Abstract
Objectives
To assess the impact of reducing the dose of antipsychotics on cognition, dopaminergic D2 receptor availability in the whole striatum, and their relationship in patients with schizophrenia, age 50 years or older
Design
Open-label prospective PET [11C]-raclopride study
Setting
A tertiary care centre outpatient setting
Participants
Thirty-seven clinically stable participants with schizophrenia or schizoaffective disorder, age 50 or above, and having been treated with olanzapine or risperidone monotherapy at the same dose for at least six months
Intervention
Gradual reduction in their olanzapine or risperidone daily dose of up to 40%
Measurements
Clinical and cognitive assessments, and [11C]-raclopride PET to determine D2 receptor availability at baseline and after the dose reduction
Main outcome measures were overall cognition and D2 receptor availability in whole striatum.
Results
Reducing the antipsychotic dose resulted in an increase in D2 receptor availability in the whole striatum and an association between D2 receptor availability and overall cognition despite lack of change in the latter. There was also an association between change in D2 receptor availability and change in overall cognition.
Conclusions
Our findings suggest that optimizing D2 receptor availability by reducing antipsychotic dose allows this system to contribute more significantly to cognitive function in patients with schizophrenia. This uncovered association could be harnessed by cognitive enhancing interventions.
Keywords: Antipsychotic, D2 receptor, Cognition, Late-Life Schizophrenia, PET
Objective
Cognitive deficits are core features in schizophrenia and relatively stable across the adult lifespan [1, 2]. They are also among the strongest predictors of functional abilities in patients with schizophrenia throughout adulthood and late in life [3, 4]. Multiple factors contribute to the cognitive deficits observed in patients with schizophrenia, including genetics [5], environmental exposures [6], and medications [7].
Antipsychotics constitute the main pharmacologic treatment for schizophrenia. Studies of their cognitive effect have had mixed results ranging from detrimental [8], to neutral [9], or positive effects [10]. Antipsychotics doses and indirect measures of dopamine D2 receptor availability have been associated with cognitive dysfunction in patients with schizophrenia. One meta-analysis found a strong inverse association between chlorpromazine-equivalent dose and performance on an information processing speed task in patients with schizophrenia [7]. In the CATIE trial, low D2 receptor availability, as estimated based on antipsychotics plasma levels, was associated with impairments on vigilance and reasoning [11]. Similarly, among 61 clinically stable mid-life patients with schizophrenia who were randomized to reducing the dose of olanzapine or risperidone by 50% or maintaining the same dose, reducing the dose was followed by better stability of overall cognition and improvement on verbal learning, both assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) [12], at six months compared to maintaining the same dose [13]. In a pilot cross-sectional PET study, we have reported the most direct evidence for an association between cognition and D2 receptor availability: in 11 clinically stable patients with schizophrenia, age 50 years or older, those with low D2 receptor availability had impaired attention compared to those with high availability [14].
[11C]-Raclopride PET assesses most robustly D2 receptor availability in the basal ganglia with clear demarcation in the dorsal caudate, putamen, and ventral striatum, together referred to as the whole striatum thereafter. These structures are associated with various cognitive functions, such as feedback-based learning (dorsal caudate), implicit procedural learning (putamen) and motivation (ventral striatum) [15–18]. The role of the striatum in cognition is supported by recurrent loops connecting it with the prefrontal cortex[19–22]. These networks are heavily modulated by dopaminergic neurotransmission[17, 19] and thus, susceptible to D2 receptor antagonism by antipsychotics.
Thus, to better understand the role of D2 receptor antagonism and cognition in patients with schizophrenia, we evaluated cognition using the RBANS at baseline and following antipsychotic dose reduction in the first prospective PET antipsychotics dose reduction study in this population [23]. Our first aim was to assess whether reducing the dose of antipsychotics results in improved overall cognition. Our second aim was to assess the relationships between D2 receptor availability in the whole striatum and overall cognition before and after the dose reduction. Notwithstanding that there are specific relationships between certain cognitive functions and specific striatal structures (e.g. feedback-based learning and dorsal caudate) [16], we opted to have the primary analysis focused on the whole striatum and overall cognition to avoid the risk of false positive results if we assess for a specific relationship between one striatal structure and one cognitive domain.
Methods
Overall Study Design
The methods of the prospective study have been described elsewhere [23]. In brief, participants had schizophrenia or schizoaffective disorder; were age 50 years or older; and had been on a stable dose of oral olanzapine or risperidone for at least six months. They were assessed at baseline with clinical and cognitive scales and a [11C]-raclopride PET scan. Then, they underwent a gradual dose reduction of their antipsychotic of up to 40% of their baseline dose or to the lowest recommended dose: 7.5 mg/day for olanzapine and 1.5 mg/day for risperidone. Clinical and cognitive assessments, and [11C]-raclopride PET scan were repeated at least two weeks after reaching the final target dose to ensure a stable antipsychotic concentration in the brain. We have reported the clinical outcomes of this study and their relationship to D2 receptor availability in a recent publication [23]. Here we report on the cognitive outcomes and their relationships to D2 receptor availability in 37 participants (the 32 of initial participants who did not relapse between the two PET scans and five new participants who also did not experience relapse).
