Abstract
Accumulating evidence has supported the fallopian tube rather than the ovary as the origin for high grade serous ovarian cancer (HGSOC). To understand the relationship between putative precursor lesions and metastatic tumors, we performed whole exome sequencing on specimens from eight HGSOC patient progression series consisting of serous tubal intraepithelial carcinomas (STIC), invasive fallopian tube lesions, invasive ovarian lesions, and omental metastases. Integration of copy number and somatic mutations revealed patient-specific patterns with similar mutational signatures and copy number variation profiles across all anatomic sites, suggesting that genomic instability is an early event in HGSOC. Phylogenetic analyses supported STIC as precursor lesions in half of our patient cohort, but also identified STIC as metastases in two patients. Ex vivo assays revealed that HGSOC spheroids can implant in the fallopian tube epithelium and mimic STIC lesions. That STIC may represent metastases calls into question the assumption that STIC are always indicative of primary fallopian tube cancers.
Keywords: ovarian cancer, genomics, phylogenetics, in situ, metastasis
Introduction
High-grade serous cancer (HGSOC) encompasses several pathological tumor entities, including ovarian, fallopian tube, and peritoneal cancer. The cell of origin of these cancers is currently unresolved, but their pattern of dissemination, clinical behavior, and chemosensitivity is very similar (1–3). For almost a century it was believed that the surface epithelium of the ovary gives rise to HGSOC. However, in 2001, a Dutch research group described preneoplastic lesions in the fallopian tubes of patients at high familial risk of HGSOC (4). Careful sectioning of the fallopian tubes in patients with HGSOC has revealed serous tubal intraepithelial carcinomas (STIC) with atypical histologic changes that resemble the invasive serous component found in 50% of all patients with HGSOC. Further molecular analysis of STIC found identical TP53 mutations in the STIC and corresponding HGSOC, as well as a similar upregulation of cell cycle and DNA repair proteins (5, 6). Because TP53 gene mutations represent one of the earliest genetic changes in HGSOC, and because they are detected in all STIC, it was then considered evident that STIC may be the precursor lesions of HGSOC. This hypothesis received additional support with the recent development of a genetic mouse model designed to determine if the fallopian tube can give rise to HGSOC. In this model, the PAX8 promoter, specific to secretory fallopian tube epithelial cells (FTECs), was used to inactivate BRCA1 (Breast Cancer 1; germline mutations in BRCA1/2 occur in about 13% of HGSOCs) and PTEN and drive expression of mutant TP53. These mice develop tumors mimicking human STIC lesions in the fallopian tube and have molecular alterations that recapitulate human HGSOC (7). If one assumes that HGSOC advances along a linear progression series from in situ tumors to invasive tumors to metastasis, as is believed of colon cancer (8), these findings support the hypothesis that HGSOC originates in the fallopian tube.
Fully understanding the relationship of STIC and HGSOC requires a comprehensive understanding of the genomic alterations underlying both STIC and HGSOC. Using next generation sequencing technologies, the TCGA and an Australian consortium has provided a snapshot of the genomic changes and signaling pathways characterizing invasive HGSOC at the ovary (2, 9). Compared to other carcinomas, HGSOC is uniquely characterized by recurrent copy number variants, with TP53 the most commonly mutated gene (10). In pancreatic and prostate cancer, among others, multi-site sequencing of primary tumors and metastases have revealed evidence of multi-step dissemination and unraveled the complex genomic trajectories of metastasis (3, 11). Although several groups have performed sequencing of HGSOC and begun to understand its metastatic trajectory throughout the peritoneal cavity (12–14), without integrative whole exome sequencing of STIC and metastatic lesions, the reconstruction of a complete metastatic trajectory for HGSOC is not possible.
We set out to characterize the dynamics of mutations and copy number variations during HGSOC dissemination, using a systematic genomic characterization of a uniform group of sporadic advanced HGSOC patients with STIC and without BRCA1/2 germline mutational changes. We hypothesized that sequencing both putative precursor lesions and metastatic HGSOC will elucidate early core events in HGSOC and the genomic characters necessary to reconstruct its step-wise progression.
