Abstract
Objective
Critical organ shortages have resulted in Ex Vivo Lung Perfusion (EVLP) gaining clinical acceptance for lung evaluation and rehabilitation to expand the use of Donation after Circulatory Death (DCD) organs for lung transplantation. We hypothesized that an innovative use of airway pressure release ventilation (APRV) during EVLP improves lung function after transplantation.
Methods
Two groups (n=4 animals/group) of porcine DCD donor lungs were procured after hypoxic cardiac arrest and a 2-hour period of warm ischemia, followed by a 4-hour period of EVLP rehabilitation with either standard conventional volume-based ventilation or pressure-based APRV. Left lungs were subsequently transplanted into recipient animals and reperfused for 4 hours. Blood gases for PaO2/FiO2 ratios, airway pressures for calculation of compliance, and percent wet weight gain during EVLP and reperfusion were measured.
Results
APRV during EVLP significantly improved left-lung oxygenation at 2-hours (561.5±83.9 vs 341.1±136.1 mmHg) and 4-hours (569.1±18.3 vs 463.5±78.4 mmHg). Similarly, compliance was significantly higher at 2-hours (26.0±5.2 vs 15.0±4.6 mL/cmH2O) and 4-hours (30.6±1.3 vs 17.7±5.9 mL/cmH2O) after transplantation. Finally, APRV significantly reduced lung edema development on EVLP based on percentage weight gain (36.9±14.6 vs 73.9±4.9%). There was no difference in additional edema accumulation 4 hours after reperfusion.
Conclusions
Pressure-directed APRV ventilation strategy during EVLP improves rehabilitation of severely injured DCD lungs. After transplant these lungs demonstrate superior lung-specific oxygenation and dynamic compliance compared to lungs ventilated with standard conventional ventilation. This strategy, if implemented into clinical EVLP protocols, could advance the field of DCD lung rehabilitation to expand the lung donor pool.
Introduction
Although the yearly number of lung transplants has increased >35-fold in the past 20 years and continues to increase, the number of organ donors has remained mostly static(1). This donor shortage is exacerbated by the fact that, in general, only 15% of lungs from multi-organ donors are deemed suitable for transplantation. Thus many patients die while waiting for a suitable donor with wait list mortality as high as 30–40%(2). Despite all current strategies implemented to increase the availability of donor lungs, the majority of potential lungs are still rejected for transplantation.
Recently, a pioneering strategy using a “lung box” for ex vivo lung perfusion (EVLP) has demonstrated significant potential to address both the quantity and quality of available organs(3). The EVLP system was originally described by Steen et al. and further modified by the Toronto group to the current ex vivo protocol using Steen solution(4, 5). This innovative method maintains the lungs in physiologically protective conditions outside the body during preservation and allows accurate evaluation of lung function as well as providing a new setting for therapeutic treatment and repair of damaged donor lungs prior to transplantation.
Stock et al. first described Airway Pressure Release Ventilation (APRV) in the 1980s(6). APRV is a pressure-directed mode of ventilation providing two levels of positive airway pressure, with the majority of time spent at the high level and a brief expiratory release at the lower pressure to facilitate ventilation(7). This mode has many reported advantages over conventional ventilation including alveolar recruitment, improved oxygenation, improved hemodynamics, and attenuation of barotrauma(8–10). Many of these advantages can be translated into the EVLP model to improve atelectatic lung recruitment, reduce pulmonary edema, and ameliorate barotrauma.
As EVLP gains popularity, it is crucial to examine the ventilation protocol used in this system. A recent paper by Terragni and colleagues demonstrate that the current EVLP ventilator settings may expose the lungs to ventilator-induced lung injury(11). The original volume-based approach was based on the ARDSNet criteria for in vivo lungs confined by the chest wall(3). The current study examines the use of APRV during EVLP to reduce barotrauma and prevent further lung injury. We hypothesized that this innovative use of APRV will improve atelectatic lung recruitment, reduce pulmonary edema, and attenuate barotrauma after lung transplantation.
Materials and Methods
Animals and Study Groups
The current study complies with the 1996 Guide for the Care and Use of Laboratory Animals as recommended by the US National Institutes of Health (NIH) and was approved by the University of Virginia Animal Care and Use Committee (ACUC). All animals received humane care during the duration of the study. Adolescent domestic swine of both sexes (24– 39 kg) underwent hypoxic cardiac arrest. After 2-hours of no-touch warm ischemia, cold preservation flush with Perfadex® (XVIVO Perfusion Inc., Englewood, CO) was performed and the lungs were procured. The experimental group (n=4) received APRV during EVLP, which was compared to a group of historical controls (n=4) receiving conventional ventilation during EVLP.
Lung Injury and Procurement
After the 2-hour warm ischemia period, procurement of donation after cardiac death lungs was completed as previously described(12). Animals were sedated and weighed followed by induction of anesthesia and intubation. Donor animals were ventilated with 100% oxygen during measurement of baseline values. Prior to cross-clamping the endotracheal tube to induce hypoxic cardiac arrest, donor animals received intravenous heparin (200 U/kg, Hospira Inc., Lake Forest, IL), and initial donor P/F ratio was obtained from a right carotid arterial blood gas (ABG) sample. After cross clamp it took a median of 18 minutes for the animals to die and there was typically some respiratory effort during this period. Death was confirmed with continuous electrocardiogram monitoring, the animal underwent 2-hours of no-touch warm ischemia. During the final 5-minutes of the warm ischemia period, ventilation was resumed and the donor underwent standard cold preservation flush with Perfadex and bilateral lung procurement.
A median sternotomy was performed and a cardioplegia cannula (Terumo Heart Inc., Ann Arbor, MI) was placed into the main pulmonary artery (PA) for delivery of Prostaglandin-E1 (500 µg, Pfizer Inc., New York, NY) followed by cold Perfadex flush. Initial flush was 1.5 liters of Perfadex supplemented with 15,000 IU of heparin after ligating the superior and inferior vena cava and venting the left atrial (LA) appendage. The trachea was cross-clamped mid-inspiration to maintain partial inflation of the lungs and the heart-lung bloc was explanted from the donor animal.
