Abstract
Rationale
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a potentially lethal arrhythmic disorder caused by mutations in the type-2 ryanodine receptor (RyR2). Mutant RyR2 cause abnormal Ca2+ leak from the sarcoplasmic reticulum (SR), which is associated with the development of arrhythmias.
Objective
To determine whether derivatives of tetracaine, a local anesthetic drug with known RyR2 inhibiting action, could prevent CPVT induction by suppression of RyR2-mediated SR Ca2+ leak.
Methods and results
Confocal microscopy was used to assess the effects of tetracaine and 9 derivatives (EL1–EL9) on spontaneous Ca2+ sparks in ventricular myocytes isolated from RyR2-R176Q/+ mice with CPVT. Whereas each derivative suppressed the Ca2+ spark frequency, derivative EL9 was most effective at the screening dose of 500 nmol/L. At this high dose, the Ca2+ transient amplitude was not affected in myocytes from WT or R176Q/+ mice. The IC50 of EL9 was determined to be 13 nmol/L, which is about 400x time lower than known RyR2 stabilizer K201. EL9 prevented the induction of ventricular tachycardia observed in placebo-treated R176Q/+ mice, without affecting heart rate or cardiac contractility.
Conclusions
Tetracaine derivatives represent a novel class of RyR2 stabilizing drugs that could be used for the treatment of the potentially fatal disorder catecholaminergic polymorphic ventricular tachycardia.
Keywords: calcium leak, catecholaminergic polymorphic ventricular tachycardia, sarcoplasmic reticulum, tetracaine, type-2 ryanodine receptor
1. Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare inherited disorder characterized by stress or exercise-induced ventricular tachycardia leading to syncope and sudden cardiac death.[1] Patients with CPVT exhibit episodes of pathognomonic bidirectional ventricular tachycardia (VT) despite having structurally normal hearts. Whereas mutations in several genes (RYR2, CASQ2, TRDN, CALM1) are known to cause CPVT, by far the most common cause is gain-of-function mutations in RyR2, the gene encoding the ryanodine receptor type-2. RyR2 regulates Ca2+ release from the sarcoplasmic reticulum (SR) in cardiomyocytes, a physiological process that is essential for normal excitation-contraction coupling. Mutant RyR2 channels have been shown to open aberrantly during diastole, leading to delayed after-depolarizations (DADs), which in turn can trigger fatal ventricular arrhythmias.[2]
Current treatment strategies of CPVT include upstream inhibition of β-adrenergic mediated-activation of RyR2 using β-adrenergic blockers, direct modulation of RyR2 channels, or symptomatic treatment of arrhythmias using an implantable cardioverter defibrillator (ICD). Because β-blockers and ICD devices often fail to prevent potentially lethal arrhythmias, there is a need to develop new drugs that correct RyR2 gain-of-function defects.[3] The first of such compounds identified using an animal model of CPVT was the 1,4-benzothiazepine derivative K201 (also known as JTV519), which suppressed ventricular tachycardia (VT) caused by SR Ca2+ leak.[4] Subsequently, Ye et al.[5] demonstrated that a K201 derivatives with enhanced electron donor properties exhibits stronger inhibition of the ryanodine receptor type-1 (RyR1) at the single channel level. Other lead compounds such as flecainide and carvedilol derivatives have also been tested for anti-arrhythmic effects in CPVT mouse models.[6, 7] Since flecainide may not be safely used in patients with structural heart disease,[8] we set out to identify another scaffold with RyR2 inhibitory properties and generate derivatives thereof in an effort to develop an improved drug therapy for CPVT.
Using the local anesthetic drug tetracaine as the scaffold, we generated novel derivatives that are able to suppress aberrant SR Ca2+ release through mutant RyR2 channels in ventricular myocytes isolated from a CPVT mouse model.[9] The most potent derivative – EL9 – was shown to have an IC50 that was less than 1/10 of the IC50 for K201. The compound EL9 was shown to suppress VT induction in CPVT mice without affecting heart rate and cardiac contractility, thus positioning it as a potential novel therapy for CPVT.
2. Material and methods
Please see Online Supplement for additional details.
Animals
Mice heterozygous for mutation R176Q in RyR2 (R176Q/+) were used at the age of 4 months.[9] Animal protocols were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine.
Synthesis of tetracaine derivatives
Nine derivatives of tetracaine (TTC) were synthesized according to methods provided in detail in the online supplement.
Ca2+ sparks measurement
Ventricular myocytes were isolated by a modified collagenase method, loaded with the Ca2+ indicator Fluo-4-AM (2 μmol/L), and imaged using a LSM510 confocal microscope (Zeiss), as previously described.[10] Once steady-state Ca2+ transient induced by 1Hz pacing was observed, pacing was stopped for 20 seconds and Ca2+ sparks were recorded. The half maximal inhibitory concentration (IC50) was calculated based on CaSF measured in the presence of compound at a series of tested concentrations.