Results
To begin to systematically understand the spatiotemporal genomics of ovarian cancer progression, we identified a cohort of eight patients who presented for primary tumor debulking surgery with advanced, metastatic HGSOC. Germline DNA was available for all patients, and the final pathology for these patients showed STIC, invasive FT, ovarian, and omental metastases (Fig. 1A). These four anatomic sites encompass the hypothetical progression series; from in situ STIC precursors to locally invasive fallopian tube lesions, then to primary ovarian metastases, and finally, to distant peritoneal omental metastases. All were chemotherapy naïve, with no germline BRCA1/2 mutations and no significant family history of ovarian or breast cancer (clinic-pathologic features Table S1). For each anatomic site, the tumor compartments were collected using laser-capture microdissection (LCM) (Fig. 1B). Microdissection was utilized to both eliminate stromal contamination and to facilitate highly-specific sequencing of small in situ STIC lesions. Ovarian tumors were microdissected from the ovary associated with the STIC lesion in cases of bilateral ovarian involvement. Because, by definition, there is limited material in microdissected STIC (Fig. 1A), the DNA isolation method was optimized to include longer digestion times and reduction of elution volumes. Whole exome sequencing (WES) and data processing were performed to assess the spatiotemporal pattern of genomic alterations during HGSOC progression (Fig. 1C, Fig. S1A). Despite low input amounts for some samples (50 ng), depth of coverage and on-target reads were similar across all anatomic sites surveyed (Fig. S1B–D, Table S2).
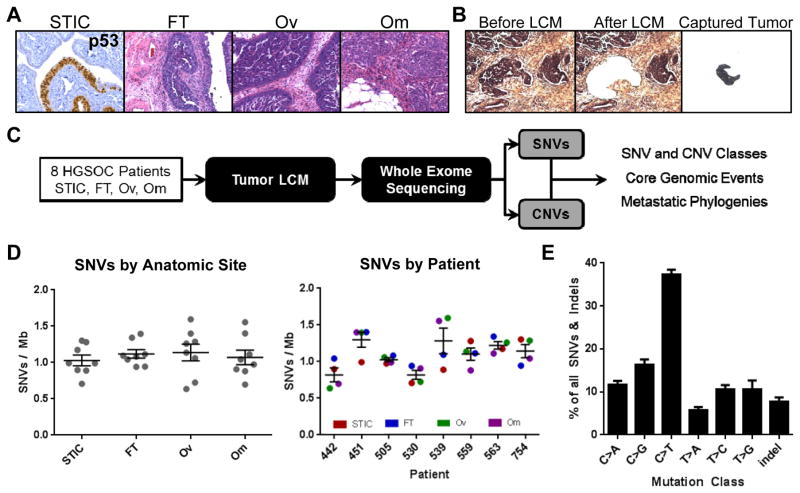
Figure 1. High grade serous ovarian cancer (HGSOC) mutational processes are established early and are patient specific.
A) Representative p53 IHC and H&E images of the hypothetical progression series of HGSOC in a patient with STIC, and invasive lesions in the fallopian tube, ovary, and abdominal omentum. B) Laser capture microdissection of the tumor compartment from omentum. C) Workflow to elucidate the spatiotemporal pattern of genomic alterations in HGSOC to capture tumor phylogenies and core events. D) Frequency of SNV by anatomic site and patient reveal that mutational burden is patient-specific rather than determined by anatomic tumor location. Average mutational burden across the patient cohort is approximately 1 somatic mutation per megabase (50 mutations total per tumor sample). E) Identification of an age-related mutational signature class characterized by high rates of C>T substitutions in all samples of the patient cohort, reveals a comparable mutational process underlying disease progression in all patients examined. STIC = serous tubal intraepithelial carcinoma; FT = invasive fallopian tube tumor; OV = invasive ovarian tumor; OM = omental metastasis.; SNV = single nucleotide variants.
An average of 1.0 SNVs per megabase (Mb) were identified, corresponding to approximately 50 de novo somatic mutations per sample, which is comparable to rates identified in the TCGA analysis of HGSOC (9, 15) (Table S3). The analysis of germline DNA did not detect BRCA1/2 mutations in the patient cohort. The majority of mutated genes in our analysis had been identified in the TCGA analysis, confirming that the patient cohort was representative of HGSOC. Mutational burden was similar across all anatomic sites. The only exception was patient 539, who had a significantly higher mutational burden in the ovarian and omental metastasis (Fig. 1D). Overall, the pattern of mutations was enriched for C>T substitutions, a signature associated with aging, mediated by spontaneous deamination of 5-methylcytosine (10). In contrast, C>A mutational rate, associated with environmental carcinogenesis (15) was low (Fig. 1E). The mutational signature was correlated with age at time of diagnosis and was patient-specific (Fig. S1E/F). No significant differences were observed by anatomic site (Fig. S1G). Mutational signatures, including rates of indel detection, were similar to those observed in the TCGA analysis of HGSOC (Fig. S1G). Patients with missense mutations in TP53 had evidence of nuclear p53 stabilization as indicated by high protein expression in the tumor. One patient (505) had a null splice site mutation and did not express p53 protein (Fig. S1H).
