Abstract
The Aamjiwnaang First Nations community is located in Canada’s ‘Chemical Valley’ situated in southwest Ontario near Sarnia. Mercury pollution in the region has been known since the 1940s but little is known about levels in the environment and area residents. The current study, using ecological and human exposure assessment methods, was conducted at the community’s request to help fill these gaps. First, Canada’s National Pollutant Release Inventory (NPRI) and the U.S. Toxics Release Inventory (TRI) were queried to investigate mercury releases from area facilities. In 2010, 700 pounds of mercury were emitted into the air, 25 pounds were released into water bodies, and 93 thousand pounds were disposed of on-site via underground injections or into landfills, and together these show continued releases into the region. Second, mercury levels were measured in stream sediment and nearby soil from sites at Aamjiwnaang (n=4) and off Reserve (n=19) in Canada and the U.S. during three seasons that spanned 2010–2011. Total mercury in sediment across all sites and sampling seasons ranged from 5.0 to 398.7μg/kg, and in soils ranged from 1.2 to 696.2μg/kg. Sediment and soil mercury levels at Aamjiwnaang were higher than the reference community, and Aamjiwnaang’s Talfourd Creek site had the highest mercury levels. Third, a biomonitoring study was performed with 43 mother-child pairs. Hair (mean±SD of all participants: 0.18±0.16μg/g) and blood (1.6±2.0μg/L) mercury levels did not differ between participants studied on- and off-Reserve, likely because of limited seafood intake (<1 serving/week). Urine mercury levels (0.5±0.8μg/L) were significantly higher (1.5–2.5 times) in mother-child pairs living on-Reserve versus those living off-Reserve. In general, the study links evidence of mercury sources, environmental fate, and human exposures, and in doing so it shows that mercury levels in ecological and human samples are similar to values found in other areas, though there are some trends and evidence of contamination at Aamjiwnaang that warrant attention.
Keywords: heavy metals, epidemiology, monitoring, Indigenous Peoples, environmental justice, community health, contamination
1.0 INTRODUCTION
Aamjiwnaang is a Chippewa First Nations community located along the St. Clair River that bisects Ontario (Canada) and Michigan (USA) in the heart of Canada’s ‘Chemical Valley’. There exists a long-history of industrial activity in the region with activities dating back to the early 19th century. Within a 25 km radius of Aamjiwnaang exist over 50 U.S. and Canadian industrial facilities that include petrochemical, polymer, and coal-fired power plants. Collectively these facilities release over 100 million kilograms of pollutants per year into the region’s environment (MacDonald and Rang, 2007).
Among the numerous pollutants released into the Aamjiwnaang environment the toxic element mercury remains of particular concern. As early as the 1940s the neighboring St. Clair River was a known hotspot for mercury contamination (US EPA, 1988). The “Mercury Crisis of 1970” was spurred by the discovery that substantial amounts of mercury were directly released by a chlor-akali plant in Sarnia into the river (OME, 1970; USEPA, 2009). During that period, mercury releases averaged approximately 30 pounds per day for upwards of two decades, and in 1969 it was estimated that as much as 75 pounds was released daily (OME 1970). Not surprisingly, resident fish populations were highly contaminated. For example, mercury levels in edible muscle usually exceeded five ppm during the 1970s (note, the current Health Canada standard is 0.5 ppm in fish), and as such commercial fishing was banned resulting in an estimated economic loss of over $1–2 million per year (OME, 1970). Since the 1970s, mercury concentrations in predatory species such as walleye have fallen to levels generally below 0.5 ppm though larger fish may still exceed consumption guidelines for children and women of childbearing age (Gewurtz, 2010). In addition to fish tissue residues, there exists some evidence of elevated mercury levels in sediment. For example, sediment mercury concentrations near Sarnia in 1985 were 4-fold higher than the US EPA’s severe effect level for fresh water invertebrates (Murdoch and Hill, 1989; Buchman, 2008).
Indigenous peoples worldwide (including Native American and First Nations populations) are particularly susceptible to chemical exposure given their reliance on country foods such as fish and seafood, which are well documented to contain contaminants such as mercury (Nriagu et al., 2012). Despite the long history of mercury pollution in the Aamjiwnaang region, there is no information on mercury exposure among area residents and little is known about current levels in the environment. Accordingly, this study was conducted at the request of the Aamjiwnaang Environment Committee to increase understanding of mercury exposure and potential risks in the area. The aims of the current study were to: 1) assess the releases of mercury from facilities within the “Chemical Valley” region; 2) characterize mercury levels in stream sediment and soil from the region; 3) determine if mothers and children living on the Aamjiwnaang Reserve had differential mercury exposures than those living off the Reserve by use of biomarkers (i.e., hair, urine, blood) for organic mercury and inorganic mercury; and 4) explore predictors of mercury exposure amongst community residents by use of surveys.
2.0 MATERIALS AND METHODS
2.1 Study Site
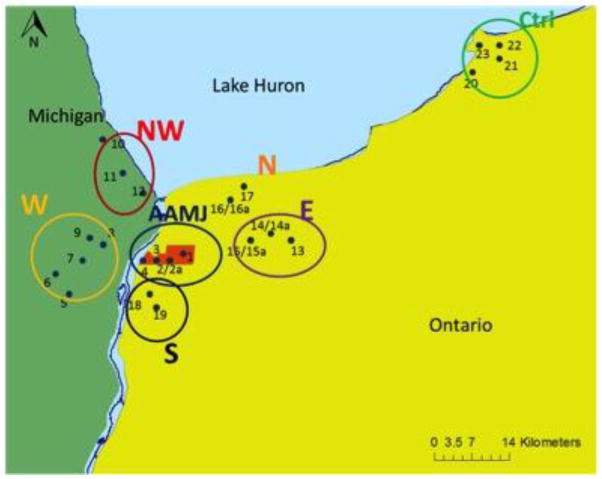
The Aamjiwnaang First Nation Reserve consists of 2850 acres of land on which ~950 members live in 244 homes (Figure 1). It is located on the southern border of the city of Sarnia (Ontario, Canada) within Lambton County. The Reserve is positioned at the junction of the St. Clair River and Lake Huron, within the Laurentian Great Lakes region of North America.
Figure 1.