Electrophysiology study
Intracardiac electrophysiology study was performed in R176Q/+ mice and WT littermates at the age of 4 months as previously described.[9, 11, 12]
Statistics
Data are presented as mean ± SEM. To compare continuous variables with a skewed distribution, the Mann-Whitney test was applied. Fisher’s exact test was used to compare categorical data. A P-value of 0.05 or less was considered statistically significant.
3. Results
3.1 Tetracaine derivatives inhibit abnormal SR Ca2+ release events in R176Q/+ myocytes
We developed a screening assay to assess whether our novel compounds can normalize excessive SR Ca2+ release through defective RyR2 channels in ventricular myocytes from R176Q/+ mice. At baseline - in the absence of β-adrenergic stimulation - the frequency of spontaneous Ca2+ sparks after a conditioning 1-Hz pacing train was higher in myocytes from R176Q/+ mice (3.4 ± 0.3 sparks/100μm/s) compared to those from WT littermates (2.0 ± 0.4; P<0.05; Supplemental Fig. 1A–B). When β-adrenergic stimulation was mimicked by application of isoproterenol (100 nmol/L for 1 hour), the difference in Ca2+ sparks frequency became more pronounced in R176Q/+ myocytes (6.8 ± 0.7) compared to WT controls (3.4 ± 0.4; P<0.05). Because arrhythmias in CPVT patients almost exclusively occur during adrenergic stress, we screened the novel compounds in cells that were exposed to isoproterenol.
To evaluate the efficacy of small molecules to suppress RyR2 activity, myocytes from R176Q/+ mice were incubated with the parent compound tetracaine (TTC) or 9 newly synthesized derivatives (EL1-9) at a concentration of 500 nmol/L for 2 hours at 37ºC. The chemical structure of these compounds is provided in Supplemental Fig. 2. Compared to the vehicle-treated cells, TTC and its 9 derivatives all reduced the Ca2+ sparks frequency (from −6% to −62%) (Supplemental Fig. 1C). Among these 10 compounds, EL9 reduced the Ca2+ sparks frequency the most (62.4 ± 6.2%; P<0.01 compared to placebo). Therefore, this compound was characterized in more detail in subsequent studies.
3.2 Tetracaine derivative EL9 reduced Ca2+ spark frequency (CaSF) in R176Q/+ myocytes
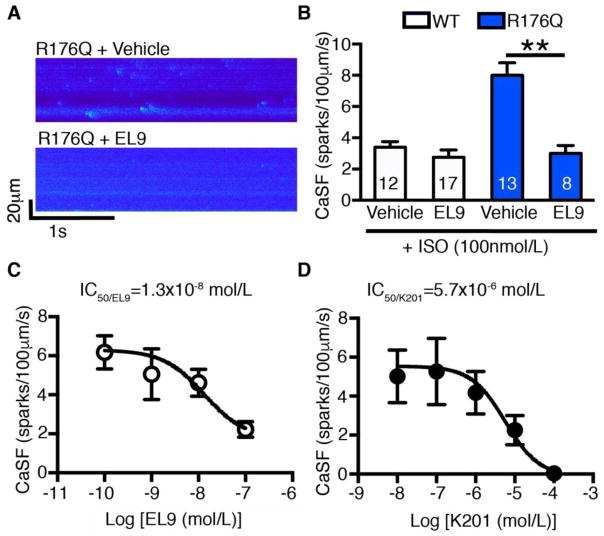
To determine whether EL9 preferentially alters activity of mutant RyR2 more than wild-type channels, we assessed the effects of this drug on spontaneous Ca2+ sparks in ventricular myocytes from WT and R176Q/+ mice (Fig. 1A). Compound EL9 used at the high screening dose of 500 nmol/L did not significantly alter the Ca2+ sparks frequency in WT myocytes (Fig. 1B). In contrast, EL9 greatly reduced Ca2+ sparks frequency in R176Q/+ myocytes (3.0 ± 0.5 sparks/100μm/s) compared to vehicle treated cells (8.0 ± 0.8; P<0.01). Similarly, EL9 also effectively reduced CaSF in a different gain-of-function RyR2 model (S2814D knockin model) as described.[13] (Supplemental Fig. 3) These data demonstrate that EL9 preferably targets the hyperactive mutant RyR2 channels, normalizing spontaneous SR Ca2+ release events to levels observed in WT mice.
Figure 1. Tetracaine derivative EL9 reduced Ca2+ spark frequency (CaSF) in R176Q/+ myocytes.