Ex vivo Lung Perfusion
After back-table preparation, a yellow cannula (XVIVO Perfusion Inc., Englewood, CO) was tied in the main PA, a green cannula (XVIVO Perfusion Inc., Englewood, CO) was sewn to the LA cuff, and a 7-0 endotracheal tube was tied into the trachea. Prior to initiation of 4 hours of EVLP, an additional 500 mL of cold Perfadex was flushed retrograde through the LA cannula. The lung block with cannulas was then weighed prior to initiation of EVLP for baseline weight.
EVLP was initiated on a perfusion circuit as previously described.(13) Circuit was primed in the standard fashion with Steen Solution, cefazolin (500 mg, APP Pharmaceuticals, Schaumburg, IL), methylprednisolone (500 mg, Pfizer Inc., New York, NY), and heparin (10,000 IU). The circuit was perfused according to the protocol described by the Toronto group with flow was initiated (0.2 mL/min) and LA pressures maintained between 0–5 mmHg(3). The perfusate was gradually warmed to 37° over the first 30 minutes as flow was titrated up to 40% of estimated cardiac output (100 mL/kg donor body weight). A standard tri-gas mixture (86% nitrogen, 8% carbon dioxide, 6% oxygen) through an Affinity membrane (Medtronic, Eden Prairie, MN) was used to deoxygenate the perfusate.
When perfusate temperature reached 35°C, ventilation was initiated with either conventional ventilation (tidal volume 8 mL/kg, respiratory rate 8 breaths/minute, positive end-expiratory pressure 5.0 cm H2O, FiO2 0.21) or APRV (Thigh 30.0 seconds, TLow 1.0 second, PHigh 10.0 cm H2O, PLow 0.0 cm H2O). PHigh was titrated to target tidal volume 6ml/kg with max PHigh of 10 cm H2O for the first hour, 15 cm H2O for the second hour, and never above 20 cm H2O the last two hours. We opted to use the longer TLow with a slightly higher PHigh to prevent Auto-PEEP which could be a major issue in open lung ventilation without a chest wall. Perfusate samples from the PA inflow and LA outflow were collected every hour following 15-minute challenge period with 1.0 FiO2 to measure the partial pressure of oxygen (PaO2). Airway pressures on conventional ventilation were measured hourly to calculate dynamic compliance.
After 4-hours of EVLP, the lungs were removed from the circuit and weighed for calculation of edema gain on EVLP. Subsequently the lung block was flushed anterograde with 500 mL of cold Perfadex. The left lung was then split and prepared on the back-table for subsequent transplantation, and the right lung was taken for fresh tissue samples and histology. Just prior to transplantion, the left lung was weighed again for baseline weight prior to reperfusion.
Left Lung Transplant and Reperfusion
Recipient animals were anesthetized and ventilated with conventional ventilation in the same manner as the donor animals above. After induction of anesthesia a central line was placed in the internal jugular vein with a Swan-Ganz catheter and an arterial line in the carotid. The animals were all maintained on conventional ventilation (tidal volume 8 mL/kg, respiratory rate 12–16 breaths/min, positive end-expiratory pressure 5 mmHg) with Isoflurane (3%) and 100% oxygen. A left lateral thoracotomy was then performed, and the animal received 5000 IU of heparin just prior to left pneumonectomy, and left lung transplant (running sutures used for end-to-end bronchial anastomosis, end-to-end PA anastomosis, and LA cuff to recipient LA appendage) as described previously(13).The median procedure time was 42 minutes with an IQR (38,44).
Post-transplant reperfusion of the left donor lung was maintained for 4 hours with conventional ventilation (tidal volume 8 mL/kg, respiratory rate 12–16 breaths/min, positive end-expiratory pressure 5 mmHg) and 100% oxygen. The left thoracotomy was left wide open with suspension from above to prevent chest wall restriction on the lung. Airway pressure measurements and carotid arterial blood gases were performed every hour during reperfusion. Superior and inferior pulmonary vein blood gas samples were obtained at 2 hours and 4 hours of reperfusion for left lung-specific PaO2/FiO2 ratio. Hemodynamic goals were pH 7.35–7.45, base excess > −5, and mean arterial pressure > 55 mmHg, which were maintained with use of normal saline, epinephrine, and sodium bicarbonate as necessary. The donor lung was explanted after 4 hours of reperfusion and weighted for measurement of edema gain during reperfusion. The animal was then euthanized.
Cytokine Measurements
After 4 hours of EVLP three fresh tissue samples were obtained (upper lobe, upper portion of the lower lobe, and lower portion of the lower lobe) from the right lung of the APRV group to establish a baseline for inflammatory markers. After 4 hours of post-transplant reperfusion three tissue samples were taken from the transplanted lung (left) of both APRV and Control groups. The fresh tissue was flash frozen in liquid nitrogen, and stored at −80°C. After homogenization with a FastPrep®-24 (MP Biomedicals, Santa Ana, CA), the total protein concentration in the supernatant of each homogenized lung tissue sample was determined with a bicinchoninic acid protein assay (Pierce, Rockford, IL). Multiplex enzyme-linked immunosorbent assay (EMD Millipore, Billerica, MA) was used to measure cytokine levels in tissue supernatant (normalized to equal protein concentrations).
Histology
After obtaining fresh tissue samples, the airways of the lower lobe were filled with 10% buffered formalin. Following overnight fixation in formalin, peripheral lung tissue samples (n=4/lung) were obtained, paraffin-embedded and sectioned. One slide from each sample was stained with hematoxylin-eosin (H&E) and a total of two slides (one from the upper most sample and one from the lower most sample) were used for immunohistochemistry evaluation of activated neutrophil infiltration.
A masked pathologist assessed the H&E stained slides for presence of lung injury. Each slide was scored on a standard scale based on polymorphonuclear cells per 40× high-powered field, alveolar edema, and interstitial inflammation as previously described.(14) The grading scale is listed in Table 1.
Table 1.
