Abstract
Objective
Aortic surgeries requiring hypothermic circulatory arrest evoke systemic inflammatory responses that often manifest as vasoplegia and hypotension. Because mast cells can rapidly release vasoactive and pro-inflammatory effectors, we investigated their role in intraoperative hypotension.
Methods
We studied 31 patients undergoing proximal aortic repair with hypothermic circulatory arrest between June 2013 and April 2015 at Duke University Medical Center. Plasma samples were obtained at different intraoperative time-points to quantify chymase, IL6, IL8, TNFa, and white blood cell CD11b expression. Hypotension was defined as the area [minutes * mmHg] below a mean arterial pressure of 55 mmHg. Biomarker responses and their association with intraoperative hypotension were analyzed by two sample t test and Wilcoxon rank sum test. Multivariable logistic regression analysis was used to examine the association between clinical variables and elevated chymase levels.
Results
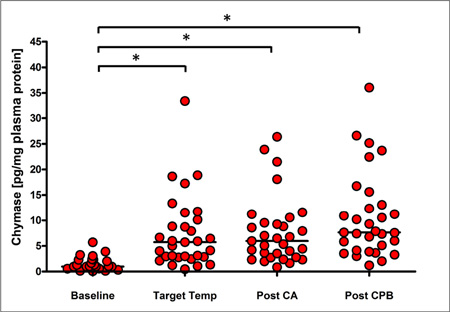
Mast cell-specific chymase increased from a median 0.97 [interquartile range, 0.01 to 1.84] pg/mg plasma protein at baseline to 5.74 [2.91 to 9.48] pg/mg plasma protein after instituting cardiopulmonary bypass, 6.16 [3.60 to 9.41] pg/mg plasma protein after completing of circulatory arrest and 7.64 [4.63 to 12.71] pg/mg plasma protein after weaning from cardiopulmonary bypass (each p<0.0001 vs baseline). Chymase was the only biomarker associated with hypotension during (p = 0.0255) and after cardiopulmonary bypass (p = 0.0221). Increased temperatures at circulatory arrest and low pre-surgical hemoglobin levels were independent predictors of increased chymase responses.
Conclusion
Mast cell activation occurs in cardiac surgery requiring cardiopulmonary bypass and hypothermic circulatory arrest and is associated with intraoperative hypotension.
Graphical abstract
Introduction
Episodes of intraoperative hypotension have been linked to adverse outcomes such as end-organ injury and mortality.[1] In cardiac surgery, complex vasomotor disturbances lead to hypotension through generalized vasoplegia and threaten adequate organ perfusion through endothelial leakage and associated tissue edema (reviewed in [2]). In fact, up to 25% of cardiac surgery patients develop a vasoplegic syndrome, a form of distributive shock that is linked to increased length of stay and mortality.[3, 4] But even in less dramatic manifestations, prolonged periods spent outside a still poorly defined optimal perfusion range affect postoperative outcomes.[5, 6] Currently, the factors that cause such hemodynamic shifts during cardiac surgery are not well understood, impeding substantial advancement of patient care beyond supportive measures.
We have recently shown in a rat model that mast cells (MCs) play an important role in early inflammatory and tissue injurious responses following deep hypothermic circulatory arrest.[7] As evidenced by their pivotal role in asthma and anaphylaxis, MCs can rapidly launch local and systemic inflammatory responses through release of potent mediators from preformed stores. These effectors can dramatically change vasomotor function (heparin, histamine, prostaglandins), endothelial integrity (proteases, prostaglandins, vascular endothelial growth factor [VEGF]), and can activate further inflammatory components (chemokines, cytokines) (reviewed in [8]). This identifies MCs as crucial regulators of vascular integrity, tone, and function and as a potent factor in the propagation of systemic inflammation.
Establishing significant MC activation in cardiac surgery constitutes a first step towards establishing a possible causative role of MCs in the development of hemodynamic disturbances and systemic inflammatory activation. Here, we present the first study that specifically addresses the activation pattern of MCs during aortic surgery by measuring intraoperative plasma chymase levels, a MC-specific protease, stored in intracellular vesicles and rapidly released upon MC stimulation. Based on our experimental data[7] and the pronounced inflammatory responses regularly observed, we targeted our study to patients undergoing proximal aortic repair necessitating hypothermic circulatory arrest (HCA). Furthermore, as a first exploration into the clinical relevance, we characterized the perioperative setting in which MC responses occur and examined their association to intraoperative hypotension.
Methods
Patient selection and data collection
As part of our ongoing research effort, we conducted a pilot study to define inflammatory responses and cognitive outcomes in patients undergoing proximal aortic arch surgery and requiring HCA between June 2013 and April 2015 at the Duke University Medical Center. The Institutional Review Board for Clinical Investigations at Duke University Medical Center approved this study and informed consent was obtained preoperatively from all patients. We included all patients ≥ 40 years of age except those with a history of symptomatic cerebrovascular disease and substantial residual deficit, alcoholism, psychiatric illness, renal failure (creatinine > 2.0 mg/dL), or those with less than a 7th grade education, or unable to complete neuropsychological testing or cranial magnetic resonance imaging. Patient data were extracted from the electronic health record software (Epic, USA) and included patient demographics, procedure type, preoperative medications, pre- and intraoperative drug administration, laboratory values, and timing of intraoperative events. Cumulative doses of vasopressor drugs were normalized to body weight and calculated from the total intraoperative doses of norepinephrine (mcg), epinephrine (mcg) and vasopressin (IU). Mean arterial pressures (MAP) from the left radial arterial line were electronically charted every minute. Values < 30 mmHg or > 250 mmHg were excluded as artifactual.