Participants
This study was conducted between January 10, 2007, and October 21, 2013 at the Centre for Addiction and Mental Health (CAMH), a large academic psychiatric centre that serves as a referral centre for a large urban and suburban population in the Greater Toronto Area, Ontario, Canada. Eligibility criteria were: (1) age 50 years or older; (2) meeting the Structured Clinical Interview for the DSM-IV-TR [24] criteria for schizophrenia or schizoaffective disorder; (3) clinical stability as operationalized by: (a) no hospitalizations for at least six months; (b) stable dose of oral olanzapine (10 mg/day or higher) or risperidone (2 mg/day or higher), as monotherapy for at least three months; and (c) a score of 3 or less on items of delusion, unusual thought content and hallucinatory behavior on the Positive And Negative Syndromes Scale (PANSS) [25] ; (4) stable dose of other psychotropic medications for mental health reasons over the last six months; (5) not meeting DSM-IV-TR criteria for substance abuse or dependence within the past six months; (6) negative urine screen for substance of abuse; (7) absence of unstable medical conditions; and (8) capacity to provide consent as determined by the MacArthur Competence Assessment Tool for Clinical Research [26];. The study was approved by CAMH Research Ethics Board and Heath Canada, and all participants provided written informed consent.
Clinical Assessments
Clinical assessments were performed at baseline and at the time of the second PET scan. They included the PANSS [25] and the Brief Psychiatric Rating Scale (BPRS) [27]. In this analysis, we used the PANSS Total Score to assess the severity of clinical symptoms and to control for these symptoms when assessing the relationship between D2 receptor availability and cognition.
Cognitive Assessments
The RBANS was administered both at baseline and at the time of the second PET scan. The RBANS has two parallel forms and is suitable for measuring change in participants’ cognitive function. The RBANS assesses Attention, Immediate Memory, Delayed Memory, Language, and Visuospatial/Constructional domains. The RBANS Total Scale was used to assess overall cognition.
Antipsychotic Dose Reduction
After the baseline assessments, olanzapine or risperidone dose was gradually reduced to a target of 60% of baseline dose with a lower limit of 7.5 mg/day for olanzapine or 1.5 mg/day for risperidone [28]. Dose was reduced weekly by 2.5 mg for olanzapine and 0.5 mg for risperidone. All other psychotropic medications were maintained at the same dose throughout the study. If a participant demonstrated an increase of 20% or more in total BPRS score from baseline, the antipsychotic dose was increased until clinical stabilization was achieved. In this report, the analysis includes only participants who tolerated the antipsychotic dose reduction, completed both PET scans, and did not require any increase in the antipsychotic dose before the second PET scan.
PET Image Acquisition and Analysis
The first [11C]-raclopride PET scan was completed at baseline and the second one was completed at least two weeks after reaching the target antipsychotic dose. The PET scans were performed 14–16 hours after taking the antipsychotic. Details on the acquisition and analysis of the PET scans were recently published [23]. D2 receptor availability was estimated using the measure of binding potential relative to the non-displaceable compartment (BPND) which was estimated using the cerebellum as the reference region. In this study the BPND of the whole striatum was used for analysis [29, 30].
Statistical Analyses
Baseline and repeat clinical, cognitive, pharmacological and PET scan variables were characterized with descriptive statistics. To test whether cognition changed in response to antipsychotic dose reduction, we performed a paired-samples t-test to compared performance on RBANS Total Scale before and after dose reduction. We then tested whether change in chlorpromazine equivalence correlated with change in BPND or change in RBANS Total Scale. To assess the relationship between D2 receptor availability and overall cognition before and after dose reduction, we performed two multiple linear regressions to assess whether BPND in the whole striatum was associated with RBANS Total Scale after controlling for age and PANSS Total Score. A series of exploratory analyses were then conducted including a series of paired-samples t-tests to assess whether any of the other clinical, cognitive, pharmacological and PET scan variables changed significantly after the dose reduction. We also conducted a series of exploratory multiple linear regressions to assess the relationships between BPND and all RBANS domains at baseline and follow-up, separately, after controlling for age and PANSS Total Score. Finally, we conducted a series of multiple linear regressions to assess associations between change in D2 receptor availability and changes in RBANS Total Scale and domains while controlling for age, change in PANSS Total Score, and baseline BPND. We applied Bonferroni correction to all p values. In all analyses, we assessed whether the following assumptions have been met: independence of residuals with Durbin-Watson statistic; no multicollinearity with VIF and tolerance statistics; linearity and homoscedasticity with the plot of standardized residuals against the standardized predicted values; and normality with the histogram and the normal probability plot. Statistical analyses were performed using IBM SPSS Statistics version 20 (IBM Corporation, Armonk, NY).