Summary of grading criteria for the Lung Injury Severity Score. Maximum score is 9 and minimum is 0.
| Score | PMN’s per HPF | Alveolar Edema | Interstitial Inflammation |
|---|---|---|---|
| 0 | <5 | <5% | None |
| 1 | 6–10 | 6–25% | Minimal |
| 2 | 11–20 | 26–50% | Moderate |
| 3 | >20 | >50% | Severe |
For neutrophil immunohistochemistry staining, mouse monoclonal anti-porcine neutrophil antibody (MBA Biomedicals, Augst, Switzerland) was the primary antibody and donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) was the secondary antibody. Standard protocol using an avidin-biotin complex followed by incubation with 3,3-diaminobenzidine tetrahydrochloride (Dako Inc., Carpentaria, CA) to produce a brown precipitate, and hematoxylin counterstain was used as previously described. (15) Microscopic photographs were taken at 40× magnification of each slide and the number of neutrophils per high-powered field (HPF) were counted by a blinded investigator.
Statistical Analysis
Student’s t-test and Fishers Exact Tests were used to determine statistical significance. Prism 7 (GraphPad Software Inc., La Jolla, CA) was used to perform statistical calculations and all data were reported as mean ± standard deviation, and p<0.05 was used for statistical significance.
Results
Lung Function and Edema
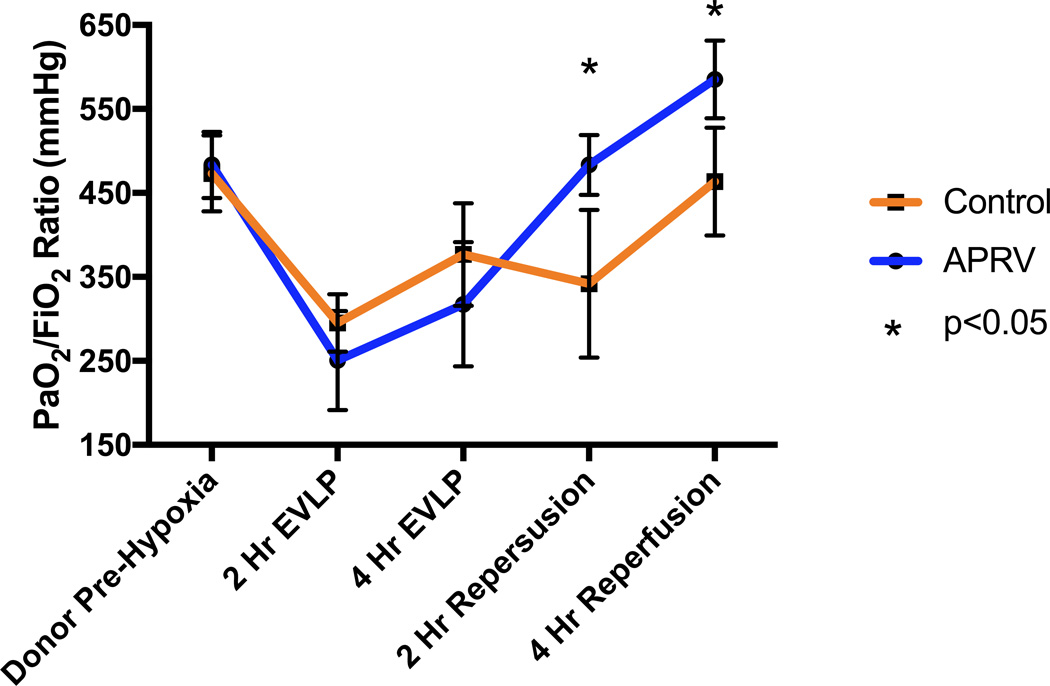
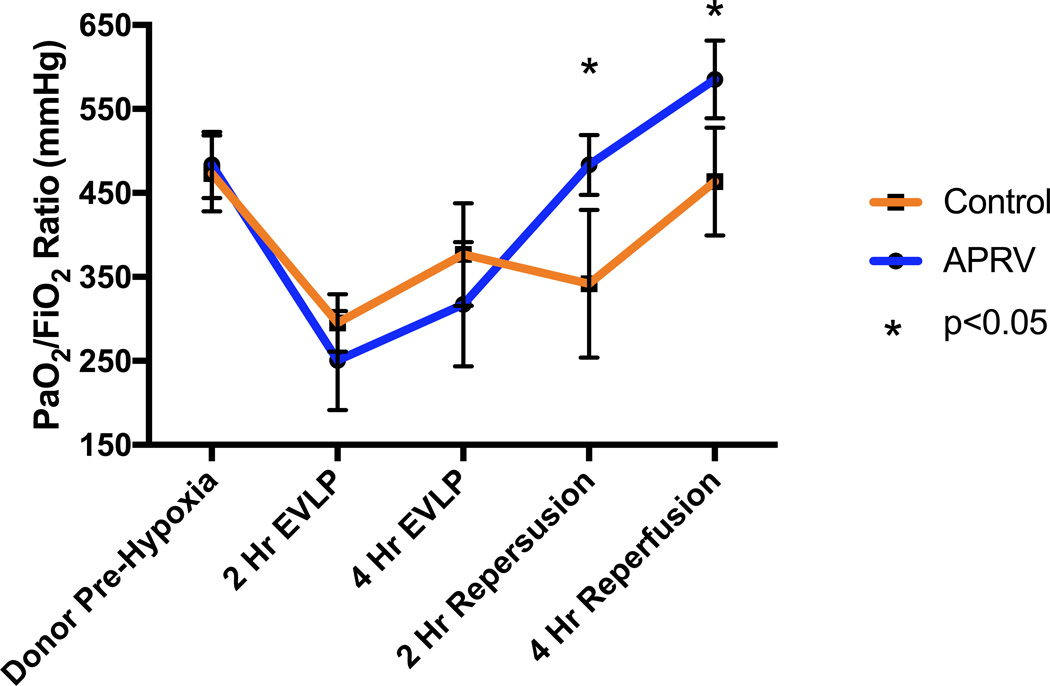
Lung specific oxygenation over the course of the experiment is illustrated in Figure 1 for each group. There was no difference in pre-hypoxia donor PaO2/FiO2 ratios (483.4±39.4 vs 473.2±45.2 mmHg, p>0.05). Additionally, during EVLP there was no difference in oxygenation at 2 or 4 hours between lungs on APRV versus those on conventional ventilation (250.5±59.0 vs 295.2±34.1 mmHg at 2-hr, 317.5±74.0 vs 376.7±61 mmHg at 4-hr, all p>0.05). However, after transplant and reperfusion a significant improvement in oxygenation at both 2 and 4 hours was observed in the group receiving APRV during EVLP (483.4±35.7 vs 341.9±87.9 mmHg at 2-hr, 585.1±46.2 vs 463.5±64.0 mmHg at 4-hr, all p<0.05).