Anesthesia, surgery and CPB
Anesthesia, surgical, and postoperative care were provided according to the standards of care established for proximal aortic arch surgery requiring HCA at Duke University Medical Center. General anesthesia was induced with midazolam and/or propofol, fentanyl, and rocuronium or cisatracurium before tracheal intubation. Anesthesia was maintained using isoflurane in an air-oxygen mixture with an inspiratory oxygen concentration of ≥ 50% and end-tidal anesthetic gas concentrations were monitored throughout the case. As part of this institutions neuroprotection protocol, all patients received 1000 mg of methylprednisolone i.v. in the preoperative area. Surgery was performed by median sternotomy with intraoperative transesophageal echocardiographic and invasive arterial blood pressure monitoring as previously described.[9] Temperature management during circulatory arrest was determined at the surgeon’s discretion. Patients undergoing moderate HCA were cooled to 20–26°C as defined by nasopharyngeal temperature, whereas, for patients undergoing deep HCA, cooling was performed until electrocerebral inactivity was demonstrated by electroencephalography. Upon completion of cooling, the circulation was halted to allow opening of the aortic arch. Adjunctive antegrade (n = 30) or retrograde cerebral perfusion (n = 1) was used for cerebral protection in all cases. After completion of the hemiarch anastomosis, CPB was reinstituted. After a 5-minute period of cold reperfusion, patients were carefully rewarmed and additionally required cardiac procedures were completed. Weaning from CPB was performed at a core temperature of 36°C. Antifi brinolytic therapy using aminocaproic acid was routinely administered intraoperatively. Transfusion decisions were made according to Society of Cardiovascular Anesthesiologists/Society of Thoracic Surgeons published guidelines[10] using surgical field observations, chest tube output, activated clotting time, platelet count, fibrinogen level, thrombelastogram data, and prothrombin and partial thromboplastin time tests. Antiplatelet therapy was held preoperatively except for 81mg aspirin and was restarted on postoperative day 1 unless active bleeding was present.
Sample collection and analysis
Study blood samples were drawn from the arterial line into EDTA tubes following induction of anesthesia (“baseline”) when reaching the target temperature before HCA (“target temp”), 5 minutes after HCA (“post HCA”), and after terminating CPB (“post CPB”) (see Figure 1). Samples were immediately transferred to ice, centrifuged, and plasma was stored at −80°C until analysis. The following ELISAs were performed: chymase (Antibody Online, Aachen, Germany), IL6, IL8, and TNFa (eBioscience, San Diego, CA). Results were normalized against the protein content of the respective sample, as determined by Bradford assay (BioRad, Hercules, CA) to account for hemodilution during CPB. In addition, leukocytes were isolated from the buffy coat following the separation of plasma. After hypotonic lysis of residual red blood cells, leukocytes were fixed and stained with APC-coupled anti-CD45 (BD Pharmigen, San Jose, CA) and FITC-coupled anti-CD11b antibody (eBioscience, San Diego, CA) and the percentage of CD11b-positive cells in the CD45-gated population was established by fluorescence-activated cell sorting (FACS). Lactate levels were obtained as part of routine blood gas analyses and the difference between the first measurement after anesthesia induction and the last before transport to the ICU was calculated.
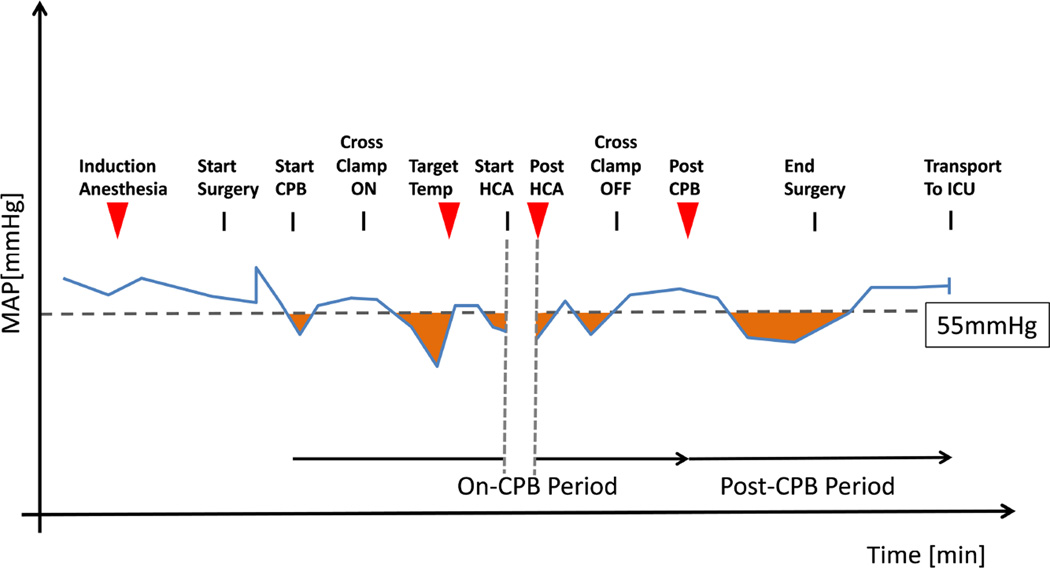
Figure 1. Sample draw time-points and hemodynamic quantitation strategy.
Relevant time-points (red triangles denote blood-draw) are graphed vs time against an idealized MAP tracing (blue line). A cutoff of 55 mmHg was chosen and the area (in minutes * mmHg) underneath the cutoff was calculated (orange shaded areas). Cumulative values were then determined for an on-CPB period (excluding HCA period) and a post-CPB period. CPB: cardiopulmonary bypass; HCA: hypothermic circulatory arrest; ICU: intensive care unit; MAP: mean arterial pressure.
Statistical Analysis
Continuous variables are presented as means (± SD) or medians (interquartile range), and categorical variables as group frequencies and percentages. Measured values of inflammatory markers at target temperature, post HCA, and post CPB were compared to baseline values using Wilcoxon signed rank test. Repeated measures analysis using generalized estimating equations (GEE) was performed to examine the trends over time for the serum measurements of chymase, IL8 and CD11b but not for IL6 and TNFa because of missing values at the post HCA and post CPB time points.