(A) Representative recordings of Ca2+ sparks in ventricular myocytes of WT and R176Q/+ mice in the presence of vehicle or EL9 (500 nmol/L). (B) Summary data showing that EL9 reduced CaSF in R176Q/+ ventricular cells compared to vehicle. (C, D) Dose-response relationship and IC50 determination of EL9 and reference compound K201 using CaSF inhibition in R176Q/+ myocytes. **P<0.01.
More in-depth analysis of the Ca2+ spark characteristics revealed that EL9 did not affect the amplitude of individual Ca2+ sparks in ventricular myocytes from WT mice (0.38 ± 0.01 F/F0), compared to vehicle controls (0.40 ± 0.01; P=0.28; Supplemental Table S1). In contrast, EL9 increased the Ca2+ spark amplitude in R176Q/+ mice (0.40 ± 0.008) compared to vehicle (0.35 ± 0.003; P=0<0.01). These results suggest that EL9 did not affect the amount of Ca2+ released during the brief rising phase of the RyR2 activation in WT myocytes.[14] On the other hand, the reduction in amplitudes seen in R176Q/+ myocytes was normalized by EL9 to levels seen in WT myocytes. Further analysis revealed that EL9 also did not alter the full duration at half-maximum, the full width at half-maximum, the decay rate (tau) and rising rate (ΔF/F0/Δt max) of the Ca2+ sparks in WT myocytes, suggesting that this compound does not have any detrimental effects on elementary Ca2+ release in normal individuals. In contrast, EL9 corrected all abnormal Ca2+ spark parameters observed in R176Q/+ myocytes to levels seen in WT mice.
3.3 Dose-response of Ca2+ spark inhibition by EL9
To get a better sense of what drug concentrations are needed to inhibit RyR2 channels, we assessed the effects of a range of doses of EL9 on Ca2+ sparks frequency in ventricular myocytes isolated from R176Q/+ mice. The dose-response relationship based on inhibition of the Ca2+ sparks frequency was determined at concentrations from 10−10 up to 10−7 mol/L (Fig. 1C). Sigmoidal dose-response curve fitting was used to determine that the half maximal inhibitory concentration (IC50) of EL9 is 1.3×10−8 mol/L. To compare the efficacy of the new compound EL9 to a previously well-characterized RyR2 inhibitor, similar studies were performed for K201. The IC50 of K201 in terms of Ca2+ sparks inhibition was found to be 5.7×10−6 mol/L, which is about 400x higher than EL9 (Fig. 1D). Thus, tetracaine derivative EL9 was found to inhibit excessive SR Ca2+ release at low nanomolar concentrations.
3.4 Absence of detrimental effects of EL9 on systolic SR Ca2+ handling
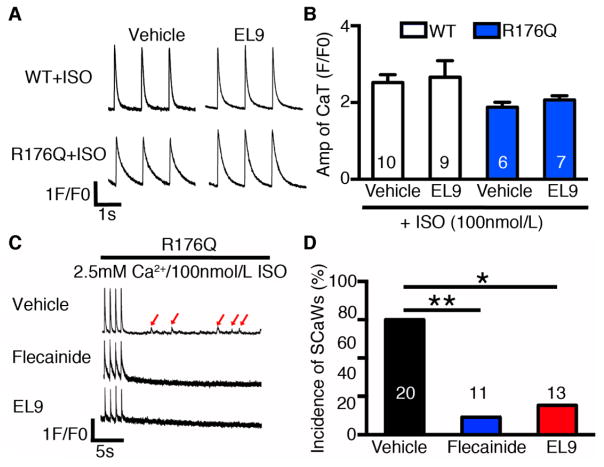
An important consideration is whether the experimental drug compound affects systolic SR Ca2+ handling, as a reduction in the Ca2+ transient amplitude would be an unwanted side-effect potentially leading to negative inotropy. To address this concern, we measured the amplitude of the Ca2+ transients induced by field stimulation at 1-Hz (Fig. 2A–B). The amplitude of the Ca2+ transient was not altered by EL9 in ventricular myocytes from WT mice (2.7 ± 0.4 F/F0) compared to vehicle controls (2.5 ± 0.2; P=0.78). Moreover, EL9 also did not alter Ca2+ transient amplitude in R176Q/+ myocytes (2.1 ± 0.1) compared to vehicle controls (1.9 ± 0.1; P=0.30). Thus, EL9 did not negatively affect the Ca2+ transient amplitude at the cellular level, which suggests no detrimental effects on cardiac function at the whole organism level.
Figure 2. Absence of detrimental effects on systolic SR Ca2+ handling and inhibition of spontaneous Ca2+ waves (SCaWs) by EL9.