Figure 1.
Changes in PaO2/FiO2 ratios of the left lung over the course of the experiment. PaO2/FiO2 ratios were significantly higher in the APRV group after transplantation at both the 2-hr and 4-hr reperfusion times.
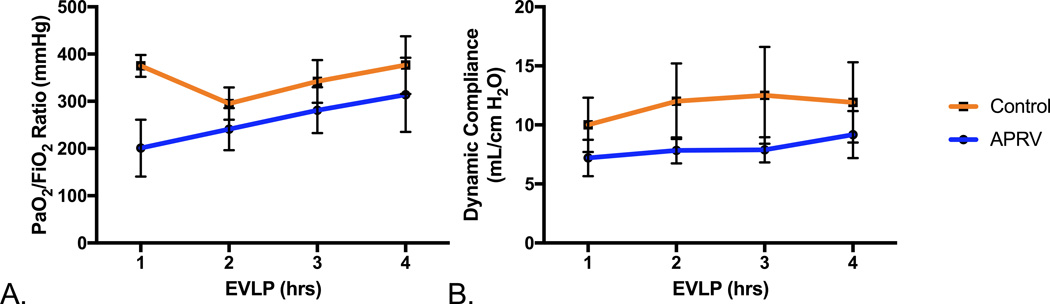
Despite starting at a higher PaO2/FiO2 ratio in the control group (375.0±23.3 vs 201.0±60.2 mmHg, p=0.02), after one hour of EVLP there was no difference in oxygenation during hours 2–4 of EVLP between the groups (all p>0.05) (Figure 2A). Oxygenation improved over the 4 hours of EVLP in both groups. Similarly there was no difference (all p>0.05) in dynamic compliance during the 4 hours of EVLP with both groups demonstrating a slight improvement (Figure 2B).
Figure 2.
Changes in PaO2/FiO2 ratios and dynamic compliance during EVLP A. No significant differences in PaO2/FiO2 ratios during EVLP were observed between the Control and APRV groups. B. Dynamic compliance throughout the 4-hours of EVLP was similar for both groups.
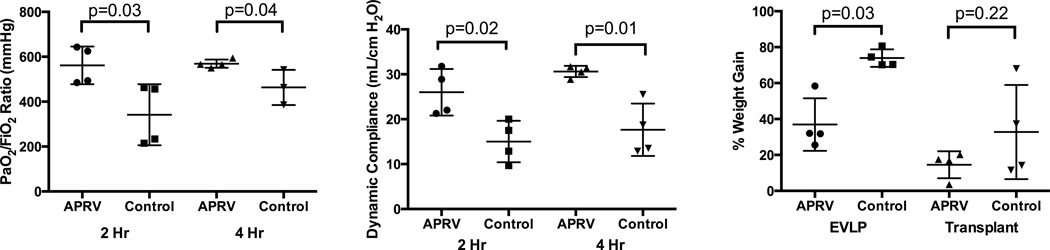
After left lung transplant, the left pulmonary vein gases in the group who had received APRV during EVLP were superior at both 2 and 4 hours versus those who had received conventional ventilation on EVLP (Figure 3A). Dynamic compliance was again significantly improved at 2 and 4 hours of reperfusion in the group receiving APRV during EVLP (Figure 3B).
Figure 3.
Lung function and edema after transplantation. PaO2/FiO2 ratios (A) and dynamic lung compliance (B) were significantly improved in the APRV group at both 2 and 4 hours of post-transplant reperfusion. C. Pulmonary edema, as a percent weight change after 4 hours of reperfusion, was significantly reduced in the APRV group during EVLP versus Control but there was no significant difference after post-transplant reperfusion.
Figure 3C demonstrates significantly less percent weight gain secondary to pulmonary edema during EVLP in the APRV group compared to the conventional ventilation group (36.9±12.7 vs 73.9±4.2%, p=0.03). However, there was no statistical difference in weight gain during reperfusion between the two groups (14.5±6.5 vs 32.8±22.7%, p=0.22).