Map of Great Lakes Basin with the Aamjiwnaang First Nation Reserve highlighted in red on the map to the right. Maps and layer data provided by Geography Network Canada, Michigan Center for Geographic Information, and Great Lakes Information Network.
2.2 Mercury Source Investigation
Canada’s National Pollutant Release Inventory (NPRI) and the U.S. Toxics Release Inventory (TRI) were queried to investigate the releases of mercury from industry within the “Chemical Valley” area for the years 2000, 2005 and 2010. Both NPRI and TRI are government programs with specific reporting requirements that provide publicly accessible databases regarding industrial uses and releases of specified chemicals. Here we investigated total on-site releases, disposals, and recycling for each facility, excluding off-site disposal and recycling since locations for these are not provided. For this report, only those facilities located within the greater Sarnia area (Canadian postal codes N7T 7H9, N7T 7N4, N7T 7H8, N7T 2l3, N7T 7H3, N7T 8H8, N7T 7MS, N7T 7J2, N7T 7M2, N7T 8A3, N7T 7J3, N7T 7K2, N7T 7W1, N7S 5N5, N7S 5N2, N7S 5M4, N7H 8H1, NON 1G0, NON 1B0, NON 1MO, and NON 1H0) and the U.S. zip codes of 48060, 48061, 48054, and 48079 were investigated.
2.3 Ecological Study
2.3.1 Ecological Study Sample Collection
Stream sediment and stream bank soil were collected from sites on the Aamjiwnaang First Nation Reserve (n=4 sites) and off the Reserve (n= 19 sites) (Table 1). For ease of geographical comparison, sites were grouped into communities in Ontario (ONT) and Michigan (MI; Table 1; Figure 2). Community 1 is The Aamjiwnaang First Nation Reserve (ONT; sites 1–4), Community 2 is located in Marysville (MI; Sites 5–6), Community 3 is Port Huron (MI; sites 7–12), Community 4 is eastern Sarnia (ONT; sites 13–15), Community 5 is northern Sarnia (ONT; sites 16–17), and Community 6 is Corunna, (ONT; sites 18–9). Community 7 is in Kettle Point (ONT; sites 20–23) and serves as a reference First Nations community as it is over 40km to the northeast of the Aamjiwnaang Reserve and its upstream waters go through a nature preserve. In three of the sites, drainage tubes were present and thus samples were collected from both upstream and downstream of the point source. To address seasonal variability samples were collected from all sites during fall 2010 (November 18–21), spring 2011 (May 2–4), and summer 2011 (August 31 – September 3).
Table 1.
Summary of study sites, and mercury levels in sediment and soil.
| Sediment Mercury (ug/kg) | Soil Mercury (ug/kg) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site # | Community | Stream Name | Intersection | latitude | longitude | elevation | Fall | Spring | Summer | Mean | Fall | Spring | Summer | Mean |
| 1 | AAMJ - Aamjiwnaang | Talfourd Creek | Scott Rd | 42.914 67 |
−82.396 737 |
176.6 | 36.9 | 36.2 | 36.7 | 36.6 | 55.0 | 56.9 | 51.3 | 54.4 |
| 2 up | Talfourd Creek | Lassalle Rd | 42.912 31 |
−82.42 2797 |
176.3 | 126.6 | 32.9 | 56.2 | 71.9 | 645.6 | 131.5 | 696.2 | 491.1 | |
| 2 down | Talfourd Creek | Lassalle Rd | 42.912 31 |
−82.42 2797 |
176.3 | 398.7 | 235.5 | 93.1 | 242.4 | |||||
| 3 | Talfourd Creek | McGregor Rd | 42.915 584 |
−82.366 836 |
188.8 | 37.3 | 32.9 | 39.6 | 36.6 | 25.5 | 45.6 | 44.5 | 38.5 | |
| 4 | Talfourd Creek | Pow Wow Grounds | 42.915 037 |
−82.452 091 |
150.4 | 43.8 | 37.2 | 43.6 | 41.5 | 43.6 | 39.2 | 61.4 | 48.1 | |
| 5 | W-Marysville | Rattle Run Creek | Wadhams Rd | 42.871 069 |
−82.558 219 |
144.7 | 38.6 | 45.5 | 43.7 | 42.6 | 37.5 | 35.8 | 53.7 | 42.3 |
| 6 | Smiths Creek | Frith Rd | 42.888 526 |
−82.577 814 |
131.4 | 19.4 | 21.8 | 16.0 | 19.1 | 20.7 | 79.6 | 36.7 | 45.7 | |
| 7 | Huffman Stream | Minnesota Rd | 42.943 705 |
−82.491 043 |
153.4 | 50.2 | 26.6 | 41.7 | 39.5 | 18.7 | 43.8 | 34.9 | 32.5 | |
| 8 | Sanborn Creek | Sanborn Park | 42.980 664 |
−82.481 048 |
180.3 | 19.4 | 13.8 | 20.9 | 18.1 | 203.1 | 30.5 | 127.1 | 120.3 | |
| 9 | Doe Creek | Brace Rd | 43.012 134 |
−82.441 755 |
175.9 | 117.2 | 137.8 | 195.6 | 150.2 | 46.6 | 40.1 | 97.6 | 61.4 | |
| 10 | NW-Port Huron | Stocks Creek | Lapeer Rd | 43.068 121 |
−82.474 522 |
156.9 | 15.8 | 18.2 | 20.9 | 18.3 | 26.8 | 45.2 | 32.1 | 34.7 |
| 11 | Unknown | Parker Rd | 43.037 199 |
−82.476 53 |
126.9 | 28.6 | 21.0 | 47.8 | 32.5 | 35.3 | 1.2 | 30.4 | 22.3 | |
| 12 | Beach Creek | Beach Rd | 43.001 898 |
−82.488 398 |
166.5 | 10.0 | 5.0 | 118.5 | 44.5 | 55.2 | 33.1 | 74.0 | 54.1 | |
| 13 | E-Eastern Sarnia | Waddell Creek | Confederation Rd | 42.958 964 |
−82.303 085 |
188.7 | 8.6 | 8.2 | 24.8 | 13.9 | 27.1 | 17.2 | 19.3 | 21.2 |
| 14 up | Perch Creek | Confederation Rd | 42.959 263 |
−82.310 465 |
190.2 | 15.3 | 15.3 | 19.3 | 16.6 | 19.7 | 29.4 | 30.0 | 26.4 | |
| 14 down | Perch Creek | Confederation Rd | 42.959 263 |
−82.310 465 |
190.2 | 24.7 | 9.3 | 22.7 | 18.9 | |||||
| 15 up | Unknown | Confederation Rd | 42.959 485 |
−82.325 95 |
