Abstract
Purpose
To compare effects of Roux-en-Y gastric bypass versus a multi-disciplinary, group-based medical diabetes and weight management program on physical fitness and behaviors.
Methods
Physical behavior and fitness was assessed in participants of the study Surgery or Lifestyle With Intensive Medical Management in the Treatment of Type 2 Diabetes(SLIMM-T2D)(NCT01073020), a randomized parallel-group trial conducted at a U.S. academic hospital and diabetes clinic with 18–24-month follow-up. Thirty-eight type 2 diabetes patients with hemoglobin A1c above 6.5% and BMI 30–42 kg/m2 were randomized to Roux-en-Y gastric bypass or medical program. A six-minute-walk-test to evaluate fitness, self-reported physical activity, standardized physical surveys, and cardiometabolic risk assessment were performed at baseline and after intervention.
Results
Both groups similarly improved six-minute-walk-test distance, with greater improvements in oxygen saturation and reduced heart rate following surgery. Self-reported physical activity improved similarly at 18–24-months following interventions, although exercise increased gradually following surgery, whereas early substantial increases in medical group were not fully sustained. Self-reported total and physical health were similar by Short Form-36, but improved more in the Impact of Weight on Quality of Life survey following surgery. Improvement in cardiovascular risk scores, HbA1c, and BMI were greater after surgery.
Conclusion
In this small, randomized study, both interventions led to therapeutic lifestyle changes and improved objective and self-reported physical fitness. Greater improvements in heart rate, oxygen saturation, and perceived impact of weight on health were seen following surgery, which could be attributable to greater weight loss. Clinical importance of these improvements with greater weight loss warrants further investigation.
Keywords: Type 2 diabetes, obesity, physical activity, physical fitness, bariatric surgery, Roux-en-Y gastric bypass
Introduction
Physical activity underlies fitness and well-being, weight maintenance or loss, diabetes prevention or management, and cardiometabolic health. Exercise lowers blood pressure(1), reduces need for blood pressure and cholesterol-lowering medications(2), and may improve mortality(3–6), although controversial in type 2 diabetes(2,7). Higher exercise capacity associates with reduced mortality, myocardial infarction, and revascularization rates(6,8). Multi-disciplinary, group-based programs effectively promote weight loss in pre-diabetes and type 2 diabetes(2,9). Increased physical activity contributes to weight loss following both non-surgical(10,11) and surgical interventions(12). Thus, physical activity and fitness are important determinants of health, but it remains uncertain how different diabetes and weight loss approaches impact longer-term change.
We compared effectiveness of metabolic surgery versus non-surgical approaches to diabetes and weight management by evaluating changes in objective measures of fitness using the six-minute walk test, and self-reported physical activity and patient-reported physical health outcomes over 18–24 months following randomization to Roux-en-y gastric bypass(RYGB) surgery or intensive medical diabetes and weight management(IMWM) interventions in obese patients with type 2 diabetes. We find two vastly different approaches to diabetes management both lead to improved objective and self-reported physical fitness and behaviors over 18–24 months, with some measures improving more following RYGB.
Patients and Methods
Trial Design, Randomization, and Intervention
To compare changes in physical activity and function following randomized allocation to RYGB versus a multidisciplinary, group-based medical diabetes and weight management program for obese patients with type 2 diabetes, we conducted a sub-study within the study Surgery or Lifestyle With Intensive Medical Management in Treatment of Type 2 Diabetes(SLIMM-T2D)(ClinicalTrials.gov Identifier:NCT01073020), a parallel-group trial with balanced (1:1) computer generated randomization allocated in blocks of four, stratified for body mass index (BMI) above or below 35 kg/m2 at an outpatient clinic and a hospital setting within one university. The protocol was approved by the Institutional Review Board. An independent data monitoring committee reviewed participant safety. Trial design, population, and one year cardiometabolic findings have been described(13). Briefly, all participants were obese(BMI 30–42 kg/m2), had established diagnosis of type 2 diabetes for ≥ 1-year, on ≥1 diabetes medication with baseline hemoglobin A1c(HbA1c) ≥ 6.5%, and considered medically appropriate for surgery or unsupervised exercise.
Participants randomized to IMWM were enrolled in WhyWAIT™(Weight Achievement and Intensive Treatment), a multidisciplinary program for weight control and intensive diabetes management(14), modeled on successful programs in diabetes treatment or prevention(2,9), with diabetes medications adjusted to simultaneously promote weight loss and glycemic goals(13,14). Key aspects of this program include twelve weekly meetings in groups of 10–15 with: a)medication adjustments, b)structured, hypocaloric diet with six initial weeks of breakfast and lunch meal replacement using Boost Glucose Control™ (Nestle Medical Nutrition Inc.) and structured dinner menus, c)cognitive behavioral intervention, d)group education, and e)up to 300 minutes/week initially of graded, balanced, individualized exercise. Exercise plans incorporated a balanced mix of aerobic, resistance, and flexibility workouts, with 12-weekly 60-minute exercise sessions under supervision. Graded exercise counseling aimed to gradually advance time and intensity of physical activity weekly(11). A maintenance phase consisted of individual monthly counseling for the remainder of the first year, with follow-up over the subsequent year according to recommendations of clinical care providers.
Laparoscopic RYGB was performed using standard procedures(17). Participants were advised to follow a balanced diet. Calorie goals were not provided. Exercise as tolerated was recommended as part of good medical care.