Results
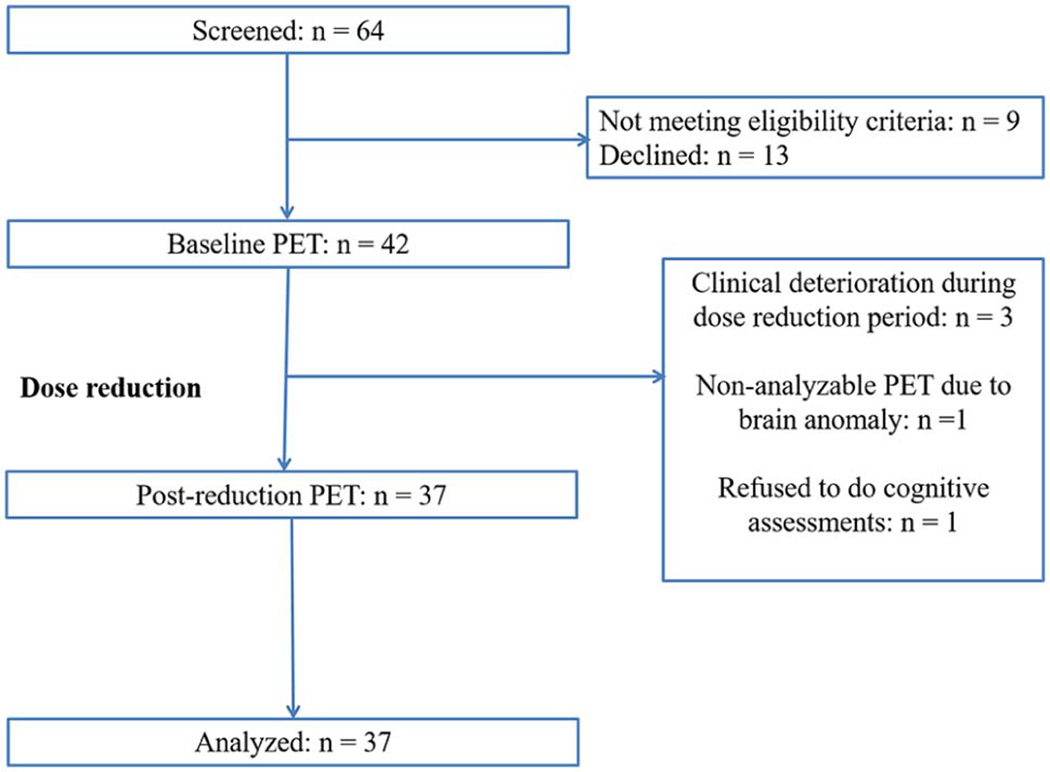
Table 1 presents the demographic and baseline and repeat clinical, cognitive, pharmacological, and PET scan characteristics of the 37 participants (22 on olanzapine and 15 on risperidone). It demonstrates that BPND increased following the dose reduction. Figure 1 presents the flow of participants in the study.
Table 1.
Clinical, Cognitive, and PET Characteristics Before and After Dose Reduction
| Characteristic | N (%) | Paired Samples t test (df) | P |
|---|---|---|---|
| Gender Female, N (%) | 11 (24.3%) | NA | NA |
| Diagnosis, N (%) | |||
| Schizophrenia | 31 (83.8%) | ||
| Schizoaffective disorder | 6 (16.2%) | ||
| Antipsychotic, N (%): | NA | NA | |
| Olanzapine | 22 (59.5%) | ||
| Risperidone | 15 (40.5%) | ||
| Mean (SD) | |||
| Age, years (range: 50–79, N = 37) | 60.2 (7.0) | NA | NA |
| Age, years (range: 50–59, N = 17) | 54.0 (3.2) | ||
| Age, years (range: 60–64), N = 7) | 61.6 (1.4) | ||
| Age, years (range: 60–64), N = 13) | 67.7 (4.1) | ||
| Dose, mg/day: | |||
| Olanzapine – Before | 20.9 (6.9) | 13.3 (21) | 0.001 |
| Olanzapine – After | 13.5 (4.4) | ||
| Risperidone – Before | 4.5 (2.6) | 6.0 (14) | 0.001 |
| Risperidone – After | 2.9 (1.6) | ||
| CPZE, mg/day: | |||
| Before | 355.4 (164.0) | 231.1 (104.2) | 0.001 |
| After | 12.27 (36) | ||
| PANSS Total Score: | |||
| Before | 59.7 (14.1) | 2.46 (36) | 0.019* |
| After | 58.8 (14.2) | ||
| Whole Striatum BPND: | |||
| Before | 1.06 (0.35) | −4.85 (36) | 0.001 |
| After | 1.31 (0.40) | ||
| Immediate Memory: | |||
| Before | 83.6 (18.6) | −1.44 (36) | 0.16 |
| After | 86.1 (17.8) | ||
| Visuospatial/Constructional: | |||
| Before | 73.1 (11.5) | −1.66 (36) | 0.11 |
| After | 75.8 (12.0) | ||
| Language: | |||
| Before | 87.2 (8.8) | 0.36 (36) | 0.72 |
| After | 86.5 (12.6) | ||
| Attention: | |||
| Before | 83.5 (15.1) | −0.80 (35) | 0.43 |
| After | 84.8 (17.0) | ||
| Delayed Memory: | |||
| Before | 77.4 (19.5) | −0.70 (36) | 0.49 |
| After | 79.1 (18.6) | ||
| RBANS Total Scale: | |||
| Before | 76.0 (12.3) | −1.54 (35) | 0.13 |
| After | 77.9 (14.8) | ||
BPND = non-displaceable Binding Potential; CPZE = Chlorpromazine Equivalence; PANSS = Positive And Negative Syndrome Scale; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
not significant after Bonferroni correction; SD = Standard Deviation
Figure 1.
CONSORT Flow Diagram.
Testing whether reducing the antipsychotic dose results in better performance on overall cognition, paired-samples t-test revealed no change on RBANS Total Scale. We also found no significant correlations between change in chlorpromazine equivalence and change in RBANS Total Scale (Pearson’s r = 0.04, N = 36, p = 0.81) or BPND (Pearson’s r = −0.10, N = 37, p = 0.55).