Cytokine Expression
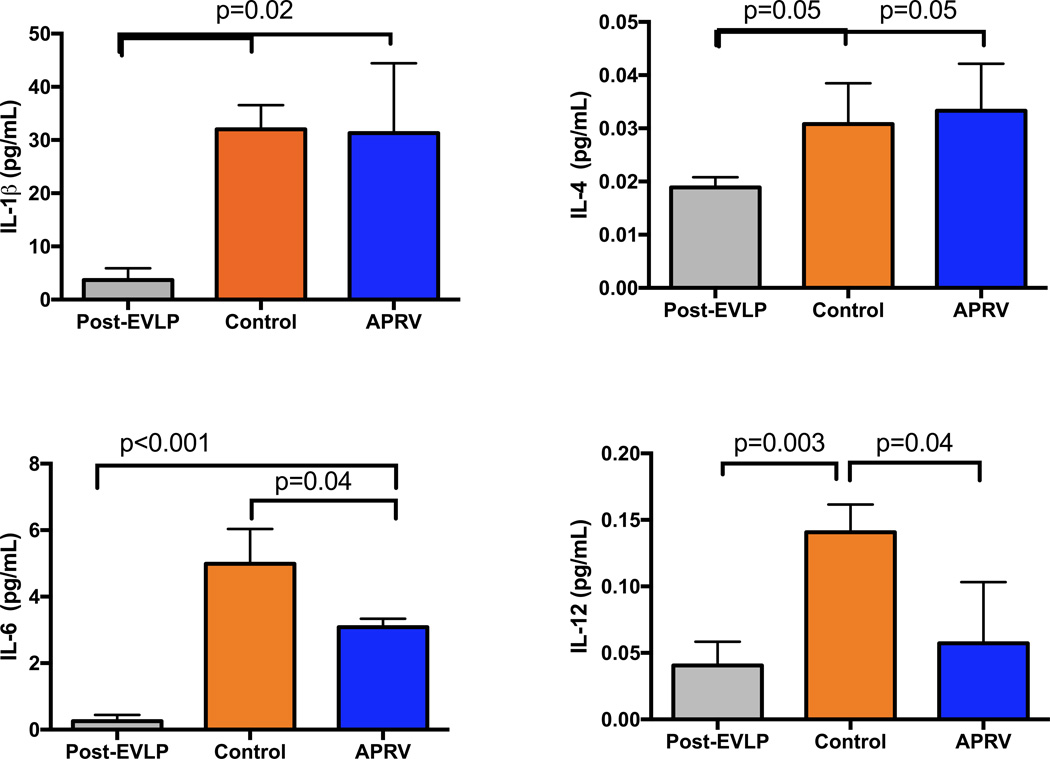
Biologically relevant pro-inflammatory cytokines were measured in lung tissue samples from right lung tissue after EVLP in group APRV as well as left lung tissue after reperfusion in the control group and APRV group (Figure 4). There is a higher expression of IL-6, IL-1β and IL-4 in the post-reperfusion tissues of both control and APRV versus the post-EVLP right lung tissue. Additionally, IL-6 expression after transplantation was significantly reduced in the APRV group versus Control (p=0.04). IL-12 expression, which was elevated after transplantation in the Control group, was significantly reduced in the APRV group (p=0.04), similar to the IL-12 levels in the post-EVLP lungs.
Figure 4.
Lung tissue expression of pro-inflammatory cytokines in left lungs of the APRV and Control groups as well as in post-EVLP right lungs. Several cytokines (IL-6 and IL-12) were significantly elevated in the Control group but were significantly reduced in the APRV group.
Lung Injury Severity Score and Neutrophil Infiltration
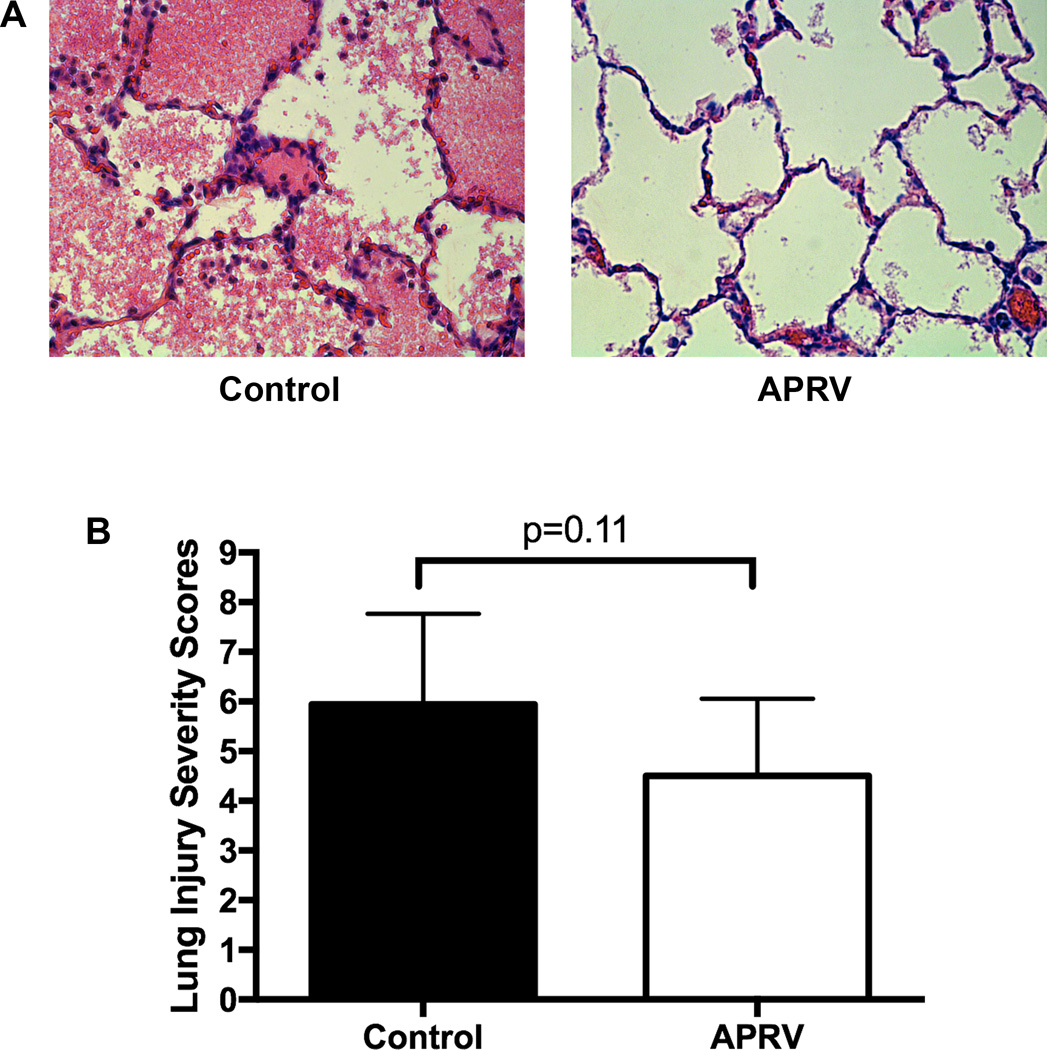
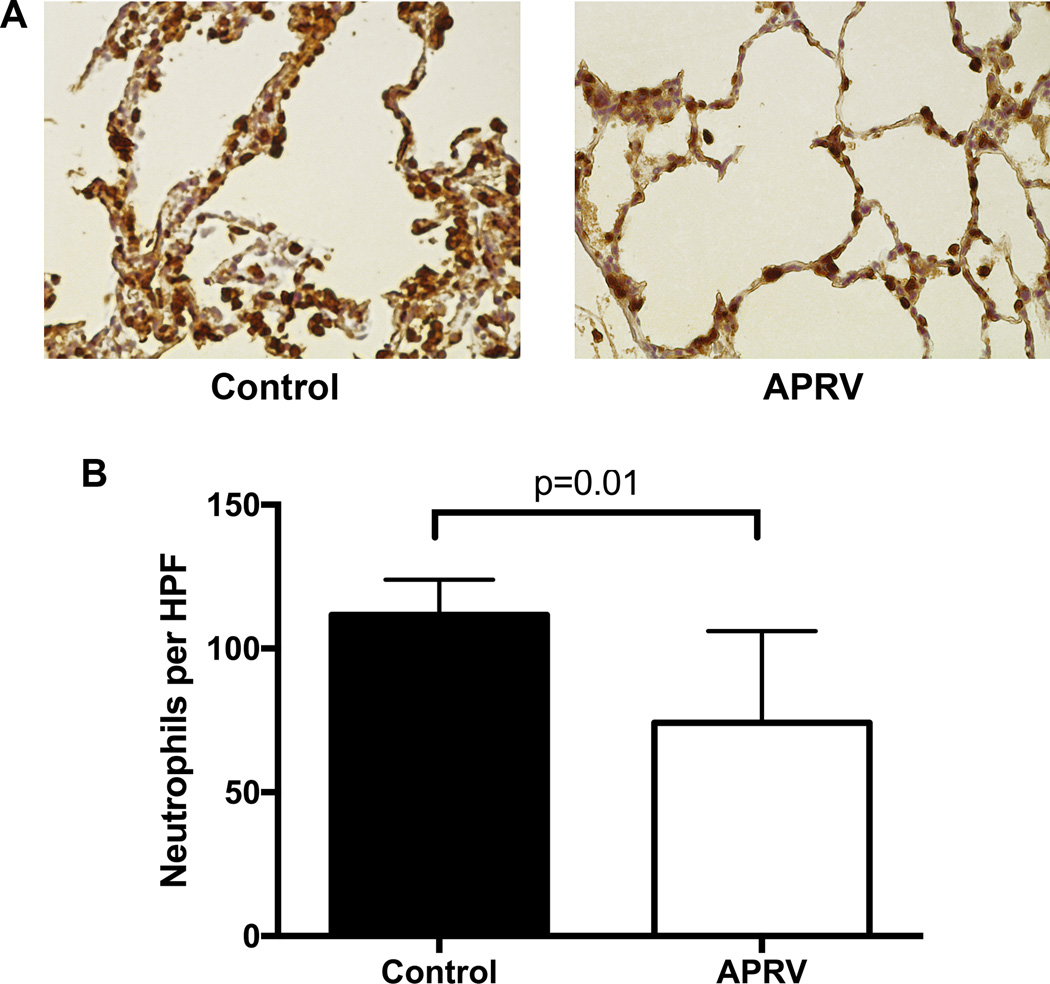
While the lung injury severity scores from 4 matched samples in each lung were lower after transplantation in the APRV group (4.5±1.3 vs 5.9±0.9, p=0.11) there was no statistical difference between the groups (Figure 5). Additionally, upon immunohistochemistry staining for neutrophils of two matched samples per lung, the APRV group had significantly less neutrophil infiltration (fewer neutrophils per high-powered field: 74.2±29.8 vs 111.7±10.0, p=0.01) versus the Control group (Figure 6).
Figure 5.
Representative lung H&E histology images from the Control and APRV groups (A). Although lower in the APRV group, lung injury severity scores were not significantly different between the Control and APRV groups (B).
Figure 6.
Representative images of immunohistochemistry staining for neutrophils in lung sections from both groups after transplantation and 4-hrs of reperfusion (A). Neutrophil counts per high-powered field (HPF) were significantly reduced in the APRV group versus Control (B).
Discussion
Using a clinically relevant porcine model of EVLP and left lung transplant after DCD procurement we demonstrated beneficial effects of APRV during EVLP on post-transplant lung performance. Lung specific oxygenation, dynamic compliance, and pulmonary edema represent the most important clinical markers of early transplant function, which were all dramatically improved in the APRV group. Additionally, biochemical markers of inflammation including IL-6 and IL-12 were significantly lower in the APRV group and closer to baseline levels observed in post-EVLP lungs prior to reperfusion. Finally, histological assessment suggests lower injury severity scores as well as reduced neutrophil infiltration in the APRV group compared to the controls.