Participants were evaluated by the clinical research team at baseline prior to intervention, at 3-months or 10% weight loss-whichever occurred first(‘early’ assessment), at 12-, and 18 to 24-months follow-up, when weight loss had stabilized. Participants were weighed and had fasting laboratory and complete physical fitness assessments.
Six-Minute Walk Test
To estimate functional capacity and general physical fitness, a six-minute walk test(13) was performed. Participants walked as far as possible in six-minutes at a pace not causing discomfort, with total walking distance recorded. Heart rate and oxygen saturation(GO2 Achieve Oximeter, Nonin Medical, Inc. Plymouth, MN) were measured immediately after exertion.
Patient-Reported Physical Activity and Health Status
Trained study-staff systematically asked participants to describe current types(walking, treadmill, elliptical, stepper, bicycle, swimming, yoga, Pilates, strength training, and other), duration(minutes-per-session), and frequency(sessions-per-week) of exercise, using validated questionnaires from Nurses’ Health Study and Health Professionals’ Follow-up Study to determine physical activity based on North American populations(15,16).
Patient-reported health status standard surveys were administered at baseline and follow-up visits. SF-36 measures general health status in two primary domains, physical and mental health component scores, and eight subscales(physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, and mental health)(17). IWQOL-Lite assesses effects of weight on quality of life overall and in five areas: physical function, self-esteem, sexual life, public distress, and work(18). This analysis focused on total and physical function scales.
Calculations
Cardiometabolic risk was calculated using the United Kingdom Prospective Diabetes Study(UKPDS) risk engine(https://www.dtu.ox.ac.uk/riskengine/download.php, accessed December 5, 2015)(19,20).
Statistical Analysis
Sample size was estimated for the primary parent-study outcome to test the null hypothesis of equal resolution of hyperglycemia(13). This sub-study included participants enrolled in the parent trial. Baseline characteristics are presented as mean±standard deviation(SD) or median(interquartile range IQR), and outcome data as mean±standard error. Categorical variables were analyzed using chi-square analysis or logistic regression. Continuous variables were analyzed using paired or unpaired Student’s t-test, or a longitudinal mixed model including treatment, time, and interaction effects. Each outcome was adjusted for baseline. Primary analysis was intention-to-treat, involving all randomly-assigned participants with at least one post-randomization assessment(modified per-protocol analysis). Pre-specified contrasts of baseline versus follow-up visit and between-group comparisons were performed at each visit. A sensitivity assessment using last observation carried forward is also presented. Results were considered significant at P<0.05. Statistical analysis was performed using STATA version 12.1(College Station, TX).
Results
Patient Demographics
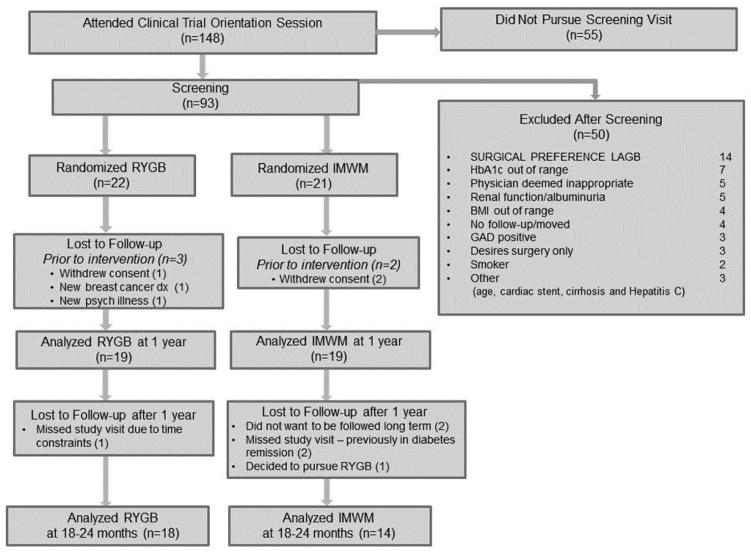
Thirty-eight participants (RYGB=19, IMWM=19) were randomized and initiated intervention between March 2010 and September 2011. Baseline characteristics were similar(Table 1). 18–24 month assessments, with mean of 22.9 months, were available in 18 RYGB (95%) and 14 IMWM (78%) participants, with two from IMWM declining participation in the six-minute walk test but completing all other assessments(Figure 1).
Table 1.
Baseline Demographics and Clinical Characteristics
| RYGB | IMWM | |
|---|---|---|
| Gender (M/F) | 6 / 13 | 9 / 10 |
| Age (yr) | 50.7 ± 7.6 | 52.6 ± 4.3 |
| Race (W/AA/As/NR) | 14 / 3 / 1 / 1 | 11 / 8 / 0 / 0 |
| BMI (kg/m2) | 36.0 ± 3.5 | 36.5 ± 3.4 |
| Weight (kg) | 104.6 ± 15.5 | 102.7 ± 17.0 |
| T2D duration (yr) | 10.6 ± 6.6 | 10.2 ± 6.1 |
| HbA1c (%) | 8.2 ± 1.4 | 8.8 ± 1.0 |
| Exercise (minutes/week)a | 37.5 (0–135) | 0.0 (0–60) |
| 6 minute walk test | ||
| Distance (meter) | 458.7 ± 65.5 | 466.7 ± 55.9 |
| Oxygen Saturation (%)b | 96.5 ± 1.4 | 96.2 ± 2.1 |
| Heart Rate (bpm)b | 106.4 ± 16.7 | 103.5 ± 16.1 |
| Quality of Life measures | ||
| IWQOL – Total | 81.5 ± 26.4 | 68.7 ± 17.7 |
| IWQOL – Physical Function | 28.7 ± 9.6 | 22.9 ± 7.0 |
| SF-36 – Total | 66.2 ± 17.7 | 71.6 ± 12.4 |
| SF-36 Physical Function | 61.3 ± 19.7 | 68.6 ± 13.2 |
All data are mean ± SD, unless noted. Groups were similar at baseline and each measure’s outcome analysis was adjusted for baseline.