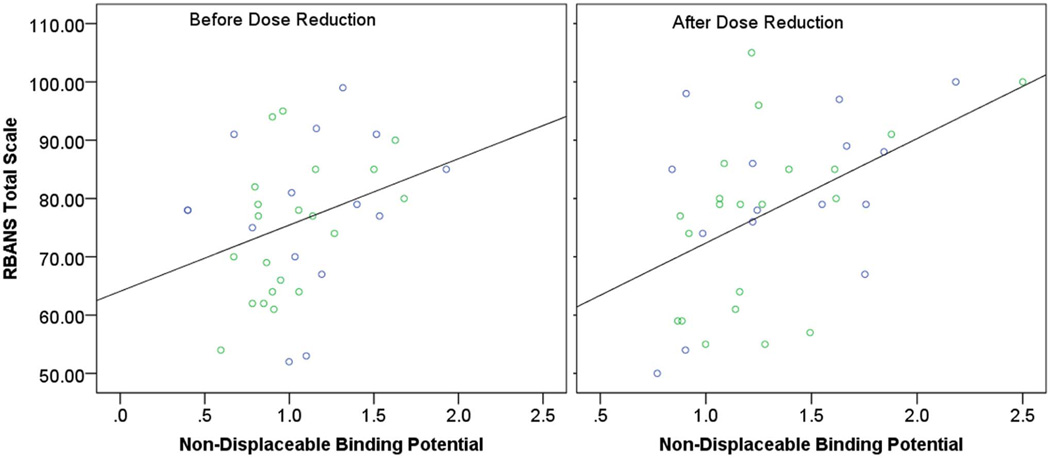
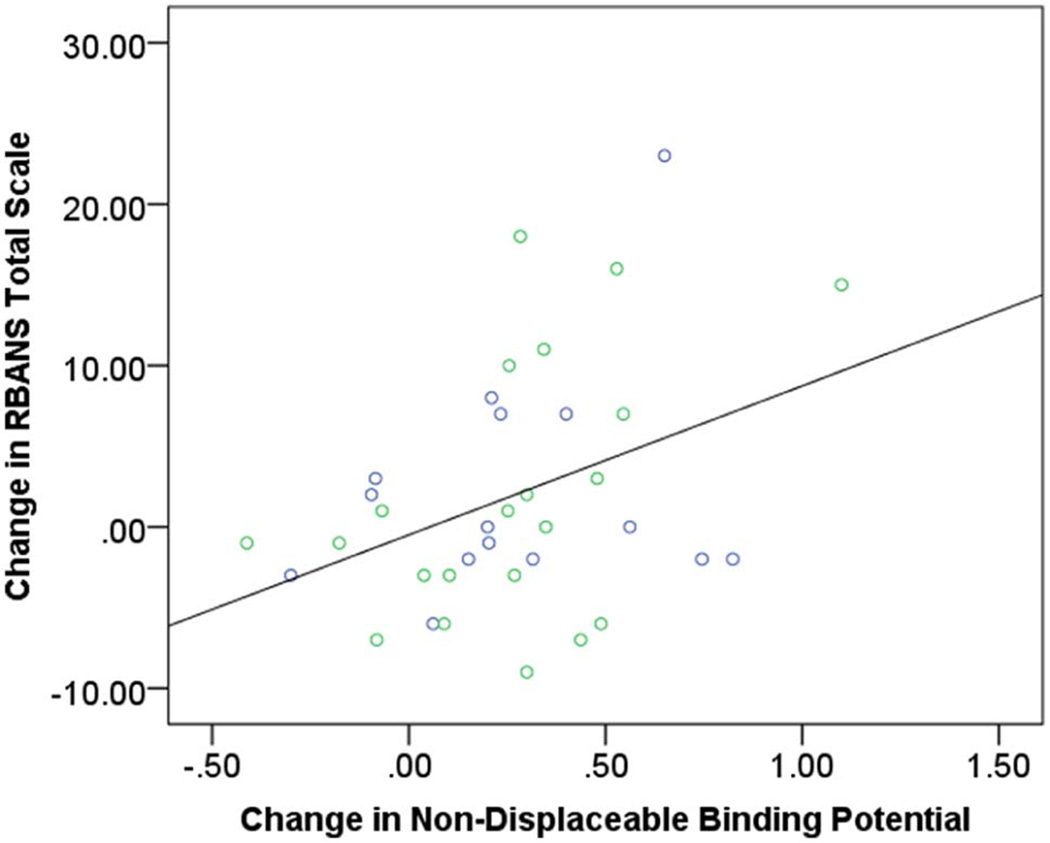
Before the dose reduction, multiple regression assessing the relationship between baseline BPND and performance on RBANS Total Scale after controlling for age and PANSS Total Score revealed no significant association (Table 2, Figure 2). There was a weak association between PANSS Total Score and performance on RBANS Total Scale but this association became non-significant after Bonferroni correction. In contrast, after the dose reduction, only BPND was associated with performance on RBANS Total Scale. This associated remained significant even after Bonferroni correction (Table 2, Figure 2). Further, multiple regression assessing the relationship between change in BPND and changes in RBANS Total Scale after controlling for age, change in PANSS Total Score, and baseline BPND revealed a significant association between change in BPND and change in RBANS Total Scale (Table 3, Figure 3).
Table 2.