(A) Representative recordings of paced Ca2+ transients in WT or R176Q/+ myocytes using 1-Hz field stimulation, in the presence of vehicle or EL9. (B) Summary data showing that EL9 did not alter the Ca2+ transient amplitude in ventricular myocytes from WT and R176Q/+ mice. (C) Representative recordings of SCaWs in R176Q/+ myocytes in the presence of vehicle, flecainide (600 nmol/L), or EL9 (500 nmol/L). (D) Incidence of SCaWs in R176Q/+ myocytes. *P<0.05, **P<0.01.
3.5 EL9 suppresses arrhythmogenic Ca2+ waves in R176Q/+ mice
Previous studies have shown that abnormal Ca2+ release via RyR2 channels promotes spontaneous Ca2+ waves (SCaWs), which can trigger ventricular arrhythmias.[9, 15] To determine whether EL9 can suppress triggered activity, we measured the SCaWs in R176Q/+ myocytes with incubation of vehicle or EL9. Since flecainide has been previously proven to be effective in reducing SCaWs in a CPVT model,[16] it was used as a positive control. While 60% of vehicle-treated R176Q/+ myocytes (n=20) developed SCaWs, 9% of flecainide (600nmol/L) treated R176Q/+ myocytes (n=11) exhibited SCaWs, similar to previous reports.[16] Similarly, 15% of EL9 (500 nmol/L) treated R176Q myocytes exhibited SCaWs (n=13), significantly lower than untreated R176Q/+ cells (P=0.015), but not different from flecainide-treated R176Q/+ cells (P=1.0) (Fig. 2C–D).
3.6 EL9 suppresses the induction of VT in R176Q/+ mice
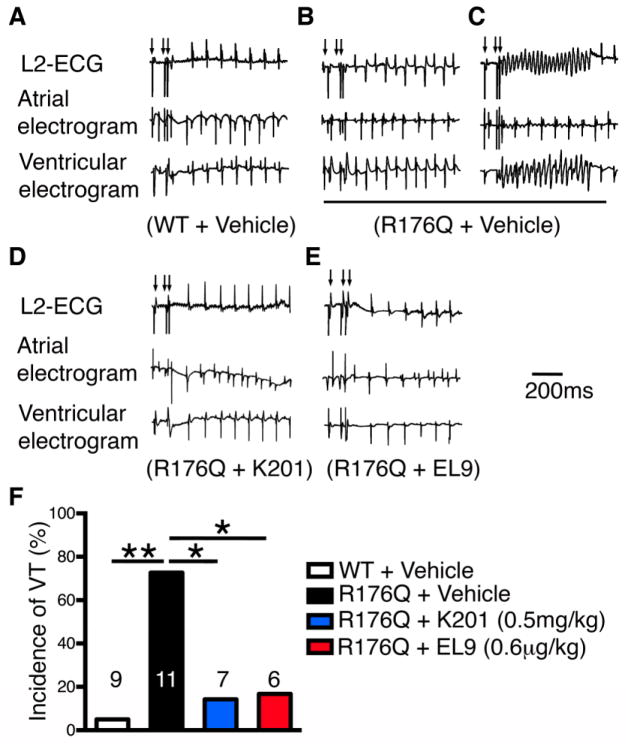
To determine whether the ability of EL9 to inhibit aberrant SR Ca2+ release events translates into the ability to block ventricular tachyarrhythmias in vivo, we performed programmed electrical stimulation studies on R176Q/+ mice. To mimic β-adrenergic stimulation that provokes arrhythmias in CPVT patients, we administered epinephrine (2 mg/kg) and caffeine (120 mg/kg) intraperitoneally, as previously described.[9] Extra-stimuli protocol was used to potentially induce ventricular tachycardia (VT). None of the vehicle treated WT mice (0 of 9) developed VT using the pacing protocols (Fig. 3A). In contrast, 70% of vehicle-treated R176Q/+ mice (7 of 10, P<0.01 vs WT) had reproducible bidirectional VT and/or polymorphic VT (see Fig. 3B–C). Each pacing protocol was performed at least twice to determine the reproducibility of VT induction.
Figure 3. EL9 suppresses the induction of ventricular tachycardia (VT) in R176Q/+ mice.
Representative recordings of surface ECG (lead 2) and intracardiac electrograms after S1–S2 extrastimuli (arrows) revealed sinus rhythm in WT mouse (A), but bidirectional (B) and sustained VT (C) in R176Q/+ mouse treated with vehicle. Treatment with K201 and EL9 suppressed the induction of VT in R176Q/+ mice (D, E). Bar graph summarizing incidence of reproducible VT in each group (F). Numbers indicate mice studied. *P<0.05, **P<0.01.