Intraoperative hypotension was defined as episodes with a MAP below 55 mmHg. This was quantified as cumulative area (in minutes * mmHg) between the actual MAP and 55mmHg, using a modification of the approach from Levin et al.[3]
Testing for an association between inflammatory marker measurements and hypotension was performed for 2 intraoperative time periods (Figure 1). The on-CPB period extended from initiation of CPB to termination of CPB, excluding the time of circulatory arrest. The post-CPB period was defined as the interval between CPB termination and the last valid MAP reading before transfer to the ICU. The MAP area below 55 mmHg from these periods was examined against the delta baseline chymase (Δ chymase) value at the target temperature measurement (for on-CPB period) and at the post CPB measurement (for post-CPB period). Upon examination of the distribution of Δ chymase measurements, we found that they had a non-normal distribution. We attempted several different methods of data transformation, but none of these transformation techniques resulted in a normal distribution or showed a linear association with MAP area below 55mmHg. This precluded us from using linear analysis models and non-linear methods such as smoothing splines did not improve model fit. Therefore, we decided to dichotomize chymase responses as outlined below. Since there is no predefined threshold for chymase, we evaluated each possible Δ chymase dichotomization cutoff for its relationship to MAP area below 55 mmHg, using either two sample t-test or Wilcoxon rank sum test. To determine the optimal cutoff, we first defined a candidate region where consistent association signals were observed between Δ chymase values and MAP area below 55mmHg. Then, we chose cutoffs that yielded significant differences and a balanced grouping between high- and low responders. The chosen cutoffs were close to (for on-CPB period) or identical to (for post-CPB period) the median. C-indices, as estimates of prediction accuracy determined by logistic regression for the chosen Δ chymase cutoff by MAP area, were 0.697 and 0.742 respectively. These Δ chymase cutoff values were then used to dichotomize our cohort into high-responders and low-responders (measured responses above or below cutoff value for respective time period).
The same search method was applied to examine the association of IL6, IL8, TNFa, and CD11b with MAP area below 55mmHg. However, no reliable cutoff could be determined using the above approach and we therefore used the median for these biomarkers.
Univariable and multivariable logistic regression analyses were applied to evaluate the association between perioperative variables and increased chymase responder status as determined at the post CPB blood draw. We examined preoperative risk factor including those from the European System for Cardiac Operative Risk Evaluation score (EuroSCORE)[11], the durations of CPB, HCA, and aortic cross-clamp, the temperature during circulatory arrest, and the amount of blood product transfusions. Variables with p < 0.05 from the univariable analyses were tested for collinearity, and entered into a multivariable logistic model. Backward stepwise selection was used for the final multivariable logistic model. Odds ratios (ORs) and corresponding 95% confidence limits are reported. All analyses were performed using the SAS version 9.3 (SAS Institute Inc, USA).
Results
A total of 31 patients (22 male, 9 female) aged 60.1 ± 12.6 years were recruited to this study. Patient characteristics and details of the procedures performed are outlined in Table 1.
Table 1.
Patient demographics and intervention details.
| Patient variables | |
| Age [years] | 61.23 ± 12.4 |
| Male | 22 (71) |
| Female | 9 (29) |
| ASA I | 0 |
| ASA II | 0 |
| ASA III | 18 (58) |
| ASA IV | 13 (41) |
| Pre-op ACE inhibitor/ARB | 13 (42) |
| Pre- op calcium blocker | 3 (10) |
| Pre- op beta blocker | 14 (45) |
| Pre- op heparin | 0 |
| Pre- op hemoglobin [g/dL] | 13.96 ± 1.53 |
| EUROscore variables | |
| Chronic pulmonary disease | 4 (13) |
| Extracardiac arteriopathy | 31 (100) |
| Neurological dysfunction | 0 |
| Serum creatinine >200mmol/L | 4 (13) |
| Poor mobility | 1 (3) |
| Pre-op EF (TEE) >50 | 20 (65) |
| Pre- op EF (TEE) 31–50 | 11 (36) |
| Pre- op EF (TEE) <30 | 0 |
| Recent myocardial infarction | 1 (3) |
| Pulmonary hypertension | 3 (10) |
| Previous cardiac surgery | 3 (10) |
| Diabetic on insulin | 0 |
| Logistic EUROscore [%] | 18.9 ± 12 |
| Surgical variables | |
| Ascending aorta only | 9 (29) |
| Ascending aorta +CABG | 2 (6) |
| Ascending aorta +valve | 20 (65) |
| Antegrade cerebral perfusion | 30 (97) |
| Retrograde cerebral perfusion | 1 (3) |
| CPB time [min] | 169.5 ± 45.4 |
| Crossclamp time [min] | 118.8 ± 35.7 |
| Circulatory arrest time [min] | 13 [11; 16] |
| NP temp at circulatory arrest [C°] | 22.4 ± 4.0 |
| Post- op EF (TEE) >50 | 21 (68) |
| Post- op EF (TEE) 31–50 | 10 (32) |
| Post- op EF (TEE) <30 | 0 |
| FFP transfusion [units] | 4 [0; 4] |
| RBC transfusion [units] | 0 [0; 1] |
| Platelet transfusion [units] | 2 [1.5; 2] |
| Cryoprecipitate transfusion [units] | 0 [0; 1] |
| Post-OP hemoglobin [g/dL] | 10.1 ± 0.84 |
Values are expressed as mean ± SD, median [interquartile range], or number (percentage).
ACE: angiotensin converting enzyme; ARB: angiotensin II receptor blocker; ASA: American Society of Anesthesiologists physical status classification system; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; EF: ejection fraction (determined by transesophageal echocardiography [TEE]); EUROscore: European System for Cardiac Operative Risk Evaluation score; FFP: fresh-frozen plasma; NP temp: nasopharyngeal temperature; RBC: red blood cell.