Determinants of Cognition Before and After Antipsychotic Dose Reduction
| Before Dose Reduction | After Dose Reduction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model | Variable | B |
SE B |
β | P | Model | B |
SE B |
β | P |
| RBANS Total Scale (Primary Analyses) | ||||||||||
|
R2 = 0.21, F(3,32) = 2.85 p = 0.053 |
Age | 0.22 | 0.31 | 0.12 | 0.48 |
R2 = 0.33, F(3,32)= 5.14 p = 0.005 |
−0.13 | 0.35 | −0.06 | 0.73 |
| PANSS | −0.32 | 0.15 | −0.36 | 0.047a | −0.28 | 0.17 | −0.27 | 0.11 | ||
| BPND | 10.03 | 5.56 | 0.29 | 0.081 | 16.70 | 5.42 | 0.46 | 0.004b | ||
| RBANS Immediate Memory | ||||||||||
|
R2 = 0.29, F(3,33) = 4.42 p = 0.010 |
Age | 0.25 | 0.44 | 0.10 | 0.57 |
R2 = 0.41, F(3,33) = 7.63 p = 0.001 |
0.02 | 0.39 | 0.009 | 0.95 |
| PANSS | −0.63 | 0.22 | −0.47 | 0.007a | −0.48 | 0.19 | −0.38 | 0.018a | ||
| BPND | 14.59 | 7.89 | 0.27 | 0.073 | 20.58 | 6.07 | 0.46 | 0.002b | ||
| RBANS Visuospatial/Constructional | ||||||||||
|
R2 = 0.01, F(3,33) = 0.15 p = 0.93 |
Age | 0.02 | 0.32 | 0.01 | 0.96 |
R2 = 0.07, F(3,33) = 0.88 p = 0.46 |
−0.11 | 0.33 | −0.07 | 0.73 |
| PANSS | 0.06 | 0.16 | 0.07 | 0.73 | −0.07 | 0.16 | −0.08 | 0.68 | ||
| BPND | 3.16 | 5.76 | 0.10 | 0.59 | 6.98 | 5.11 | 0.23 | 0.18 | ||
| RBANS Language | ||||||||||
|
R2 = 0.27, F(3,33) = 4.06 p = 0.015 |
Age | −0.40 | 0.21 | −0.32 | 0.07 |
R2 = 0.18, F(3,33) = 2.41 p = 0.09 |
−0.44 | 0.32 | −0.24 | 0.18 |
| PANSS | −0.08 | 0.11 | −0.13 | 0.46 | −0.09 | 0.16 | −0.11 | 0.57 | ||
| BPND | 8.99 | 3.80 | 0.35 | 0.024a | 9.08 | 5.07 | 0.29 | 0.08 | ||
| RBANS Attention | ||||||||||
|
R2 = 0.04, F(3,32) = 0.48 p = 0.70 |
Age | 0.11 | 0.42 | 0.05 | 0.80 |
R2 = 0.21, F(3,32) = 2.81 p = 0.051 |
−0.18 | 0.43 | −0.07 | 0.69 |
| PANSS | −0.23 | 0.21 | −0.21 | 0.27 | −0.21 | 0.21 | −0.17 | 0.34 | ||
| BPND | 1.83 | 7.55 | 0.04 | 0.81 | 16.41 | 6.69 | 0.39 | 0.02a | ||
| RBANS Delayed Memory | ||||||||||
|
R2 = 0.20, F(3,33) = 2.70 p = 0.06 |
Age | 0.63 | 0.49 | 0.23 | 0.21 |
R2 = 0.28, F(3,33) = 4.17 p = 0.013 |
−0.16 | 0.45 | −0.06 | 0.72 |
| PANSS | −0.58 | 0.24 | −0.42 | 0.023a | −0.36 | 0.22 | −0.28 | 0.11 | ||
| BPND | 11.15 | 8.80 | 0.20 | 0.21 | 18.05 | 7.04 | 0.39 | 0.015a | ||
B = parameter estimate; SE B = standard error of B; β = standardized B; BPND = non-displaceable Binding Potential; F = F-test; P = p-value based on a t-test and the degrees of freedom as indicated by the error degrees of freedom of the F-test; PANSS = Positive And Negative Syndrome Scale total score; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status;
significant without Bonferroni correction but not significant if Bonferroni correction is applied;
significant with or without Bonferroni correction.
Figure 2. RBANS Total Scale and Whole Striatum non-displaceable Binding Potential.
Left panel: Before Antipsychotic Dose Reduction. R2 = 0.10, p = 0.054. Right panel: After Antipsychotic Dose Reduction. R2 = 0.24, p = 0.002. Green circles represent participants on olanzapine. Blue circles represent participants on risperidone.
Table 3.