Traditionally PaO2/FiO2 ratios as a measure of lung oxygenation capacity has been used as the most important predictor of successful lung transplantation, however, our group and others have demonstrated the importance of lung compliance(3, 16). In the present study both of these markers of lung function improved after reperfusion in the group receiving APRV during EVLP. The reduction in pulmonary edema during EVLP is likely the main cause of this difference. By keeping the alveoli continuously recruited with APRV during EVLP, the surface tension reduces fluid translocation(10, 17). It is important to note that even after transplant and reperfusion with conventional ventilation, lungs undergoing APRV on EVLP did not pick up additional edema during the reperfusion period. These clinical parameters demonstrate that APRV recruitment on EVLP has post-transplant benefits and should be considered for translation into human studies.
The biochemical profile of these lungs after EVLP and subsequent transplant represents a complex interaction between donor lung ischemia-reperfusion injury with initiation of EVLP and subsequent recipient cell activation and injury after transplant and reperfusion. IL-6 is part of the TNF-α activated pro-inflammatory pathway that has been noted by several groups including our own to be a component of ischemia-reperfusion injury after lung transplant(16, 18–22). However, Farivar and colleagues demonstrated reduced endothelial disruption and neutrophil sequestration with recombinant IL-6 in a rat ischemia-reperfusion model of lung transplant highlighting the signal orchestration that can lead to both pro- and anti-inflammatory effects of this cytokine(23). In the present study, IL-6 levels are significantly higher in the transplanted lungs compared to post-EVLP lungs but much lower in the APRV treated group versus the control group after reperfusion. While tissue level expression of IL-6 is correlated with increased neutrophil counts, EVLP may wash out the intermediary TNF-α signal. Additionally, IL-12 has been cited as a pro-inflammatory cytokine in the inflammosome pathway(24, 25). Our group has previously demonstrated benefit from IL-12 deregulation through the adenosine 2B pathway in a DCD lung EVLP model (Charles AATS 2016 Submitted JTCVS). In the present study, IL-12 expression in the APRV group is similar to baseline post-EVLP levels and significantly less than the control group after transplant.