TP53 was the only gene mutated in every patient and anatomic site (Fig. 2A, Fig. S2A) and the only gene identified as significantly mutated across more than one patient by MutSig, an algorithm that corrects for high mutational rates in late replicating and poorly transcribed genes (16). Common mutations in CSMD3 and other genes were not significant. Each patient had the same TP53 mutation in all anatomic sites (Table S4) while other mutations were limited to a subset of the patients or anatomic sites (Fig. S2A). Mutant allele frequencies (MAF) for TP53 were high at all sites (0.7188–1.000), suggesting early loss-of-heterozygosity at the TP53 locus, highlighting the advantages of microdissection for obtaining high tumor purity (Table S4). In general, an increase in MAF across the progression from STIC to omental metastases was observed (Fig. S2B).
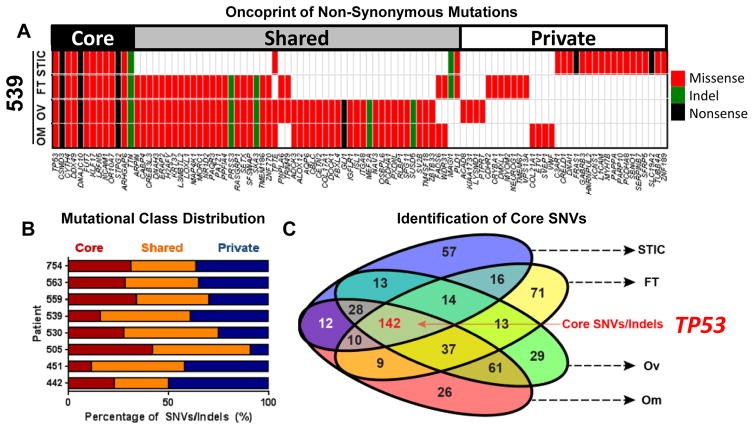
Figure 2. Core, recurrent SNVs in HGSOC are restricted to TP53 mutations.
A) Oncoprint of all non-synonymous mutations in patient 539 reveals mutation of TP53 and distribution of mutations into core, shared, and private classes. B) A minority of SNVs and insertions/deletions (indels) are present in all samples (“core”). The majority of mutations are shared between 2–3 anatomic sites (“shared”), or present in only one site (“private”) C) Euler diagram of all non-synonymous SNVs and indels. Core SNVs/indels present in all anatomic sites are highlighted in red.
To identify candidate driver molecular changes, mutations were assigned to three classes: 1) core mutations, present in all four anatomic sites; 2) shared mutations, present in two or three anatomic sites; and 3) private mutations unique to a single anatomic site. In all patients, a minority of SNVs were “core” mutations, likely to participate in the early transformation of normal cells (Fig. 2B). Core and private mutations had identical mutational signatures (Fig. S2C). A clear pattern emerged when we examined mutational class by anatomic site: the STIC and FT sites had a significantly higher proportion of “private” mutations. Gene ontology (GO) analysis of putative core mutations across all patients (Fig. 2C) revealed enrichment of three separate pathways involved in cell adhesion (Fig. S2D), suggesting that mutation of genes involved in mediating contact with extracellular matrices (ECMs) or other cells may be an early event in HGSOC progression.
HGSOC has a disproportionately high number of copy number variants (CNVs) compared to other cancers (10). The genomic location of CNVs in our data set were highly concordant with the TCGA analysis of HGSOC (9), including frequent amplification of chromosome 1q, 3q, and 8q and deletion of 4p, 4q, and 8p (Fig. 3A, Table S5). Across all anatomic sites, patient-specific copy-number profiles were apparent, including amplification of cytoband 8q24 in patient 754 (Fig. 3B), but every patient had distinct amplifications and deletions. Because the deletion of DNA damage repair genes plays a role in the etiology of HGSOC (9, 17), we specifically investigated copy number profiles for DNA damage repair and response genes. Indeed, frequent deletion of genes implicated in homologous recombination (BRCA2, FANCB), base excision repair (APEX2, NEIL3), non-homologous end-joining (WRN), and DNA damage signaling (RAD23A) was observed (Fig. 3C, Fig. S3A). Although the extent and frequency of amplifications and deletions varied significantly across patients (Fig. 3D), we observed no significant differences between anatomic sites (Fig. 3E). We did not detect any metastasis specific genes. Interestingly, the genomic instability evident in omental and ovarian sites was already present in the fallopian tube and STIC (Fig. 3E).