Next, we tested the effects of K201 – a known RyR2 stabilizer – on the inducibility of CPVT. The drug was injected in R176Q/+ mice (0.5mg/kg, i.p) 15 min prior to programmed electrical stimulation. Consistent with prior findings in another mouse model of CPVT,[4] we found that K201 suppressed the induction of VT in R176Q/+ mice (1 of 7 mice; P<0.05 vs vehicle; Fig. 3D). Since the new compound EL9 requires much lower concentrations to suppress aberrant RyR2-mediated Ca2+ release events compared to K201, we sought to evaluate whether EL9 can inhibit the development of CPVT in R176Q mice at a dose 400x lower that of K201 (0.6 μg/kg). Similar to K201, EL9 was injected intraperitoneally 15 minutes prior to programmed electrical stimulation. In contrast to vehicle-treated R176Q mice, only 17% of EL9-treated R176Q/+ mice (1 of 6 P<0.05 vs vehicle, Fig. 3E) developed VT.
3.7 Effects of EL9 on heart rate and ventricular function of R176Q/+ mice
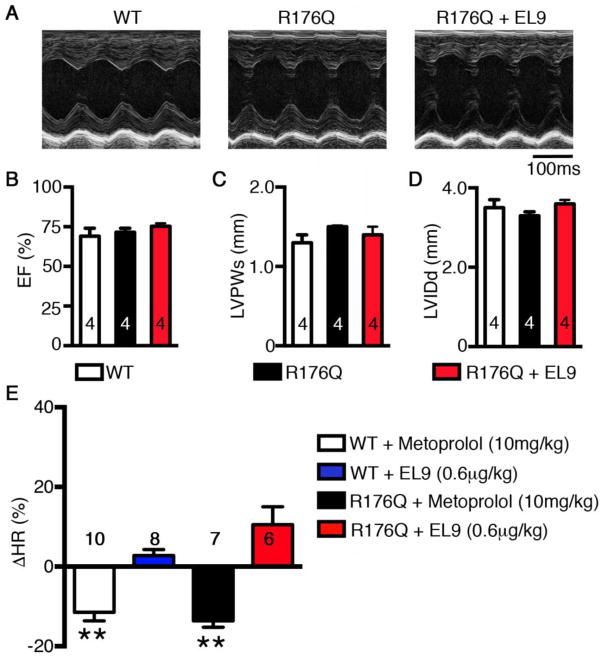
An ideal RyR2 modulating compound should suppress CVPT-associated aberrant diastolic SR Ca2+ release events (Ca2+ sparks) without altering the action potential/voltage-triggered Ca2+ transient, which is essential for normal excitation-contraction coupling and cardiac contractility.[17] To examine the potential effects of EL9 on ventricular contractility, we first determined the potential acute, short-term effects of EL9 on WT mice using echocardiography. The ejection fraction (EF%), left-ventricular wall thickness at end-systole (LVPWs), and internal diameter at end-diastole (LVIDd), among others, were evaluated before and 15 minutes after injection of EL9 (0.6 μg/kg i.p.). There were no significant differences in any of the major contractile or structural parameters following short-term EL9 administration (Supplemental Table S2).
Next, we assessed the long-term effects of EL9 on ventricular function in R176Q/+ mice. Echocardiography was performed on WT and R176Q/+ mice, as well as R176Q/+ mice treated with EL9 (0.6 μg/kg/hr for 6 days) (Fig. 4A). There were no significant differences between WT and R176Q/+ mice in terms of the EF%, LVPWs, and LVIDd (Fig. 4B–C). Thus, EL9 did not affect short-term or long-term ventricular function in mice.
Figure 4. Effects of EL9 on heart rate and ventricular function of R176Q/+ mice.
(A) Representative M-mode echocardiogram tracings obtained from WT, R176Q/+, and R176Q/+ mice treated with EL9 (0.6 μg/kg/hr for 6 days). Summary bar graphs showing no differences among these groups in terms of (B) Ejection fraction (EF), (C) left-ventricular posterior wall-thickness at end-systole (LVPWs), and (D) left-ventricular internal diameter at end-diastole (LVIDd). (E) The change in heart rate (ΔHR %) in WT and R176Q /+ mice treated with metoprolol (10 mg/kg) or EL9 (0.6 μg/kg). **P<0.01 vs pre-injection.