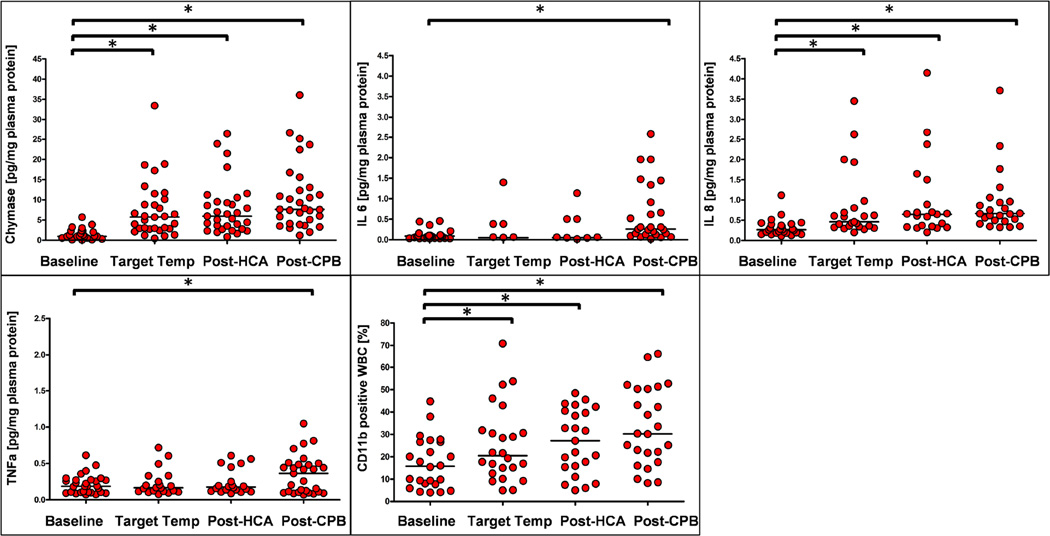
Measurements of inflammatory responses are shown in Figure 2 and detailed in Supplemental Table 1. MC-specific chymase increased significantly from a baseline median of 0.97 [interquartile range (IQR), 0.01 to 1.84] pg/mg plasma protein, to a median of 5.74 [IQR, 2.92 to 9.48] pg/mg plasma protein with institution of CPB and cooling. Chymase levels did not increase substantially with reperfusion after HCA (median 6.16 [IQR, 3.60 to 9.41] pg/mg plasma protein), but rose slightly to median 7.64 [IQR, 4.63 to 12.71] pg/mg plasma protein after completing CPB (p < 0.0001 vs baseline for all time-points). IL8 showed a similar response, increasing from median 0.27 [IQR, 0.19 to 0.40] pg/mg plasma protein at baseline to median 0.46 [IQR, 0.35 to 0.75], 0.65 [IQR, 0.35 to 0.89], and 0.67 [IQR, 0.51 to 1.04] pg/mg plasma protein at target temperature, post HCA and post CPB respectively (p < 0.0001 vs baseline for all time-points). Similarly, we found that neutrophil activation occurred early during surgery, with the percentage of CD11b-positive,CD45-positive cells increasing from median 15.7 [IQR, 7.9 to 26.6] % at baseline to median 20.5 [IQR, 14.4 to 30.9], 27.1 [IQR, 15.6 to 40], and 30.2 [IQR, 20.7 to 50.3] % at target temperature, post HCA and post CPB respectively (p < 0.0001 vs baseline for all time-points). In contrast, TNFa and IL6 rose only at the later time-points of surgery, ie, TNFa from median 0.19 [IQR, 0.11 to 0.28] pg/mg plasma protein at baseline to median 0.39 [IAR, 0.11 to 0.49] pg/mg at post CPB (p< 0.0001 vs baseline) and IL6 from median 0.08 [IQR, 0.03 to 0.11] pg/mg plasma protein at baseline to 0.28 [IQR, 0.13 to 1.02] pg/mg plasma protein post-CPB (p< 0.0001 vs baseline). Repeated measure analysis of chymase, IL8, and CD11b levels taken at different intraoperative time points significantly increased over time from baseline. (Supplemental Figure 1).
Figure 2. Intraoperative responses of inflammatory markers.
Mast cell-specific chymase (A), IL6 (B), IL8 (C), TNFa (D), and the percentage of CD11b positive white blood cells (E) were determined at baseline, when reaching target temperature on cardiopulmonary bypass (Target Temp), after completion of hypothermic circulatory arrest (Post HCA), and after completion of cardiopulmonary bypass (Post CPB). * increase vs baseline p≤0.05 by 2-sample t test.
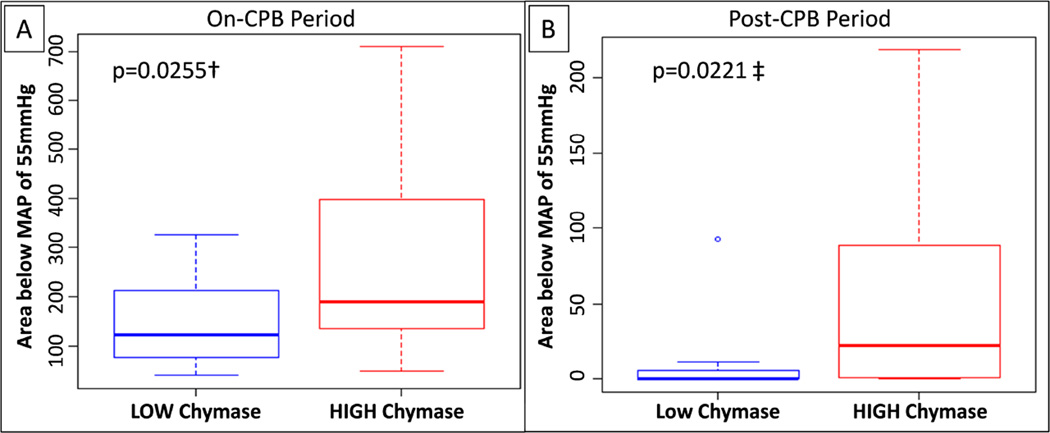
We next examined whether chymase responses were associated with increased intraoperative hypotension. As shown in Figure 3, chymase high-responders displayed a significantly increased MAP area below 55 mmHg during CPB, when compared to low-responders (median 188 [IQR, 140 to 392] minutes × mmHg vs. median 123 [IQR, 76 to 212] minutes × mmHg; p = 0.0255). Similarly, hypotensive episodes were significantly more pronounced after terminating CPB in chymase high-responders, compared to low-responders (median 22.2 [IQR, 1.1 to 88.6] minutes × mmHg vs. 0.4 [IQR, 0 to 5.1] minutes × mmHg; p = 0.0221).
Figure 3. Association of plasma chymase responses and intraoperative hypotension.
Delta-baseline chymase responses were dichotomized according to the cutoffs chosen for the entire cohort, using chymase levels at the target-temperature blood draw for the on-CPB period (A) and at the post-CPB blood draw for the post-CPB period (B). Hypotensive episodes were quantified by calculating the cumulative area of MAPs below a cutoff of 55 mmHg (in min × mmHg). † marks 2-sample t test, and ‡ marks Wilcoxon rank sum test.