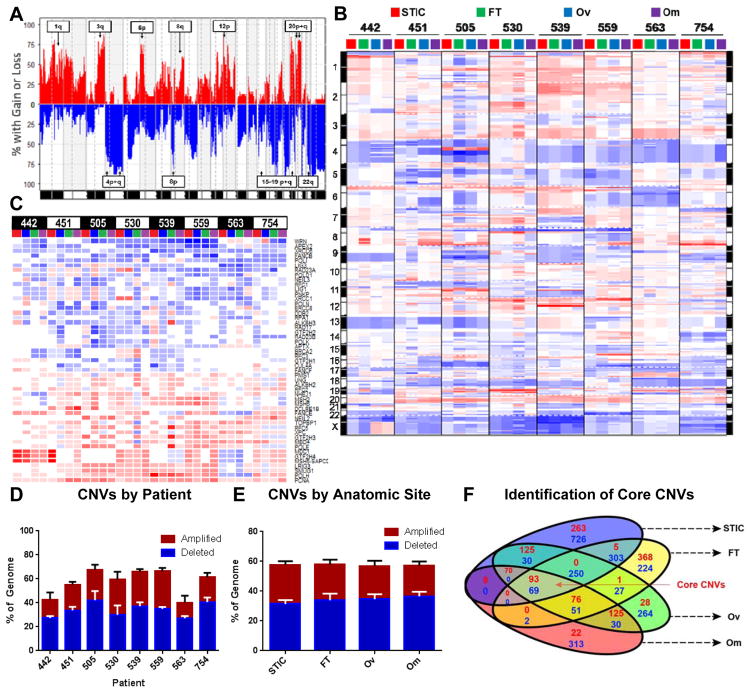
Figure 3. Genomic instability is a core feature of ovarian cancer that frequently involves DNA-damage repair genes.
A) Frequency plot of copy number variations (CNVs) across all patients and all four anatomic sites. Annotated arm level events were also identified as significant in the TCGA analysis of HGSOC. For all plots in Figure 3, amplifications are red and deletions are blue. B) Genomic aberration plot of CNVs across all anatomic sites and patients imply high degree of genomic instability in all anatomic sites. Chromosome numbers are indicated on margin. Amplifications are red; deletions are blue. C) CNV status of significantly altered DNA repair pathway genes (from REPAIRtoire) reveals common deletion of DNA damage response and repair genes known to be involved in HGSOC. Amplifications are red; deletions are blue. D/E) Extent of genomic instability is patient-specific (D) and does not vary by anatomic site (E). F) GISTIC identification and analysis of significantly altered genes identifies conserved core amplifications and deletions. Number of amplifications in red and deletions in blue.
Next, we utilized GISTIC, an algorithm to identify significant, recurrent amplifications and deletions (18), to detect core CNVs collaborating with TP53 to drive HGSOC (Fig. S3B/C). The Euler plot in Fig. 3F integrates significantly amplified or deleted genes at each anatomic site, identifying several genomic events known to be involved in HGSOC tumorigenesis, including CCNE1 amplification, NF1 deletion, and other genes with previously uncharacterized roles in HGSOC biology. These core genomic events consisted of 93 amplified and 69 deleted genes, which represented less than 5% of all significantly amplified and deleted genes. Significantly amplified processes included genes involved in the regulation of transcription and RNA metabolism, as well as several microRNAs (Fig. S3D, Table S6).
