Abstract
Adenosine is an endogenous nucleoside that modulates important physiological processes, such as vasodilation, in the central nervous system. A rapid, 2–4 seconds, mode of adenosine signaling has been recently discovered, but the relationship between this type of adenosine and blood flow change has not been characterized. In this study, adenosine and oxygen changes were simultaneously measured using fast-scan cyclic voltammetry. Oxygen changes occur when there is an increase in local cerebral blood flow and thus are a measure of vasodilation. About 34% of adenosine transients in the rat caudate-putamen are correlated with a subsequent transient change in oxygen. The amount of oxygen was correlated with the concentration of adenosine release and larger adenosine transients (over 0.4 μM) always had subsequent oxygen changes. The average duration of adenosine and oxygen transients were 3.2 seconds and 3.5 seconds, respectively. On average, the adenosine release starts and peaks 0.2 seconds prior to the oxygen. The A2a antagonist, SCH442416, decreased the number of both adenosine and oxygen transient events by about 32%. However, the A1 antagonist, DPCPX, did not significantly affect simultaneous adenosine and oxygen release. The nitric oxide (NO) synthase inhibitor L-NAME also did not affect the concentration or number of adenosine and oxygen release events. These results demonstrate that both adenosine and oxygen release are modulated via A2a receptors. The correlation of transient concentrations, time delay between adenosine and oxygen peaks, and effect of A2a receptors suggests adenosine modulates blood flow on a rapid, sub-second time scale.
Keywords: Adenosine, Oxygen, in vivo, blood flow, Fast scan cyclic voltammetry, FSCV
Introduction
Adenosine is an endogenous nucleoside involved in many important biochemical processes throughout the body, including energy balance. Adenosine formation increases during times of accelerated neuronal activity (Benington and Heller, 1995; Obal and Krueger, 2003), and it can be released from cells, becoming an extracellular signal that more energy is being consumed. One way adenosine signals for more energy to be delivered is by acting as a vasodilator, increasing blood flow (Phillis, 1989; Pelligrino et al., 2011). Tissue metabolism has a continuous need for oxygen, which usually is delivered by blood flow (Lowry et al., 1997a). In stressful conditions, when energy consumption is high, vasodilation occurs and the amount of oxygen delivered to the tissue is actually higher than during normoxic conditions. Previous studies showed that the concentration of adenosine in extracellular space of the brain dramatically increases during conditions such as ischemia (Winn et al., 1981b; Van Wylen et al., 1986; Paul et al., 2011). Endogenous adenosine acts as a vasodilator to increase cerebral blood flow (Winn et al., 1981a), an effect primarily mediated by A2a receptors located on blood vessels (Shryock and Belardinelli, 1997; Belardinelli et al., 1998); however, the relationship between adenosine and oxygen and the extent to which A2a receptors regulating both adenosine and blood flow changes are not fully known.
Local blood flow increases are measured in several ways. One is hydrogen clearance (Demchenko et al., 1998; Barbu et al., 2015), where platinum electrodes are used to measure the clearance of inhaled H2. Another method for estimating blood flow is measuring changes in cerebrovascular resistance using laser Doppler spectroscopy (Kusano et al., 2010). Changes in vessel diameter are also measured to investigate cerebral vasodilation after pharmacological administration (Ngai et al., 2001). Blood flow changes can be estimated by monitoring oxygen level changes using blood-oxygen level dependent (BOLD) imaging (Morton et al., 2002; Mulderink et al., 2002). The increase in oxygen can also be measured electrochemically, using either amperometry or fast-scan cyclic voltammetry (FSCV) (Zimmerman et al., 1992; Lowry et al., 1997a). FSCV is useful because it can measure both neurotransmission and oxygen levels. For example, FSCV has been used to measure dopamine or norepinephrine levels simultaneously with oxygen levels, using a modified waveform that scans down to −1.4 V to measure oxygen reduction (Venton et al., 2003; Bucher et al., 2014). To measure adenosine and oxygen levels, the FSCV waveform can be modified to scan up to 1.5 V and down to −1.4 V to both oxidize adenosine and reduce oxygen (Cechova and Venton, 2008).
Previous studies have shown that adenosine accumulates in times of stress, such as ischemia, and can induce vasodilation and change cerebral blood flow. Recently, a new type of rapid adenosine release has been characterized in the caudate-putamen and prefrontal cortex regions in vivo (Nguyen et al., 2014). This spontaneous, transient adenosine release is fast, lasting only 2 – 5 seconds in the extracellular space (Nguyen et al., 2015). Transient adenosine release can modulate dopamine neurotransmission on a rapid time scale (Ross and Venton, 2015). However, it is not known if this rapid mode of adenosine release has an effect on cerebral blood flow.
The goal of this study was to determine the relationship between transient adenosine and changes in cerebral blood flow. We tested the hypothesis that spontaneous, transient adenosine is correlated with vasodilation in local areas of the brain. Adenosine and oxygen transients were measured simultaneously in caudate-putamen of anesthetized rats using FSCV. Oxygen release was detected after about 34% of adenosine transients. The concentrations of adenosine and oxygen changes were correlated and big adenosine transients were always followed by an oxygen transient. The A2a antagonist, SCH442416, decreased the number of adenosine and oxygen transients and increased the time interval between each transient release but A1 antagonist, DPCPX, did not. Thus, endogenous transient adenosine was correlated with transient local vasodilation and both were modulated through A2a receptors.
Materials and Methods
Chemicals and Drugs
All components of the phosphate buffered saline (PBS) solution (in mM: 3.0 KCl, 10.0 NaH2PO4, 2.0 Na2SO4, 1.2 MgCl2, 131.25 NaCl and 1.2 CaCl2, with pH adjusted to 7.4) were purchased from Fisher Scientific (Fair Lawn, NJ, USA) and used for electrode calibration. Adenosine was purchased from Sigma-Aldrich (Milwaukee, WI, USA). A 10.0 mM stock solution of adenosine was prepared in 0.1 M perchloric acid (HClO4) and stored in the refrigerator. A 1.0 μM adenosine solution was made in PBS on the day of use. All aqueous solutions were prepared using deionized water (Milli-Q Biocel; Millipore, Billerica, MA, USA).