In addition, chymase high-responders displayed a trend towards increased cumulative usage of vasopressor drugs (p = 0.07) as well as increased intraoperative lactate production (p = 0.121) (Supplemental Figure 2).
Subsequently, we also examined the association between, IL6, IL8, TNFa, and CD11b and intraoperative hypotension and found that there was no statistically significant association between any of these markers and hypotension during on-CPB or post-CPB periods (Supplemental Table 2).
We next aimed at better defining the conditions related to increased chymase responses. In univariable analysis, several preoperative and intraoperative variables were significantly associated with increased Δ chymase levels at the end of CPB (Table 2). In multivariable analysis, pre-operative hemoglobin, and nasopharyngeal temperature at circulatory arrest emerged as independent predictors of increased chymase responses. As such, for every g/dL increase of preoperative hemoglobin, the odds for an increased chymase response was 0.32 and for every degree Celsius increase at circulatory arrest 1.36 times the original odds.
Table 2.
Univariable and multivariable analysis.
| Univariable Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio |
95 CI LL | 95 CI UL | p value | Odds Ratio |
95 CI LL |
95 CI UL |
p value |
| Gender (M vs F) | 0.35 | 0.06 | 1.68 | 0.2009 | ||||
| Age [years] | 1.03 | 0.98 | 1.10 | 0.2836 | ||||
| Pre- op calcium blocker | 2.31 | 0.20 | 53.10 | 0.5142 | ||||
| Pre- op ACE inhibitor/ARB | 4.50 | 1.03 | 22.98 | 0.0544 | ||||
| Pre- op beta blocker | 0.39 | 0.09 | 1.63 | 0.2045 | ||||
| ASA (4 vs 3) | 0.86 | 0.20 | 3.61 | 0.8326 | ||||
| Chronic renal insufficiency | 1.08 | 0.12 | 10.07 | 0.9449 | ||||
| Previous cardiac surgery | 2.31 | 0.20 | 53.10 | 0.5142 | ||||
| Pre- op EF (TEE) (>50 vs 31–50) |
2.00 | 0.17 | 45.99 | 0.5887 | ||||
| PMH allergy/autoimmune Disease |
4.33 | 0.90 | 25.54 | 0.0788 | ||||
| Hemoglobin pre- op [g/dL] | 0.32 | 0.11 | 0.69 | 0.0157 | 0.32 | 0.08 | 0.78 | 0.0370 |
| WBC count pre- op [109/L] | 0.75 | 0.40 | 1.33 | 0.3341 | ||||
| Platelet count pre- op [109/L] | 1.00 | 0.99 | 1.02 | 0.5729 | ||||
| Pre-induction MAP [mmHg] | 0.97 | 0.92 | 1.02 | 0.2424 | ||||
| Additional valve surgery | 8.36 | 1.61 | 65.89 | 0.0198 | ||||
| CPB time [min] | 1.00 | 0.99 | 1.02 | 0.6479 | ||||
| Crossclamp time [min] | 1.00 | 0.98 | 1.02 | 0.8484 | ||||
| HCA time [min] | 1.06 | 0.95 | 1.20 | 0.2998 | ||||
| NP temp at circ arrest [°C] | 1.34 | 1.08 | 1.75 | 0.0146 | 1.36 | 1.07 | 1.89 | 0.0276 |
| MAP area on-CPB [mmHg*min] | 1.00 | 1.00 | 1.01 | 0.2116 | ||||
| Post- op EF (TEE) (>50 vs 31–50) |
2.40 | 0.50 | 13.79 | 0.2896 | ||||
| Transfusion RBC [units] | 4.71 | 1.53 | 24.07 | 0.0259 | ||||
| Transfusion FFP [units] | 0.96 | 0.69 | 1.31 | 0.7916 | ||||
| Transfusion Platelets [units] | 1.45 | 0.53 | 4.50 | 0.4842 | ||||
| Transfusion Cryoprecipitate [units] |
0.78 | 0.300 | 1.94 | 0.5929 | ||||
| Hemoglobin post- op [g/dL] | 0.97 | 0.40 | 2.30 | 0.9381 | ||||
| WBC count 24h post- op [1000/µl] |
1.07 | 0.90 | 1.30 | 0.4534 | ||||
| WBC count 48h post- op [1000/µl] |
1.08 | 0.93 | 1.29 | 0.3318 | ||||
| Baseline chymase [pg/mg plasma protein] |
1.66 | 1.01 | 3.16 | 0.0745 | ||||
| Delta-chymase target-temp [pg/mg plasma protein] |
1.43 | 1.12 | 2.05 | 0.0193 | ||||
| Delta-IL6 target-temp [pg/mg plasma protein] |
1.60 | 0.20 | 11.41 | 0.5989 | ||||
| Delta-IL6 post-CPB [pg/mg plasma protein] |
1.51 | 0.70 | 4.15 | 0.3202 | ||||
| Delta-IL8 target-temp [pg/mg plasma protein] |
0.97 | 0.31 | 3.44 | 0.9616 | ||||
| Delta-IL8 tost-CPB [pg/mg plasma protein] |
0.96 | 0.65 | 1.41 | 0.8379 | ||||
| Delta-TNFa target-temp [pg/mg plasma protein] |
1.68 | 0.00 | 3683.40 | 0.8898 | ||||
| Delta-TNFa post-CPB [pg/mg plasma protein] |
14.04 | 0.20 | 2571.36 | 0.2519 | ||||
| CD11b target-temp [fold increase over baseline] |
1.19 | 0.28 | 5.36 | 0.8169 | ||||
| CD11b post-CPB [fold increase over baseline] |
0.90 | 0.52 | 1.39 | 0.6318 | ||||
As outcome served an increased chymase response determined at the post-CPB blood draw. Hosmer-Lemeshow goodness-of-fit: Chi-squared, 10; df, 2; p-value=0.10.