Minutes of exercise was reported as median (25th percentile – 75th percentile)
Oxygen saturation and heart rate were measured immediately following the 6-minute walk.
Abbreviations in order of appearance: M –male, F –female, W - white, AA - African American, As - Asian, NR - not reported, BMI - body mass index, T2D - type 2 diabetes, HbA1c - hemoglobin A1c, BPM –beats per minute, IWQOL - Impact of Weight on Quality of Life-Lite, SF-36-Short Form-36
Figure 1. Enrollment, Randomization, and Retention of the Study Participants.
Consort diagram detailing enrollment, randomization, and retention of participants. All participants randomized and initiating intervention in the primary trial were eligible for the ancillary investigation on physical activity in this report. Abbreviations used in reasons for exclusion after screening: LAGB indicates laparoscopic adjustable gastric band; HbA1c, hemoglobin A1c; BMI, body mass index; GAD, antiglutamic acid decarboxylase antibody-positive.
Six-Minute Walk Test
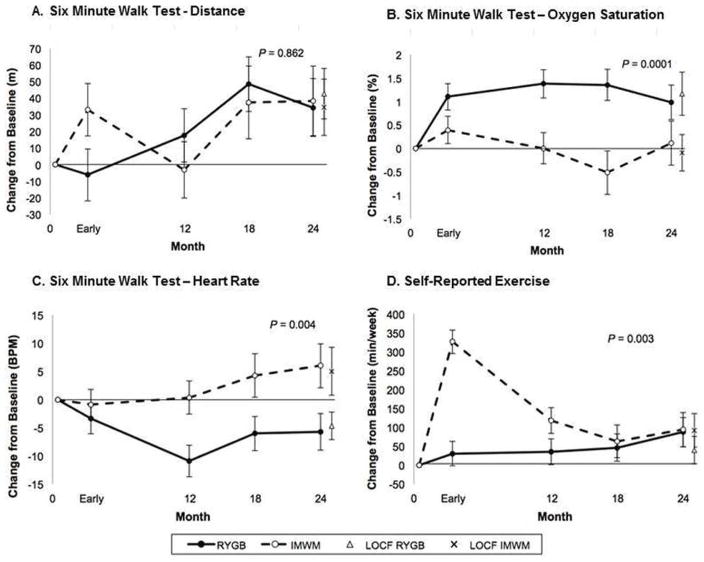
There was no difference in improvement in covered distance during six-minute walk test between RYGB and IMWM (group effect, P=0.862) over 18–24 months(Figure 2A). RYGB improved covered distance from baseline to 18–24 months (34.2±17.4 meters, P=0.049) similar to IMWM improvements (38.5±20.9 meters, P=0.067) with no difference between groups at 18–24 month assessment (P=0.880).
Figure 2. Changes in Six Minute Walk Test and Self-Reported Exercise Measures.
(A) Six Minute Walk Test – Distance. Difference in walking distance in meters, (B) Six Minute Walk Test – Oxygen Saturation. Change in oxygen saturation (SpO2, %), and (C) Six Minute Walk Test – Heart Rate. Change in heart rate in beats per minute, immediately after 6-minute walk test and (D) Self-Reported Exercise. Self-reported exercise in minutes per week, at early, 12-month, 18-month, and 24-month time points compared between patients participating in the intensive medical and weight management or undergoing RYGB surgery. Note: “Early” time point is defined in “Trial Design, Randomization, and Intervention” section as after participants lost 10% of their baseline weight or at the 3-month mark, whichever occurred first. Mean and standard error are shown. (●) Roux-en-Y gastric bypass (RYGB), (○) Intensive medical weight management (IMWM), (X) Last observation carried forwards (LOCF) for RYGB group, (◇) Last observation carried forwards (LOCF) for IMWM group. Abbreviations: beats per minute (BPM), meters (m), minutes per week (min/week). P-values represent the difference between groups over 24 months in mixed model analysis.
Overall, change in oxygen saturation by pulse oximeter was higher following RYGB (P=0.0001). Group differences were driven by early and sustained improvement in oxygen saturation within RYGB (0.98±0.37%, P=0.007) while IMWM remained unchanged from baseline (0.12±0.47%, P=0.806)(Figure 2B).
Heart rate immediately following six-minute walk, was lower in RYGB throughout follow-up (P=0.004)(Figure 2C). At 18–24 months, RYGB tended to have lower heart rate compared to baseline by −5.7±3.3 beats/minute (P=0.080) versus a numeric increase (+6.0±3.9 beats/minute, P=0.124) in IMWM, such that at 18–24 month assessments difference between groups was significant (P=0.021).
