Abstract
Localized provoked vulvodynia (LPV) is a common, chronic, and disabling condition; patients experience profound pain and a diminished quality of life. The etiologic origins of vulvodynia are poorly understood, yet recent evidence suggests a link to site-specific inflammatory responses. Fibroblasts isolated from the vestibule of LPV patients are sensitive to pro-inflammatory stimuli and copiously produce pain-associated pro-inflammatory mediators (IL‐6 and PGE2). Although LPV is a multifactorial disorder, understanding vulvar inflammation and targeting the inflammatory response should lead to treatment advances, especially for patients exhibiting signs of inflammation. NFkB (already targeted clinically) or other inflammatory components may be suitable therapeutic targets.
Keywords: fibroblast, vulvodynia, vulvar pain, vestibulitis, inflammation, Dectin‐1, NFkB, IL‐6, PGE2
Introduction
Vulvodynia is a prevalent form of chronic vulvar pain, affecting as many as 28% of women within their lifetime1. Population studies estimate roughly 8% of women in the United States currently suffer from vulvodynia2,3. Recognized in 1987 as “vulvar vestibulitis,” vulvodynia is now defined as persistent vulvar pain in the absence of any obvious disease pathology, such as active microbial infection or dermatological conditions4. This represents a chief obstacle in diagnosing and treating vulvodynia. Vulvodynia can be sub-classified into “localized” or “generalized”, the former affecting at least a portion of the vulvar vestibule or the clitoris, and the latter affecting the vulva as a whole5. The pain, often described as “knife-like,” burning, stinging, rawness, irritation, or itching can be provoked by touch (e.g. during tampon insertion or sexual intercourse), can be unprovoked, or mixed6. Localized provoked vulvodynia (LPV) is most common, especially in premenopausal women6,7, while generalized vulvodynia is more common in peri- and postmenopausal women7. Here, we will focus on LPV, which is more prevalent and has received greater attention in the literature.
The elusive origins of vulvodynia and the unsurprising treatment shortcomings
Overview
Although many theories have been proposed to explain the occurrence of vulvodynia, including gene polymorphisms8,9, psychological disorders10, inflammation/dysregulation of inflammatory pathways11-17, histories of yeast18,19 or human papilloma virus (HPV) infection20, sexual/childhood abuse21,22, and childbirth/pelvic floor muscle dysfunction23,24, there is no consensus regarding the precise cause(s) of disease. It is now generally accepted that vulvodynia is a multifactorial disorder influenced by several contributing factors; multidisciplinary therapies have been most effective in reducing/managing chronic vulvar pain and are currently the recommended line of treatment5,25.
Topical Therapies
Treatment failures stem from a limited understanding of the disease pathology and the factors that precipitate pain5. Current treatment strategies follow a “trial and error” approach guided mainly by expert opinion rather than an evidence-based approach from randomized clinical trials (RCTs)26. Under this strategy, the degree of therapeutic intervention increases as symptoms fail to remit or worsen. Initial intervention involves minimizing environmental irritants to the vulva, such as the cessation of detergent use, wearing exclusively cotton underwear, and refraining from wearing tight clothing5. These measures are often followed by or combined with the use of topical agents to relieve pain, namely anesthetics (e.g. lidocaine) applied nightly or immediately prior to intercourse5. Other topical therapies (with questionable efficacy) include estrogen7, fibroblast lysates27, moisturizers, muscle relaxers (e.g. baclofen)28, capsaicin29,30, and topical tricyclic antidepressants (e.g. amitriptyline)31 or anticonvulsants (e.g. gabapentin)32.
Oral Medications
When these lines of defense show no appreciable change, oral medications may be prescribed, which fall into two general categories: antidepressants and anticonvulsants5. Tricyclic antidepressants (TCAs), such as amitriptyline, nortriptyline, and desipramine target pain and depression (associated with vulvodynia)33,34, while TCAs also have proven neuropathic pain relieving effects35. However, a recent placebo-controlled RCT found that desipramine alone or in combination with lidocaine performed no better than placebo36. Other antidepressants, such as serotonin reuptake inhibitors, are also largely ineffective for vulvodynia26. Although early theories suggested that unexplained vulvar pain represents a strictly psychological disorder10, depression, hypervigilence, and catastrophizing are now regarded as evolving as the chronic pain state persists5. Nevertheless, many patients report symptom improvement when receiving treatment that targets the psychological sequelae of vulvodynia, such as cognitive behavioral therapy5,6,25. Another option is the use of oral anticonvulsants (e.g. gabapentin), which may be especially useful for patients with pelvic floor dysfunction37-40. However, pelvic floor dysfunction is likely secondary to and not the cause of vulvodynia1,6. Nonetheless, gabapentin has other indications for use; double-blind placebo-controlled studies indicate gabapentin is effective in relieving neuropathic pain26. Currently, the first multicenter RCT is underway to examine the efficacy of oral gabapentin38. Prior studies suggest that gabapentin may improve self-reported symptoms, but these investigations lacked placebo controls39,40. Another confounding factor is that several studies have shown a significant improvement in vulvodynia symptoms with placebo6,36. The degree of placebo effect is correlated with the level of desire to get better and the strength of belief that the proposed treatment may be effective6.
Physical Therapy
Physical therapy and biofeedback have also shown some success5,6. These techniques can be applied to the treatment of both localized and generalized vulvodynia and can be particularly effective when there is concomitant vaginismus, a physical/psychological pain condition that may reflect hypertonicity of the pelvic floor muscles23,24. Physical therapy is aimed at improving pelvic floor tone and increasing the patient's awareness of her pelvic floor muscles to ease reflex guarding and muscle spasm6. Biofeedback also focuses on developing self-awareness to control or minimize vulvar pain and typically involves the use of an electromyography (EMG) unit that is inserted into the vagina, which allows the patient to measure the force of her pelvic floor contractions through the use of Kegel-like exercises41,42.
Psychological Approaches
Psychological, sexual, and behavioral therapies have also been reported to be successful in reducing pain5. However, few RCTs have investigated the impact of such therapies; only one RCT has demonstrated that psychological and cognitive behavioral therapies (CBT) are effective treatments for vulvodynia43. Although it is now generally accepted that psychological distress and depression are secondary to vulvodynia, the literature supports the use of psychological, sexual, and behavioral therapy to treat vulvodynia symptoms6. Nonetheless, these therapies generally do not address the underlying disease mechanisms. Childhood/sexual abuse may be a risk factor for vulvodynia development and adjunctive counseling may be indicated, if discovered21,22. Because psychological distress and depression can arise either following past traumatic experience, such as sexual abuse, or secondary to the chronic pain of vulvodynia itself, the literature supports the adjunctive use of psychological, sexual, and behavioral therapy to treat vulvodynia symptoms6. Nonetheless, these therapies generally do not address the underlying peripheral disease mechanisms.