ACE: angiotensin converting enzyme; ARB: angiotensin II receptor blocker; ASA: American Society of Anesthesiologists physical status classification system; CA: circulatory arrest; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; Delta-: delta baseline; EF: ejection fraction (determined by transesophageal echocardiography [TEE]); EUROscore: European System for Cardiac Operative Risk Evaluation score; FFP: fresh-frozen plasma; HCA: hypothermic circulatory arrest; MAP: mean arterial pressure; NP temp: nasopharyngeal temperature; PMH: past medical history; NP temp: naspharyngeal temperature; RBC: red blood cell; WBC: white blood cell; 95 CI LL: 95% confidence interval lower limit, 95 CI UL: 95% confidence interval upper limit.
Discussion
This study of 31 patients undergoing proximal aortic repair with CPB and HCA demonstrates for the first time, significant intraoperative release of the MC-specific degranulation product, chymase. Importantly, MC responses, but not other inflammatory markers such as IL6, IL8, TNFa, or CD11b expression in white blood cells, were associated with significant intraoperative hypotension. Given the profound inflammatory effects of MC products in asthma and anaphylaxis, this work identified MCs as potentially crucial effector of inflammatory and tissue injurious signaling in cardiac surgery. Designed as a hypothesis-forming study, our data strongly encourages future research to determine whether modulation of MC activity can be utilized to improve outcomes in this setting. Importantly, well-established drugs that block MC degranulation exist and have been successfully used in animal models.[7]
Systemic inflammation following cardiac surgery is a multifactorial process, and may have profound adverse effects on injured and normal tissue. Despite compelling evidence for relevant MC activation in animal models of trauma,[12] hemorrhagic shock,[13] ischemia/reperfusion (I/R) injury [14], but also rat HCA [7] and porcine ECMO [15], corresponding human studies have been lacking. MCs are a ubiquitously present population of potent inflammatory surveillance cells that rapidly respond to inflammatory triggers and tissue stress signals with release of pre-formed and de-novo synthesized proinflammatory, vasoactive, and tissue-disruptive effectors (reviewed in [8]). However, previous attempts to quantify MC activation during adult or pediatric cardiac surgery have yielded incoherent results.[16–18] Fayaz et al observed rapid histamine release upon initiation of CPB, but dismissed MCs as source because of concurrently decreasing MC-tryptase levels. Based on our own unpublished observations, we think that tryptase binding to heparin may interfere with detection in the setting of CPB. Instead, in our hands chymase constituted a robust marker of MC activation during cardiac surgery. Interestingly, these chymase measurements showed, that in contrast to the rat model,[7] MC responses in patients were vigorous after implementation of CPB and cooling, but marginal after HCA. Increased age and comorbidities may make patients (vs. young, healthy rats) more sensitive to the inflammatory and circulatory challenges of extracorporeal perfusion. We are currently examining the implications of these findings by examining MC activation in coronary artery bypass grafting procedures. Furthermore, it is noteworthy, that MC responses occurred in patients receiving 1000mg methylprednisolone as part of a neuroprotection protocol. Chymase, but also a significant portion of early cytokine release stems from degranulation of preformed stores,[15] a process that appears unaffected by steroids.[19]
As the first report on MC activation in a cardiac surgical setting, the present study offers important insights into a possible role for MCs in perioperative responses. Most importantly, this work demonstrated an association between amplified MC responses and intraoperative hypotension. Circulatory instability is often the first clinical manifestations of inflammatory events and presents as a predominantly distributive hypotension with inadequate responses to volume expansion. In cardiac surgery 10% – 20% of patients develop vasoplegic syndrome, which substantially impacts immediate clinical care and postoperative outcomes.[3, 4] While this study was not designed to support conclusions on whether MC responses are cause or effect of hypotension, available data supports the notion that MC activation occurs early and promotes inflammation and vasomotor disturbances. Here, our previous work in a rat model of HCA has shown that pretreatment with the MC stabilizer cromolyn sodium, reduced local and systemic inflammation.[7] In addition, MC products have a critical effect on vascular functions. Due to their predominant perivascular distribution, MC mediators may almost immediate impact on vascular structures and trigger endothelial barrier break down, plasma leakage, and edema (VEGF, TNFa, the eicosanoids LTC4, LTD4, and LTE4, the proteases tryptase, and chymase) and cause vasomotor disturbances (histamine and chymase).[8]
CPB and HCA constitutes an extremely complex context and multiple events such as I/R [14], complement activation,[20] and cytokine release[21] may stimulate MCs. Here, our uni- and multivariable analyses, provide additional evidence of the clinical setting in which MC activation occurs.
As such, increased chymase responses were associated with higher target temperatures at circulatory arrest. A number of recent works have highlighted advantages of performing circulatory arrest procedures at higher temperatures (most recently [22, 23]). However, the consequences on I/R injury and systemic inflammatory responses are not yet fully defined.[9, 24] In fact, conflicting results have been reported on the temperature-dependency of cytokine release in cardiac cases.[25, 26] Consistent with a previous report[13] our data suggests that MC degranulation is blunted at low temperatures, but, as with Rasmussen et al,[26] other inflammatory markers were not affected (data not included). Our previous work in a rat model of HCA revealed substantial intestinal I/R injury.[7] Therefore, while ante- or retrograde cerebral perfusion is thought to confer some degree of brain protection, a concern remains as to I/R injury of abdominal organs. Increased MC activation at higher HCA temperatures may reflect suboptimal ischemic protection at these sites, but further studies are needed to define resultant injury patterns better.
We also observed that low pre-operative hemoglobin levels were independent predictors of above-median MC responses. Pre-operative anemia reduces oxygen delivery and thus increases the risk of end-organ injury during cardiac surgery.[27] It is possible that both anemia and HCA temperature impact MC activation and related perioperative inflammatory responses through their role in I/R injury. On the other hand, RBC transfusions were associated with increased chymase responses in univariable, but not in multivariable analysis. There is ample evidence that RBC transfusion may directly contribute to adverse clinical outcomes in cardiac surgery.[28, 29] While the mechanisms by which transfusions elicit injurious responses remain unknown, there appears to be a distinct inflammatory component.[30] Apart from anaphylactic transfusion reactions, MC activation has been reported due to immunoglobulin E oligomers in donor plasma[31], however future studies will have to determine if MCs are also involved in mediating a more general inflammatory response to transfusion.
