BMC Microbiol. 2001;1:7. doi: 10.1186/1471-2180-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Jones BD, Brun YV. A set of ftsZ mutants blocked at different stages of cell division in Caulobacter. Molecular Microbiology. 2001;40:347–360. doi: 10.1046/j.1365-2958.2001.02395.x. [DOI] [PubMed] [Google Scholar]
- 45**.Söderström B, Mirzadeh K, Toddo S, Heijne von G, Skoglund U, Daley DO. Coordinated disassembly of the divisome complex in Escherichia coli. Molecular Microbiology. 2016;101:425–438. doi: 10.1111/mmi.13400. The authors use imaging approaches to demonstrate that FtsZ disassembles from the division site prior to the completion of compartmentalization. This argues against a force generating role for FtsZ during late constriction. [DOI] [PubMed] [Google Scholar]
- 46.Söderström B, Skoog K, Blom H, Weiss DS, Heijne von G, Daley DO. Disassembly of the divisome in Escherichia coli: evidence that FtsZ dissociates before compartmentalization. Molecular Microbiology. 2014;92:1–9. doi: 10.1111/mmi.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taschner PE, Huls PG, Pas E, Woldringh CL. Division behavior and shape changes in isogenic ftsZ, ftsQ, ftsA, pbpB, and ftsE cell division mutants of Escherichia coli during temperature shift experiments. J Bacteriol. 1988;170:1533–1540. doi: 10.1128/jb.170.4.1533-1540.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarz U, Asmus A, Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969;41:419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- 49.Spratt BG. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci US A. 1975;72:2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spratt BG. Properties of the penicillin-binding proteins of Escherichia coli K12. Eur J Biochem. 1977;72:341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- 51.Leaver M, Domínguez-Cuevas P, Coxhead JM, Daniel RA, Errington J. Life without a wall or division machine in Bacillus subtilis. Nature. 2009;457:849–853. doi: 10.1038/nature07742. [DOI] [PubMed] [Google Scholar]
- 52.Ouellette SP, Karimova G, Subtil A, Ladant D. Chlamydia co-opts the rod shape-determining proteins MreB and Pbp2 for cell division. Molecular Microbiology. 2012 doi: 10.1111/j.1365-2958.2012.08100.x. [DOI] [PubMed] [Google Scholar]
- 53.Pilhofer M, Aistleitner K, Biboy J, Gray J, Kuru E, Hall E, Brun YV, Vannieuwenhze MS, Vollmer W, Horn M, et al. Discovery of chlamydial peptidoglycan reveals bacteria with murein sacculi but without FtsZ. Nat Commun. 2013;4:2856. doi: 10.1038/ncomms3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liechti GW, Kuru E, Hall E, Kalinda A, Brun YV, VanNieuwenhze M, Maurelli AT. A new metabolic cell-wall labelling method reveals peptidoglycan in Chlamydia trachomatis. Nature. 2014;506:507–510. doi: 10.1038/nature12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Frandi A, Jacquier N, Théraulaz L, Greub G, Viollier PH. FtsZ-independent septal recruitment and function of cell wall remodelling enzymes in chlamydial pathogens. Nat Commun. 2014;5:4200. doi: 10.1038/ncomms5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jacquier N, Frandi A, Pillonel T, Viollier PH, Viollier P, Greub G. Cell wall precursors are required to organize the chlamydial division septum. Nat Commun. 2014;5:3578. doi: 10.1038/ncomms4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iyer-Biswas S, Wright CS, Henry JT, Lo K, Burov S, Lin Y, Crooks GE, Crosson S, Dinner AR, Scherer NF. Scaling laws governing stochastic growth and division of single bacterial cells. Proc Natl Acad Sci USA. 2014;111:15912–15917. doi: 10.1073/pnas.1403232111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huls PG, Vischer NO, Woldringh CL. Delayed nucleoid segregation in Escherichia coli. Molecular Microbiology. 1999;33:959–970. doi: 10.1046/j.1365-2958.1999.01535.x. [DOI] [PubMed] [Google Scholar]
- 59.Aarsman MEG, Piette A, Fraipont C, Vinkenvleugel TMF, Nguyen-Distèche M, Blaauwen den T. Maturation of the Escherichia coli divisome occurs in two steps. Molecular Microbiology. 2005;55:1631–1645. doi: 10.1111/j.1365-2958.2005.04502.x. [DOI] [PubMed] [Google Scholar]
- 60**.Liu B, Persons L, Lee L, de Boer PAJ. Roles for both FtsA and the FtsBLQ subcomplex in FtsN-stimulated cell constriction in Escherichia coli. Molecular Microbiology. 2015;95:945–970. doi: 10.1111/mmi.12906. This study, along with 61** and 62**, identifies mutations in late divisome proteins that promote premature initiation of constriction ([60**] and [61**] or that hyperactivate constriction through either accelerated constriction or premature initiation [62**]. Collectively, they provide strong support for a role of the late divisome and its associated peptidoglycan-metabolic activity in driving constriction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61**.Tsang M-J, Bernhardt TG. A role for the FtsQLB complex in cytokinetic ring activation revealed by an ftsLallele that accelerates division. Molecular Microbiology. 2015;95:925–944. doi: 10.1111/mmi.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62**.Modell JW, Kambara TK, Perchuk BS, Laub MT. A DNA Damage-Induced, SOS-Independent Checkpoint Regulates Cell Division in Caulobacter crescentus. PLoS Biol. 2014;12:e1001977. doi: 10.1371/journal.pbio.1001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banerjee S, Scherer NF, Dinner AR. Shape dynamics of growing cell walls. Soft Matter. 2016;12:3442–3450. doi: 10.1039/c5sm02991k. [DOI] [PubMed] [Google Scholar]
- 64.Proctor SA, Minc N, Boudaoud A, Chang F. Contributions of Turgor Pressure, the Contractile Ring, and Septum Assembly to Forces in Cytokinesis in Fission Yeast. Curr Biol. 2012 doi: 10.1016/j.cub.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Varma A, Young KD. FtsZ collaborates with penicillin binding proteins to generate bacterial cell shape in Escherichia coli. J Bacteriol. 2004;186:6768–6774. doi: 10.1128/JB.186.20.6768-6774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buske P, Levin PA. A flexible C-terminal linker is required for proper FtsZ assembly in vitro and cytokinetic ring formation in vivo. Molecular Microbiology. 2013 doi: 10.1111/mmi.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67**.Sundararajan K, Miguel A, Desmarais SM, Meier EL, Casey Huang K, Goley ED. The bacterial tubulin FtsZ requires its intrinsically disordered linker to direct robust cell wall construction. Nat Commun. 2015;6:7281. doi: 10.1038/ncomms8281. Using mutant variants of FtsZ in C. crescentus, these authors establish a link between the C-terminal disordered linker of FtsZ and the regulation of cell wall metabolism that appears to transcend a simple scaffolding role for FtsZ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68*.Zhou Z, Munteanu EL, He J, Ursell T, Bathe M, Huang KC, Chang F. The contractile ring coordinates curvature-dependent septum assembly during fission yeast cytokinesis. Molecular Biology of the Cell. 2015;26:78–90. doi: 10.1091/mbc.E14-10-1441. Working in fission yeast, the authors use a combination of genetics, imaging, and modeling to demonstrate that the actomyosin contractile ring provides a mechanism to rectify septum shape by mechanical stimulation of the cell wall synthetic machinery. [DOI] [PMC free article] [PubMed] [Google Scholar]