Injectable Agents
When the aforementioned approaches do not appreciably improve symptoms, some women may try injected agents6. Such therapies are less applicable to generalized vulvodynia, as the injection site(s) is usually limited to the vulvar vestibule and areas immediately surrounding the introitus37,44-46. Botulinum toxin A has been most extensively investigated as a possible injectable treatment for vulvodynia37,44-47. Botulinum toxin is a neurotoxin derived from bacterial pathogen, Clostridium botulinum37. In addition to reduction of superimposed pelvic floor muscle spasm, botulinum toxin may also possess efficacy for vulvodynia due to its ability to inhibit substance P release, a neurotransmitter associated with inflammation and pain47. Despite promising results in case studies, the only RCT examining the efficacy of botulinum toxin A failed to show a significant improvement in symptoms versus placebo46. Injected corticosteroids may also improve pain profiles in women with vulvodynia, which has been attributed to their potential anti-inflammatory effects26. However, further investigation is necessary to confirm their effectiveness.
Surgical Intervention
Vestibulectomy, a surgical procedure to remove all or part of the vulvar vestibule, is currently regarded as an effective therapy for vulvodynia, yet it is typically reserved as a final measure due to its disfiguring qualities, invasive nature, and risk for both short term and long term surgical complications (e.g. unsatisfying appearance, decreased lubrication, sensitive scar tissue)48. The relative success of surgical intervention has been largely been evaluated using data from case reports, which suggest a roughly 90% pain improvement and satisfaction rate after vestibulectomy 48,49. However, vestibulectomy is probably less effective for generalized vulvodynia, and a handful of cases have reported intensified post-recovery pain symptoms48-50. Patients receiving surgery may also experience inclusion cyst development or pain recurrence/persistence at a rate 0-13% and will therefore undergo more than one surgery48.
As in all chronic pain conditions, long-standing vulvodynia is associated with a complex layering of neuropathology that includes supraspinal influences of depression, anxiety, hypervigilance, and catastrophization51,52. What is key and unique to the treatment of vulvodynia is the efficacy of targeted vestibular therapy. Clinical research support comes from a number of directions. First, a recent systematic review of vulvodynia treatment has shown a “complete relief of vulvodynia pain” effect size of 67% for surgical excision of the vestibule53. This clearly surpasses other published therapeutic modalities. Second, one of the few, well designed RCTs compared the therapeutic effectiveness of three modalities: surgical excision of the vestibule, cognitive behavioral therapy (which theoretically targets supraspinal neuropathology), and pelvic floor physiotherapy (targeting pelvic floor musculature)50. Although all treatments reduced vulvodynia pain and dysfunction, surgical excision was demonstrated to be most effective. The fact that removal of the vestibular tissue is effective in reducing pain suggests that inherent factors associated with the vulvar vestibule influence disease; the vulvar vestibule is derived from a different embryonic origin compared to the exterior vulva and vagina54. To place in perspective, this does not conclude that surgical excision of the vestibular tissue is the only effective future approach. Rather, medically-targeted intracellular intervention to the unexcised vestibule is both feasible and likely to correct pain in a majority of vulvodynia cases.
Summary
Although a number of vulvodynia causes have been theorized, no definitive mechanism has been defined and no therapy is effective in permanently eliminating all patient-reported symptoms. Current evidence suggests that several contributing factors and potentially overlapping mechanisms/etiologies are involved in generating chronic vulvar pain. Therefore, there is an urgent need to develop improved treatment strategies. Using both basic and clinical research strategies to better elucidate the origins of disease should lead to vast improvements in the available therapeutic tools, enabling clinicians to target the underlying causes of vulvodynia, directing our efforts toward primary prevention.
Inflammation revisited: evidence that vulvodynia may have an inflammatory basis
History of terminology
The original term vestibulitis eludes to the potential inflammatory origins of the disease; “itis” typically denotes an inflammatory condition55. The use of this term was supported by evidence that inflammatory cell infiltrates (e.g. mast cells) and inflammatory mediators were present in the vestibular tissue of women with vulvodynia56,57. However, inflammatory cells are also present in “healthy” women, indicating that this may be a normal state not associated with disease pathology58. Therefore, this condition was reclassified as vulvodynia in 2003, effectively removing the inflammatory classification and placing emphasis on allodynia (pain to light touch)55. However, recent studies have revisited the potential inflammatory origins and suggest that a less classical inflammatory presentation may contribute to chronic vulvar pain11-17.
New evidence implicating inflammation in vulvodynia
Although both LPV patients and healthy women show signs of infiltrating inflammatory cells, the relative abundance and organization of these cells may differ between patients and controls. A recent paper demonstrated that women with LPV have higher densities of immune cells in the vulvar vestibule16. LPV patients presented with greater numbers of B lymphocytes and mature mucosal IgA-plasma cells, while B and T cells were arranged into germinal centers in cases that were absent in controls16. However, similar to much earlier observations, cases and controls both showed the presence of antigen-presenting dendritic cells, macrophages, and mast cells, which were of roughly equivalent abundance16,58. In addition, LPV patients may have elevated levels of CD4 positive T cells, which are often recruited by allergic or infectious triggers17. Overall, the vestibular area appears to have a localized immune system that contributes to inflammation. Therefore, targeting inflammation may represent a valuable resource for the development of more efficacious therapies for vulvodynia, although, as for any therapy, it may not be equally effective for all LPV patients, based on individual disease profiles.
There is an established link between pain and inflammation; inflammation and pro-inflammatory mediators have been long associated with allodynia12,59-67. Allodynia is generally indistinguishable from neural pain fiber (nociceptor) sensitization and is often stimulated by the release of intradermal or subcutaneous pro‐inflammatory factors, including IL‐6 and prostaglandin E2 (PGE2)60,61. Such factors are frequently elevated in chronic pain conditions62,64, and elevated expression of PGE2 and IL‐6 provokes allodynia in both human and animal studies59,66, while suppression of these pro-inflammatory mediators alleviates allodynia68,69. We have determined that human fibroblasts isolated from painful vulvar sites produce elevated levels of IL‐6 and PGE2 compared to fibroblasts isolated from non-painful sites12,13. Furthermore, pro-inflammatory mediator production is elevated in fibroblasts isolated from vulvodynia patients compared to those isolated from “healthy” controls.