8-Cyclopentyl-1, 3-dipropylxanthine (DPCPX, 6 mg/kg, Sigma-Aldrich) and 2-(2- Furanyl)-7-[3-(4-methoxyphenyl) propyl]-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5- amine (SCH442416, 3 mg/kg, Tocris, UK) were dissolved in dimethyl sulfoxide (DMSO, Amresco, Solon, OH, USA), whereas Nω-Nitro-L-arginine methyl ester hydrochloride ( L-NAME, 100 mg/kg, Sigma-Aldrich) was dissolved in 1 mL heated saline and all drugs were administered intraperitoneally (i.p). These doses were selected based on previous experiments that were used in the literature (Orrú et al.; Venton et al., 2003; Nguyen et al., 2014).
Fabrication of Carbon-Fiber Microelectrodes and Fast Scan Cyclic Voltammetry
Carbon-fiber microelectrodes were fabricated by aspirating a 7 μm diameter T-650 carbon-fiber (Cytec Engineering Materials, West Patterson, NJ, USA) into a glass capillary (1.2 mm x 0.68 mm; A-M System, Inc., Seqium, WA, USA) and pulling it in a vertical micropipette puller (model PE-21; Narishige, Tokyo, Japan). The extended carbon fiber was cut with a scalpel to a length of around 100 μm. Prior to use, electrodes were soaked in 2-propanol for at least 10 minutes and then backfilled with 1 M KCl.
Fast-scan cyclic voltammetry (FSCV) was used to detect adenosine and oxygen with sub-second temporal resolution. The cyclic voltammetric waveform was generated and the resulting signals were computer controlled by Tar Heel CV or HDCV (from Mark Wightman, UNC at Chapel Hill), written in LabVIEW (National Instruments, Austin, TX, USA). A Dagan Chem Clamp potentiostat (Dagan Corporation; Minneapolis, MN, USA) was used to apply the potential and measure the current. For simultaneous detection of adenosine and oxygen, the electrode was scanned from 0 V to 1.45 V, then −1.4 V and back to 0 V every 100 milliseconds at 450 V/s. The reference electrode was a Ag/AgCl electrode. All color plots and cyclic voltammograms were background subtracted to remove any non-Faradic currents, by subtracting the average of 10 CVs collected no more than 10 seconds before the event.
Flow-Injection Apparatus and in vitro Calibrations
A flow-injection apparatus was used to calibrate the electrode in concentrations of adenosine and oxygen after in vivo experimentation (Strand and Venton, 2008). Electrodes were calibrated with 1.0 μM adenosine in PBS buffer. To calibrate oxygen concentration, air- and nitrogen-saturated PBS buffers were used. Oxygen concentrations were measured in various mixtures of nitrogen- and air-saturated PBS with volume ratios of 2:1, 5:1, 10:1, and 15:1 using D.O. 6+ dissolved oxygen meter (Eutech Instruments Pte Ltd. Singapore). The concentration of oxygen in each solution was compared to nitrogen-saturated PBS, which contains no oxygen. Peak reduction currents for oxygen were used for calibration, shown in Fig. S1.
Animals and Surgery
Male Sprague-Dawley rats (250 – 350 g; Charles-River, Wilmington, MA, USA) were housed on a 12:12-h light/dark cycle with food and water provided ad libitum. Rats were initially anesthetized with isoflurane (1 mL/100 g rat weight) and then injected with urethane (1.5 g/kg, i. p.). The surgical site was shaved and the rat was placed in a stereotaxic frame. Bupivacaine (0.25 mL) (Sensorcaine® MPF, APP Pharmaceuticals, LLC; Schaumburg, IL, USA) was administered under the skin for local anesthesia. Holes were drilled for electrode placement after the skull was exposed using a stereotaxic drill. Electrodes were placed in the caudate-putamen (+ 1.2 mm anterior-posterior (AP), + 2.0 mm mediolateral (ML), and − 4.5 mm dorsoventral (DV). A Ag/AgCl reference electrode was inserted on the contralateral side of brain. The body temperature of the rat was maintained at 37 °C using an isothermal pad (FHC, Bowdoin, ME, USA). All experiments were approved by the Institutional Animal Care and Use Committee of the University of Virginia.
Data Collection and Analysis
Electrodes were implanted and equilibrated for at least 30 minutes with the applied waveform prior to data collection. Data were excluded if less than 10 transients were observed within the initial 30 minutes. If robust transients were not found, a new electrode was inserted and up to five electrodes were tried for each animal. After transients were identified, the electrode placement was optimized and data were collected for four hours.
Principal component regression analysis was used to identify adenosine and oxygen transients. A training set was obtained using the five largest and most definitive adenosine transients for each rat in vivo (Nguyen et al., 2014). For oxygen, a training set was compiled for the five different concentrations of oxygen that were measured in vitro. The oxidation peak of adenosine and the reduction peak of oxygen were filtered using a Fourier transform 1 Hz filter to reduce noise.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA), or OriginPro 7.5 (Origin Lab Corporation, Northampton, MA, USA). A student’s t-test was used to compare the effects of the drugs on pre- and post-drug response of the number and concentrations of adenosine and oxygen transients. The distribution of inter-event times of adenosine and oxygen transients was analyzed using a Kolmogorov-Smirnov (KS) test. Some data points for adenosine and oxygen inter-event times are not shown in order to better show the lower inter-event times where changes are more obvious after drug administration. However, all data for inter-event times were used for statistical analyses. Statistical significance was designated at p < 0.05 and all data are presented as mean ± standard error of the mean (SEM) or standard deviation (SD).