Many opponents of APRV ventilation during EVLP argue that because of the absence of the chest wall there is minimal recoil force and the lung is at high risk of barotrauma. While this is certainly true, we found that by focusing on peak pressure (PHigh) we were able to maintain a lower airway pressure with better lung recruitment in the APRV mode. To confirm that we were not causing further injury, we performed histological assessment of the lung tissue to evaluate a standard lung injury severity score previously reported(14, 18, 26). We demonstrate no statistical difference in lung injury scores with a slight improvement in the APRV group compared to the controls. Additionally, neutrophil activation and translocation is a common phenomenon after lung injury(27), and we demonstrated reduced neutrophil infiltration in the APRV group by immunohistochemistry. These results refute the theory of increased lung injury and barotrauma with APRV during EVLP.
The limitations of this study include the preclinical porcine model and inherent variability between farm raised animals. The control group and APRV group experiments were both performed by the same team of surgeons over a 3-month time period with the controls being used previously as a baseline in another study due to high cost of Steen. The two-hour period of donor warm ischemia prior to procurement resulted in significant lung injury that was adequately rehabilitated on EVLP (mean end PaO2/FiO2 ratio above 300) but would have likely been declined for human transplant. The EVLP protocol used in the NOVEL trial dictates a standard oxygen challenge with calculation of dynamic compliance using peak pressure, tidal volume, and PEEP in conventional mode (3). For this reason we used a conventional mode for five minutes every hour for measurement of compliance. During this time there was massive loss of recruitment and an increase in peak pressure above previous PHigh despite a tidal volume setting below the volumes previously achieved with APRV. Further studies will be needed to address this issue prior to translation into human studies so that a surrogate of compliance can be calculated using PHigh, PEEP, and corresponding tidal volume to alleviate the need for mode changes. Finally, imaging and bronchoscopy are used to aid in evaluation of the lungs during the perfusion period in the clinical setting however in our studies these modalities were not used.
In conclusion, a pressure-directed APRV ventilation strategy during EVLP improves rehabilitation of severely injured DCD lungs. After transplantation these lungs demonstrate superior lung specific oxygenation and dynamic compliance compared to lungs ventilated with standard conventional ventilation. This strategy, if implemented into current clinical EVLP protocols, could advance the field of DCD lung rehabilitation to expand the lung donor pool.
Supplementary Material
Video: University of Virginia Porcine Lung Procurement and Transplant Procedure: Deceased Donor Harvest (00:09), EVLP Cannulation (03:30), Left Lung Back-table Preparation (06:20), Recipient Pneumonectomy (08:09), Left Lung Transplant (11:50), Left Pulmonary Vein Sampling (12:59)
Central Image.
APRV during EVLP improves Oxygenation post-transplant despite no difference on EVLP.
Perspective.
The use of EVLP for lung evaluation and rehabilitation prior to transplantation has been touted as a solution to the critical organ shortage. Volume-based ventilation using ARDSNet criteria leads to further lung injury during EVLP, which is attenuated with APRV. The current study represents a paradigm shift in EVLP necessitating reexamination of current clinical practice.
Central Message.
Current EVLP ventilation strategies contribute to lung injury that can be attenuated with Airway Pressure Release Ventilation.
Acknowledgments
We thank the University of Virginia Research Histology Core for efficient preparation of histology slides. Special thanks to Tony Herring, Cindy Dodson, and Sheila Hammond for their technical support and commitment to completion of this project.
The National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number T32HL007849 and R01HL119218 supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
List of Abbreviations
- EVLP
ex vivo Lung Perfusion
- DCD
Death after Cardiac Death Donor
- APRV
Airway Pressure Release Ventilation
- IRI
Ischemia Reperfusion Injury
- ABG
Arterial Blood Gas
- PEEP
Positive End Expiratory Pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: WTS Annual Meeting, June 24th, 2016 in Waikoloa, Hawaii
There are no financial disclosures or conflicts of interest from any of the authors.
References
- 1.Christie JD, Edwards LB, Kucheryavaya AY, Aurora P, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-seventh official adult lung and heart-lung transplant report--2010. J Heart Lung Transplant. 2010;29(10):1104–1118. doi: 10.1016/j.healun.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 2.De Meester J, Smits JM, Persijn GG, Haverich A. Listing for lung transplantation: life expectancy and transplant effect, stratified by type of end-stage lung disease, the Eurotransplant experience. J Heart Lung Transplant. 2001;20(5):518–524. doi: 10.1016/s1053-2498(01)00241-8. [DOI] [PubMed] [Google Scholar]
- 3.Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M, et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant. 2008;27(12):1319–1325. doi: 10.1016/j.healun.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjoberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76(1):244–252. doi: 10.1016/s0003-4975(03)00191-7. discussion 52. [DOI] [PubMed] [Google Scholar]