Other research groups have also shown that pro-inflammatory mediators are present/elevated in the vestibule of women with vulvodynia. One recent report examining pro-inflammatory mediator expression in the vestibular tissue of cases and controls detected tumor necrosis factor-α (TNF-α; a pro-inflammatory mediator) more readily in women with vulvodynia15, which is consistent with previous findings indicating that TNF-α and IL1-β are elevated in vulvodynia patients70. This study provides histological evidence suggesting that pro-inflammatory mediator production is elevated in the vestibule of vulvodynia patients15, which agrees with findings from our group that indicate vestibular fibroblasts from cases produce elevated levels of pro-inflammatory mediators11-13.
Clinically pain mapping the vulva of an affected patient finds that a mere 3 cm distance separates painful vestibular sites from the non-painful exterior vulva13. Despite the proximity to the external vulva, the vestibular tissue is derived from the endoderm,54 and this tissue likely holds distinct immunologic properties11-13. Furthermore, the vestibule of women with vulvodynia may also be hyperinnervated compared to pain-free controls71,72. However, hyperinnervation may lack specificity for vulvodynia, because it has been associated with itching in atopic dermatitis73, and neuropathic pain is usually linked to nerve loss, rather than increased nerve density74. We propose that hyperinnervation likely does not represent the pathophysiological foundation of vulvodynia, although it may play a role in this condition. Specifically, there may be an important relationship between hyperinnvervation and the inflammatory response; nerve fibers express receptors for recognizing inflammatory stimuli and produce pro-inflammatory mediators, while inflammatory stimuli may promote nerve growth (demonstrated in a mouse model of vulvodynia), and increased nerve density can exacerbate the inflammatory response18,75-77.
Stimuli associated with the development of vulvodynia
Another problem in linking LPV to inflammation is that the identification of precursors to vulvodynia onset has been subject to patient recall and represents a major hurtle in elucidating the origins of disease6,78,79. Patient interviews have generated long lists of possible catalysts, reflecting the complex etiology of LPV, which include childbirth, pregnancy, stress, diet, vulvovaginal infections, sexual/physical abuse, and injury, many of which have not been reliably associated with the onset of vulvar pain6,78,79. However, one consistent precipitating stimulus has been cited in greater than 70% of vulvodynia patients, which is a history of recurrent yeast infections80. The empirical evidence linking yeast infection to vulvodynia is limited, although in a mouse model, repeated vulvovaginal infection with Candida albicans, a common etiological agent of vulvovaginal yeast infection, results in contact hypersensitivity and pain, even after infection clearance18. Furthermore, a study in humans showed that vulvodynia patients are more likely to react to a patch test with C. albicans than “healthy” women19. Nonetheless, one important caveat to consider is that in most cases yeast infection is self-diagnosed and treated with topical over-the-counter preparations, offering the potential for misdiagnosis; self-reported yeast infection could represent other gynecological conditions or infections81. Therefore, it is plausible that additional organisms may play a role in this inflammatory response, while there is a clear role for Candida species. Mucous membranes are particularly vulnerable to microbial infection and utilize a number of often overlapping defense systems for responding to noxious stimuli including, fungi, bacteria, and viruses82.
Critics of the infectious origins theory have noted that women with vulvodynia do not present with yeast infection1,5,26. Furthermore, treatment with antifungals does not resolve the symptoms of vulvodynia83, although previous recurrent infection may be sufficient to elicit chronic pain18. However, patient vestibular fibroblasts respond to very low doses of C. albicans (less than 100 yeast cells), while pain-free external vulvar cells fail to respond11. Such a low dose of yeast is unlikely to be detected by our current clinical diagnostic methods (e.g. culture or DNA probe) and is typically not associated with active infection84. This may explain why women with vulvodynia do not present with yeast infection. Doses required to elicit a response in control fibroblasts and in the external vulvar fibroblasts of LPV patients were roughly 1000-fold greater, more consistent with infectious loads11. These findings suggest that the vulvar vestibule of women with vulvodynia is inherently sensitive to yeast; subclinical infection with C. albicans may be sensed by these fibroblasts to generate a maladaptive immune response. We propose that this represents dysregulation of a normally beneficial response that would typically help to maintain a healthy vulvovaginal flora.
Mechanisms for vulvar inflammation
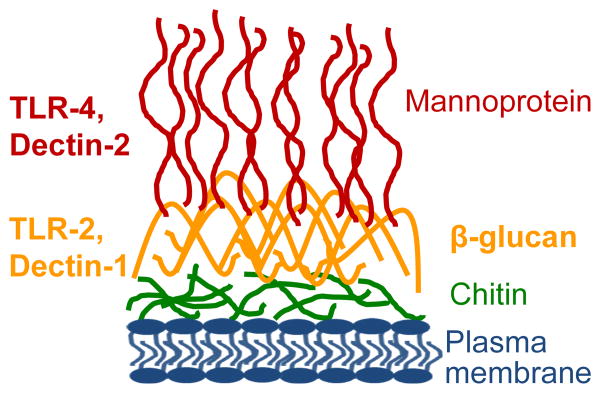
Research into the mechanisms that might govern inflammation led our group focus on Dectin‐1, a well-characterized yeast responsive pattern recognition receptor (PRR) that recognizes fungal β‐glucan11,85. The current literature suggests that β‐glucan is abundant during chronic infection86,87, while it is also probable that fibroblasts would be able to sense β‐glucan during infection, because C. albicans debrides the epithelium through protease secretion and invasion88,89. In turn, the underlying fibroblasts should be exposed to invading yeast and their products. We found that vestibular fibroblasts from vulvodynia patients express slightly elevated protein levels of Dectin‐1 compared to controls11. At the same time, Dectin‐1 is modestly elevated in vestibular versus external vulvar fibroblasts. Although we have not yet definitively demonstrated that increased receptor abundance accounts for heightened sensitivity, this is a plausible explanation we plan to investigate further. However, additional receptors (e.g. TLR‐2, TLR‐4; Figure 1) may also be involved in the heightened response to yeast or other microbial triggers, as we identified other active PRRs on vestibular fibroblasts. PRRs have been implicated in host recognition of a wide range pathogen-associated molecular patterns (PAMPs) expressed by yeast and even bacterial and viral species85,90. We suspect that the combined abundance and activity of these receptors influences the production of pro-inflammatory mediators, ultimately determining the overall pain profile.