Results
Adenosine and oxygen cyclic voltammetry in vivo
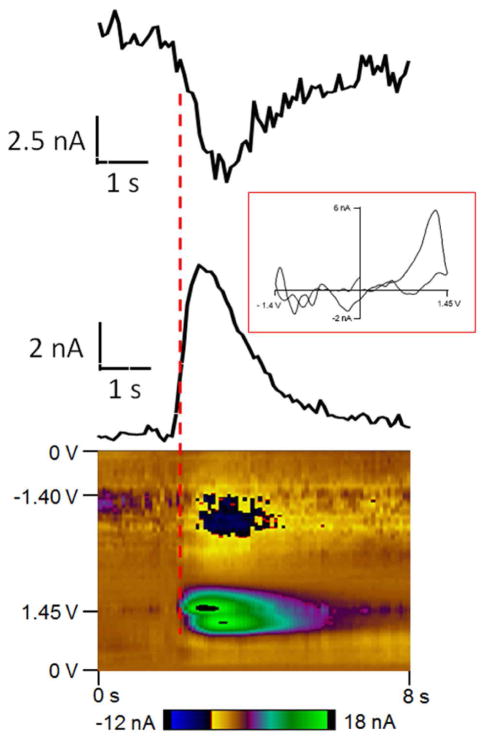
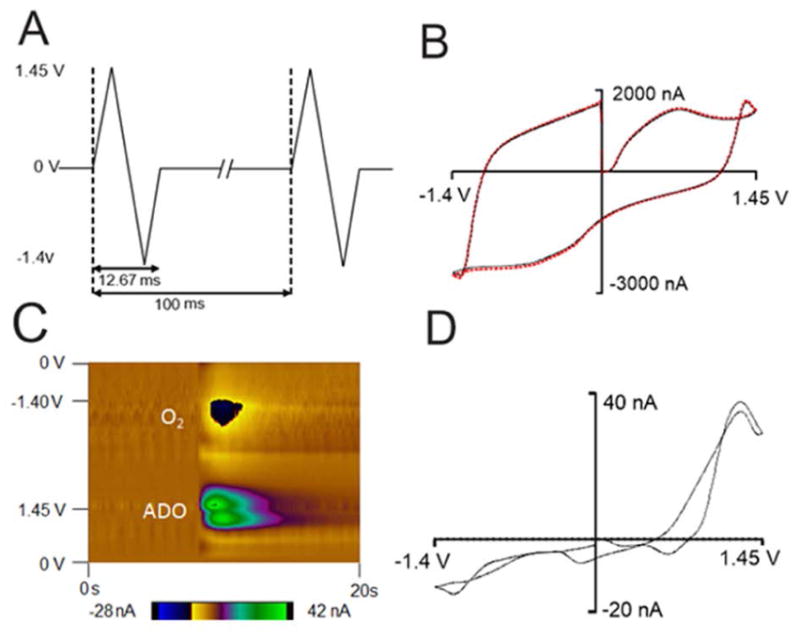
Fast-scan cyclic voltammetry at a carbon-fiber microelectrode was used to simultaneously detect fast changes in adenosine and oxygen with sub-second temporal resolution. The potential was scanned from 0 V to 1.45 V, then to −1.4 V and back to 0 V at 450 V/s every 100 milliseconds as shown in Figure 1A. The double layer charging at the electrode produces a large but stable background current and can be subtracted out to obtain a background-subtracted cyclic voltammogram (Figure 1B and 1D). Oxidation of adenosine results in two oxidation peaks in the background-subtracted cyclic voltammogram. With this waveform, the primary oxidation of adenosine occurs right after the switching potential at 1.27 V on the cathodic scan because of slow electron transfer. A secondary oxidation occurs at 1.26 V on the anodic scan. The reduction of oxygen occurs at −1.3 V on the cathodic scan. A representative color plot is shown in Figure 1C. In the color plot, time is plotted on the abscissa, electrode potential is plotted on the ordinate, and the current is depicted in false color. The green/purple ovals represent the adenosine oxidation currents. The top oval is the primary oxidation and the bottom oval is the secondary oxidation of adenosine. The secondary oxidation is slightly delayed in time from the primary oxidation because it is due to the oxidative byproduct formed after the primary oxidation. For oxygen, the dark blue area around −1.3 V is the reduction of oxygen. Because the oxidation of adenosine and reduction of oxygen occur at different potentials, adenosine and oxygen release can be detected simultaneously.
Figure 1.
Correlation of adenosine and oxygen
To determine whether the oxygen signal was delayed from the adenosine signal, the starting points of adenosine and oxygen peaks were measured and compared (Figure 2). Current versus time traces are plotted at the primary oxidation potential of adenosine and the reduction potential of oxygen, which help identify the timing of the events. In this example, adenosine starts to rise about 0.1 second before oxygen starts to change. The CV shows that the first CV that has a clear adenosine signal does not have an oxygen peak that is discernible from the noise. On average, adenosine started to increase 0.2 ± 0.03 seconds before oxygen, indicating the adenosine transient occurs before the release of oxygen.
Figure 2.
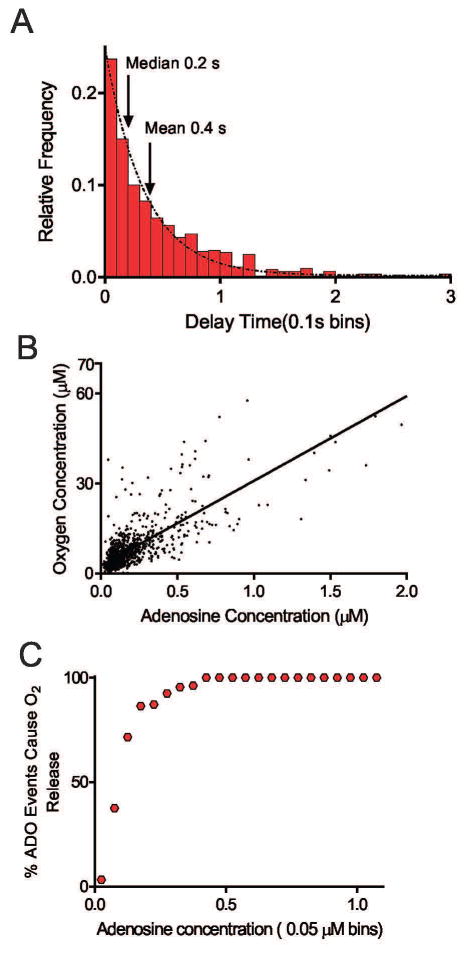
Additionally, the time difference between the peak adenosine and oxygen concentration was calculated for each set of transients and plotted in 0.1 second bins (Figure 3A). Further supporting that adenosine comes first, the mean and median of delay time for the oxygen peak were 0.4 seconds and 0.2 seconds, respectively, which means if an oxygen transient was observed, the oxygen usually peaked 0.2 seconds after the adenosine peak.
Figure 3.
Figure 3B shows a correlation plot of the concentration of spontaneous adenosine vs. the oxygen concentration. Data are only plotted for adenosine transients that occurred with an oxygen transient. There is a linear relationship between the transient adenosine concentration and the oxygen concentration. The correlation coefficient is 0.63, indicating that 63 percent of the variance in oxygen concentration is due to the variance in spontaneous transient adenosine concentration.