Study Limitations
Our small-scale study was designed as an exploratory study to identify the presence of MC activation in our cohort and to characterize the clinical setting in which MC activation occurs. To overcome some of the challenges of our limited cohort while still attempting to explore the clinical relevance of MC activation, we analyzed intraoperative hypotension because a dense dataset of MAP measurements was available through electronic charting. It must be noted that we do not routinely use pulmonary artery catheters in these cases. While, independent evaluation by a board-certified echocardiographer pre- and post-procedure did not reveal a significant deterioration of cardiac function, we thus could not calculate vascular resistance as a measure vasoplegia.[4] Instead, we defined hypotension as a MAP below 55 mmHg, which according to various reports from cardiac and non-cardiac surgery settings showed a strong association between the time spent below this threshold and perioperative adverse outcomes. [1, 32, 33]
In addition, we found that the distribution of biomarker measurements did not allow analysis using linear models and we therefore resorted to dichotomizing chymase levels into high- and low-responders. While we strongly believe that the robust association between hypotension and elevated chymase levels supports the clinical relevance of perioperative MC activation as a concept, this approach may limit generalizability of our study findings.
Lastly, data on clinical risk factors, medication use, and laboratory values were retrospectively retrieved from electronic medical records. Thus, the effects of certain risk factors and medications may have been biased. In addition, specimen volume was too limited in some patients to measure all of the inflammatory markers.
Conclusion
We demonstrated, for the first time, significant MC activation during proximal aortic repair surgery requiring circulatory arrest. Multivariable logistic regression analysis indicated that increased circulatory arrest temperatures and low preoperative hemoglobin contribute to such MC activation. Importantly, MC activation was significantly associated with relevant intraoperative hypotension.
Supplementary Material
Part 1: Case presentation, cannulations including right axillary cannulation and cross clamp.
Part 2: Moderate hypothermic circulatory arrest with antegrade cerebral perfusion, transverse aortic arch reconstruction (Hemi-Arch) and preparation of the aortic root for Reconstruction.
Part 3: T. David-V valve-sparing aortic root replacement with coronary reconstruction and anastomosis of aortic arch graft and aortic root graft.
Repeated measures analysis to examine the trends over time for measurements of chymase (A), IL8 (B), and CD11b (C). The decision to transform the time scale was based on the curve of mean measurement over time as indicated by the black line in each panel. Estimate denotes the coefficient of ‘time’ and denotes the slope of the median. Wald: Wald-type rank test, Pr(>|W|: p-value.
Indicators of relevant hypotension are increased in above-median chymase responders (post-CPB). Cumulative dose of vasopressors (A) was calculated by adding total intraoperative doses of epinephrine (mcg), vasopressin (IU), and norepinephrine (mcg) and adjusted for each patient`s body weight. Lactate levels (B) are represented as difference between last and first intraoperative lactate level (mg/dL).
Acknowledgments
The authors would like to thank Laura Mitrescu, BSc, for technical support and Betsy W. Hale, BSc, and Austin Traylor, BSc (Data Analysts, Department of Anesthesiology, Duke University Medical Center, Durham, NC) for their help with data retrieval. We also thank Kathy Gage, BS (Research Development Associate, Department of Anesthesiology, Duke University Medical Center) for editorial help. The research was supported in part by grants HL126891 (JAK) and HL109971, HL096978, and HL108280 (JPM) from the National Institutes of Health and grant 15SDG25080046 (JAK) from the American Heart Association.
Glossary of Abbreviations
- CPB
cardiopulmonary bypass
- EuroSCORE
European System for Cardiac Operative Risk Evaluation score
- LTC4/D4/E4
leukotriene C4/D4/E4
- MC
Mast cell
- HCA
hypothermic circulatory arrest
- ICU
intensive care unit
- IU
international units
- IL6/8
interleukin 6/8
- I/R
ischemia/reperfusion
- MAP
mean arterial pressure
- OR
odds ratio
- TNFa
tumor necrosis factor alpha
- SD
standard deviation
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflict of interest.
Citations
- 1.Walsh M, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507–515. doi: 10.1097/ALN.0b013e3182a10e26. [DOI] [PubMed] [Google Scholar]
- 2.Ruel M, et al. Vasomotor dysfunction after cardiac surgery. Eur J Cardiothorac Surg. 2004;26(5):1002–1014. doi: 10.1016/j.ejcts.2004.07.040. [DOI] [PubMed] [Google Scholar]
- 3.Levin MA, et al. Early on-cardiopulmonary bypass hypotension and other factors associated with vasoplegic syndrome. Circulation. 2009;120(17):1664–1671. doi: 10.1161/CIRCULATIONAHA.108.814533. [DOI] [PubMed] [Google Scholar]
- 4.Sun X, et al. Is vasoplegic syndrome more prevalent with open-heart procedures compared with isolated on-pump CABG surgery? Cardiovasc Revasc Med. 2011;12(4):203–209. doi: 10.1016/j.carrev.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Ono M, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg. 2014;147(1):483–489. doi: 10.1016/j.jtcvs.2013.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aronson S, et al. Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology. 2010;113(2):305–312. doi: 10.1097/ALN.0b013e3181e07ee9. [DOI] [PubMed] [Google Scholar]
- 7.Karhausen J, et al. Intestinal mast cells mediate gut injury and systemic inflammation in a rat model of deep hypothermic circulatory arrest. Crit Care Med. 2013;41(9):e200–e210. doi: 10.1097/CCM.0b013e31827cac7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kunder CA, St John AL, Abraham SN. Mast cell modulation of the vascular and lymphatic endothelium. Blood. 2011;118(20):5383–5393. doi: 10.1182/blood-2011-07-358432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lima B, et al. Results of proximal arch replacement using deep hypothermia for circulatory arrest: is moderate hypothermia really justifiable? Am Surg. 2011;77(11):1438–1444. [PMC free article] [PubMed] [Google Scholar]