191.6 | 22.2 | 18.0 | 20.3 | 20.2 | 26.0 | 37.2 | 42.5 | 35.2 | |
| 15 down | Unknown | Confederation Rd | 42.959 485 |
−82.325 95 |
191.6 | 19.3 | 16.7 | 25.1 | 20.3 | |||||
| 16 up | N-North Sarnia | Wawanosh Creek | London Line | 42.983 744 |
−82.339 674 |
213.9 | 53.9 | 68.1 | 9.8 | 43.9 | 48.0 | 2.6 | 30.7 | 27.1 |
| 16 down | Wawanosh Creek | London Line | 42.983 744 |
−82.339 674 |
213.9 | 26.4 | 16.8 | 10.6 | 17.9 | |||||
| 17 | Wawanosh Creek | Michigan Line | 43.007 951 |
−82.316 793 |
216.6 | 56.4 | 34.6 | 68.1 | 53.0 | 38.1 | 63.0 | 58.9 | 53.3 | |
| 18 | S-Corunna | Marsh Creek | Hill St | 42.888 015 |
−82.428 402 |
172.2 | 49.5 | 65.9 | 69.3 | 61.5 | 58.3 | 49.4 | 14.3 | 40.7 |
| 19 | Baby Creek | Rockeby Line | 42.863 815 |
−82.435 473 |
182.7 | 58.9 | 68.5 | 67.1 | 64.8 | 66.1 | 5.2 | 42.6 | 37.9 | |
| 20 | Ctrl-Kettle Point | Shashwanda Creek | Lakeshore Rd | 42.877 59 |
−82.446 016 |
149.8 | 10.7 | 27.1 | 19.7 | 19.2 | 18.3 | 11.7 | 20.0 | 16.7 |
| 21 | Ipperwash Creek | Ipperwash Rd | 43.193 063 |
−81.968 367 |
163.8 | 13.2 | 13.3 | 19.7 | 15.4 | 20.7 | 35.1 | 30.1 | 28.6 | |
| 22 | Unknown | Ipperwash Rd | 43.204 963 |
−81.973 949 |
174.8 | 6.4 | 7.3 | 9.8 | 7.8 | 5.9 | 4.8 | 1.7 | 4.2 | |
| 23 | Unknown | Lakeshore Rd | 43.162 005 |
−82.018 245 |
205.9 | 10.1 | 10.0 | 23.7 | 14.6 | 54.3 | 42.9 | 40.2 | 45.8 | |
Figure 2.
Communities from which soil and sediment samples were collected. The Aamjiwnaang First Nation (AAMJ) is indicated in red (sampling sites 1–4), and sampled communities are located in each direction from the Reserve as indicated: NW: Port Huron, MI, situated north west of Aamjiwnaang; W: Marysville, MI, situated west; S: Corunna, Ont, situated to the south; N: northern Sarnia, Ont, situated north; E: eastern Sarnia, Ont, situated east; and Ctrl: Kettle Point, Ont, the control community. Maps and layer data provided by Geography Network Canada, Michigan Center for Geographic Information, and Great Lakes Information Network.
For sediment, a ~10 gram grab sample was collected into a Whirlpack bag at the point of entry as well as at two consecutive 10 meter intervals upstream of the entry point thus resulting in 3 sediment samples per site. Water quality measures (temperature, pH, and conductivity) were recorded at each stream site using a YSI 556MPS probe (Yellow Springs, OH) and GPS coordinates were recorded using a Garmin Oregon 450 (Olathe, KS; Table 1). For soil, a site was identified approximately 5 meters from the stream bank and from that site five subsamples of soil were collected from the middle and four corners of an approximate 12×12” square. Sediment and soil samples were shipped to the laboratory and frozen until analysis.
2.3.2 Total Mercury Analysis
Approximately 5 grams of sediment or soil was dried at 60°C for 72 hours. Approximately 0.1 grams of sample was analyzed for total mercury content using a Direct Mercury Analyzer 80 (DMA80; Milestone, Shelton, CT) using EPA Method 7473 as outlined by others (Basu et al., 2010; Nam and Basu, 2011). All samples were batch processed and analyzed in triplicate, after each seasonal collection.
Accuracy (within 20% of expected values; mean recovery: 107%) and precision (within 7% relative standard deviation; mean RSD was 2%) were measured using standard reference materials including National Institute of Standards and Technology (NIST) San Joaquin Soil (NIST 2709), NIST Trace Elements in Soil (NIST 2586), and National Research Council (NRC) Canada Dogfish Liver (NRC DOLT-4). The theoretical method detection limit (TMDL; 3 times the standard deviation of the mean blank value) ranged from 0.01 to 0.67 ng mercury. Samples for which concentrations were below limit of detection are noted in the results section with the measured value being retained.
2.3.3 Statistical Analysis of Ecological Study
For all sites, the three sediment subsamples were averaged together to produce an overall site value. Statistics were performed using this mean value for sediment. For soil, individual site values for soil mercury concentration were analyzed. Data were generally not normally distributed and thus non-parametric statistical tests were used. Differences among communities and among seasons were evaluated using Kruskal-Wallis and further investigated using Mann-Whitney tests. Associations between soil and sediment mercury concentrations with water quality measures were performed using Spearman correlations. For water quality parameters and mercury concentrations, comparisons of interest focused primarily on differences between communities within the “Chemical Valley” region and between the Aamjiwnaang First Nation and the reference community. Each stream site was compared to benchmark values as addressed in the text. All statistics were performed in SPSS (Version 19.0; Armonk, New York) and a p-value of <0.05 was used to denote statistically significant differences.