To further understand how often adenosine was released with an oxygen event, a plot was made of binned adenosine concentration vs. percentage of transient adenosine events with an accompanying oxygen event (Figure 3C). The minimum concentration of adenosine that was ever released with an oxygen event was 0.02 μM, which is higher than 0.01 μM, the lowest concentration of adenosine that we can detect. When the concentration of adenosine was 0.15 μM, about 50 percent of adenosine events were released with oxygen, and when the concentration of adenosine was 0.4 μM or higher, all adenosine events had oxygen transients that followed. Overall, because most adenosine release events were low concentration, 34% of measured adenosine events were accompanied by an oxygen transient.
The duration of adenosine and oxygen changes were both fast, demonstrating that that adenosine and oxygen are available to mediate responses in the extracellular space for only a few seconds. The durations of adenosine and oxygen release events were 3.2 ± 1.9 seconds and 3.5 ± 1.9 seconds, respectively. High concentration release events of adenosine or oxygen had a longer duration because it takes a longer time to clear higher concentrations (Ross et al., 2014).
Adenosine and Oxygen Transients Over Time
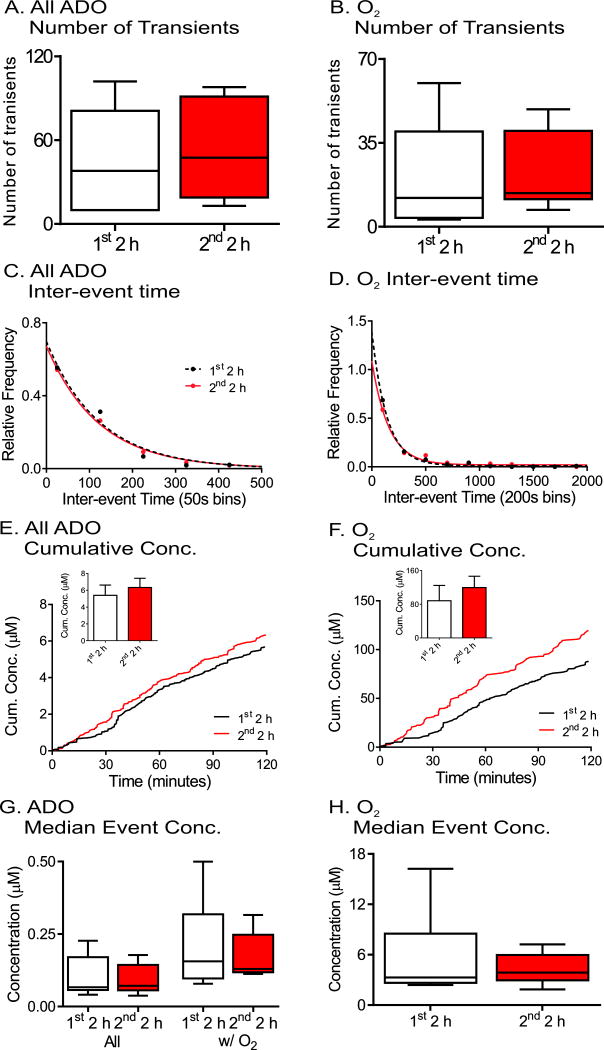
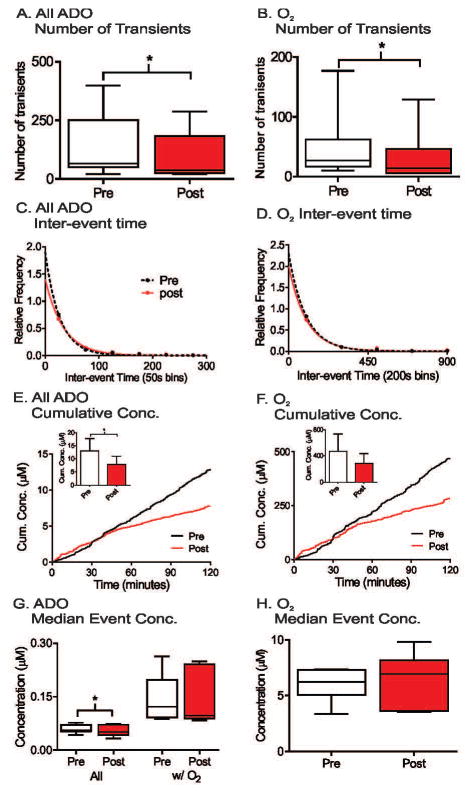
Spontaneous adenosine and oxygen events were continuously measured for four hours and comparison made between the first two hours and second two hours as a control for pharmacology experiments. The number of transients was averaged for each animal, and the plot shows box and whisker plots with the median and quartiles in the box and the range marked by the whiskers (Fig. 4A and B). The variance is large due to differences from animal to animal and there were no significant changes in the number of adenosine or oxygen transients between first two hour and second two hour time periods (n = 6, paired t-test, p = 0.13 and p = 0.89, respectively). To examine the frequency, the time between consecutive transients was plotted as a histogram (Figure 4C and 4D). The adenosine transients occur closer together than the oxygen transients because not all adenosine transients were followed by oxygen release. The distribution of inter-event times for the first two hours and second two hours were compared using the Kolmogorov- Smirnov (KS) test and were not significantly different (n = 6 animals, p = 0.51 and p = 0.46, respectively). The average cumulative concentration of released adenosine was plotted by adding the peak concentrations of adenosine over time and then the concentrations were divided by the number of animals. While the average cumulative concentration traces of both all adenosine and oxygen transients show higher concentration in second two hours (Figure 4E and 4F), the maximum cumulative concentrations, which is maximum cumulative concentration from each animal, were not significant for adenosine or oxygen transients (student’s paired t-test, n = 6 animals, p = 0.064 and p = 0.087, respectively). The median event concentration was calculated per animal and box and whisker plots are plotted in Figure 4G and 4H for all adenosine, adenosine transients with oxygen (Ad w/O2) and oxygen concentrations. There were no significant effects of time on the median concentrations of transients (paired t-test, n = 6, p = 0.099, p = 0.22, and p = 0.43, respectively). Thus, the number of transients, inter-event time, or concentrations of adenosine and oxygen transients do not change over four hours of data collection. In addition, a four hour control experiment was also performed by injecting the vehicle, DMSO, after 2 hours. The administration of DMSO did not affect the number of transients, inter-event time, or concentration of adenosine or oxygen transients (Figure S2).