To elucidate the metastatic trajectories of HGSOC in each patient, a phylogenetic clustering approach was used (11). This analysis revealed three distinct classes of dissemination: I) “Basal STIC,” in which STIC represented the basal branch (most similar to germline DNA of the patient) of a hierarchical, step-wise dissemination process. II) “Parallel” dissemination in which no clear hierarchal dissemination pattern was evident and all branches developed simultaneously from one precursor. III) “STIC Metastases,” in which other anatomic sites were hierarchically more basal than the STIC lesions, implying that in these patients, STIC represented metastases to the fallopian tube (Fig. 4A, Fig. S4, Table S7). Histologically, the STIC from these cases was indistinguishable from the other basal STIC, although one of these cases presented with intraluminal HGSOC spheroids in the fallopian tube (Fig. 4B). Intraluminal HGSOC spheroids were present in three of the eight patients in our cohort, including one patient in whom spheroids adhered to the apical surface of the fallopian tube epithelium (Fig. 4C).
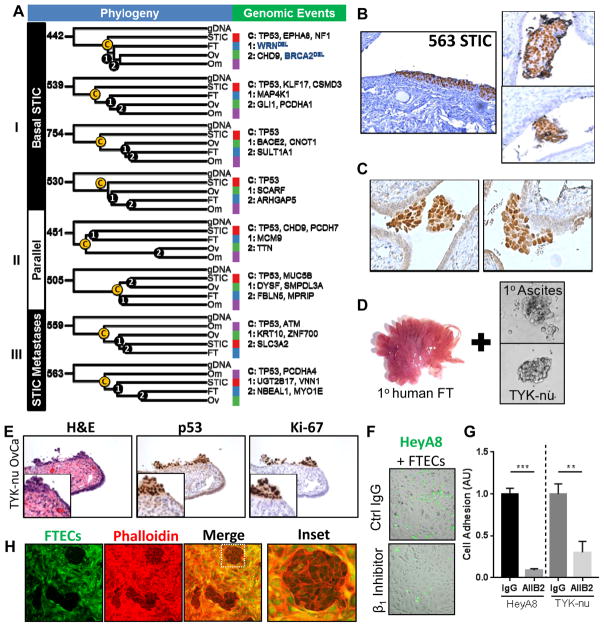
Figure 4. Phylogenetic analyses of ovarian cancer progression reveal diverse metastatic processes and evidence of intraepithelial metastasis.
A) Phylogenetic trees of each patient with key genomic events (SNVs, Indels, CNVs) that characterize each branching event annotated (C (“core”), 1, or 2). Deletions are in blue. B) Representative p53 staining of a “STIC Metastasis” in patient #563 (left), as well as intraluminal HGSOC spheroids within the same fallopian tube (right). C) P53 staining of intraluminal HGSOC spheroids adhering to the epithelium of the fallopian tube (patient 530) D) Human fallopian tube fimbriae and spheroids that were co-cultured. E) Co-culture of human fallopian tube explants with TYK-nu ovarian cancer spheroids mimic STICs with clearance of normal epithelium and implantation of tumor cells expressing Ki-67 and nuclear p53. F/G) Adhesion of fluorescently-labeled HGSOC cells (green) to primary FTEC monolayer (brightfield) after 15 minutes. Pretreatment with β1-integrin blocking antibody (AIIB2) attenuated adhesion of HeyA8 and TYK-nu cells to FTEC monolayer. H) TYK-nu ovarian cancer spheroids (phalloidin only) clear a primary human fallopian tube epithelial monolayer (phalloidin and CellTracker Green) after 12 hours.
While intraepithelial metastases of HGSOC to the fallopian tube have not been described, they have been observed in other tumor types, including endometrial and colon cancer (12, 19, 20). Intraluminal HGSOC spheroids found within the FT are common findings in HGSOC patients (21). Because metastasis to the fallopian tube was an unexpected finding, we sought to determine if HGSOC cells are able to implant into the fallopian tube epithelium. A model of intraepithelial metastasis was developed, in which full thickness primary human fallopian tubes were co-cultured with ovarian cancer spheroids (Fig. 4D, Fig. S5A). In this ex vivo model, the normal fallopian tube epithelial morphology was preserved, including high expression of PAX8 and junctional expression of β-catenin and E-cadherin (22) (Fig. S5B). Histological examination of the fallopian tube-cancer cell explants revealed the presence of implanted tumor cells that histologically resembled STIC and expressed the established molecular markers of STIC, including p53, Ki-67, and stathmin (Fig. 4E, S5C) (5, 6, 12, 23). HT-29 colon adenocarcinoma cells were also capable of implanting in the fallopian tube epithelium and could be distinguished by a lack of PAX8 immunoreactivity (Fig. S5D). In addition, using a similar approach, we found that HGSOC spheroids were also capable of adhering to ex vivo omental explants (Fig. S5E), but did not adhere to endometrial explants after extended culture (data not shown).