2.4 Human Biomarker Study
2.4.1 Human Participants
Institutional Review Board (IRB) approval was obtained (HUM00029363), and permission was obtained from the Aamjiwnaang Band Council via a Band Council Resolution (2008/2009–28). Mother-child pairs (n=43) were recruited from the Reserve and surrounding areas. Written informed consent was obtained from each maternal caregiver for her and her child’s participation, along with the child’s assent. Participants were met either in their home or at the Aamjiwnaang First Nation Health Center.
2.4.2 Surveys and Human Biomarkers
A written survey was filled out by each maternal caregiver to capture self-reported demographics, diet, health, and household information. Caregivers were asked to provide the same information for their children. Fish consumption, by species, over the most recent six months was addressed along with consumption of local produce and game. Participant recollection of diet was reinforced by a 24-hour recall survey. The seafood Hg content was obtained from species-specific Hg concentrations reported by the U.S. Food and Drug Administration Monitoring Program in the most recent years as detailed by others (Goodrich et al., 2016) Participants were also asked to detail information regarding personal dental amalgams to better increase understanding of inorganic mercury exposures.
From both mothers and children, blood, hair and urine were obtained. Venous whole blood samples (reflecting potential biomarkers of exposures to both organic and inorganic mercury) were collected into BD Vacutainer tubes certified for trace metals analysis, spot urine sample (reflecting potential biomarkers of exposures to inorganic mercury) were collected, and scalp hair samples (reflecting potential biomarkers of exposures to organic mercury) were obtained from each participant using stainless steel scissors and the proximal end was designated. All samples were stored at −20°C in a locked freezer at the Aamjiwnaang First Nation Health Center before being relocated on ice to the laboratory where they were kept frozen at −80°C in a secure facility.
2.4.3 Human Biomarker Analysis
Human biospecimen samples were analyzed for Hg content using the DMA-80 as described by others (Basu et al, 2014). Briefly, urine and blood samples were vortexed, and 500–1000ul was then placed into a quartz sampling boat. For hair, a 2 cm segment cut from the proximal end was washed with acetone, rinsed three times with Milli-Q water, dried overnight and ~2–5mg was placed into a nickel sampling boat. Accuracy (within 20% of expected values; mean recovery: 92.4% for urine, 95.1% for blood, 93.0% for hair) and precision (within 7% relative standard deviation; mean RSD was 5.6% for urine, 5.1% for blood, 8.7% for hair) were determined using standard reference materials including QMEQAS084-01(urine) and QMEQAS09B-02 (blood) from the Institut National de Santé Publique du Québec (INSPQ), CRM 13 (human hair) National Institute for Environmental Studies, Japan, and DOLT-4 (dogfish liver) National Research Council Canada. The theoretical method detection limit (TMDL; 3 times the standard deviation of the mean blank value) was 0.03 ng Hg for urine, 0.06 ng Hg for blood, and 0.04 ng Hg for hair. Samples for which concentrations were below limit of detection are noted in the results section with the measured value being retained.
2.4.4 Statistical Analysis of Human Exposure Study
Biomarker concentrations were not normally distributed and transformations did not achieve normality, and thus non-parametric tests were performed. Primary comparisons of interest were differences in mercury biomarkers between mothers and children living on and off Reserve. Values are reported as mean ± standard deviation, unless otherwise indicated, and a p-value of <0.05 was used to denote statistically significant differences.
3. RESULTS
3.1 Source Results
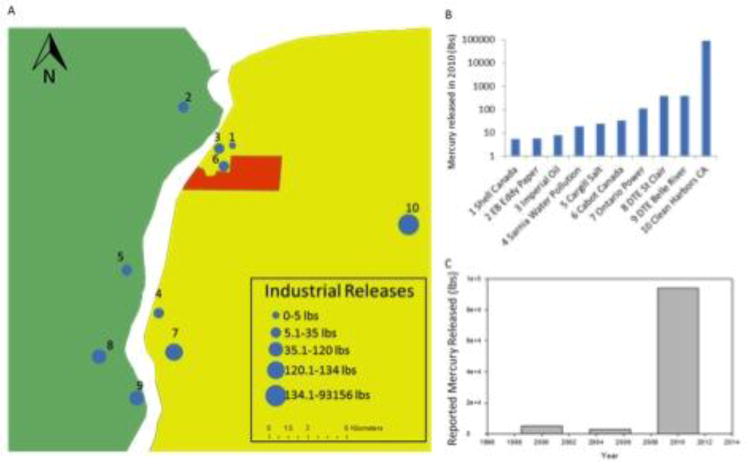
An analysis of the TRI and NPRI databases revealed that nearly 94 thousand pounds of mercury were released or processed within the “Chemical Valley” area as reported for 2010 (Figure 3). Of these releases, 700 pounds were emitted into the air, 25 pounds were released directly into water bodies, and upwards of 93 thousand pounds were disposed of on-site either via underground injections or into landfills. The primary sources of mercury included wastewater treatment plants, waste treatment and disposal facilities, and coal-fired power plants. Since the year 2000, releases of mercury from facilities within the ‘Chemical Valley’ region have shown a general increase as determined from reviewing the reported results from 2005 and 2010.
Figure 3. Mercury releases reported by facilities within “Chemical Valley”.
Data was obtained from Canada’s National Pollutant Release Inventory (NPRI) and the U.S. Toxics Release Inventory (TRI). (A) Map of facilities that reported releasing mercury within the ‘Chemical Valley’ region in the 2010 reporting year. Mercury releasing facilities are represented on the map by dots and are numbered to correspond with Panel B. Facilities with large dots representing those that released more mercury according to the legend. (B) Bar graph of mercury released (lbs) by the region’s top 10 facilities. (C) Total mercury releases within ‘Chemical Valley’ in the years 2000, 2005, and 2010 based on NPRI and TRI data.