Determinants of Change in Cognition with Antipsychotic Dose Reduction
| Model | Variable | B | SE B | P | P |
|---|---|---|---|---|---|
| Δ RBANS Total Scale | |||||
|
R2 = 0.27, F(4,31) = 2.84 p = 0.04 |
Age | −0.30 | 0.17 | −0.27 | 0.09 |
| Δ PANSS Total Score | −0.32 | 0.60 | −0.09 | 0.57 | |
| Δ BPND | −10.46 | 3.85 | −0.43 | 0.011 | |
| Baseline BPND | 4.24 | 3.47 | 0.20 | 0.23 | |
| Δ Immediate Memory | |||||
|
R2 = 0.10, F(4,32) = 0.89 p = 0.48 |
Age | −0.10 | 0.26 | −0.07 | 0.69 |
| Δ PANSS Total Score | −0.24 | 0.85 | −0.05 | 0.78 | |
| Δ BPND | −10.15 | 5.83 | −0.30 | 0.09 | |
| Baseline BPND | 3.29 | 5.37 | 0.11 | 0.55 | |
| Δ Visuospatial/Constructional | |||||
|
R2 = 0.06, F(4,32) = 0.51 p = 0.73 |
Age | −0.23 | 0.24 | −0.16 | 0.35 |
| Δ PANSS Total Score | 0.15 | 0.82 | 0.03 | 0.86 | |
| Δ BPND | −4.92 | 5.58 | −0.16 | 0.38 | |
| Baseline BPND | −1.40 | 5.14 | −0.05 | 0.79 | |
| Δ Language | |||||
|
R2 = 0.06, F(4,32) = 0.47 p = 0.76 |
Age | −0.05 | 0.28 | −0.03 | 0.86 |
| Δ PANSS Total Score | 0.59 | 0.95 | 0.11 | 0.54 | |
| Δ BPND | −3.66 | 6.50 | −0.10 | 0.58 | |
| Baseline BPND | −4.94 | 5.99 | −0.15 | 0.42 | |
| Δ Attention | |||||
|
R2 = 0.20, F(4,31) = 1.90 p = 0.14 |
Age | −0.22 | 0.22 | −0.16 | 0.33 |
| Δ PANSS Total Score | −0.91 | 0.72 | −0.21 | 0.22 | |
| Δ BPND | −6.31 | 5.03 | −0.21 | 0.22 | |
| Baseline BPND | 7.90 | 4.54 | 0.29 | 0.09 | |
| Δ Delayed Memory | |||||
|
R2 = 0.13, F(4,32) = 1.21 p = 0.33 |
Age | −0.61 | 0.35 | −0.29 | 0.09 |
| Δ PANSS Total Score | −0.06 | 1.17 | 0.009 | 0.96 | |
| Δ BPND | −7.29 | 8.0 | −0.16 | 0.37 | |
| Baseline BPND | 9.16 | 7.36 | 0.22 | 0.22 | |
B = parameter estimate; SE B = standard error of B; β = standardized B; F = F-test; P = p-value based on a t-test and the degrees of freedom as indicated by the error degrees of freedom of the F-test;
Δ= Change; BPND = non-displaceable Binding Potential; Baseline BPND = BPND before dose reduction; PANSS = Positive And Negative Syndrome Scale; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status
Figure 3. Change in RBANS Total Scale and Change in Whole Striatum non-displaceable Binding Potential.
R2 = 0.15, p = 0.022. Green circles represent participants on olanzapine. Blue circles represent participants on risperidone.
Exploratory paired-samples t-tests demonstrated that participants experienced significant decreases in antipsychotics doses and increase whole striatum BPND, but no significant changes in PANSS Total Score or performances on individual RBANS domains after Bonferroni correction (Table 2).
The exploratory multiple regressions with RBANS domains revealed only one significant association after Bonferroni correction and this was between BPND and Immediate Memory after the dose reduction (Table 2).
The multiple regressions assessing the relationships between changes in RBANS domains and change in BPND, after controlling for age, change in PANSS Total Score, and baseline BPND revealed no significant associations (Table 3).
In all analyses, assumptions of independence of residuals, no multicollinearity, linearity and homoscedasticity, and normality were met.
Conclusions
In this first prospective PET imaging antipsychotic dose reduction among patients with schizophrenia age 50 years and older, we found that reducing the dose of the antipsychotic results in increasing D2 receptor availability in the striatum. It also results in the emergence of an association between D2 receptor availability and overall cognition, despite the lack of change in overall cognition.
The dopaminergic system has been implicated in several cognitive functions. In the frontal cortex, dopaminergic neurotransmission is associated with executive functions [31]. In the hippocampus, it is associated with learning and memory [32]. In the basal ganglia, and in particular the dorsal caudate, dopaminergic neurotransmission supports feedback-based learning [16]. Our study provides direct evidence that increasing D2 receptor availability in the striatum of older patients with schizophrenia results in a significant contribution of D2 receptor availability to overall cognition. And this contribution seems to be mostly driven by the domain of verbal learning. These findings are consistent with indirect evidence from the data of the CATIE based study that assessed the relationship between cognition and estimated D2 receptor availability. In that study, estimated D2 receptor availability was comparable to what we found at baseline in our study, and there was no association between estimated D2 receptor availability and any cognitive function [11]. These findings suggest that the contribution of the dopaminergic system to cognition is blocked by high doses of olanzapine and risperidone; after the dose reduction, the D2 receptors become more available to play a cognitive role.
Despite finding D2 receptor availability increased, we did not find that our participants improved as a group in cognition. We also did not find any correlation between change in dose and change in D2 receptor availability or cognition. Thus, our findings do not provide direction to clinicians to reduce the dose and expect cognitive benefits. Several reasons could have accounted for this negative finding. First, some participants experienced a decline in performance – and for some of those participants their D2 availability decreased as well. Second, the antipsychotic dose may need to be decreased further to observe cognitive benefit. In fact, in the previous dose reduction study that demonstrated cognitive improvement following dose reduction[13], the baseline doses of olanzapine and risperidone were comparable to the doses after reduction in our study. Third, increasing D2 receptor availability may not be sufficient to lead to robust cognitive enhancement in the absence of a cognitive intervention that engages these receptors and related dopaminergic neurotransmission. Our results support testing such interventions following a dose reduction given that D2 receptor availability becomes associated with cognition after D2 receptor availability increased. Further, one could speculate clinically that reducing the dose and monitoring cognition over a period of several months as patients engage in their daily cognitive activities could result in cognitive benefits.