Physical Activity
Change from baseline in self-reported exercise differed between RYGB and IMWM over 18–24 months (P=0.003)(Figure 2D). At early assessment, RYGB had no change in self-reported exercise (P=0.345), while IMWM increased exercise over 300% (P<0.0001). Over two-years, RYGB slowly increased self-reported activity (by 87.3±38.6 minutes/week, P=0.024), while early increases within IMWM were incompletely sustained but remained increased from baseline (93.8±44.9 minutes/week, P=0.037). At 18–24 months, there was no difference in improvement between groups (P=0.913). At end-of-study, 41% of RYGB reported exercising equal to or more than the American Diabetes Association recommended 150 minutes/week(21), compared to 30% within IMWM (P=0.561).
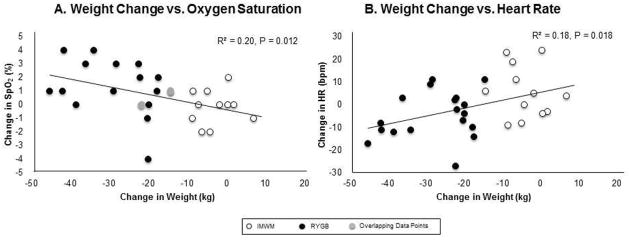
To assess relationships between weight change and improvement in measures of cardiorespiratory fitness(oxygen saturation and heart rate at end of 6-minute walk testing), we performed linear regression between these variables in the combined cohort at 18–24-months. Weight loss significantly correlated with improvement in oxygen saturation (R2=0.20; P=0.012) and reduction in heart rate (R2=0.18;P=0.018) after 6-minute-walk test(Figures 3A–3B, respectively).
Figure 3. Association Between Weight Change and Improvement in Measures of Cardiorespiratory Fitness.
(A) Weight Change vs. Oxygen Saturation. Association between change in weight and change in oxygen saturation immediately following six-minute walk test between 18 and 24 months from intervention in both groups. (B) Weight Change vs. Heart Rate. Association between change in weight and heart rate immediately following six-minute walk test between 18 and 24 months from intervention in both study groups. (●) Roux-en-Y gastric bypass (RYGB), (○) Intensive medical weight management (IMWM), (●) Overlapping data from two participants, one from RYGB, one from IMWM.
Patient-Reported Outcomes of Physical Health
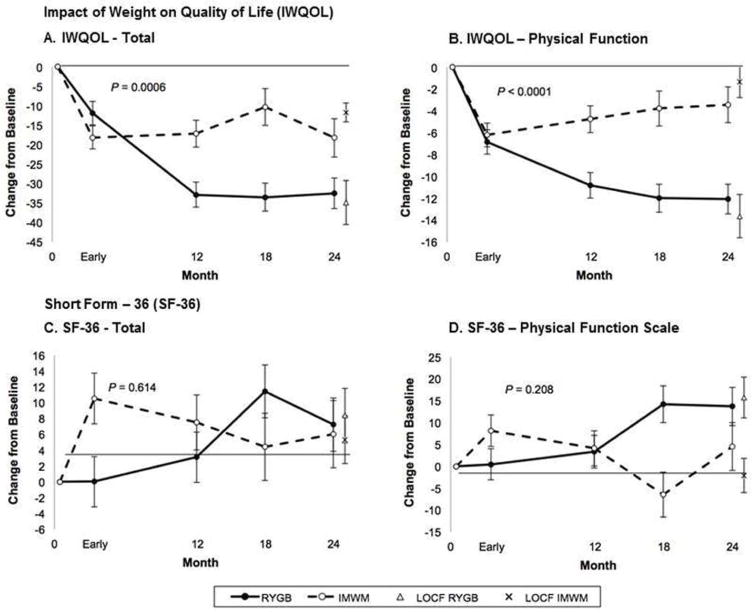
Overall, groups differed for IWQOL-Lite scores with lower/better values in RYGB than IMWM over 18–24 months for both total and physical function subscale scores (P=0.0006 and P<0.0001, Figure 4A–4B, respectively). For total IWQOL-Lite score, RYGB had a 32.5±4.0-point (P<0.0001) reduction at 18–24-months, compared to a lesser, yet still highly significant decrease of 18.3±5.0 (P<0.0001) from baseline within IMWM. Improvement was greater for RYGB than IMWM at 18–24 months (P=0.025). The physical function subscale mirrored this improvement with a within-group decrease of 12.1±1.4 (P<0.0001) for RYGB compared to the 3.4±1.6 (P=0.04) points decrease in IMWM, with significant difference between groups at 18–24 months (P=0.0001).
Figure 4. Patient Reported Outcomes of Physical Health.
Change in patient-reported impact of weight on quality of life (IWQOL) and Short Form-36 (SF-36) total scores and physical function domains over time in Roux-en-Y gastric bypass (RYGB) and intensive medical weight management (IMWM) groups. (A) IWQOL total score and (B) IWQOL physical functioning subscale, and (C) SF-36 total score and (D) SF-36 physical function subscale at early, 12-month, 18-month, and 24-month time points in both study groups. Note: “Early” time point is defined in “Trial Design, Randomization, and Intervention” section as after participants lost 10% of their baseline weight or at the 3 month mark, whichever occurred first. Mean and standard error are shown. (●) Roux-en-Y gastric bypass (RYGB), (○) Intensive medical weight management (IMWM), (X) Last observation carried forwards (LOCF) for RYGB group, (◇) Last observation carried forwards (LOCF) for IMWM group. P-values represent the difference between groups over 24 months in mixed model analysis.