3.2 Ecological Results
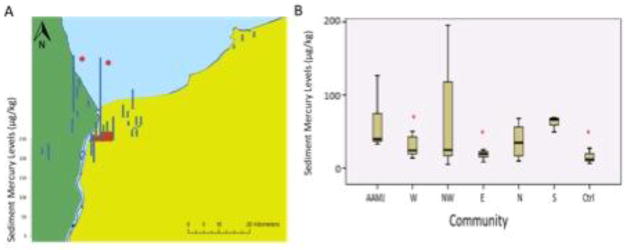
Sediment samples were collected from each of the 23 stream sites during three seasons. The geometric means of all sediment concentrations at all sites across each collection timeframe ranged from 5.0 to 398.7 μg/kg dry weight, d.w. (Figure 4). In general, mercury concentrations across communities were variable though despite the variability there were significant differences in mercury sediment concentrations among communities. Sediment mercury at Aamjiwnaang (85.8 ± 88.8 μg/kg) was significantly higher than the reference community (14.2 ± 4.7 μg/kg across the four Sites) and eastern Sarnia (18.0 ± 2.7 μg/kg across the five Sites). Site 2 on the Aamjiwnaang First Nation Reserve (242.4 ± 152.1 μg/kg) had the highest concentrations of sediment mercury (Table 2; Figure 4). The collections across the seasons were not significantly different from each other.
Figure 4. Sediment mercury concentrations (ug/kg).
A) Map of sediment mercury concentrations (ug/kg) from each of the study sites. Each site is represented by its average mercury concentration across the three sampling sessions. Asterisks denote a site at which mercury levels were above the US EPA lowest effect level for freshwater invertebrates. B) Box and whisker plot of sediment mercury concentrations (ug/kg) from the 7 study communities. The horizontal line represents the median, the bar represents first through third quartiles, and the whiskers represent the minimum and maximum values. Asterisks denote communities that differed significantly from Aamjiwnaang.
Table 2.
Total mercury levels in hair, blood, and urine of mother-child pairs, as well as in household soil from the Aamjiwnaang and the off-reserve population.
| Mean | Std. Deviation | 10th | 25th | 50th | 75th | 90th | ||
|---|---|---|---|---|---|---|---|---|
| Hair Hg (μg/g) | Children (On-Reserve) | 0.13 | 0.13 | 0.02 | 0.04 | 0.09 | 0.15 | 0.29 |
| Children (Off-Reserve) | 0.13 | 0.08 | 0.04 | 0.07 | 0.10 | 0.16 | 0.28 | |
| Mothers (On-Reserve) | 0.22 | 0.13 | 0.07 | 0.12 | 0.21 | 0.29 | 0.43 | |
| Mothers (Off-Reserve) | 0.28 | 0.27 | 0.05 | 0.09 | 0.17 | 0.40 | 0.83 | |
| Blood Hg (μg/L) | Children (On-Reserve) | 1.96 | 2.03 | 0.22 | 0.44 | 0.90 | 3.82 | 5.69 |
| Children (Off-Reserve) | 0.75 | 1.40 | 0.09 | 0.14 | 0.28 | 0.63 | 3.82 | |
| Mothers (On-Reserve) | 1.61 | 1.96 | 0.26 | 0.37 | 0.88 | 1.71 | 4.64 | |
| Mothers (Off-Reserve) | 1.98 | 2.21 | 0.26 | 0.59 | 1.09 | 2.85 | 6.63 | |
| Urinary Hg (μg/L) | Children (On-Reserve) | 0.57 | 1.11 | 0.10 | 0.16 | 0.30 | 0.50 | 1.16 |
| Children (Off-Reserve) | 0.23 | 0.20 | 0.04 | 0.11 | 0.13 | 0.38 | 0.57 | |
| Mothers (On-Reserve) | 0.66 | 0.74 | 0.15 | 0.24 | 0.39 | 1.00 | 1.38 | |
| Mothers (Off-Reserve) | 0.44 | 0.41 | 0.09 | 0.17 | 0.27 | 0.72 | 1.19 | |
| Soil Hg (μg/kg) | On-Reserve Households | 99.6 | 127.7 | 35.8 | 44.9 | 54.1 | 79.4 | 519.7 |
| Off-Reserve Households | 81.1 | 70.1 | 37.4 | 48.7 | 56.7 | 105.0 | 181.5 |
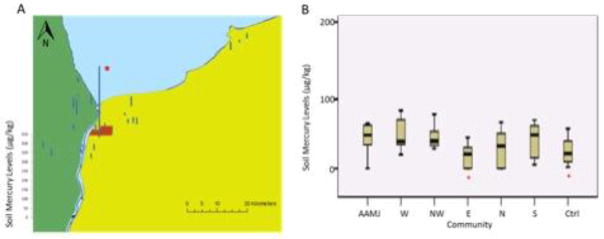
Soil samples were collected near streams from each of the 23 study sites (Table 2). Similar trends were seen in soil as were seen in sediment, and there was a significant correlation between sediment and soil mercury concentrations across all sites and seasons (rs =0.835, p<0.001). The geometric means of all soil samples ranged from 1.2 to 696.2 μg/kg (Figure 5). When comparing soils across communities, significant differences were detected. Soil mercury concentrations were significantly higher on Reserve (158.0 ± 222.1 μg/kg) than the reference community (26.2 ± 20.9 μg/kg)and eastern Sarnia (27.6 ± 7.1 μg/kg). Soil mercury levels were significantly higher at Talfourd Creek that runs through Aamjiwaang (Site #2; 491.1 ± 312.5 μg/kg)) than all other sites, and the measured concentrations here exceeded the NOAA benchmark value for plant and invertebrate health (Buchman, 2008). Soil mercury levels did not vary according to season.
Figure 5. Soil mercury concentrations (ug/kg).
A) Map of soil mercury concentrations from each of the study sites. Each site is represented by its average mercury concentration across the three sampling sessions. Asterisks denote a site at which mercury levels were above the US EPA ecological soil screening levels for plant health. B) Box and whisker plot of sediment mercury concentrations from the 7 study communities. The horizontal line represents the median, the bar represents first through third quartiles, and the whiskers represent the minimum and maximum values. Asterisks denote communities that differed significantly from Aamjiwnaang.
In terms of water quality, there were no differences in water temperature, pH, or conductivity among communities, but they did vary across the seasons (mean seasonal values - fall: 6.1°C, 8.3 pH, 0.4 S; spring: 10.6°C, 9.4 pH, 0.5 S; summer: 20.8°C, 9.7 pH, 1.6 S). Water temperature and conductivity measures were highest in the summer, and water pH was highest in spring. Among all sites and seasons, stream water temperature was positively correlated to sediment mercury concentrations (rs=0.454). Soil mercury concentrations were not associated with the measured water quality parameters.