Finally, we evaluated whether EL9 affects cardiac conduction and heart rate (HR), since it is conceivable that inhibition of RyR2 might affect nodal function or conduction velocities.[18] We monitored the ECG in WT and R176Q mice before and after a single injection of EL9 or metoprolol (a positive control), over a period of 15-minutes. Metoprolol (10 mg/kg) reduced HR by 11.5 ± 2.1% in WT mice and by 13.6 ± 1.6% in R176Q mice (P<0.01 vs pre-injection in both strains of mice; Fig. 4E). In contrast, EL9 (0.6 μg/kg) did not significantly affect HR of WT mice (−2.8 ± 1.5%) or R176Q mice (10.5 ± 4.5%) compared to pre-injection rates (Fig. 4E). Additional analysis of the ECG parameters revealed that administration of EL9 (quantified 15 min after injection) did not significantly alter PR, QRS, or QTc intervals in WT or R176Q/+ mice (Supplemental Table S3). Together, these data suggest that EL9 is safe in vivo, because it does not affect cardiac conduction or ventricular contractility.
4. Discussion
In this study, we characterized novel derivatives of tetracaine, a local anesthetic drug with known RyR2 inhibiting features.[19] We uncovered that one of the members of this new class of compounds, referred to as EL9, effectively suppresses aberrant SR Ca2+ release events in ventricular myocytes isolated from mice carrying the pro-arrhythmogenic R176Q/+ mutation in RyR2.[9, 20] Moreover, the compound was shown to prevent the induction of ventricular tachyarrhythmias in the R176Q/+ mice, in the absence of cardiovascular side-effects. Therefore, tetracaine derivative EL9 may represent a promising lead compound for the development of more effective drug therapies for CPVT.
4.1 Selective RyR2 inhibition as a treatment for CPVT
If left untreated, CPVT is associated with mortality rates of up to 30–50% by the age of 40 years.[21] Since the initial description of CPVT, β-blockers have been the cornerstone of therapy of this disorder. In some high-risk CPVT cases, left cardiac sympathetic denervation is used when β-blocker and implantable cardioverter defibrillator (ICD) therapy are refused or contraindicated. However, resection of the sympathetic chain is associated with side-effects in the majority of patients.[22] In 2009, the class 1c antiarrhythmic drug flecainide was shown to suppress ventricular arrhythmias patients with CPVT.[6] The 2013 guidelines now recommend flecainide treatment for CPVT patients with recurrent syncope or polymorphic VT while treated with β-blockers.[23] The proposed mechanism of flecainide in CPVT is a direct inhibiting effect on RyR2 in addition to the well-known sodium channel-blocking effects, although some groups have claimed that the beneficial effects of flecainide are solely attributable to the sodium channel blocking effects of the drug.[24] Nevertheless, it is undisputed that flecainide can block cardiac sodium channels. The use of flecainide is contraindicated in patients with sick sinus syndrome or significant conduction delay, 2nd or 3rd degree heart block or bundle branch block, acquired or congenital long QT syndrome, and patients with a history of torsade de pointes.[25]
Since CPVT is most commonly caused by mutations in RyR2, or associated proteins such as calmodulin and calsequestrin, drugs have been developed to specifically target this Ca2+ release channel complex.[2] Initial studies focused on a 1,4-benzothiazepine derivative K201.[26] K201 was shown to prevent the induction of lethal ventricular tachycardias in a mouse model of CPVT.[4] However, K201 exhibits many off-target effects including inhibition of the delayed rectifier K+-current, which may cause prolonged QT intervals and increase risk of torsade de pointes.[27] Moreover, K201 was shown to alter the gating of the L-type Ca2+ channel, which could interfere with excitation-contraction coupling.[28]
Lehnart et al.[29] demonstrated that derivatives of K201 with a lower IC50 and better safety profile could suppress arrhythmias in a mouse model of CPVT. For example, compound S107 - an orally available 1,4-benzothiazepine derivative with high RyR2 activity and no significant off-target effects – was shown to suppress ventricular arrhythmias in a R2474S heterozygous knock-in mouse model of CVPT. At the single channel level, S107 reduced the open probability of RyR2 channels from heterozygous mutant mice, but did not inhibit RyR2 from WT mice. The molecular mechanism underlying the therapeutic effects of 1,4-benzothiazepine derivatives like K201 and S107 is believed to be enhanced binding of FK506-binding protein 12.6 (FKBP12.6) to the mutant RyR2 channel complex.[4, 29] In fact, K201 derivatives such as S107 were identified using a screening assay focused on enhanced FKBP12.6 binding to RyR2, which is a major distinction from our current class of tetracaine derivatives.