In comparison with the other two studies [11] [13] that indirectly estimated D2 receptor availability using antipsychotics plasma levels and cognition, our study provides further insight into this relationship using a direct in-vivo quantification of D2 receptor availability. In the CATIE study [11], mean age of participants was 41 year-old (range: 18–62) vs. 60 year-old (range: 50–79) in our study; and mean dose of risperidone was 4 mg and that of olanzapine was 20 mg vs. 4.5 mg and 21 mg, respectively, in our study. In this CATIE study, estimated D2 occupancy was not associated with performance on any cogntive measure. In the mid-life dose reduction study [13], mean age of participants was 40 year-old (range: 20–71) and mean doses of risperidone and olanzapine were 4 mg and 14 mg, respectively, before the dose reduction. In constrat, the doses were 2 mg and 7 mg for risperidone and olanzapine after the dose reduction vs. 3 mg and 13.5 mg in our study. Thus, while the samples in these two studies partially overlap with our sample on age, it is clear that our study focuses uniquely on a geriatric sample that was still receiving an antipsychotic dose that is comparable to that observed in younger samples. Further, these two studies taken together with ours suggest that further studies of dose reduction and cognition needs to aim to about 2 mg for risperidone and 7 mg for olanzapine.
Our study has several limitations. First, our participants were 50 years old or older, hence our results may not be generalizable to younger patients. However, our findings are consistent with what has been described in two younger samples [11, 13]. Second, our PET radiotracer is reliable assessing D2 receptor availability in the basal ganglia compared to the cortex or to other dopaminergic receptors that also play a cognitive role (e.g. D1 receptors). Future studies could assess dopaminergic neurotransmission and cognition using different tracers that are reliable to assess cortical dopaminergic receptors. Finally, while our sample is relatively large compared to other PET studies, it is still relatively small for a cognitive study and our findings would need to be replicated in studies with larger samples.
In conclusion, our study shows for the first time the direct impact of increasing D2 receptor availability on the relationship between striatal D2 dopaminergic function and cognition in patients with schizophrenia. Our study highlights the need to address the multiple intrinsic and extrinsic factors when studying the cognitive profile of these patients.
Acknowledgments
Dr. Rajji receives research support from Brain Canada, Brain and Behavior Research Foundation, CAMH Foundation, a Canada Research Chair in Neurostimulation for Cognitive Disorders, Canadian Foundation for Innovation (CFI), Canadian Institutes of Health Research (CIHR), Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, the US National Institute of Health (NIH), and the W. Garfield Weston Foundation. Dr. Rajji reports no competing interests. Dr. Mulsant receives research support from Brain Canada, CAMH Foundation, CFI, CIHR, and NIH. During the past five years, he has received medications for NIH-funded clinical trials from Bristol-Myers Squibb, Eli Lilly, and Pfizer. He directly own stocks of General Electric (less than $5,000). Dr. Nakajima has received fellowship grants from CIHR, Japan Society for the Promotion of Science, and Nakatomi Foundation, and manuscript fees from Dainippon Sumitomo Pharma and Kyowa Hakko Kirin. Dr. Suzuki has received manuscript and speaker’s fees from Astellas, Dainippon Sumitomo, Eli Lilly, Elsevier Japan, Janssen, Meiji Seika, Otsuka, and Weily Japan. Dr. Uchida has received grants from Astellas Pharmaceutical, Eisai, Otsuka Pharmaceutical, GlaxoSmithKline, Shionogi, Dainippon-Sumitomo Pharma, Eli Lilly, Mochida Pharmaceutical, Meiji-Seika Pharma, and Yoshitomi Yakuhin and speaker’s honoraria from Otsuka Pharmaceutical, Eli Lilly, Shionogi, GlaxoSmithKline, Yoshitomi Yakuhin, Dainippon-Sumitomo Pharma, Meiji-Seika Pharma, Abbvie, MSD, and Janssen Pharmaceutical within the past two years. Dr. Pollock has received support from Brain Canada, CAMH Foundation, CIHR, and NIH. He was also a faculty member of the Lundbeck International Neuroscience Foundation (LINF) (final meeting was April 2010). Dr. Graff-Guerrero has received support from Brain Canada, Brain & Behavior Research Foundation, CFI, CIHR, NIH, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, Ontario Mental Health Foundation (OMHF), Consejo Nacional de Ciencia y Tecnologia (CONACyT), Instituto de Ciencia y Tecnología del DF (ICyTDF).