Figure 4.
A1, A2a, and NO Modulation of Oxygen Response
To investigate the relationship between adenosine and blood flow changes, we tested the effects of A1 and A2a receptor drugs on simultaneous adenosine and oxygen transients. For all pharmacological experiments, two hours of baseline data were collected and then the drug was administered and data were collected for additional two hours.
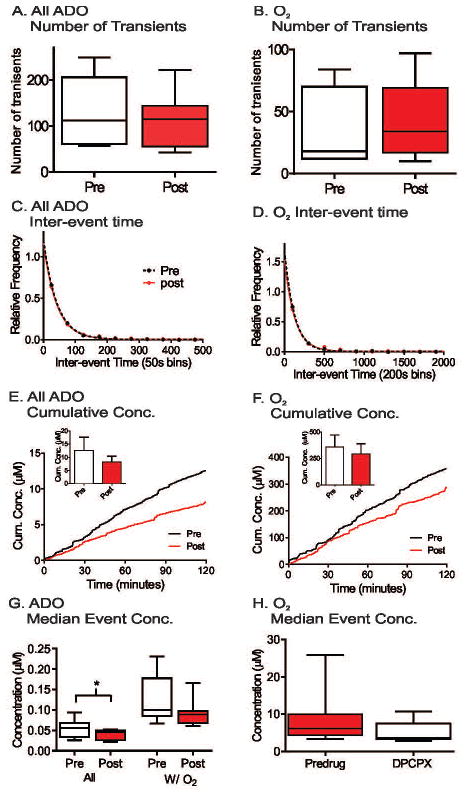
The A1 receptor antagonist, DPCPX, was administered at 6 mg/kg, i.p. The number of adenosine and oxygen transients did not significantly changed after DPCPX, although there was a trend towards more oxygen transients (Figure 5A and 5B, student’s paired t-test, n = 8 animals, p = 0.47 and p = 0.055, respectively). Administration of DPCPX did not significantly change the underlying inter-event time distributions for either transient adenosine or oxygen release (Figure 5C and 5D, KS test, n= 8 animals, p = 0.69 and p = 0.87, respectively). The average cumulative concentration of both adenosine and oxygen transients were lower after DPCPX administration (Figure 5E and 5F), but the differences of maximum cumulative concentrations were not significant (student’s paired t-test, n = 8 animals, p = 0.31 and p = 0.46, respectively). There was a significant decrease in median event concentration for all spontaneous adenosine transients (student’s paired t-test, n = 8 animals, p = 0.016) (Figure 5G), but DPCPX did not significantly affect the median event concentration of Ad w/O2 or oxygen transients (student’s paired t-test, n = 8 animals, p = 0.093 and p = 0.086, respectively) (Figure 5G and 5H). Thus, A1 receptor blockade with DPCPX did not change the number, concentration or inter-event time of simultaneous adenosine and oxygen release but only decreased the median concentration of all adenosine transients.
Figure 5.
SCH442416 (3 mg/kg, i.p.), an A2a receptor antagonist, was administered to test the extent to which A2a receptors mediated the oxygen transients. SCH442416 significantly decreased the average number of adenosine transients from 132 to 90 (paired t- test, n = 7 animals, p = 0.023) and oxygen transients from 49 to 33 (paired t-test, n = 7 animals, p = 0.030). In addition, SCH442416 increased the mean inter-event time for adenosine transients from 53 to 77 seconds and for oxygen from 129 to 216 seconds. The underlying inter-event time distributions of both adenosine and oxygen transients were significantly different than pre-drug (n = 7 animals, KS test, p = 0.0016 and p = 0.044, respectively) (Figure 6C and 6D). The average cumulative concentration of both adenosine and oxygen transients were lower after SCH442416 administration, but only the maximum cumulative concentrations of adenosine transient significantly decreased (paired t-test, n = 7 animals, p = 0.030); There is no significant change in maximum cumulative concentrations of oxygen (paired t-test, n = 7 animals, p = 0.17). SCH442416 significantly decreased the median adenosine event concentration of all adenosine transients, but not the median concentration of Ad w/O2 and oxygen (paired t-test, n = 7, p = 0.041, p = 0.73, and p = 0.66, respectively). Therefore, blocking A2a receptors did not change the concentration of oxygen release, but both adenosine and oxygen were released less frequently.
Figure 6.
To test the extent to which the oxygen signals were dependent on nitric oxide (NO) nitric oxide (NO) synthase was inhibited using L-NAME(100 mg/kg, i.p)(Iadecola et al., 1994; Venton et al., 2003). There were no significant differences between before and after L-NAME in terms of number of transients, inter-event time, or concentration of adenosine or oxygen transients (Figure S3). These results confirm that NO does not play a role in simultaneous adenosine and oxygen release and therefore, this transient vasodilation does not rely on NO.
Discussion
Here we demonstrate that spontaneous, transient changes in adenosine and oxygen are correlated in the caudate-putamen. Transient changes in oxygen release are an indicator of increased blood flow. Adenosine transients were followed by oxygen release about one-third of the time, and the concentration of adenosine and evoked oxygen changes are correlated. Typically, the oxygen release started and peaked about 0.2 seconds after the adenosine transient. A1 receptors do not have an effect on simultaneous adenosine and oxygen release while A2a receptors modulated the number of adenosine and oxygen transients. Thus, spontaneous adenosine transients are correlated with oxygen release, particularly when the adenosine release is large, suggesting adenosine modulates blood flow on a rapid, sub-second time scale.
Transient adenosine and blood flow changes on a rapid time scale
Transient release of adenosine has recently been characterized, showing that adenosine can signal on a rapid time scale (Nguyen et al., 2014, 2015; Nguyen and Venton, 2015). Transient adenosine modulates phasic dopamine release, but the modulation only occurs when adenosine is present (Ross and Venton, 2015). However, the extent to which this rapid mode of adenosine signaling can transiently mediate vasodilation was unknown. Most previous studies of adenosine mediating blood flow correlated changes that lasted for minutes. For example, cerebral blood flow increased at 4th minute of intravenous infusion of adenosine (Soricelli et al., 1995). One of the faster studies of adenosine build up found that cerebral adenosine was elevated within 5 seconds after the onset of ischemia, and they hypothesized that this rapid rise of adenosine caused vasodilation associated with ischemia (Winn et al., 1979).