Other agents thought to normalize RyR2 dysfunction in CPVT include dantrolene, an agent traditionally used for the treatment of malignant hyperthermia owing to its effects on the skeletal muscle ryanodine receptor type 1 (RyR1). Dantrolene has been shown to inhibit VT in a mouse model of CVPT.[30] Although dantrolene might prevent VT in mouse models of CPVT, there are concerns that the compound is more selective for RyR1 compared to RyR2. Finally, non-β-blocking carvedilol analogues were shown to be effective in suppressing arrhythmogenic Ca2+ waves and β-adrenergic stress-induced VT in mice with CPVT.[31] Unlike S-carvedilol, which possessing β-blocking activity, the R-carvedilol enantiomer was shown to normalize RyR2 activity without causing bradycardia and hypotension.
4.2 Tetracaine derivatives for CPVT treatment
In this study, we used an unbiased pharmaceutical approach to develop novel derivatives of tetracaine (a well-known local anesthetic with some RyR2 inhibiting activity). The new tetracaine derivatives were synthesized and structure-activity relationship (SAR) studies were performed to identify compounds that inhibit mutant RyR2 channels at nanomolar concentrations. We demonstrated that derivative EL9 suppresses aberrant SR Ca2+ release events in ventricular myocytes isolated from R176Q/+ mice at concentrations 400-fold lower than the RyR2 inhibitor K201.
Using a low dose of 0.6 μg/mg administered intraperitoneally, we demonstrated that EL9 effectively prevented the induction of ventricular tachycardia in a mouse model of CPVT. On the other hand, there was no evidence of altered chronotropy or inotropy in R176Q/+ mice treated with EL9, suggesting that WT RyR2 channels are not inhibited at therapeutic drug levels. Although patch-clamp studies of Na+ channels in isolated myocytes were not performed, it is unlikely that EL9 alters Na+ channel gating because the QRS complex was not altered on the ECG of WT and R176Q/+ mice treated with EL9 (Table 2). These data together demonstrate that EL9 is a safe antiarrhythmic agent that could be further evaluated in large-animal safety studies in preparation for future clinical studies.
5. Conclusions
This study provides a description of a novel class of antiarrhythmic compounds that target the SR Ca2+ release channel RyR2. Among derivatives of tetracaine, compound EL9 was shown to inhibit aberrant Ca2+ sparks in ventricular myocytes isolated from R176Q/+ mice with CPVT. Moreover, the compound was shown to effectively prevent the induction of lethal ventricular arrhythmias in vivo. Compound EL9 works at an IC50 in the nanomolar range, and does not exhibit cardiac side-effects that compromise cardiac rhythmicity or contractility. Therefore, compound EL9 is a promising lead compound for further drug development efforts that could potentially provide novel treatment options for the orphan disease CPVT in the future.
Supplementary Material
Acknowledgments
The authors wish to acknowledge Sandra Shotwell, Douglas Kawahara, and Douglas Boatman for their contributions to the project, and Mouse Phenotyping Core at Baylor College of Medicine for assistance to the echocardiography experiment.
Funding
This work was supported by grants from the National Institutes of Health [R01-HL089598, R01-HL091947, R01-HL117641, R41-HL129570 to X.H.T.W., R56HL131649 to N.L., and R42-HL114206 to J.J.A.], and the American Heart Association [13EIA14560061 to X.H.T.W., and 14SDG20080008 to N.L.].
Non-standard abbreviations
- CPVT
Catecholaminergic polymorphic ventricular tachycardia
- RyR2
Ryanodine receptor type-2
- SCaWs
Spontaneous Ca2+ waves
- SR
Sarcoplasmic reticulum
Footnotes
Disclosure
Drs. Abramson, Strongin, Salama and Wehrens are co-founders and co-owners of Elex Biotech, a small biotech company dedicated to the development of drug molecules for the treatment of heart disease.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Priori SG, Chen SR. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–83. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, et al. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–40. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 3.van der Werf C, Zwinderman AH, Wilde AA. Therapeutic approach for patients with catecholaminergic polymorphic ventricular tachycardia: state of the art and future developments. Europace. 2012;14:175–83. doi: 10.1093/europace/eur277. [DOI] [PubMed] [Google Scholar]