- 5.Yeung JC, Cypel M, Waddell TK, van Raemdonck D, Keshavjee S. Update on donor assessment, resuscitation, and acceptance criteria, including novel techniques--non-heart-beating donor lung retrieval and ex vivo donor lung perfusion. Thorac Surg Clin. 2009;19(2):261–274. doi: 10.1016/j.thorsurg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Stock MC, Downs JB, Frolicher DA. Airway pressure release ventilation. Crit Care Med. 1987;15(5):462–466. doi: 10.1097/00003246-198705000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Henzler D. What on earth is APRV? Crit Care. 2011;15(1):115. doi: 10.1186/cc9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daoud EG, Farag HL, Chatburn RL. Airway pressure release ventilation: what do we know? Respir Care. 2012;57(2):282–292. doi: 10.4187/respcare.01238. [DOI] [PubMed] [Google Scholar]
- 9.Kollisch-Singule M, Emr B, Smith B, Roy S, Jain S, Satalin J, et al. Mechanical breath profile of airway pressure release ventilation: the effect on alveolar recruitment and microstrain in acute lung injury. JAMA Surg. 2014;149(11):1138–1145. doi: 10.1001/jamasurg.2014.1829. [DOI] [PubMed] [Google Scholar]
- 10.Smith BJ, Lundblad LK, Kollisch-Singule M, Satalin J, Nieman G, Habashi N, et al. Predicting the response of the injured lung to the mechanical breath profile. J Appl Physiol (1985) 2015;118(7):932–940. doi: 10.1152/japplphysiol.00902.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terragni PP, Fanelli V, Boffini M, Filippini C, Cappello P, Ricci D, et al. Ventilatory Management During Normothermic Ex Vivo Lung Perfusion: Effects on Clinical Outcomes. Transplantation. 2016;100(5):1128–1135. doi: 10.1097/TP.0000000000000929. [DOI] [PubMed] [Google Scholar]
- 12.LaPar DJ, Laubach VE, Emaminia A, Crosby IK, Hajzus VA, Sharma AK, et al. Pretreatment strategy with adenosine A2A receptor agonist attenuates reperfusion injury in a preclinical porcine lung transplantation model. J Thorac Cardiovasc Surg. 2011;142(4):887–894. doi: 10.1016/j.jtcvs.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulloy DP, Stone ML, Crosby IK, Lapar DJ, Sharma AK, Webb DV, et al. Ex vivo rehabilitation of non-heart-beating donor lungs in preclinical porcine model: delayed perfusion results in superior lung function. J Thorac Cardiovasc Surg. 2012;144(5):1208–1215. doi: 10.1016/j.jtcvs.2012.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagner CE, Pope NH, Charles EJ, Huerter ME, Sharma AK, Salmon MD, et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J Thorac Cardiovasc Surg. 2015 doi: 10.1016/j.jtcvs.2015.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, LaPar DJ, Steidle J, Emaminia A, Kron IL, Ailawadi G, et al. Adenosine signaling via the adenosine 2B receptor is involved in bronchiolitis obliterans development. J Heart Lung Transplant. 2010;29(12):1405–1414. doi: 10.1016/j.healun.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone ML, Sharma AK, Mas VR, Gehrau RC, Mulloy DP, Zhao Y, et al. Ex Vivo Perfusion With Adenosine A2A Receptor Agonist Enhances Rehabilitation of Murine Donor Lungs After Circulatory Death. Transplantation. 2015 doi: 10.1097/TP.0000000000000830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kollisch-Singule M, Jain S, Andrews P, Smith BJ, Hamlington-Smith KL, Roy S, et al. Effect of Airway Pressure Release Ventilation on Dynamic Alveolar Heterogeneity. JAMA Surg. 2016;151(1):64–72. doi: 10.1001/jamasurg.2015.2683. [DOI] [PubMed] [Google Scholar]
- 18.Emaminia A, Lapar DJ, Zhao Y, Steidle JF, Harris DA, Laubach VE, et al. Adenosine A(2)A agonist improves lung function during ex vivo lung perfusion. Ann Thorac Surg. 2011;92(5):1840–1846. doi: 10.1016/j.athoracsur.2011.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian W, Liu Y, Zhang B, Dai X, Li G, Li X, et al. Infusion of mesenchymal stem cells protects lung transplants from cold ischemia-reperfusion injury in mice. Lung. 2015;193(1):85–95. doi: 10.1007/s00408-014-9654-x. [DOI] [PubMed] [Google Scholar]
- 20.Chang JE, Kim HJ, Yi E, Jheon S, Kim K. Reduction of ischaemia-reperfusion injury in a rat lung transplantation model by low-concentration GV1001. Eur J Cardiothorac Surg. 2016 doi: 10.1093/ejcts/ezw135. [DOI] [PubMed] [Google Scholar]
- 21.Lu W, Si YI, Ding J, Chen X, Zhang X, Dong Z, et al. Mesenchymal stem cells attenuate acute ischemia-reperfusion injury in a rat model. Exp Ther Med. 2015;10(6):2131–2137. doi: 10.3892/etm.2015.2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abreu Mda M, Pazetti R, Almeida FM, Correia AT, Parra ER, Silva LP, et al. Methylene blue attenuates ischemia--reperfusion injury in lung transplantation. J Surg Res. 2014;192(2):635–641. doi: 10.1016/j.jss.2014.07.043. [DOI] [PubMed] [Google Scholar]
- 23.Farivar AS, Merry HE, Fica-Delgado MJ, McCourtie AS, Mackinnon-Patterson BC, Mulligan MS. Interleukin-6 regulation of direct lung ischemia reperfusion injury. Ann Thorac Surg. 2006;82(2):472–478. doi: 10.1016/j.athoracsur.2006.03.060. [DOI] [PubMed] [Google Scholar]
- 24.Teng MW, Bowman EP, McElwee JJ, Smyth MJ, Casanova JL, Cooper AM, et al. IL-12 and IL-23 cytokines: from discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21(7):719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 25.Cero FT, Hillestad V, Loberg EM, Christensen G, Larsen KO, Skjonsberg OH. IL-18 and IL-12 synergy induces matrix degrading enzymes in the lung. Exp Lung Res. 2012;38(8):406–419. doi: 10.3109/01902148.2012.716903. [DOI] [PubMed] [Google Scholar]
- 26.Reece TB, Ellman PI, Maxey TS, Crosby IK, Warren PS, Chong TW, et al. Adenosine A2A receptor activation reduces inflammation and preserves pulmonary function in an in vivo model of lung transplantation. J Thorac Cardiovasc Surg. 2005;129(5):1137–1143. doi: 10.1016/j.jtcvs.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Laan M, Cui ZH, Hoshino H, Lotvall J, Sjostrand M, Gruenert DC, et al. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162(4):2347–2352. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video: University of Virginia Porcine Lung Procurement and Transplant Procedure: Deceased Donor Harvest (00:09), EVLP Cannulation (03:30), Left Lung Back-table Preparation (06:20), Recipient Pneumonectomy (08:09), Left Lung Transplant (11:50), Left Pulmonary Vein Sampling (12:59)