Figure 1. The fungal cell wall and its paradigm receptors.
This illustration depicts the C. albicans cell wall, which is comprised of four major components: mannoprotein, β‐glucan, chitin, and the plasma membrane. Although, the mannoprotein layer is primarily exposed, β‐glucan and chitin are accessible at bud scars during cell division, and β‐glucan is actively secreted by C. albicans during chronic infection. We have focused on receptors involved in mannoprotein and glucan recognition (listed on the left), as they are major components of zymosan, which has also been established to elicit a strong response in human vulvar fibroblasts.
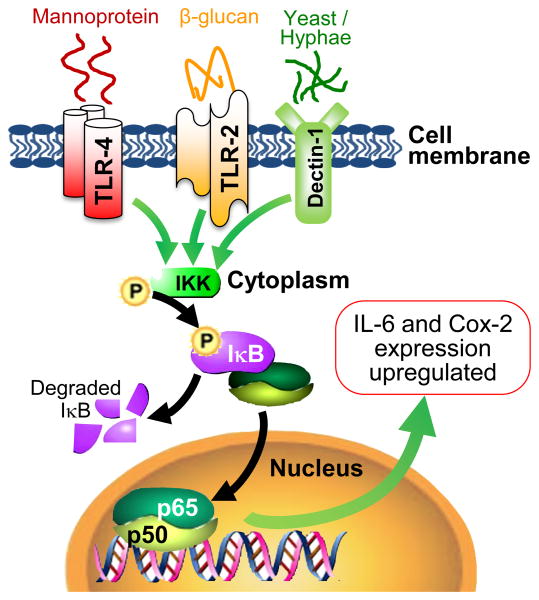
At present, this PRR-mediated response (summarized in Figure 2) is the only intracellular mechanism described for vulvodynia11. We acknowledge that more investigation will be required to completely elucidate the mechanisms of disease and definitively demonstrate that inflammation plays a causative role in vulvodynia. However, our research has uncovered potential targets for the development of additional LPV therapeutics. Not only have we shown that Dectin‐1 is more abundant on fibroblasts isolated from painful areas, but the activity of Dectin‐1 contributes to the production of pro-inflammatory mediators; blocking the function or expression of Dectin‐1 results in a significant decrease in IL‐6 and PGE2 production11. Dectin‐1 can signal through the NFkB pathway, a key pathway triggered during inflammation, which activates the transcription of pro-inflammatory mediators (e.g. IL‐6 and Cox‐2, involved in PGE2 production)91-93. Although NFkB activation has not been previously investigated in vulvar fibroblasts, our recent work demonstrates that the NFkB pathway is activated in cells stimulated with zymosan or live yeast11. Furthermore, inhibiting NFkB essentially abrogates pro-inflammatory mediator secretion in vulvar fibroblasts11. Therefore, we have already identified at least two potential targets for the development of new therapeutics: Dectin‐1 and NFkB. We expect our current line of investigation to identify other potentially more specific and selective targets.
Figure 2. Inflammatory PPR-mediated mechanisms implicated in vulvodynia.
This illustration depicts the transcriptional activation of pro-inflammatory mediators when Dectin‐1 and other PRRs (e.g. TLR‐2 and TLR‐4) signal through the NFkB pathway. Dectin‐1 senses live yeast and zymosan to elicit the production and release of pro-inflammatory mediators (IL‐6 and PGE2). Signaling through Dectin‐1 results in phosphorylation of NFkB inhibitors, which are subsequently degraded via proteolysis to allow NFkB subunits (associated with canonical pathway) to translocate to the nucleus to activate transcription of IL‐6 and Cox‐2 (rate-limiting enzyme in PGE2 synthesis). We have discovered the presence of TLR‐2 and TLR‐4 on vulvar fibroblasts; these PRRs have been shown to signal through NFkB in other cells types, although their function in vulvar fibroblasts has not been confirmed. We are investigating the role of these and other PRRs in sensing and responding to subclinical levels of yeast and other stimuli that may contribute to chronic vestibular inflammation.
Towards better therapeutics
Vulvodynia is a prevalent condition with severe consequences for afflicted women and their partners. However, treatments for vulvodynia fall short, and patients may not receive adequate relief, or symptoms may recur, even after undergoing invasive treatment (e.g. vestibulectomy)48-50. Multidisciplinary approaches have been most effective and all available evidence suggests these will continue to be5,6,25. However, taking into account the limited number of RCTs and the placebo effect, it is difficult to discern what the overall best course of treatment will be for a given woman26,36,38. This results in multiple visits to various physicians and therapists, during which time the patient may perceive that her situation is hopeless or is not being adequately addressed5,6. This only serves to augment the psychosomatic components of the disease33,34.
The monetary cost of vulvodynia care in the United States is greater than $8000/patient for a six-month course of treatment and bears with it an annual national burden in excess of $31 billion94. The sudden onset and crippling effects of vulvodynia, combined with its prevalence, translates to countless women willing to try nearly any therapy, regardless of cost or proven efficacy6. However, some measures taken by patients to “wash away” symptoms may only exacerbate them6. Therefore, it is imperative that we carefully examine the underlying mechanisms of disease and develop improved rationales for new therapies or enhanced formulations of current therapies. It is not our viewpoint that the current treatment modalities are invalid; most clinically implemented therapies do have at least some empiric evidence to support their use5,6,26. However, we envision a future where these therapies can be better implemented, along with new therapies targeting the inflammatory origins of disease. We believe that recent evidence is sufficient to implicate an inflammatory mechanism that serves a role in generating or amplifying vulvar pain. Therefore, the addition of strategies aimed at modulating this response will likely improve pain symptoms in women with vulvodynia.
Conclusion
By accepting inflammation as a possible contributing factor to the occurrence of vulvodynia, we open a new set of possibilities for the treatment and management of this disabling condition. Although we do not advocate for a terminology change (a return to vestibulitis) or drastic changes to how practitioners treat vulvodynia, we contend that researchers and clinicians alike should be aware that inflammation likely plays a role in this condition. Further investigation into how inflammation may influence LPV could lead to the development of new therapeutics or even the improved application of currently accepted and utilized therapies.
Acknowledgments
We thank Stephen J. Pollock for his assistance with figure design.
Funding: Relevant research performed at the University of Rochester is supported by NIH-NICHD R01 HD069313 and by NIH-NCATS CTSI grant TL1 TR000096.