There was no between-group difference in either SF-36 total score or its physical function scale over the study (P=0.614 and P=0.208, Figure 4C–4D, respectively). For total SF-36 total score, RYGB improved from baseline (7.2±3.4 points, P=0.032) compared to a smaller increase for IMWM (6.0±4.2 points, P=0.156), although at 18–24 months no statistical difference between groups was observed (P=0.827). The physical function scale paralleled the total SF-36 survey, with increase from baseline at 18–24 months in RYGB (13.7±4.4 points, P=0.002) compared to no change (4.5±5.5 points, P=0.409) in IMWM, with no between-group differences (P=0.193).
Cardiometabolic Measures
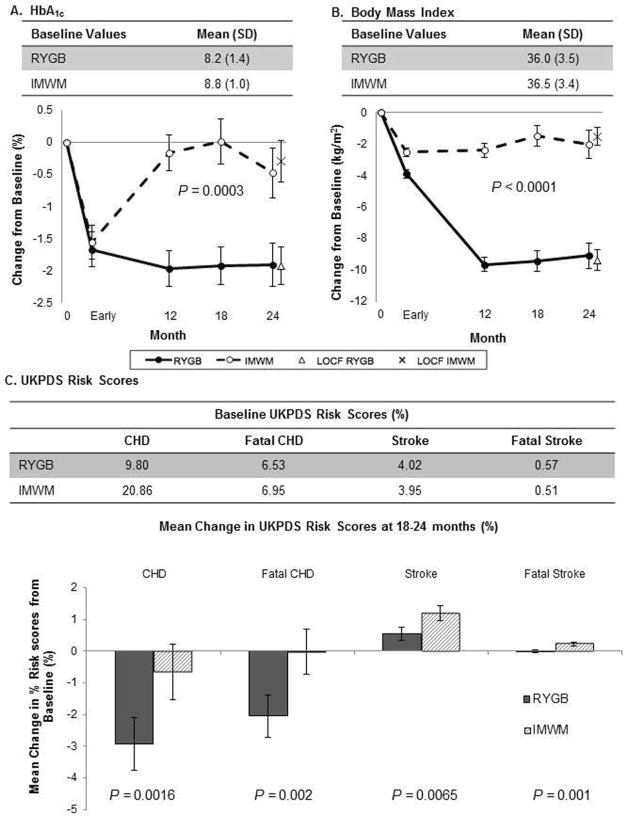
RYGB achieved a greater reduction in HbA1c (P=0.0003)(Figure 5A) and weight(P<0.0001)(Figure 5B) over 18–24 months. RYGB lowered HbA1c early, with sustained reductions of 1.9±0.3% (P<0.0001) at 18–24 months. Early improvements in HbA1c within IMWM were not fully sustained (−0.5±0.4%, P=0.222), resulting in greater reduction in HbA1c at end-of-study for RYGB (P=0.006). RYGB had nadir weight at 12-months, which was largely sustained at 18–24 months with BMI reduction of −9.1±0.8 kg/m2 (P<0.0001). IMWM sustained early weight loss, reducing BMI to a lesser degree of −2.0±0.9 kg/m2 (P=0.022), with significant differences between groups at 18–24 months (P<0.0001).
Figure 5. Cardiometabolic Changes Following RYGB and Intensive Medical Weight Management.
(A) Change in Hemoglobin A1c and (B) Body Mass Index at early, 12-month, 18-month, and 24-month time points in patients randomized to RYGB or the intensive medical and weight management program. Note: “Early” time point is defined in “Trial Design, Randomization, and Intervention” section as after participants lost 10% of their baseline weight or at the 3 month mark, whichever occurred first. Mean and standard error are shown. (●) Roux-en-Y gastric bypass (RYGB), (○) Intensive medical weight management (IMWM), (X) Last observation carried forwards (LOCF) for RYGB group, (◇) Last observation carried forwards (LOCF) for IMWM group. P-values represent the difference between groups over 24 months in mixed model analysis. (C) UKPDS Risk Scores at 18–24 months in the two study cohorts. Baseline scores are indicated in the table at top, and change scores (baseline to 18–24 months) in the bar graphs below. P-values indicate differences in change scores between groups.
Additionally, patients randomized to RYGB had lower UKPDS risk scores for coronary heart disease (CHD, P=0.0016), fatal CHD (P=0.002), stroke (P=0.0065), and fatal stroke (P=0.001) at 18–24 months(Figure 5C).
Adverse Events
No participant experienced severe hypoglycemia. Surgical arm serious adverse events included ischemic heart disease with coronary artery bypass surgery, cholelithiasis, new breast cancer, nephrolithiasis, acute kidney injury, exacerbated depression, panniculectomy, hip arthroplasty, and cervical osteophyte resection. In the nonsurgical arm, one participant had a resuscitated cardiac arrest, and three participants had presyncopal events.
Discussion
This study evaluated whether obese persons with type 2 diabetes undergoing structured, multi-disciplinary, group-based exercise counseling and medical intensification of diabetes control would achieve and maintain greater improvement in physical activity and fitness than patients without intensive exercise counseling undergoing RYGB. While objective measures of fitness improved similarly in both groups, quantified by distance walked during six-minutes, there were greater improvements in oxygen saturation and heart rate following RYGB, which correlated with greater weight loss after surgery. Self-reported physical activity increased dramatically during the early intensive phase of the IMWM program, but was incompletely sustained whereby self-reported physical activity increased to a similar level in both groups over longer term.