- 4.Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, et al. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science. 2004;304:292–6. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 5.Ye Y, Yaeger D, Owen LJ, Escobedo JO, Wang J, Singer JD, et al. Designing calcium release channel inhibitors with enhanced electron donor properties: stabilizing the closed state of ryanodine receptor type 1. Mol Pharmacol. 2012;81:53–62. doi: 10.1124/mol.111.074740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe H, Chopra N, Laver D, Hwang HS, Davies SS, Roach DE, et al. Flecainide prevents catecholaminergic polymorphic ventricular tachycardia in mice and humans. Nat Med. 2009;15:380–3. doi: 10.1038/nm.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Q, Xiao J, Jiang D, Wang R, Vembaiyan K, Wang A, et al. Carvedilol and its new analogs suppress arrhythmogenic store overload-induced Ca2+ release. Nat Med. 2011;17:1003–9. doi: 10.1038/nm.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 9.Kannankeril PJ, Mitchell BM, Goonasekera SA, Chelu MG, Zhang W, Sood S, et al. Mice with the R176Q cardiac ryanodine receptor mutation exhibit catecholamine-induced ventricular tachycardia and cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:12179–84. doi: 10.1073/pnas.0600268103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li N, Wang T, Wang W, Cutler MJ, Wang Q, Voigt N, et al. Inhibition of CaMKII phosphorylation of RyR2 prevents induction of atrial fibrillation in FKBP12.6 knockout mice. Circ Res. 2012;110:465–70. doi: 10.1161/CIRCRESAHA.111.253229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Wehrens XH. Programmed electrical stimulation in mice. J Vis Exp. 2010:1730. doi: 10.3791/1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–79. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 15.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–20. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilliard FA, Steele DS, Laver D, Yang Z, Le Marchand SJ, Chopra N, et al. Flecainide inhibits arrhythmogenic Ca2+ waves by open state block of ryanodine receptor Ca2+ release channels and reduction of Ca2+ spark mass. J Mol Cell Cardiol. 2010;48:293–301. doi: 10.1016/j.yjmcc.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehrens XH. The molecular basis of catecholaminergic polymorphic ventricular tachycardia: what are the different hypotheses regarding mechanisms? Heart Rhythm. 2007;4:794–7. doi: 10.1016/j.hrthm.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King JH, Zhang Y, Lei M, Grace AA, Huang CL, Fraser JA. Atrial arrhythmia, triggering events and conduction abnormalities in isolated murine RyR2-P2328S hearts. Acta Physiol (Oxf) 2013;207:308–23. doi: 10.1111/apha.12006. [DOI] [PubMed] [Google Scholar]
- 19.Gyorke S, Lukyanenko V, Gyorke I. Dual effects of tetracaine on spontaneous calcium release in rat ventricular myocytes. J Physiol. 1997;500( Pt 2):297–309. doi: 10.1113/jphysiol.1997.sp022021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, et al. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–14. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 22.Waddell-Smith KE, Ertresvaag KN, Li J, Chaudhuri K, Crawford JR, Hamill JK, et al. Physical and Psychological Consequences of Left Cardiac Sympathetic Denervation in Long-QT Syndrome and Catecholaminergic Polymorphic Ventricular Tachycardia. Circ Arrhythm Electrophysiol. 2015;8:1151–8. doi: 10.1161/CIRCEP.115.003159. [DOI] [PubMed] [Google Scholar]
- 23.Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–63. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Bannister ML, Thomas NL, Sikkel MB, Mukherjee S, Maxwell C, MacLeod KT, et al. The mechanism of flecainide action in CPVT does not involve a direct effect on RyR2. Circ Res. 2015;116:1324–35. doi: 10.1161/CIRCRESAHA.116.305347. [DOI] [PubMed] [Google Scholar]
- 25.Razavi M. Safe and effective pharmacologic management of arrhythmias. Tex Heart Inst J. 2005;32:209–11. [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneko N, Matsuda R, Toda M, Shimamoto K. Inhibition of annexin V-dependent Ca2+ movement in large unilamellar vesicles by K201, a new 1,4-benzothiazepine derivative. Biochim Biophys Acta. 1997;1330:1–7. doi: 10.1016/s0005-2736(97)00132-6. [DOI] [PubMed] [Google Scholar]
- 27.Kiriyama K, Kiyosue T, Wang JC, Dohi K, Arita M. Effects of JTV-519, a novel anti-ischaemic drug, on the delayed rectifier K+ current in guinea-pig ventricular myocytes. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:646–53. doi: 10.1007/s002100000230. [DOI] [PubMed] [Google Scholar]
- 28.Kohno M, Yano M, Kobayashi S, Doi M, Oda T, Tokuhisa T, et al. A new cardioprotective agent, JTV519, improves defective channel gating of ryanodine receptor in heart failure. American journal of physiology Heart and circulatory physiology. 2003;284:H1035–42. doi: 10.1152/ajpheart.00722.2002. [DOI] [PubMed] [Google Scholar]
- 29.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, et al. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–45. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, et al. Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J. 2010;74:2579–84. doi: 10.1253/circj.cj-10-0680. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Zhou Q, Smith CD, Chen H, Tan Z, Chen B, et al. Non-beta-blocking R-carvedilol enantiomer suppresses Ca2+ waves and stress-induced ventricular tachyarrhythmia without lowering heart rate or blood pressure. Biochem J. 2015;470:233–42. doi: 10.1042/BJ20150548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.