Our method is unique in that both adenosine and oxygen changes can be measured simultaneously with sub-second temporal resolution. The results clearly demonstrate that transient adenosine and oxygen release are correlated. The increase in adenosine always precedes the oxygen increase, with a median delay time between the peak adenosine and oxygen concentrations of 0.2 seconds. The appearance and clearance of both adenosine and oxygen are fast, with an average duration of only 3 seconds. Other physiological stimuli, such as a tail pinch, cause vasodilation that lasts for minutes (Lowry et al., 1997b). Changes in oxygen due to electrical stimulations have been measured, including a first peak that is not mediated by adenosine, which occurs within 3 – 4 seconds after stimulation, and a second peak mediated by adenosine that occurs 10 – 30 seconds after the stimulation and lasts for 20 seconds(Venton et al., 2003). Therefore, our data here is the fastest reported correlation between adenosine release and shifts in blood flow and the duration of the oxygen changes are much shorter than with other stimuli.
The concentration of spontaneous adenosine and oxygen changes were also correlated. Previous studies also found a correlation between the concentration of adenosine and the dilation magnitude of pial artery (Shin et al., 2000; Hein et al., 2013). While about one-third of adenosine transients were accompanied by changes in oxygen, high concentration adenosine transients (over 0.4 μM) were always followed by oxygen release. Low concentrations of adenosine were not always correlated with oxygen release, likely because the event did not cause sufficient receptor activation. However, some smaller adenosine transients did cause oxygen release. Our electrodes only measure in one discrete location, and it is possible that for some smaller amounts of adenosine, the electrode was not at the center of the release, making the event look smaller. Also, there may be differences in global blood flow that would affect local blood flow and consequently modulate delivery of oxygen (Shimosegawa et al., 1995).
Role of Adenosine Receptors in Mediating Adenosine and Blood Flow Changes
The effects of adenosine are mediated by four G-protein coupled receptors, A1, A2a, A2b, and A3 (Fredholm et al., 2001). A1 and A2a receptors are highly expressed in the caudate-putamen of the brain (Fredholm et al., 2000; Cieślak et al., 2008). A1 receptors mediate the inhibitory modulation of phasic dopamine release by transient adenosine in the caudate-putamen (Ross and Venton, 2015). Both stimulated and spontaneous adenosine release are self-regulated by A1 receptors, which have autoreceptor characteristics (Cechova et al., 2010; Nguyen et al., 2014). Here, our study showed that blocking A1 receptors decreased the concentration of adenosine transients but did not significantly affect on the number of transients or inter-event times. In contrast, our previous study found a significant decrease in the inter-event time of transient adenosine release but no change in concentration after DPCPX (Nguyen et al., 2014). That study used a different FSCV waveform that did not sweep to the low potentials necessary for oxygen reduction. While the effects were slightly different here, they are consistent with A1 receptors being inhibitory and self-regulating transient adenosine. The concentration of oxygen did not decrease because the concentration of adenosine that correlated with oxygen did not decrease; i.e. the large adenosine transients were still correlated with oxygen release. The number of oxygen transients was the same, indicating that A1 receptors do not mediate vasodilation.
A2a receptors are highly expressed on cerebral blood vessels (O’Regan, 2005; Pelligrino et al., 2010; Mills et al., 2011), and are located on endothelial cells (Coney and Marshall, 1998). In addition, A2a receptors play a protective role in hypoxia/ischemia, as A2a antagonists protect against brain damage after hypoxia (Phillis, 1989; Monopoli et al., 1998; Melani et al., 2003). In the brain, the density of A2a receptors in the basal ganglia is about 20 times greater than other brain regions (Svenningsson, 1999; Lopes et al., 2004). The number of both adenosine and oxygen transients were reduced by the A2a antagonist, SCH442416, while the concentration of adenosine transients that caused oxygen and oxygen transients stayed the same. These results suggest that A2a receptors have self-regulating properties by modulating adenosine concentrations (Cechova et al., 2010). While the link between A2a receptors on endothelial cells regulating blood flow had been previously established, this work shows that the A2a antagonist can function by lowering the amount of adenosine transients and therefore oxygen transients.
Our results definitely show that adenosine and oxygen transients in the brain are correlated, especially for large adenosine transients which are always accompanied by oxygen transients. This leads to the question of whether the adenosine transients cause the oxygen changes. The delay time of 0.2 seconds between the start of the adenosine transient and the oxygen transient demonstrates that the adenosine transients come first. The distance between blood vessels in the brain is about 50 μm, and diffusion of adenosine to a blood vessel, 25 μm at most, would take less than 0.5 seconds. Thus, the time delay is of the correct order of magnitude for adenosine diffusion. The proposed mechanism of adenosine causing vasodilation and oxygen increases is by acting at A2a receptors on endothelial cells, and the expected result that would confirm this is a decrease in the number of oxygen transients compared to adenosine transients. Instead, the observed result is that the A2a antagonist causes adenosine and oxygen transients to decrease proportionally, a 32% decrease for both. This proportional decrease is harder to interpret; it does not definitively show that the adenosine transients were working at the A2a receptor, but it does confirm that the receptor is important for regulating adenosine transients and thus oxygen transients. Therefore, there are two possible explanations for the data: (1) adenosine transients cause the oxygen transients or (2) a large metabolic event causes the release of both adenosine and other vasodilatory molecules that lead to the oxygen increases. We tested L-NAME to block NO production to test the hypothesis that NO might also play a role in these oxygen changes(Venton et al., 2003). No effect was seen, indicating that these oxygen changes were not due to inducible nitric oxygen synthase made NO. However, there are other sources of NO and other vasodilatory signals such as pH and CO2 that have not been ruled out (Hylland et al., 1996; Venton et al., 2003; Yoon et al., 2012). Nevertheless, the bulk of the evidence points towards adenosine causing the oxygen changes although the exact mechanism has not been delineated. A more definitive approach in the future would be to try these experiments in mice and test the effects of global A2a knock outs or even specific A2a KO just on the blood vessels to test causation.