3.3 Participant Demographics
In total, 43 mother-child pairs residing on the Aamjiwnaang First Nation Reserve and in the surrounding “Chemical Valley” area were recruited. Of these, three pairs were lost to follow-up for unknown reasons. Most (87%, n=37) mothers living on Reserve were of First Nations descent, compared with 23% (n=6) of mothers living off the Reserve. Most (76.5%) mothers living off Reserve were Caucasian, compared with 8.7% of mothers living on the Reserve. There was not a significant difference in education (highest level achieved) and age (36.7±8.9 on-Reserve vs. 31.9 ± 5.9 off-Reserve) between mothers living on and off Reserve.
Child participation was limited to those aged 4–14 years. On and off-Reserve child participants did not show any differences between age and sex. Mean ages of children living on (9.0 ± 2.9) and off Reserve (7.5 ± 2.5). Among on-Reserve children, 41% were male compared with 46% off Reserve. All on-Reserve children were of First Nation descent. Of the off-Reserve mother and child participants, 24% were First Nation, 70% were Caucasian, and 6% self-reported as other nationalities.
3.4 Mercury Exposure Biomarkers
Total mercury was measured in each sample of hair, blood and urine analyzed (Table 2). In hair, mercury concentrations in children (126.7 ± 112.9 μg/kg) were significantly lower than in their mothers (242.1 ± 188.6 μg/kg) from both on and off the Reserve. Hair mercury levels did not differ between participants studied on and off the Reserve. There were no relationships between participant age or sex on hair mercury levels.
For blood, we were unable to obtain samples from eight children (n=4 on and off-Reserve each) and six mothers (n=2 on-Reserve, n=4 off-Reserve). Mean blood mercury levels in children studied from on the Reserve were ~2.6× higher than those sampled off-Reserve but this was not to a level of statistical significance and possibly limited by sample size. Concentrations of blood mercury in children were not correlated with levels in their mothers. There were no age-related differences in blood mercury levels for mothers or children. Across all children, blood mercury levels in girls (2.23 ± 2.26 μg/L) was found to be significantly higher than in boys (0.73 ± 0.91 μg/L). Blood and hair mercury levels were significantly correlated in mothers (rs= 0.429), but not in children (rs=0.145).
Here, the mean consumption of seafood was 0.96 (± 1.12) servings per week in mothers and 0.62 (±1.01) in children. The top three consumed items were tuna (canned and canned light), halibut, and shrimp. When the mercury content in each seafood was related to the survey information, the calculated mean intake of mercury was 0.03 ± 0.08 μg/kg bw/d in children and 0.05 ± 0.13 μg/kg bw/d in the mothers. Estimated mercury intake through fish consumption (μg/kg bw/d) was significantly correlated to hair mercury in children (rs=0.440) and in mothers (rs = 0.356), but not with the other biomarkers.
Concentrations of total mercury in urine were found to be significantly higher in both on-Reserve mothers and children compared to those living off-Reserve (Table 2). There were no significant sex-related differences in urinary mercury concentrations in either on or off-Reserve children. There was no correlation between urine mercury levels and number of personal dental amalgams.
One soil sample was obtained from each participant’s home and analyzed for total mercury content. In doing so, there was no difference in soil mercury levels in homes sampled on-Reserve and off-Reserve. None of the soil samples exceeded the Canadian Soil Quality Guideline for inorganic mercury in residential areas (6,600 μg/kg).
4.0 DISCUSSION
The primary aim of this study was to increase understanding of mercury exposures in the Aamjiwnaang First Nation Reserve. The closure of the Dow Chemical chlor alkali plant in the 1970s marked the cease of the largest source of mercury release into water bodies, though analysis of the TRI and NPRI databases show that mercury is still being released in the region (e.g., in 2010, 700 pounds were emitted into the air and 25 pounds released into water bodies) with values increasing over the period covered by our analyses of the reported results. The mercury released into air is likely in the inorganic form and upwards of 50% may be deposited locally or regionally (US EPA 1997). The amount of mercury released is not inconsequential. For example, across Canada in 2010 facilities reported to NPRI releasing 3,429 kg of mercury compounds into the air. The releases from the “Chemical Valley” would amount to ~9.2% of these Canadian releases with nearly half of this (i.e., 350 pounds or 159 kg per year) possibly falling in the local region.
Having documented that mercury is still being released in the region, the second aim of this study was to compare levels of mercury in sediment and soil in sites located on the Aamjiwnaang Reserve to sites in the surrounding “Chemical Valley”, including a reference community. We found significantly higher levels of mercury in sediment and soil on Reserve than in the reference site during three different sampling seasons. Though, when we studied soils from people’s homes there was no difference between those sampled on and off the Reserve, and none of the samples exceeded the Canadian Soil Quality Guideline for inorganic mercury in residential areas. In the past, a mercury cell chlor-alkali plant was among the facilities located near the Aamjiwnaang First Nation Reserve, and the mercury released from this facility is most likely still present in the region. Elevated concentrations of mercury in soils and sediments located in close proximity to such plants have previously been observed with upwards of 262 μg/kg directly near the source and 0.12 μg/kg within 1km of the source (Gonzalez, 1991). One site on Reserve (Site #2, Talfourd Creek) seems to be a mercury hotspot and was found to contain mercury concentrations that exceeded the National Oceanic and Atmospheric Administration benchmark levels for the protection of ecological receptors in both the stream sediment and nearby soil samples (Buchman, 2008). Talfourd Creek has always been a site of concern in terms of contamination, and further monitoring of this stream should be performed at regular intervals especially since mercury exposure in soil may be realized via direct ingestion (geophagy), hand-to-mouth contact, and from soil on skin, clothing, and food (Rajaee et al., 2015).. One site in Port Huron (MI) also contained high levels of mercury in sediment that warrants further investigation. While all other sites in the study had sediment substrates comprised of clay and clay with rocks, this site had much more detritus within the sediment. Mercury absorbed in allochthonous detritus is a possible explanation for the elevated mercury concentrations at this site (Herrick et al., 1982), along with possibly increased atmospheric deposition from nearby industries.