Oxygen saturation does not limit six-minute walk distance following RYGB, where despite greater improvements in oxygen saturation over time after surgery, total distance covered during the walk test did not improve more. Oxygen saturation depends on pulmonary function, circulatory capacity, and end-organ oxygen extraction and demand. Oxygen saturation integrates health status across these domains. Difference in oxygen saturation and heart rate between groups may partly be attributed to differences in achieved weight. Lower oxygen saturation after six-minute walk test occurs in obese compared to lean dogs(22).
Exercise underlies prevention and treatment of diabetes and its co-morbidities, including obesity and cardiovascular disease(23–25). Physical activity increased following bariatric surgery in the Longitudinal Assessment of Bariatric Surgery(26,27), but changes were not compared with randomized intensive non-surgical intervention. Although previous studies demonstrate a graded dose-response effect for exercise training on fitness(28) we were not able to demonstrate this relationship in between-group comparison as both groups increased objective and self-reported activity similarly and magnitude of weight loss, not physical activity, more closely related to the quantitative measures of fitness of improved oxygen saturation and lower heart rate.
The pattern of change in self-reported exercise differed between the groups. Early assessment occurred within a short postoperative time interval with reduced physical activity during surgical recovery while IMWM were participating in weekly group and supervised exercise sessions. Participants in the IMWM group may have reported higher physical activity due to successes of weekly interventions to increase amount of exercise performed, but there may also be a tendency for bias within the group to appear compliant with program recommendations. Nonetheless, both groups appear to plateau over 18–24-months in quantitative measures of fitness assessed by distance during the walk test, oxygen saturation and heart rate, albeit with greater improvement in the latter indices following RYGB.
Patient-reported outcomes pertinent to impact of weight on quality of life improved similarly early after both interventions, but continued to improve only in RYGB. In contrast, total quality of life and physical function assessed by SF-36 were similar between groups over follow-up despite multiple greater improvements in objective measures following surgery. This may be due to lasting effects of education and support received during the IMWM program, which edifies the patient and prompts them to perceive maintaining physical ability in spite of weight regain, discordant to the programmatic teaching of the importance of physical fitness and weight maintenance or further loss for sustained improved health. Discordance between the IWQOL-Lite and SF-36 may be due to focus of the first survey on the specific role of weight on quality of life, and the second broader aspects of health. However in general, SF-36 more closely mimics self-reported changes in activity.
It is important to put the IMWM program in context as RYGB was not compared to standard of care, and physical fitness and activity were assessed following what would be considered optimal multi-disciplinary group-based program intervention. The magnitude and sustained weight loss in the IMWM group would be considered clinically successful weight management, comparable to that in the Diabetes Prevention Program and LookAHEAD studies(2,9), albeit less than for RYGB. Randomization and IMWM intervention occurred anteceding Food and Drug Administration approval of new weight loss agents including lorcaserin, the phentermine/topiramate combination, and liraglutide and similarly predating approval or widespread availability of sodium-glucose co-transporter 2 (SGLT2) inhibitors and GLP-1 agonists respectively, which can improve both weight and glycemia. It is possible physical fitness and activity would improve more with these therapeutics. Glycemic improvements were similar between groups early following intervention but sustained only in RYGB, despite the use of fewer anti-diabetic medications(13). Anecdotally, participants in the IMWM group often indicated feeling equipped with the knowledge to regain control of their diabetes through lifestyle measures and commitment to change, which appeared in some instances to delay intensification of medical management. Intensification of pharmacologics may explain the second downward trend in HbA1c beyond 18 months within IMWM.
Exercise and pharmacological interventions appear to have similar morbidity outcomes for secondary prevention of coronary heart disease(29). In contrast UKPDS risk scores for stroke and fatal coronary heart disease were lower following RYGB than IMWM(13), similar to reported non-randomized studies(30,31). The UKPDS risk equation accounts for many conventional vascular risk factors, but does not include weight loss, improved glycemic control, or changes in cardiorespiratory fitness. Thus, the UKDPS risk engine may underestimate long-term benefits of RYGB if physical activity and fitness independently contribute to further reduce cardiovascular risk.
This work is limited by the small number of participants. Participants were systematically questioned about type and duration of exercise in the week prior to study visits, which may not accurately reflect week-to-week variability. Pedometers or accelerometers were not used. Patients may over-report physical activity or feeling more active(32) knowing they are expected to be more active, a bias applicable to both groups. Consistent with accuracy of self-reports, objective measures of fitness assessed by distance during six-minute walk test parallel self-reported activity. Though not a focus of this study, the dietary approaches to diabetes and weight management differed fundamentally, with substantial differences between groups in regard to appetite, satiety, caloric intake, and degree of malabsorption in RYGB.
In conclusion, this study characterizes fitness and physical activity following randomization to RYGB or IMWM using subjective and objective measures. Despite 3-months of group exercise sessions and intensive coaching in IMWM, we demonstrate comparable improvements in fitness and level of physical activity, as assessed by total covered distance during six-minute walk test, self-reported physical activity, and SF-36 total and physical function scores two years following randomization to RYGB or an intensive diabetes and weight management program. Improvements in oxygen saturation and heart rate are greater after surgery, potentially related in part to the greater magnitude weight lost. Similarly, quantitative patient-reported outcomes show greater improvements in IWQOL-Lite total and physical function scores. Finally, our results demonstrate cardiometabolic risk estimates improved more after surgery. Understanding and removing barriers to develop and sustain physical activity may improve clinical management in obese patients with type 2 diabetes.