Sources of Support:
This work was partially supported by Canadian Institutes of Health Research (MOP-9794) to D. C. M.) and the National Institutes of Health (R01MH084886-01A2 to D. C. M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Rajji TK, et al. Cognitive performance of individuals with schizophrenia across seven decades: A study using the MATRICS Consensus Cognitive Battery. American Journal of Geriatric Psychiatry. 2013;21(2):108–118. doi: 10.1016/j.jagp.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Green MF, et al. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the “right stuff”? Schizophrenia Bulletin. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 4.Kalache SM, et al. The Impact of Aging Cognition, and Symptoms on Functional Competence in Individuals With Schizophrenia Across the Lifespan. Schizophrenia Bulletin. 2015;41(2):374–381. doi: 10.1093/schbul/sbu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilder RM, et al. The genetics of cognitive impairment in schizophrenia: a phenomic perspective. Trends in Cognitive Sciences. 2011;15(9):428–435. doi: 10.1016/j.tics.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Os J, Kenis G, Rutten BPF. The environment and schizophrenia. Nature. 2010;468(7321):203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 7.Knowles EEM, David AS, Reichenberg A. Processing Speed Deficits in Schizophrenia: Reexamining the Evidence. American Journal of Psychiatry. 2010;167(7):828–835. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- 8.Vinogradov S, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. American Journal of Psychiatry. 2009;166(9):1055–1062. doi: 10.1176/appi.ajp.2009.09010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keefe RSE, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE trial. Archives of General Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 10.Harvey PD, et al. Effect of lurasidone on neurocognitive performance in patients with schizophrenia: A short-term placebo- and active-controlled study followed by a 6-month double-blind extension. European Neuropsychopharmacology. 2013;23(11):1373–1382. doi: 10.1016/j.euroneuro.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Sakurai H, et al. Dopamine D-2 Receptor Occupancy and Cognition in Schizophrenia: Analysis of the CATIE Data. Schizophrenia Bulletin. 2013;39(3):564–574. doi: 10.1093/schbul/sbr189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randolph C, et al. The repeatable battery for the assessment of neuropsychological status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 13.Takeuchi H, et al. Effects of Risperidone Olanzapine Dose Reduction on Cognitive Function in Stable Patients With Schizophrenia: An Open-Label Randomized, Controlled. Pilot Study. Schizophrenia Bulletin. 2013;39(5):993–998. doi: 10.1093/schbul/sbt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Uchida H, et al. D-2 Receptor Blockade by Attention Deficits in Risperidone Correlates With Late-Life Schizophrenia. Journal of Clinical Psychopharmacology. 2009;29(6):571–575. doi: 10.1097/JCP.0b013e3181bf4ea3. [DOI] [PubMed] [Google Scholar]
- 15.Arsalidou M, Duerden EG, Taylor MJ. The Centre of the Brain: Topographical Model of Motor Cognitive, Affective and Somatosensory Functions of the Basal Ganglia. Human Brain Mapping. 2013;34(11):3031–3054. doi: 10.1002/hbm.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Progress in Neurobiology. 2008;86(3):141–155. doi: 10.1016/j.pneurobio.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Foerde K, Shohamy D. The role of the basal ganglia in learning and memory: Insight from Parkinson’s disease. Neurobiology of Learning and Memory. 2011;96(4):624–636. doi: 10.1016/j.nlm.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brovelli A, et al. Differential roles of caudate nucleus and putamen during instrumental learning. Neuroimage. 2011;57(4):1580–1590. doi: 10.1016/j.neuroimage.2011.05.059. [DOI] [PubMed] [Google Scholar]
- 19.Helmich RC, et al. Spatial Remapping of Cortico-striatal Connectivity in Parkinson’s Disease. Cerebral Cortex. 2010;20(5):1175–1186. doi: 10.1093/cercor/bhp178. [DOI] [PubMed] [Google Scholar]
- 20.Robinson JL, et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60(1):117–129. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter CS, et al. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158(9):1423–1428. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- 22.Seidman LJ, et al. Neuropsychology of the prodrome to psychosis in the NAPLS consortium: relationship to family history and conversion to psychosis. Arch Gen Psychiatry. 2010;67(6):578–588. doi: 10.1001/archgenpsychiatry.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Graff-Guerrero A, et al. Evaluation of Antipsychotic Dose Reduction in Late-Life Schizophrenia: A Prospective Dopamine D2/3 Receptor Occupancy Study. JAMA Psychiatry. 2015 doi: 10.1001/jamapsychiatry.2015.0891. In Press. [DOI] [PubMed] [Google Scholar]
- 24.First MB. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 25.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 26.Grisso T, Appelbaum PS, HillFotouhi C. The MacCAT-T: A clinical tool to assess patients’ capacities to make treatment decisions. Psychiatric Services. 1997;48(11):1415–1419. doi: 10.1176/ps.48.11.1415. [DOI] [PubMed] [Google Scholar]
- 27.Overall JE, Gorham DR. The Brief Psychiatric Rating-Scale. Psychological Reports. 1962;10(3):799–812. [Google Scholar]
- 28.Alexopoulos GS, et al. Using antipsychotic agents in older patients. J Clin Psychiatry. 2004;65(Suppl 2):5–99. discussion 100–102; quiz 103-4. [PubMed] [Google Scholar]
- 29.Mawlawi O, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab. 2001;21(9):1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Martinez D, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23(3):285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- 31.Cools R, D’Esposito M. Inverted-U-Shaped Dopamine Actions on Human Working Memory and Cognitive Control. Biological Psychiatry. 2011;69(12):E113–E125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hansen N, Manahan-Vaughan D. Dopamine D1/D5 Receptors Mediate Informational Saliency that Promotes Persistent Hippocampal Long-Term Plasticity. Cerebral Cortex. 2014;24(4):845–858. doi: 10.1093/cercor/bhs362. [DOI] [PMC free article] [PubMed] [Google Scholar]