Footnotes
Details of Ethics Approval: All human subjects' research performed at the University of Rochester was approved by the University of Rochester Institutional Review Board (RSRB # 42136), and all human subjects gave their informed written consent.
Disclosure of Interests: No conflicts of interest to disclose. The ICMJE disclosure forms are available as online supporting information.
Contribution to Authorship: MLF wrote the bulk of the paper. DCF, RPP, and ADB refined text and provided significant intellectual contributions. No competing interests. Work funded by NIH-NICHD R01 HD069313.
References
- 1.Groysman V. Vulvodynia: new concepts and review of the literature. Dermatol Clin. 2010 Oct;28(4):681–96. doi: 10.1016/j.det.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 2.Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: population-based estimates from 2 geographic regions. American journal of obstetrics and gynecology. 2014 Jan;210(1):40 e1–8. doi: 10.1016/j.ajog.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reed BD, Harlow SD, Sen A, Legocki LJ, Edwards RM, Arato N, et al. Prevalence and demographic characteristics of vulvodynia in a population-based sample. American journal of obstetrics and gynecology. 2012 Feb;206(2):170 e1–9. doi: 10.1016/j.ajog.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haefner HK. Report of the International Society for the Study of Vulvovaginal Disease terminology and classification of vulvodynia. Journal of lower genital tract disease. 2007 Jan;11(1):48–9. doi: 10.1097/01.lgt.0000225898.37090.04. [DOI] [PubMed] [Google Scholar]
- 5.Haefner HK, Collins ME, Davis GD, Edwards L, Foster DC, Hartmann ED, et al. The vulvodynia guideline. Journal of lower genital tract disease. 2005 Jan;9(1):40–51. doi: 10.1097/00128360-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Sadownik LA. Etiology, diagnosis, and clinical management of vulvodynia. Int J Womens Health. 2014;6:437–49. doi: 10.2147/IJWH.S37660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Safi ZA, Santoro N. Menopausal hormone therapy and menopausal symptoms. Fertility and sterility. 2014 Apr;101(4):905–15. doi: 10.1016/j.fertnstert.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Babula O, Linhares IM, Bongiovanni AM, Ledger WJ, Witkin SS. Association between primary vulvar vestibulitis syndrome, defective induction of tumor necrosis factor-alpha, and carriage of the mannose-binding lectin codon 54 gene polymorphism. American journal of obstetrics and gynecology. 2008 Jan;198(1):101 e1–4. doi: 10.1016/j.ajog.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 9.Foster DC, Sazenski TM, Stodgell CJ. Impact of genetic variation in interleukin-1 receptor antagonist and melanocortin-1 receptor genes on vulvar vestibulitis syndrome. The Journal of reproductive medicine. 2004 Jul;49(7):503–9. [PubMed] [Google Scholar]
- 10.Lynch PJ. Vulvodynia as a somatoform disorder. The Journal of reproductive medicine. 2008 Jun;53(6):390–6. [PubMed] [Google Scholar]
- 11.Falsetta ML, Foster DC, Woeller CF, Pollock SJ, Bonham AD, Haidaris CG, et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. American journal of obstetrics and gynecology. 2015 12 Feb; doi: 10.1016/j.ajog.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster D, Falsetta M, Woeller C, Pollock S, Song K, Bonham A, et al. Site-specific mesenchymal control of inflammatory pain to yeast challenge in vulvodynia afflicted and pain-free women. Pain. 2015 Mar;156(3):386–96. doi: 10.1097/01.j.pain.0000460320.95267.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster DC, Piekarz KH, Murant TI, LaPoint R, Haidaris CG, Phipps RP. Enhanced synthesis of proinflammatory cytokines by vulvar vestibular fibroblasts: implications for vulvar vestibulitis. American journal of obstetrics and gynecology. 2007 Apr;196(4):346 e1–8. doi: 10.1016/j.ajog.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 14.Harlow BL, He W, Nguyen RH. Allergic reactions and risk of vulvodynia. Annals of epidemiology. 2009 Nov;19(11):771–7. doi: 10.1016/j.annepidem.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seckin-Alac E, Akhant SE, Bastu E, Tuzlalik S, Yavuz E. Elevated tissue levels of tumor necrosis factor-alpha in vulvar vestibulitis syndrome. Clinical and experimental obstetrics & gynecology. 2014;41(6):691–3. [PubMed] [Google Scholar]
- 16.Tommola P, Butzow R, Unkila-Kallio L, Paavonen J, Meri S. Activation of vestibule-associated lymphoid tissue in localized provoked vulvodynia. American journal of obstetrics and gynecology. 2015 Apr;212(4):476 e1–8. doi: 10.1016/j.ajog.2014.10.1098. [DOI] [PubMed] [Google Scholar]
- 17.Leclair CM, Leeborg NJ, Jacobson-Dunlop E, Goetsch MF, Morgan TK. CD4-positive T-cell recruitment in primary-provoked localized vulvodynia: potential insights into disease triggers. Journal of lower genital tract disease. 2014 Apr;18(2):195–201. doi: 10.1097/LGT.0b013e3182a55591. [DOI] [PubMed] [Google Scholar]
- 18.Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, Milagrosa ZE, et al. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci Transl Med. 2011 Sep 21;3(101):101ra91. doi: 10.1126/scitranslmed.3002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramirez De Knott HM, McCormick TS, Do SO, Goodman W, Ghannoum MA, Cooper KD, et al. Cutaneous hypersensitivity to Candida albicans in idiopathic vulvodynia. Contact Dermatitis. 2005 Oct;53(4):214–8. doi: 10.1111/j.0105-1873.2005.00685.x. [DOI] [PubMed] [Google Scholar]
- 20.Turner ML, Marinoff SC. Association of human papillomavirus with vulvodynia and the vulvar vestibulitis syndrome. The Journal of reproductive medicine. 1988 Jun;33(6):533–7. [PubMed] [Google Scholar]
- 21.Edwards L, Mason M, Phillips M, Norton J, Boyle M. Childhood sexual and physical abuse. Incidence in patients with vulvodynia. The Journal of reproductive medicine. 1997 Mar;42(3):135–9. [PubMed] [Google Scholar]