Bullets.
Type 2 diabetes patients randomized to gastric bypass surgery or multi-disciplinary, group-based medical diabetes and weight management based interventions similarly improved fitness, measured by six-minute-walk-test distance, self-reported physical activity, and total and physical health scores by Short Form-36 over 18–24 months follow-up.
Improvements in oxygen saturation and heart rate recovery following six-minute-walk-test, Impact of Weight on Quality of Life survey responses, HbA1c, BMI, and cardiometabolic risk scores were greater after surgery.
Acknowledgments
We would like to thank Katherine Kelly, BA, and members of the Why WAIT program team: Osama Hamdy, MD; Nuha El Sayed, MD; Iris Marquis, NP; Jacqueline Shahar, RCEP, CDE; Michael See, EP; Gillian Arathuzik, RD, CDE; Amanda Kirpitch, RD, CDE; John Zrebiec, LICSW, CDE; Pam Needle, RN, CDE; Rebecca Lungo, NP; and Joan Beaton, BA.
Funding: The National Institute of Health, and National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda, MD) (RC-DK086918, R56-DK095451) principally supported the trial, with additional support from P30-DK036836, PCORI CE1304-6756, and Covidien (Mansfield, MA) for surgery in patients with BMI below 35 kg/m2, the Herbert Graetz Fund, the Marietta Blau Grant from the Österreichischer Austausdienst, Lifescan (Milpitas, CA), Nestlé Medical Nutrition Inc., (Florham Park, FL), and Novo Nordisk (Bagsværd, Denmark). No funding source had a role in trial design, conduct, data analysis, or manuscript preparation.
Abbreviations
- BMI
body mass index
- HbA1c
hemoglobin A1c
- IMWM
intensive medical diabetes and weight management program
- IWQOL-Lite
Impact of Weight on Quality of Life
- IQR
interquartile range
- LOCF
last observation carried forward
- RYGB
Roux-en-Y gastric bypass
- SF-36
Short Form-36
- SD
standard deviation
- SLIMM-T2D
Surgery or Lifestyle With Intensive Medical Management in the Treatment of Type 2 Diabetes
- T2D
Type 2 diabetes
- UKPDS
United Kingdom Prospective Diabetes Study
Additional Abbreviations in Tables
- M
male
- F
female
- W
white
- AA
African American
- As
Asian
- NR
not reported
- BPM
beats per minute
Footnotes
please note these affiliations represent time when the work was performed. Some co-authors have subsequently moved to new positions.
Protocol: Available on request.
Disclosures: Authors have nothing to disclose.
Author contributions: Authors JP, SAD, MW, AGF, KF, FH, AV, and ABG contributed to study visit conduct. DCS contributed to statistical analysis. All authors contributed to manuscript preparation and have approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Figueira FR, Umpierre D, Cureau FV, Zucatti AT, Dalzochio MB, Leitao CB, Schaan BD. Association between Physical Activity Advice Only or Structured Exercise Training with Blood Pressure Levels in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Sports Med. 2014 doi: 10.1007/s40279-014-0226-2. [DOI] [PubMed] [Google Scholar]
- 2.Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, Coday M, Crow RS, Curtis JM, Egan CM, Espeland MA, Evans M, Foreyt JP, Ghazarian S, Gregg EW, Harrison B, Hazuda HP, Hill JO, Horton ES, Hubbard VS, Jakicic JM, Jeffery RW, Johnson KC, Kahn SE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montez MG, Murillo A, Nathan DM, Patricio J, Peters A, Pi-Sunyer X, Pownall H, Reboussin D, Regensteiner JG, Rickman AD, Ryan DH, Safford M, Wadden TA, Wagenknecht LE, West DS, Williamson DF, Yanovski SZ. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. The New England journal of medicine. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, Williams DE, Geiss L. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 4.Sadarangani KP, Hamer M, Mindell JS, Coombs NA, Stamatakis E. Physical activity and risk of all-cause and cardiovascular disease mortality in diabetic adults from Great Britain: pooled analysis of 10 population-based cohorts. Diabetes care. 2014;37:1016–1023. doi: 10.2337/dc13-1816. [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. The New England journal of medicine. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 6.Church TS, Cheng YJ, Earnest CP, Barlow CE, Gibbons LW, Priest EL, Blair SN. Exercise capacity and body composition as predictors of mortality among men with diabetes. Diabetes care. 2004;27:83–88. doi: 10.2337/diacare.27.1.83. [DOI] [PubMed] [Google Scholar]
- 7.Schellenberg ES, Dryden DM, Vandermeer B, Ha C, Korownyk C. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Annals of internal medicine. 2013;159:543–551. doi: 10.7326/0003-4819-159-8-201310150-00007. [DOI] [PubMed] [Google Scholar]