In conclusion, this study provides evidence that spontaneous, transient adenosine are correlated with transient oxygen changes. The subsequent oxygen changes occur quickly, usually within about 0.2 seconds, and the clearance is fast, lasting only about 3.5 seconds. Our study also shows that A2a receptors regulate this transient vasodilation, by modulating the number of adenosine transients that are correlated with oxygen transients. Because adenosine is rapidly metabolized,(Haskó et al., 2008) adenosine signaling is likely local rather than systemic and could transiently affect local blood flow (Nguyen et al., 2015). Future research will focus on whether transient adenosine cause local vasodilation and increase oxygen release.
Supplementary Material
Acknowledgments
This research was supported by a grant from NIH (R01NS076875) to BJV.
Footnotes
Conflict of Interest
The authors declare no competing financial interests.
References
- Barbu A, Jansson L, Sandberg M, Quach M, Palm F. The use of hydrogen gas clearance for blood flow measurements in single endogenous and transplanted pancreatic islets. Microvasc Res. 2015;97:124–129. doi: 10.1016/j.mvr.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Belardinelli L, Shryock JC, Snowdy S, Zhang Y, Monopoli A, Lozza G, Ongini E, Olsson RA, Dennis DM. The A2A adenosine receptor mediates coronary vasodilation. J Pharmacol Exp Ther. 1998;284:1066–1073. [PubMed] [Google Scholar]
- Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45:347–360. doi: 10.1016/0301-0082(94)00057-o. [DOI] [PubMed] [Google Scholar]
- Bucher ES, Fox ME, Kim L, Kirkpatrick DC, Rodeberg NT, Belle AM, Wightman RM. Medullary norepinephrine neurons modulate local oxygen concentrations in the bed nucleus of the stria terminalis. J Cereb Blood Flow Metab. 2014;34:1128–1137. doi: 10.1038/jcbfm.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechova S, Elsobky AM, Venton BJ. A1 receptors self-regulate adenosine release in the striatum: evidence of autoreceptor characteristics. Neuroscience. 2010;171:1006–1015. doi: 10.1016/j.neuroscience.2010.09.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechova S, Venton BJ. Transient adenosine efflux in the rat caudate-putamen. J Neurochem. 2008;105:1253–1263. doi: 10.1111/j.1471-4159.2008.05223.x. [DOI] [PubMed] [Google Scholar]
- Cieślak M, Komoszyński M, Wojtczak A. Adenosine A(2A) receptors in Parkinson’s disease treatment. Purinergic Signal. 2008;4:305–312. doi: 10.1007/s11302-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coney AM, Marshall JM. Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J Physiol. 1998;509(Pt 2):507–518. doi: 10.1111/j.1469-7793.1998.507bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demchenko IT, Boso AE, Natoli MJ, Doar PO, O’Neill TJ, Bennett PB, Piantadosi CA. Measurement of cerebral blood flow in rats and mice by hydrogen clearance during hyperbaric oxygen exposure. Undersea Hyperb Med. 1998;25:147–152. [PubMed] [Google Scholar]
- Fredholm BB, Arslan G, Halldner L, Kull B, Schulte G, Wasserman W. Structure and function of adenosine receptors and their genes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:364–374. doi: 10.1007/s002100000313. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TW, Xu W, Ren Y, Kuo L. Cellular signalling pathways mediating dilation of porcine pial arterioles to adenosine A2A receptor activation. Cardiovasc Res. 2013;99:156–163. doi: 10.1093/cvr/cvt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylland P, Nilsson GE, Lutz PL. Role of nitric oxide in the elevation of cerebral blood flow induced by acetylcholine and anoxia in the turtle. J Cereb Blood Flow Metab. 1996;16:290–295. doi: 10.1097/00004647-199603000-00014. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab. 1994;14:175–192. doi: 10.1038/jcbfm.1994.25. [DOI] [PubMed] [Google Scholar]
- Kusano Y, Echeverry G, Miekisiak G, Kulik TB, Aronhime SN, Chen JF, Winn HR. Role of adenosine A2 receptors in regulation of cerebral blood flow during induced hypotension. J Cereb Blood Flow Metab. 2010;30:808–815. doi: 10.1038/jcbfm.2009.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA. Binding of the prototypical adenosine A(2A) receptor agonist CGS 21680 to the cerebral cortex of adenosine A(1) and A(2A) receptor knockout mice. Br J Pharmacol. 2004;141:1006–1014. doi: 10.1038/sj.bjp.0705692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry JP, Boutelle MG, Fillenz M. Measurement of brain tissue oxygen at a carbon paste electrode can serve as an index of increases in regional cerebral blood flow. J Neurosci Methods. 1997a;71:177–182. doi: 10.1016/s0165-0270(96)00140-9. [DOI] [PubMed] [Google Scholar]
- Lowry JP, Boutelle MG, Fillenz M. Measurement of brain tissue oxygen at a carbon past electrode can serve as an index of increases in regional cerebral blood flow. J Neurosci Methods. 1997b;71:177–182. doi: 10.1016/s0165-0270(96)00140-9. [DOI] [PubMed] [Google Scholar]
- Melani A, Pantoni L, Bordoni F, Gianfriddo M, Bianchi L, Vannucchi MG, Bertorelli R, Monopoli A, Pedata F. The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003;959:243–250. doi: 10.1016/s0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- Mills JH, Alabanza L, Weksler BB, Couraud P-O, Romero IA, Bynoe MS. Human brain endothelial cells are responsive to adenosine receptor activation. Purinergic Signal. 2011;7:265–273. doi: 10.1007/s11302-011-9222-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monopoli A, Lozza G, Forlani A, Mattavelli A, Ongini E. Blockade of adenosine A2A receptors by SCH 58261 results in neuroprotective effects in cerebral ischaemia in rats. Neuroreport. 1998;9:3955–3959. doi: 10.1097/00001756-199812010-00034. [DOI] [PubMed] [Google Scholar]
- Morton DW, Maravilla KR, Meno JR, Winn HR. Systemic Theophylline Augments the Blood Oxygen Level--Dependent Response to Forepaw Stimulation in Rats. AJNR Am J Neuroradiol. 2002;23:588–593. [PMC free article] [PubMed] [Google Scholar]