- 10.Society of Thoracic Surgeons Blood Conservation Guideline Task, F et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 11.Nashef SA, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 12.Cai C, et al. Mast cells play a critical role in the systemic inflammatory response and end-organ injury resulting from trauma. J Am Coll Surg. 2011;213(5):604–615. doi: 10.1016/j.jamcollsurg.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyan GN, et al. Induced hypothermia during resuscitation from hemorrhagic shock attenuates microvascular inflammation in the rat mesenteric microcirculation. Shock. 2014;42(6):518–524. doi: 10.1097/SHK.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang MQ, et al. The role of mast cells in ischemia and reperfusion injury. Inflamm Res. 2014;63(11):899–905. doi: 10.1007/s00011-014-0763-z. [DOI] [PubMed] [Google Scholar]
- 15.McILwain RB, et al. Plasma concentrations of inflammatory cytokines rise rapidly during ECMO-related SIRS due to the release of preformed stores in the intestine. Lab Invest. 2010;90(1):128–139. doi: 10.1038/labinvest.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fayaz KM, et al. Histamine release during adult cardiopulmonary bypass. Anaesthesia. 2005;60(12):1179–1184. doi: 10.1111/j.1365-2044.2005.04368.x. [DOI] [PubMed] [Google Scholar]
- 17.Withington DE, Elliot M, Man WK. Histamine release during paediatric cardiopulmonary bypass. Agents Actions. 1991;33(1–2):200–202. doi: 10.1007/BF01993167. [DOI] [PubMed] [Google Scholar]
- 18.Valen G, et al. Open heart surgery increases the levels of histamine in arterial and coronary sinus blood. Agents and actions. 1994;41(1–2):11–16. doi: 10.1007/BF01986386. [DOI] [PubMed] [Google Scholar]
- 19.Cohan VL, et al. Dexamethasone does not inhibit the release of mediators from human mast cells residing in airway, intestine, or skin. Am Rev Respir Dis. 1989;140(4):951–954. doi: 10.1164/ajrccm/140.4.951. [DOI] [PubMed] [Google Scholar]
- 20.Ali H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett. 2010;128(1):36–45. doi: 10.1016/j.imlet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan AP, et al. Histamine releasing factors and cytokine-dependent activation of basophils and mast cells. Adv Immunol. 1991;50:237–260. doi: 10.1016/s0065-2776(08)60826-3. [DOI] [PubMed] [Google Scholar]
- 22.Algarni KD, et al. Profound hypothermia compared with moderate hypothermia in repair of acute type A aortic dissection. J Thorac Cardiovasc Surg. 2014;148(6):2888–2894. doi: 10.1016/j.jtcvs.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 23.Tsai JY, et al. Moderate hypothermia during aortic arch surgery is associated with reduced risk of early mortality. J Thorac Cardiovasc Surg. 2013;146(3):662–667. doi: 10.1016/j.jtcvs.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Luehr M, et al. Modern temperature management in aortic arch surgery: the dilemma of moderate hypothermia. Eur J Cardiothorac Surg. 2014;45(1):27–39. doi: 10.1093/ejcts/ezt154. [DOI] [PubMed] [Google Scholar]
- 25.Menasche P, et al. A potential mechanism of vasodilation after warm heart surgery. The temperature-dependent release of cytokines. J Thorac Cardiovasc Surg. 1994;107(1):293–299. [PubMed] [Google Scholar]
- 26.Rasmussen BS, et al. The release of systemic inflammatory mediators is independent of cardiopulmonary bypass temperature. J Cardiothorac Vasc Anesth. 2007;21(2):191–196. doi: 10.1053/j.jvca.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 27.Magruder JT, et al. Nadir Oxygen Delivery on Bypass and Hypotension Increase Acute Kidney Injury Risk After Cardiac Operations. Ann Thorac Surg. 2015;100(5):1697–1703. doi: 10.1016/j.athoracsur.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 28.Karkouti K. Transfusion and risk of acute kidney injury in cardiac surgery. Br J Anaesth. 2012;109(Suppl 1):i29–i38. doi: 10.1093/bja/aes422. [DOI] [PubMed] [Google Scholar]
- 29.Shaw RE, et al. Blood transfusion in cardiac surgery does increase the risk of 5-year mortality: results from a contemporary series of 1714 propensity-matched patients. Transfusion. 2014;54(4):1106–1113. doi: 10.1111/trf.12364. [DOI] [PubMed] [Google Scholar]
- 30.Ferraris VA, Ballert EQ, Mahan A. The relationship between intraoperative blood transfusion and postoperative systemic inflammatory response syndrome. Am J Surg. 2013;205(4):457–465. doi: 10.1016/j.amjsurg.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 31.Abe T, et al. Immunoglobulin E oligomers identified in blood components activate mast cells: relevance to anaphylactic transfusion reaction. Transfusion. 2011;51(11):2327–2336. doi: 10.1111/j.1537-2995.2011.03126.x. [DOI] [PubMed] [Google Scholar]
- 32.Kertai MD, et al. Bispectral Index Monitoring, Duration of Bispectral Index Below 45, Patient Risk Factors, and Intermediate-term Mortality after Noncardiac Surgery in the B-Unaware Trial. Anesthesiology. 2011;114(3):545–556. doi: 10.1097/ALN.0b013e31820c2b57. [DOI] [PubMed] [Google Scholar]
- 33.Monk TG, et al. Anesthetic management and one-year mortality after noncardiac surgery. Anesthesia and Analgesia. 2005;100(1):4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Part 1: Case presentation, cannulations including right axillary cannulation and cross clamp.
Part 2: Moderate hypothermic circulatory arrest with antegrade cerebral perfusion, transverse aortic arch reconstruction (Hemi-Arch) and preparation of the aortic root for Reconstruction.
Part 3: T. David-V valve-sparing aortic root replacement with coronary reconstruction and anastomosis of aortic arch graft and aortic root graft.
Repeated measures analysis to examine the trends over time for measurements of chymase (A), IL8 (B), and CD11b (C). The decision to transform the time scale was based on the curve of mean measurement over time as indicated by the black line in each panel. Estimate denotes the coefficient of ‘time’ and denotes the slope of the median. Wald: Wald-type rank test, Pr(>|W|: p-value.
Indicators of relevant hypotension are increased in above-median chymase responders (post-CPB). Cumulative dose of vasopressors (A) was calculated by adding total intraoperative doses of epinephrine (mcg), vasopressin (IU), and norepinephrine (mcg) and adjusted for each patient`s body weight. Lactate levels (B) are represented as difference between last and first intraoperative lactate level (mg/dL).