- 22.Khandker M, Brady SS, Stewart EG, Harlow BL. Is chronic stress during childhood associated with adult-onset vulvodynia? Journal of women's health. 2014 Aug;23(8):649–56. doi: 10.1089/jwh.2013.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gentilcore-Saulnier E, McLean L, Goldfinger C, Pukall CF, Chamberlain S. Pelvic floor muscle assessment outcomes in women with and without provoked vestibulodynia and the impact of a physical therapy program. The journal of sexual medicine. 2010 Feb;7(2 Pt 2):1003–22. doi: 10.1111/j.1743-6109.2009.01642.x. [DOI] [PubMed] [Google Scholar]
- 24.Reissing ED, Brown C, Lord MJ, Binik YM, Khalife S. Pelvic floor muscle functioning in women with vulvar vestibulitis syndrome. Journal of psychosomatic obstetrics and gynaecology. 2005 Jun;26(2):107–13. doi: 10.1080/01443610400023106. [DOI] [PubMed] [Google Scholar]
- 25.Nunns D, Mandal D, Byrne M, McLelland J, Rani R, Cullimore J, et al. Guidelines for the management of vulvodynia. The British journal of dermatology. 2010 Jun;162(6):1180–5. doi: 10.1111/j.1365-2133.2010.09684.x. [DOI] [PubMed] [Google Scholar]
- 26.Eppsteiner E, Boardman L, Stockdale CK. Vulvodynia. Best practice & research Clinical obstetrics & gynaecology. 2014 Oct;28(7):1000–12. doi: 10.1016/j.bpobgyn.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. Journal of lower genital tract disease. 2012 Oct;16(4):427–36. doi: 10.1097/LGT.0b013e31825a2274. [DOI] [PubMed] [Google Scholar]
- 28.Keppel Hesselink JM, Kopsky DJ, Sajben NL. Vulvodynia and proctodynia treated with topical baclofen 5 % and palmitoylethanolamide. Archives of gynecology and obstetrics. 2014 Aug;290(2):389–93. doi: 10.1007/s00404-014-3218-4. [DOI] [PubMed] [Google Scholar]
- 29.Murina F, Radici G, Bianco V. Capsaicin and the treatment of vulvar vestibulitis syndrome: a valuable alternative? MedGenMed : Medscape general medicine. 2004;6(4):48. [PMC free article] [PubMed] [Google Scholar]
- 30.Steinberg AC, Oyama IA, Rejba AE, Kellogg-Spadt S, Whitmore KE. Capsaicin for the treatment of vulvar vestibulitis. American journal of obstetrics and gynecology. 2005 May;192(5):1549–53. doi: 10.1016/j.ajog.2004.10.626. [DOI] [PubMed] [Google Scholar]
- 31.Pagano R, Wong S. Use of amitriptyline cream in the management of entry dyspareunia due to provoked vestibulodynia. Journal of lower genital tract disease. 2012 Oct;16(4):394–7. doi: 10.1097/LGT.0b013e3182449bd6. [DOI] [PubMed] [Google Scholar]
- 32.Boardman LA, Cooper AS, Blais LR, Raker CA. Topical gabapentin in the treatment of localized and generalized vulvodynia. Obstetrics and gynecology. 2008 Sep;112(3):579–85. doi: 10.1097/AOG.0b013e3181827c77. [DOI] [PubMed] [Google Scholar]
- 33.Bond KS, Weerakoon P, Shuttleworth R. A literature review on vulvodynia and distress. Sex Relatsh Ther. 2012;27(1):46–62. [Google Scholar]
- 34.Bergeron S, Likes WM, Steben M. Psychosexual aspects of vulvovaginal pain. Best practice & research Clinical obstetrics & gynaecology. 2014 Oct;28(7):991–9. doi: 10.1016/j.bpobgyn.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 35.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Archives of neurology. 2003 Nov;60(11):1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 36.Foster DC, Kotok MB, Huang LS, Watts A, Oakes D, Howard FM, et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstetrics and gynecology. 2010 Sep;116(3):583–93. doi: 10.1097/AOG.0b013e3181e9e0ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeon Y, Kim Y, Shim B, Yoon H, Park Y, Shim B, et al. A retrospective study of the management of vulvodynia. Korean journal of urology. 2013 Jan;54(1):48–52. doi: 10.4111/kju.2013.54.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown CS, Foster DC, Wan JY, Rawlinson LA, Bachmann GA, Gabapentin Study G. Rationale and design of a multicenter randomized clinical trial of extended release gabapentin in provoked vestibulodynia and biological correlates of response. Contemporary clinical trials. 2013 Sep;36(1):154–65. doi: 10.1016/j.cct.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leo RJ. A systematic review of the utility of anticonvulsant pharmacotherapy in the treatment of vulvodynia pain. The journal of sexual medicine. 2013 Aug;10(8):2000–8. doi: 10.1111/jsm.12200. [DOI] [PubMed] [Google Scholar]
- 40.Spoelstra SK, Borg C, Weijmar Schultz WC. Anticonvulsant pharmacotherapy for generalized and localized vulvodynia: a critical review of the literature. Journal of psychosomatic obstetrics and gynaecology. 2013 Sep;34(3):133–8. doi: 10.3109/0167482X.2013.823942. [DOI] [PubMed] [Google Scholar]
- 41.McKay E, Kaufman RH, Doctor U, Berkova Z, Glazer H, Redko V. Treating vulvar vestibulitis with electromyographic biofeedback of pelvic floor musculature. The Journal of reproductive medicine. 2001 Apr;46(4):337–42. [PubMed] [Google Scholar]
- 42.Naess I, Bo K. Pelvic floor muscle function in women with provoked vestibulodynia and asymptomatic controls. International urogynecology journal. 2015 4 Mar; doi: 10.1007/s00192-015-2660-6. [DOI] [PubMed] [Google Scholar]
- 43.Masheb RM, Kerns RD, Lozano C, Minkin MJ, Richman S. A randomized clinical trial for women with vulvodynia: Cognitive-behavioral therapy vs. supportive psychotherapy. Pain. 2009 Jan;141(1-2):31–40. doi: 10.1016/j.pain.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goldstein AT, Burrows LJ, Kellogg-Spadt S. Intralevator injection of botulinum toxin for the treatment of hypertonic pelvic floor muscle dysfunction and vestibulodynia. The journal of sexual medicine. 2011 May;8(5):1287–90. doi: 10.1111/j.1743-6109.2011.02270.x. [DOI] [PubMed] [Google Scholar]
- 45.Pelletier F, Parratte B, Penz S, Moreno JP, Aubin F, Humbert P. Efficacy of high doses of botulinum toxin A for treating provoked vestibulodynia. The British journal of dermatology. 2011 Mar;164(3):617–22. doi: 10.1111/j.1365-2133.2011.10235.x. [DOI] [PubMed] [Google Scholar]