To mechanistically understand the interactions between fallopian tube epithelial cells (FTEC) and HGSOC cells, we established in vitro models of FTEC-cancer cell adhesion and epithelial clearance. Human FTECs (22) expressing PAX8 and preserving tight junctions (β-catenin expression, Fig. S6A/B) were co-cultured with HGSOC spheroids and adhesion measured. The cancer cells adhered to a monolayer of primary fallopian tube epithelium within 15 minutes in a β1-integrin-dependent manner (Fig. 4F/G, Fig. S6C) and cleared the FTEC and fully integrated in the monolayer within 12 hours (Fig. 4H). Combined, these in vitro and ex vivo experiments suggest that ovarian cancer cells are capable of adhering to and integrating with fallopian tube epithelium, suggesting that a portion of putative precursor STIC are, instead, metastatic mimics.
Discussion
We set out to study the dynamics of genomic alterations with the goal of understanding the relationships of STIC to the metastatic trajectory of HGSOC. Although we were initially challenged by the intrinsically low sample amounts of STIC, we show here that WES of low-input, in situ specimens is feasible and can be integrated into established workflows. That this study was performed with archival paraffin blocks indicates that the sources of samples used for analysis of core genomic events or phylogenetic reconstruction can be expanded to paraffin blocks which are widely available. The laser-capture microdissection of the tumor compartment allowed the collection of pure tumor samples, allowing us to make firm conclusions about genetic changes without the stromal contamination observed in other studies (3, 9).
We find that multi-site sequencing is useful for identifying candidate driver genomic events, with putative core mutations representing a minority of mutational events at any one site. In our study the mutation rate and mutation signature, as well as the CNV rates of STIC and HGSOC were consistent and unique to each patient. All samples had mutations at the TP53 locus, including all STIC lesions, demonstrating that the patients examined here are representative of HGSOC (5, 24). Every patient had very different genomic changes, consistent with the perception of HGSOC as a very heterogeneous disease that is not easily defined by a specific mutational change. There were no significant alterations between anatomic sites within a single patient, suggesting that the biologic processes underlying genomic instability, both CNVs and SNVs, are established early during disease progression. The extent and nature of mutations and CNVs is such that even “basal STIC” possess the same genomic instability and all the features of invasive HGSOC. During the progression to a disseminated HGSOC, the STIC have co-evolved with the tumor, thus increasing the extent of CNVs and SNVs. Common core events include mutation of TP53, frequent amplification of CCNE1, and deletion of DNA damage repair and signaling genes. In addition, we found a previously unappreciated widespread (70–100%) and early loss of heterozygosity (LOH) at the TP53 locus. This is in stark contrast to TP53 LOH rates in other cancers (15), indicating an exceptionally strong selective advantage for loss of the wild-type TP53 allele in HGSOC.
Notably, we found evidence that a portion of STIC represent metastases to the fallopian tube epithelium, rather than the origin of the tumor. Another study had considered the possibility of metastasis to the fallopian tube from HGSOC and uterine cancers (12). Targeted sequencing of multiple, anatomically distinct STIC in ovarian cancer patients has identified identical TP53 mutations in these synchronous in situ lesions, suggesting that they may represent clonal metastases within the fallopian tube (5). In the absence of germline TP53 mutation, the acquisition of identical TP53 mutations in two synchronous STIC is highly unlikely. Intraepithelial metastasis is thought to also occur in lung cancer, where cells metastasize to the bronchial epithelium (25). Although we found that non-gynecologic cancers may metastasize to the fallopian tube, these implants do not gain expression of FTEC markers and can be immunohistochemically differentiated from HGSOC. There is also evidence that the fallopian tube epithelium is a receptive site for metastasis of non-gynecologic cancers (19, 20).
The peritoneal cavity, including the fallopian tubes and ovaries, is a continuous system bathed in peritoneal fluid, which may facilitate complex patterns of dissemination. In both cases of “STIC metastasis” the omentum represented the most basal tumor site. The clinical presentation of primary peritoneal carcinoma, in which HGSOC is detected primarily in non-ovarian anatomical sites (26), suggests that the omentum and peritoneal mesothelium represents a microenvironment that fosters the establishment and growth of early metastases (27, 28). Based on expression of molecular markers and the lack of putative precursor lesions (26), it would be surprising for the peritoneal mesothelium of the omentum to represent the precursor of HGSOC. Endosalpingiosis to the omentum occurs frequently (15%) of women in an unselected cohort (29, 30). Normal oviductal tissue displaced to the omentum or peritoneum (endosalpingiosis) could undergo transformation thus mimicking an omental primary tumor or a primary peritoneal cancer with serous histology (30, 31). Alternatively, it is possible that the STIC metastases either originated from a precursor lesion within the fallopian tube or that a STIC precursor lesion colonized the peritoneum and later back-metastasized to the tube (11).