- Mulderink TA, Gitelman DR, Mesulam M-M, Parrish TB. On the use of caffeine as a contrast booster for BOLD fMRI studies. Neuroimage. 2002;15:37–44. doi: 10.1006/nimg.2001.0973. [DOI] [PubMed] [Google Scholar]
- Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol. 2001;280:H2329–H2335. doi: 10.1152/ajpheart.2001.280.5.H2329. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. Characterization of spontaneous, transient adenosine release in the caudate-putamen and prefrontal cortex. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Ross AE, Ryals M, Lee ST, Venton BJ. Clearance of rapid adenosine release is regulated by nucleoside transporters and metabolism. Pharmacol Res Perspect. 2015;3 doi: 10.1002/prp2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Venton BJ. Fast-scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release. Comput Struct Biotechnol J. 2015;13:47–54. doi: 10.1016/j.csbj.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Regan M. Adenosine and the regulation of cerebral blood flow. Neurol Res. 2005;27:175–181. doi: 10.1179/016164105X21931. [DOI] [PubMed] [Google Scholar]
- Obal F, Krueger JM. Biochemical regulation of non-rapid-eye-movement sleep. Front Biosci. 2003;8:d520–d550. doi: 10.2741/1033. [DOI] [PubMed] [Google Scholar]
- Orrú M, Quiroz C, Guitart X, Ferré S. Pharmacological evidence for different populations of postsynaptic adenosine A2A receptors in the rat striatum. Neuropharmacology. 61:967–974. doi: 10.1016/j.neuropharm.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Elsinga PH, Ishiwata K, Dierckx RaJO, van Waarde a. Adenosine A(1) receptors in the central nervous system: their functions in health and disease, and possible elucidation by PET imaging. Curr Med Chem. 2011;18:4820–4835. doi: 10.2174/092986711797535335. [DOI] [PubMed] [Google Scholar]
- Pelligrino DA, Vetri F, Xu H-L. Purinergic mechanisms in gliovascular coupling. Semin Cell Dev Biol. 2011;22:229–236. doi: 10.1016/j.semcdb.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrino DA, Xu H-L, Vetri F. Caffeine and the control of cerebral hemodynamics. J Alzheimers Dis. 2010;20(Suppl 1):S51–S62. doi: 10.3233/JAD-2010-091261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis JW. Adenosine in the control of the cerebral circulation. Cerebrovasc Brain Metab Rev. 1989;1:26–54. [PubMed] [Google Scholar]
- Ross AE, Nguyen MD, Privman E, Venton BJ. Mechanical stimulation evokes rapid increases in extracellular adenosine concentration in the prefrontal cortex. J Neurochem. 2014;130:50–60. doi: 10.1111/jnc.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AE, Venton BJ. Adenosine transiently modulates stimulated dopamine release in the caudate-putamen via A1 receptors. J Neurochem. 2015;132:51–60. doi: 10.1111/jnc.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimosegawa E, Kanno I, Hatazawa J, Fujita H, Iida H, Miura S, Murakami M, Inugami A, Ogawa T, Itoh H. Photic stimulation study of changing the arterial partial pressure level of carbon dioxide. J Cereb Blood Flow Metab. 1995;15:111–114. doi: 10.1038/jcbfm.1995.12. [DOI] [PubMed] [Google Scholar]
- Shin HK, Shin YW, Hong KW. Role of adenosine A2B receptors in vasodilation of rat pial artery and cerebral blood flow autoregulation. Am J Physiol Hear Circ Physiol. 2000;278:H339–H344. doi: 10.1152/ajpheart.2000.278.2.H339. [DOI] [PubMed] [Google Scholar]
- Shryock JC, Belardinelli L. Adenosine and Adenosine Receptors in the Cardiovascular System: Biochemistry, Physiology, and Pharmacology. Am J Cardiol. 1997;79:2–10. doi: 10.1016/s0002-9149(97)00256-7. [DOI] [PubMed] [Google Scholar]
- Soricelli A, Postiglione A, Cuocolo A, De Chiara S, Ruocco A, Brunetti A, Salvatore M, Ell PJ. Effect of Adenosine on Cerebral Blood Flow as Evaluated by Single-Photon Emission Computed Tomography in Normal Subjects and in Patients With Occlusive Carotid Disease: A Comparison With Acetazolamide. Stroke. 1995;26:1572–1576. doi: 10.1161/01.str.26.9.1572. [DOI] [PubMed] [Google Scholar]
- Strand AM, Venton BJ. Flame etching enhances the sensitivity of carbon-fiber microelectrodes. Anal Chem. 2008;80:3708–3715. doi: 10.1021/ac8001275. [DOI] [PubMed] [Google Scholar]
- Svenningsson P. Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol. 1999;59:355–396. doi: 10.1016/s0301-0082(99)00011-8. [DOI] [PubMed] [Google Scholar]
- Van Wylen DG, Park TS, Rubio R, Berne RM. Increases in cerebral interstitial fluid adenosine concentration during hypoxia, local potassium infusion, and ischemia. J Cereb Blood Flow Metab. 1986;6:522–528. doi: 10.1038/jcbfm.1986.97. [DOI] [PubMed] [Google Scholar]
- Venton BJ, Michael DJ, Wightman RM. Correlation of local changes in extracellular oxygen and pH that accompany dopaminergic terminal activity in the rat caudate-putamen. J Neurochem. 2003;84:373–381. doi: 10.1046/j.1471-4159.2003.01527.x. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio GR, Berne RM. The role of adenosine in the regulation of cerebral blood flow. J Cereb Blood Flow Metab. 1981a;1:239–244. doi: 10.1038/jcbfm.1981.29. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine production in the rat during 60 seconds of ischemia. Circ Res. 1979;45:486–492. doi: 10.1161/01.res.45.4.486. [DOI] [PubMed] [Google Scholar]
- Winn HR, Rubio R, Berne RM. Brain adenosine concentration during hypoxia in rats. Am J Physiol. 1981b;241:H235–H242. doi: 10.1152/ajpheart.1981.241.2.H235. [DOI] [PubMed] [Google Scholar]
- Yoon S, Zuccarello M, Rapoport RM. pCO(2) and pH regulation of cerebral blood flow. Front Physiol. 2012;3:365. doi: 10.3389/fphys.2012.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JB, Kennedy RT, Wightman RM. Evoked neuronal activity accompanied by transmitter release increases oxygen concentration in rat striatum in vivo but not in vitro. J Cereb Blood Flow Metab. 1992;12:629–637. doi: 10.1038/jcbfm.1992.87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.