- 46.Petersen CD, Giraldi A, Lundvall L, Kristensen E. Botulinum toxin type A-a novel treatment for provoked vestibulodynia? Results from a randomized, placebo controlled, double blinded study. The journal of sexual medicine. 2009 Sep;6(9):2523–37. doi: 10.1111/j.1743-6109.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 47.Tieu KD, MacGregor JL. Successful treatment of vulvodynia with botulinum toxin A. Archives of dermatology. 2011 Feb;147(2):251–2. doi: 10.1001/archdermatol.2010.443. [DOI] [PubMed] [Google Scholar]
- 48.Tommola P, Unkila-Kallio L, Paavonen J. Surgical treatment of vulvar vestibulitis: a review. Acta obstetricia et gynecologica Scandinavica. 2010 Nov;89(11):1385–95. doi: 10.3109/00016349.2010.512071. [DOI] [PubMed] [Google Scholar]
- 49.Tommola P, Unkila-Kallio L, Paavonen J. Long-term follow up of posterior vestibulectomy for treating vulvar vestibulitis. Acta obstetricia et gynecologica Scandinavica. 2011 Nov;90(11):1225–31. doi: 10.1111/j.1600-0412.2011.01248.x. [DOI] [PubMed] [Google Scholar]
- 50.Bergeron S, Binik YM, Khalife S, Pagidas K, Glazer HI, Meana M, et al. A randomized comparison of group cognitive--behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain. 2001 Apr;91(3):297–306. doi: 10.1016/S0304-3959(00)00449-8. [DOI] [PubMed] [Google Scholar]
- 51.Pukall CF, Binik YM, Khalife S, Amsel R, Abbott FV. Vestibular tactile and pain thresholds in women with vulvar vestibulitis syndrome. Pain. 2002 Mar;96(1-2):163–75. doi: 10.1016/s0304-3959(01)00442-0. [DOI] [PubMed] [Google Scholar]
- 52.Sutton KS, Pukall CF, Chamberlain S. Pain ratings, sensory thresholds, and psychosocial functioning in women with provoked vestibulodynia. Journal of sex & marital therapy. 2009;35(4):262–81. doi: 10.1080/00926230902851256. [DOI] [PubMed] [Google Scholar]
- 53.Andrews JC. Vulvodynia interventions--systematic review and evidence grading. Obstetrical & gynecological survey. 2011 May;66(5):299–315. doi: 10.1097/OGX.0b013e3182277fb7. [DOI] [PubMed] [Google Scholar]
- 54.Puppo V. Embryology and anatomy of the vulva: the female orgasm and women's sexual health. European journal of obstetrics, gynecology, and reproductive biology. 2011 Jan;154(1):3–8. doi: 10.1016/j.ejogrb.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 55.Moyal-Barracco M, Lynch PJ. 2003 ISSVD terminology and classification of vulvodynia: a historical perspective. The Journal of reproductive medicine. 2004 Oct;49(10):772–7. [PubMed] [Google Scholar]
- 56.Chaim W, Meriwether C, Gonik B, Qureshi F, Sobel JD. Vulvar vestibulitis subjects undergoing surgical intervention: a descriptive analysis and histopathological correlates. European journal of obstetrics, gynecology, and reproductive biology. 1996 Sep;68(1-2):165–8. doi: 10.1016/0301-2115(96)02502-x. [DOI] [PubMed] [Google Scholar]
- 57.Prayson RA, Stoler MH, Hart WR. Vulvar vestibulitis. A histopathologic study of 36 cases, including human papillomavirus in situ hybridization analysis. The American journal of surgical pathology. 1995 Feb;19(2):154–60. doi: 10.1097/00000478-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Lundqvist EN, Hofer PA, Olofsson JI, Sjoberg I. Is vulvar vestibulitis an inflammatory condition? A comparison of histological findings in affected and healthy women. Acta dermato-venereologica. 1997 Jul;77(4):319–22. doi: 10.2340/0001555577319322. [DOI] [PubMed] [Google Scholar]
- 59.Cui JG, Holmin S, Mathiesen T, Meyerson BA, Linderoth B. Possible role of inflammatory mediators in tactile hypersensitivity in rat models of mononeuropathy. Pain. 2000 Dec 1;88(3):239–48. doi: 10.1016/S0304-3959(00)00331-6. [DOI] [PubMed] [Google Scholar]
- 60.Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. British journal of pharmacology. 1992 Nov;107(3):660–4. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eliav E, Benoliel R, Herzberg U, Kalladka M, Tal M. The role of IL-6 and IL-1beta in painful perineural inflammatory neuritis. Brain, behavior, and immunity. 2009 May;23(4):474–84. doi: 10.1016/j.bbi.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Firestein GS, Alvaro-Gracia JM, Maki R. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. Journal of immunology. 1990 May 1;144(9):3347–53. [PubMed] [Google Scholar]
- 63.Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002 Mar;96(1-2):57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- 64.Cavalli F, Mucci MP, Cociancich L, Micheli W, Bacarini L, Cisternino M. Prostaglandin E2 liberation in the synovial fluid induced by organo-iodinated contrast media. Interrelations with the genesis of post-arthrographic pain. La Radiologia medica. 1987 Dec;74(6):512–5. [PubMed] [Google Scholar]
- 65.Kawabata A. Prostaglandin E2 and pain--an update. Biological & pharmaceutical bulletin. 2011;34(8):1170–3. doi: 10.1248/bpb.34.1170. [DOI] [PubMed] [Google Scholar]
- 66.Lin CR, Amaya F, Barrett L, Wang H, Takada J, Samad TA, et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. The Journal of pharmacology and experimental therapeutics. 2006 Dec;319(3):1096–103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 67.Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daymond TJ, Rowell FJ. Reduction of prostaglandin E2 concentrations in synovial fluid of patients suffering from rheumatoid arthritis following tiaprofenic acid or indomethacin treatment. Drugs. 1988;35(Suppl 1):4–8. doi: 10.2165/00003495-198800351-00004. [DOI] [PubMed] [Google Scholar]
- 69.Zanjani TM, Sabetkasaei M, Mosaffa N, Manaheji H, Labibi F, Farokhi B. Suppression of interleukin-6 by minocycline in a rat model of neuropathic pain. European journal of pharmacology. 2006 May 24;538(1-3):66–72. doi: 10.1016/j.ejphar.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 70.Foster DC, Hasday JD. Elevated tissue levels of interleukin-1 beta and tumor necrosis factor-alpha in vulvar vestibulitis. Obstetrics and gynecology. 1997 Feb;89(2):291–6. doi: 10.1016/S0029-7844(96)00447-4. [DOI] [PubMed] [Google Scholar]