Though it has been known for upwards of four decades that mercury is a pollutant of concern in the Aamjiwnaang region, no biomarker study has been performed to gauge individual exposures. Here, while all participants had detectable levels of mercury in their hair, blood, and urine, none of the levels differed from the average Canadian citizen, and they did not exceed any health guidance values (Legrand et al., 2010). Methylmercury exposures, as gauged by measuring levels in hair and blood, among the Aamjiwnaang mothers were similar to those seen in other First Nations populations across Canada where a subsistence diet is consumed. For example, a relevant comparison may be with the 18 communities that participated in the Ontario portion of the extensive pan-Canadian First Nations Food, Nutrition and Environment Study (Chan et al., 2014). In this survey, 744 adults were eligible to have hair measured for mercury during the fall of 2011 and 2012. This work showed that, across all communities surveyed, the average hair mercury level was 0.64 ug/g in all adults and 0.40 ug/g in women of childbearing age. This survey also included an exposure assessment that related mercury content in traditional foods with intake surveys to calculate a mean estimated intake of mercury of 0.03 ug/kg bw/d in women of child bearing age. This value is similar to what we report here.
In terms of urinary mercury, we are unaware of levels from another Canadian First Nations community to make comparisons with. Here, urinary total mercury levels were higher among mothers and children residing on-Reserve than off-Reserve. A similar relationship was found among adults residing at varying distances from an active chlor-alkali plant in Poland (Jarosinska et al., 2006). Average concentrations found among and near Aamjiwnaang were above those seen in Poland for both the chlor-alkali and reference zones (mean chlor-alkali =0.40 μg/L, mean reference = 0.21 μg/L). In addition to the former chlor alkali plant located within “Chemical Valley” there are multiple active sources of inorganic mercury such as coal-fired power plants, carbon black production, and a hazardous waste facility (Figure 2). In comparison to a Native American Tribe located in California near a cinnabar mine, the urinary mercury ranged much lower among the Aamjiwnaang (0.4 – 12.5 μg/L; Harnly et al., 1997). Urine concentrations among on and off-Reserve children averaged below those seen across the U.S. (mean=0.25 μg/L; CDC, 2012). On-Reserve mothers averaged concentrations above those seen in the U.S., though off-Reserve mothers did not (mean=0.46 μg/L).
The project showed that there were slight differences in biomarker concentrations between mothers and their children living on the Reserve in comparison to those living off the Reserve, though the latter are still located relatively near the industrial complexes. Hair mercury concentrations were correlated to estimated mercury intake through fish consumption as expected since methylmercury exposure occurs mainly through ingestion of contaminated fish (Clarkson, 1993). Children living off Reserve showed higher hair mercury concentrations that did children living on the Reserve. This find was unexpected, as First Nations Peoples generally eat more fish than the general public (Harris and Harper, 1997). However, in the current study, we found that a significantly higher percentage of our children living off-Reserve reported eating fish than did children living on-Reserve.
First Nations people living a traditional lifestyle would be expected to consume local produce and game, fostering healthy diets and cultural connections. Such lifestyles could also potentially put First Nations populations at increased risk of being exposed to environmental pollutants such as mercury (Harris and Harper, 1997; Chan et al., 2010). However, the current study suggests that members of the Aamjiwnaang First Nation are experiencing a dietary and cultural shift, as seen in many other Native and First Nations Peoples (Kuhnlein and Receveur, 1996). Aamjiwnaang members reported eating less locally grown foods and fish in recent years. In our study, 88% of mothers and 64% of children reported anxiety and/or fear associated with contaminants released by surrounding facilities (data not shown). Reports of anxiety among the Aamjiwnaang associated with living near the petrochemical center have been previously documented (Luginaah et al., 2010). A shift in diet, along with chemical, physiological, and emotional stressors are of health concern to the community.
This study was performed as a community-based participatory research project in conjunction with the Aamjiwnaang First Nation’s Environment Committee. Even with community collaboration, recruitment was challenged making sample size a limitation. However, mercury was measured in a diverse range of sample media including human biomarkers and ecological samples that had not yet been well characterized in this community. Other limitations of the study included sampling bias as participants were self-selected volunteers though we advertised the study in the broader Aamjiwnaang and Sarnia communities through a variety of methods, and recall bias as participants provided self-reported health and dietary information. This was a cross-sectional study and as such may not reflect exposures during other periods, though our work on sediment and soil showed that values are quite stable. Regardless of these limitations, we used diverse and robust field and laboratory methods in order to address the study aims which were driven by community concerns.
5.0 CONCLUSIONS
This study was performed in response to requests made by, and in collaboration with the Aamjiwnaang First Nation’s Environment Committee. By studying diverse environmental samples and human biospecimens, and utilizing ecological and epidemiological methods, we were able to characterize mercury levels in the “Chemical Valley” area. While mercury levels were generally similar to values found elsewhere, there were some apparent trends and evidence of contamination that warrant continued monitoring. Notable was the higher mercury levels found in Talfourd Creek which runs through Aamjiwnaang. The study also showed that, despite some evidence of elevated mercury levels in the environment, that mercury exposures amongst area residents was relatively low. This likely reflects reduced dietary intakes of fish among Aamjiwnaang member as has been observed in other First Nations communities.
Acknowledgments
We thank Sharilyn Johnston, Christine Rogers, Wilson Plain Jr. and members of the Aamjiwnaang Environment Committee for their guidance and support. We thank Mozhgon Rajaee and other lab members for technical assistance, and Caitlyn Kowalsky and Lawrence Kowalsky for assistance with human subjects research. DK was support by a University of Michigan Rackham Graduate Research Fellowship and by the U.S. National Institutes of Environmental Health Sciences (NIEHS) Environmental Toxicology Research Training Grant (T32 ES07062). The research was funded by the Great Lakes Commission’s GLAD program, the Michigan Institute of Clinical Health Research’s CTSA program (UL1RR024986), and the University of Michigan School of Public Health.