- 8.Hung RK, Al-Mallah MH, McEvoy JW, Whelton SP, Blumenthal RS, Nasir K, Schairer JR, Brawner C, Alam M, Keteyian SJ, Blaha MJ. Prognostic Value of Exercise Capacity in Patients With Coronary Artery Disease: The FIT (Henry Ford ExercIse Testing) Project. Mayo Clinic proceedings. 2014;89:1644–1654. doi: 10.1016/j.mayocp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 9.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM Diabetes Prevention Program Research G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewbank PP, Darga LL, Lucas CP. Physical activity as a predictor of weight maintenance in previously obese subjects. Obesity research. 1995;3:257–263. doi: 10.1002/j.1550-8528.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 11.Jakicic JM, Marcus BH, Lang W, Janney C. Effect of exercise on 24-month weight loss maintenance in overweight women. Archives of internal medicine. 2008;168:1550–1559. doi: 10.1001/archinte.168.14.1550. discussion 1559–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egberts K, Brown WA, Brennan L, O’Brien PE. Does exercise improve weight loss after bariatric surgery? A systematic review. Obesity surgery. 2012;22:335–341. doi: 10.1007/s11695-011-0544-5. [DOI] [PubMed] [Google Scholar]
- 13.Halperin F, Ding SA, Simonson DC, Panosian J, Goebel-Fabbri A, Wewalka M, Hamdy O, Abrahamson M, Clancy K, Foster K, Lautz D, Vernon A, Goldfine AB. Roux-en-Y Gastric Bypass Surgery or Lifestyle With Intensive Medical Management in Patients With Type 2 Diabetes: Feasibility and 1-Year Results of a Randomized Clinical Trial. JAMA surgery. 2014;149:716–726. doi: 10.1001/jamasurg.2014.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamdy O, Carver C. The Why WAIT program: improving clinical outcomes through weight management in type 2 diabetes. Current diabetes reports. 2008;8:413–420. doi: 10.1007/s11892-008-0071-5. [DOI] [PubMed] [Google Scholar]
- 15.Chasan-Taber S, Rimm EB, Stampfer MJ, Spiegelman D, Colditz GA, Giovannucci E, Ascherio A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–86. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, Rosner B, Kriska A, Willett WC. Reproducibility and validity of a self-administered physical activity questionnaire. International journal of epidemiology. 1994;23:991–999. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 17.McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Medical care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation. 2002;11:157–171. doi: 10.1023/a:1015081805439. [DOI] [PubMed] [Google Scholar]
- 19.Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA, Holman RR. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke; a journal of cerebral circulation. 2002;33:1776–1781. doi: 10.1161/01.str.0000020091.07144.c7. [DOI] [PubMed] [Google Scholar]
- 20.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in Type II diabetes (UKPDS 56) Clin Sci (Lond) 2001;101:671–679. [PubMed] [Google Scholar]
- 21.Association AD. Foundations of Care: Education, Nutrition, Physical Activity, Smoking Cessation, Psychosocial Care, and Immunization. Diabetes care. 2015;38:S20–S30. doi: 10.2337/dc15-S007. [DOI] [PubMed] [Google Scholar]
- 22.Manens J, Ricci R, Damoiseaux C, Gault S, Contiero B, Diez M, Clercx C. Effect of body weight loss on cardiopulmonary function assessed by 6-minute walk test and arterial blood gas analysis in obese dogs. Journal of veterinary internal medicine / American College of Veterinary Internal Medicine. 2014;28:371–378. doi: 10.1111/jvim.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Church T. Exercise in obesity, metabolic syndrome, and diabetes. Progress in cardiovascular diseases. 2011;53:412–418. doi: 10.1016/j.pcad.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Dela F, Prats C, Helge JW. Exercise interventions to prevent and manage type 2 diabetes: physiological mechanisms. Medicine and sport science. 2014;60:36–47. doi: 10.1159/000357334. [DOI] [PubMed] [Google Scholar]
- 25.Roberts CK, Little JP, Thyfault JP. Modification of insulin sensitivity and glycemic control by activity and exercise. Medicine and science in sports and exercise. 2013;45:1868–1877. doi: 10.1249/MSS.0b013e318295cdbb. [DOI] [PubMed] [Google Scholar]
- 26.Jacobi D, Ciangura C, Couet C, Oppert JM. Physical activity and weight loss following bariatric surgery. Obesity reviews : an official journal of the International Association for the Study of Obesity. 2011;12:366–377. doi: 10.1111/j.1467-789X.2010.00731.x. [DOI] [PubMed] [Google Scholar]
- 27.King WC, Hsu JY, Belle SH, Courcoulas AP, Eid GM, Flum DR, Mitchell JE, Pender JR, Smith MD, Steffen KJ, Wolfe BM. Pre- to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2 (LABS-2) Surgery for obesity and related diseases : official journal of the American Society for Bariatric Surgery. 2012;8:522–532. doi: 10.1016/j.soard.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. Jama. 2007;297:2081–2091. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 29.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577. doi: 10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams TD, Gress RE, Smith SC, Halverson RC, Simper SC, Rosamond WD, Lamonte MJ, Stroup AM, Hunt SC. Long-term mortality after gastric bypass surgery. The New England journal of medicine. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 31.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. The New England journal of medicine. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 32.Bond DS, Jakicic JM, Unick JL, Vithiananthan S, Pohl D, Roye GD, Ryder BA, Sax HC, Wing RR. Pre- to postoperative physical activity changes in bariatric surgery patients: self report vs. objective measures. Obesity (Silver Spring) 2010;18:2395–2397. doi: 10.1038/oby.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]