- 71.Bohm-Starke N, Hilliges M, Falconer C, Rylander E. Increased intraepithelial innervation in women with vulvar vestibulitis syndrome. Gynecologic and obstetric investigation. 1998;46(4):256–60. doi: 10.1159/000010045. [DOI] [PubMed] [Google Scholar]
- 72.Tympanidis P, Terenghi G, Dowd P. Increased innervation of the vulval vestibule in patients with vulvodynia. The British journal of dermatology. 2003 May;148(5):1021–7. doi: 10.1046/j.1365-2133.2003.05308.x. [DOI] [PubMed] [Google Scholar]
- 73.Tominaga M, Takamori K. Itch and nerve fibers with special reference to atopic dermatitis: therapeutic implications. The Journal of dermatology. 2014 Mar;41(3):205–12. doi: 10.1111/1346-8138.12317. [DOI] [PubMed] [Google Scholar]
- 74.Rowbotham MC, Yosipovitch G, Connolly MK, Finlay D, Forde G, Fields HL. Cutaneous innervation density in the allodynic form of postherpetic neuralgia. Neurobiology of disease. 1996;3(3):205–14. doi: 10.1006/nbdi.1996.0021. [DOI] [PubMed] [Google Scholar]
- 75.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nature neuroscience. 2012 Aug;15(8):1063–7. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chakrabarty A, McCarson KE, Smith PG. Hypersensitivity and hyperinnervation of the rat hind paw following carrageenan-induced inflammation. Neuroscience letters. 2011 May 9;495(1):67–71. doi: 10.1016/j.neulet.2011.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Margolis KG, Stevanovic K, Karamooz N, Li ZS, Ahuja A, D'Autreaux F, et al. Enteric neuronal density contributes to the severity of intestinal inflammation. Gastroenterology. 2011 Aug;141(2):588–98. 98 e1–2. doi: 10.1053/j.gastro.2011.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reed BD, Legocki LJ, Plegue MA, Sen A, Haefner HK, Harlow SD. Factors associated with vulvodynia incidence. Obstetrics and gynecology. 2014 Feb;123(2 Pt 1):225–31. doi: 10.1097/AOG.0000000000000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arnold LD, Bachmann GA, Rosen R, Kelly S, Rhoads GG. Vulvodynia: characteristics and associations with comorbidities and quality of life. Obstetrics and gynecology. 2006 Mar;107(3):617–24. doi: 10.1097/01.AOG.0000199951.26822.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Donders G, Bellen G. Characteristics of the pain observed in the focal vulvodynia syndrome (VVS) Med Hypotheses. 2012 Jan;78(1):11–4. doi: 10.1016/j.mehy.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 81.Hoffstetter SE, Barr S, LeFevre C, Leong FC, Leet T. Self-reported yeast symptoms compared with clinical wet mount analysis and vaginal yeast culture in a specialty clinic setting. The Journal of reproductive medicine. 2008 Jun;53(6):402–6. [PubMed] [Google Scholar]
- 82.Nasu K, Narahara H. Pattern recognition via the toll-like receptor system in the human female genital tract. Mediators of inflammation. 2010;2010:976024. doi: 10.1155/2010/976024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bornstein J, Livnat G, Stolar Z, Abramovici H. Pure versus complicated vulvar vestibulitis: a randomized trial of fluconazole treatment. Gynecologic and obstetric investigation. 2000;50(3):194–7. doi: 10.1159/000010309. [DOI] [PubMed] [Google Scholar]
- 84.Brown CJ, Wong M, Davis CC, Kanti A, Zhou X, Forney LJ. Preliminary characterization of the normal microbiota of the human vulva using cultivation-independent methods. Journal of medical microbiology. 2007 Feb;56(Pt 2):271–6. doi: 10.1099/jmm.0.46607-0. [DOI] [PubMed] [Google Scholar]
- 85.Bourgeois C, Kuchler K. Fungal pathogens-a sweet and sour treat for toll-like receptors. Frontiers in cellular and infection microbiology. 2012;2:142. doi: 10.3389/fcimb.2012.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chaffin WL. Candida albicans cell wall proteins. Microbiol Mol Biol Rev. 2008 Sep;72(3):495–544. doi: 10.1128/MMBR.00032-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Taff HT, Nett JE, Zarnowski R, Ross KM, Sanchez H, Cain MT, et al. A Candida biofilm-induced pathway for matrix glucan delivery: implications for drug resistance. PLoS Pathog. 2012;8(8):e1002848. doi: 10.1371/journal.ppat.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giraldo P, von Nowaskonski A, Gomes FA, Linhares I, Neves NA, Witkin SS. Vaginal colonization by Candida in asymptomatic women with and without a history of recurrent vulvovaginal candidiasis. Obstetrics and gynecology. 2000 Mar;95(3):413–6. doi: 10.1016/s0029-7844(99)00577-3. [DOI] [PubMed] [Google Scholar]
- 89.Taylor BN, Staib P, Binder A, Biesemeier A, Sehnal M, Rollinghoff M, et al. Profile of Candida albicans-secreted aspartic proteinase elicited during vaginal infection. Infection and immunity. 2005 Mar;73(3):1828–35. doi: 10.1128/IAI.73.3.1828-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nochi T, Kiyono H. Innate immunity in the mucosal immune system. Current pharmaceutical design. 2006;12(32):4203–13. doi: 10.2174/138161206778743457. [DOI] [PubMed] [Google Scholar]
- 91.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes & development. 2004 Sep 15;18(18):2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 92.Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor perspectives in biology. 2009 Dec;1(6):a001651. doi: 10.1101/cshperspect.a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geijtenbeek TB, Gringhuis SI. Signalling through C-type lectin receptors: shaping immune responses. Nature reviews Immunology. 2009 Jul;9(7):465–79. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie Y, Shi L, Xiong X, Wu E, Veasley C, Dade C. Economic burden and quality of life of vulvodynia in the United States. Current medical research and opinion. 2012 Apr;28(4):601–8. doi: 10.1185/03007995.2012.666963. [DOI] [PubMed] [Google Scholar]