References
- Basu N, Abare M, Buchanan S, Cryderman D, Nam D-H, Sirkin S, Schmidtt S, Hu H. A combined ecological and epidemiologic investigation of exposure to metals amongst Indigenous Peoples near the Marlin Mine in Western Guatemala. Sci Total Environ. 2010;409:70–77. doi: 10.1016/j.scitotenv.2010.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu N, Tutino R, Zhang Z, Cantonwine D, Goodrich J, Somers E, Rodriguez L, Schnaas L, Solano M, Mercado A, Peterson K, Sanchez B, Hernández-Avila M, Hu H, Tellez-Rojo M. Mercury Levels in Pregnant Women, Children, and Seafood from Mexico City. Environmental Research. 2014;135:63–69. doi: 10.1016/j.envres.2014.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beuter A, Edwards R. Effect of chronic exposure to meythylmercury on eye movements in Cree subjects. Int Arch Occup Environ Health. 2004;77(2):97–107. doi: 10.1007/s00420-003-0480-3. [DOI] [PubMed] [Google Scholar]
- Buchman M. NOAA OR&R Report 08-1: NOAA Screening Quick Reference Tables. Seattle, WA: Office of Response and Restoration Division, National Oceanic and Atmospheric Administration; 2008. p. 34. [Google Scholar]
- Centers for Disease Control, Department of Health and Human Services. Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, September 2012. CDC; Atlanta, GA: 2012. [Accessed 29 Oct 2012]. p. 308. Available via http://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Sep2012.pdf. [Google Scholar]
- Chan L, Receveur O, Batal M, David W, Schwartz H, Ing A, Fediuk K, Black A, Tikhonov C. First Nations Food, Nutrition and Environment Study (FNFNES): Results from Ontario (2011/2012) Ottawa: University of Ottawa; 2014. Print. [Google Scholar]
- Clarkson T. Mercury: major issues in environmental health. Environ Health Perspect. 1993;100:31–38. doi: 10.1289/ehp.9310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewurtz S, Bhavsar S, Jackson D, Fletcher R, Awad E, Moody R, Reiner E. Temporal and spatial trends of organochlorines and mercury in fishes from the St. Clair River/Lake St. Clair corridor, Canada. J Great Lakes Res. 2010;3(1):100–112. [Google Scholar]
- Gonzalez H. Mercury pollution caused by a chlor-alkali plant. Water, Air, & Soil Pollut. 1991;56(1):83–93. [Google Scholar]
- Goodrich JM, Chou H-N, Gruninger S, Franzblau A, Basu N. Exposures of Dental Professionals to Elemental Mercury and Methylmercury. Journal of Exposure Science and Environmental Epidemiology. 2016;26:78–85. doi: 10.1038/jes.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnly M, Seidel S, Rojas P, Fornes R, Flessel P, Smith D, Kreutzer R, Goldman L. Biological monitoring for mercury within a community with soil and fish contamination. Environ Health Perspect. 1997;105(4):424–429. doi: 10.1289/ehp.97105424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Harper B. A Native American exposure scenario. Risk Analysis. 1997;17(6):789–795. doi: 10.1111/j.1539-6924.1997.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Herrick C, Goodman E, Guthrie C, Blythe R, Hendriz G, Smith R, Galloway J. A model of mercury contamination in a woodland stream. J Ecol Model. 1982;15:1–28. [Google Scholar]
- Jarosinka D, Barregard L, Biesiada M, Muszynska-Graca M, Dabkowska B, Denby B, Pacyna J, Fudala J, Zielonka U. Urinary mercury in adults in Poland living near a chloralkali plant. Science of the Total Environment. 2006;368:335–343. doi: 10.1016/j.scitotenv.2005.09.069. [DOI] [PubMed] [Google Scholar]
- Kuhnlein H, Receveur O. Dietary change and traditional food systems of indigenous peoples. Ann Rev Nutr. 1996;16:417–442. doi: 10.1146/annurev.nu.16.070196.002221. [DOI] [PubMed] [Google Scholar]
- Legrand M, Feeley M, Tikhonov C, Schoen D, Li-Muller A. Methylmerury blood guidance value for Canada. Can J Public Health. 2010;101(1):28–31. doi: 10.1007/BF03405557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginaah I, Smith K, Lockridge A. Surrounded by chemical valley and ‘living in a bubble’. The case of the Aamjiwnaang First Nation, Ontario. J Environ Plan Man. 2010;53(3):353–370. [Google Scholar]
- MacDonald E, Rang S. Exposing Canada’s chemical valley: An investigation of cumulative air pollution emissions in the Sarnia, Ontario Area. Ecojustice. 2007 [Google Scholar]
- McDowell M, Dillon C, Osterloh J, Bolger P, Pellizzari E, Fernando R, Montes de Oca R, Schober S, Sinks T, Jones R, Mahaffey K. Hair mercury levels in children and women of child bearing age: Reference range data from NHANES 1999–2000. Env Health Perspect. 2004;112(11) doi: 10.1289/ehp.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch A, Hill K. Distribution of mercury in Lake St. Clair and the St. Clair River sediments. Water Poll Res J of Can. 1989;24(1):1–21. [Google Scholar]
- Nam D-H, Basu N. Rapid methods to detect organic mercury and total selenium in biological samples. Chem Central Journal. 2011;5:3. doi: 10.1186/1752-153X-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nriagu J, Basu N, Charles S. Chp 15 - Environmental Justice: The Mercury Connection. In: Bank M, editor. Mercury in the Environment: Pattern and Process. University of California Press; Berkeley, California: 2012. [Google Scholar]
- Rajaee M, Long R, Renne E, Basu N. Mercury exposure assessment and spatial distribution in a Ghanaian small-scale gold mining community. International Journal of Environmental Research and Public Health. 2015;12(9):10755–10782. doi: 10.3390/ijerph120910755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OME. Summary Report on Mercury Pollution of the St. Clair River System. Ontario Water Resources Commission, Ontario Ministry of the Environment; 1970. [Google Scholar]
- U.S. Environmental Protection Agency. Detroit River-Western Lake Erie Basin Indicator Project, Indicator: Mercury in Lake St. Clair Walleye. Washington DC: U.S. Environmental Protection Agency; 2009. [Google Scholar]
- U.S. Environmental Protection Agency. Mercury study report to congress. Volume 3: Fate and transport of mercury in the environment. Washington DC: U.S. Environmental Protection Agency; 1997. [Google Scholar]
- U.S. Environmental Protection Agency. Upper Great Lakes Connecting Channels Study. Washington DC: U.S. Environmental Protection Agency; 1988. [last accessed June 20, 2015]. http://www.epa.gov/greatlakes/uglcc/ [Google Scholar]