The data presented herein may lead us to develop our perception of STIC further: STIC in HGSOC could be primary or metastatic. Previous studies observed precursor lesions exclusively in the fallopian tube of BRCA mutation carriers undergoing prophylactic salpingo-oophorectomy (32), while our study focused on sporadic HGSOC. As clinical studies have begun to investigate the utility and safety of salpingectomy in high-risk patients (33–35), it will be imperative to understand if metastasis to the fallopian tube occurs in the context of germline BRCA mutations. Lastly, the frequent observation of STIC detected after neo-adjuvant treatment (occurring in 50% of all cases) could represent a late metastasis to a chemoresistant niche (36). Therefore a better understanding of the cross-talk between cell types unique to intraepithelial fallopian tube metastases could be both relevant clinically and important biologically.
Materials and Methods
Surgical treatment
All patients had newly diagnosed advanced, metastatic high-grade serous ovarian carcinoma (HGSOC) and were undergoing primary debulking surgery by a gynecologic oncologist (SDY, EL) at the University of Chicago (Table S1). All tissues were collected with informed consent under approved, University of Chicago IRB protocols and in accordance with the Declaration of Helsinki.
LCM and DNA extraction
10 μm sections of each tissue were cut onto PEN-MembraneSlides (Leica), deparaffinized with xylenes, rehydrated through graded alcohols, and stained with hematoxylin. Tumor components from each tissue were microdissected with a Leica LMD 6500 (up to 5 serial sections per sample). In cases of bilateral ovarian involvement, tumor was microdissected from the ovary of the same laterality as the STIC lesion. DNA was extracted with a QIAamp DNA FFPE Tissue Kit (Qiagen), with proteinase K digestion extended to 12 hours and sequential elution of DNA in 20 μl volumes following 5 minute incubations.
Sequencing and alignment
Libraries were prepared per standard protocols (Agilent SureSelect Human All Exon v5) and sequenced on an Illumina HiSeq 2500 ultra high-throughput sequencing system (100 bp, paired-end). Sequence quality was assessed using FastQC v0.11.2. Adapters were then removed and overlapping mates were merged using SeqPrep. Processed reads were then aligned to the human genome (hg19) using Novoalign v3.02.07 and filtered to remove low quality alignments and PCR duplicates with sambamba v0.5.4 (37). Alignment metrics were gathered using the CalculateHsMetrics tool from Picard v1.129 and the Agilent SureSelect target files. Filtered alignments were then refined as suggested by the Broad Institute’s “Best Practices” (38) using GATK v3.4 (39).
SNV/Indel Analysis
Refined alignments were then used to detect somatic mutations across the various combinations of normal (gDNA) and tumor samples within a patient. To reduce the number of false positives, three somatic mutation detection (SMD) tools were used: MuTect v1.1.4 (40), VarScan2 v2.3.9 (41), and Virmid v1.2.0 (42). Important differences between these tools include 1) Virmid and VarScan2 can detect loss of heterozygosity (LOH); 2) Virmid and VarScan2 can detect germline mutations; and 3) only VarScan2 attempts to call indels.
All detected somatic variants (including indels) were filtered to remove low quality, low coverage, and ambiguous genotype calls using in-house scripts (43). Filtered variants were then annotated using Annovar (44) and further filtered to remove variants located in intergenic, upstream/downstream, intronic, UTR5/3, or non-coding regions. Next, results across the 3 SMD tools were combined and merged at loci where the alleles were concordant or the mutation was observed in more than one anatomic site of the same patient. For one patient, manual review of TP53 sequencing alignments with the Integrated Genome Viewer was used to confirm mutation of TP53 at all anatomic sites. To calculate mutational rates, per-locus coverage was estimated using GATK’s DepthOfCoverage tool. Then, per-sample estimates of total target size were estimated by counting the number of loci with both normal and tumor sample coverage ≥8×. Next, using exonic and splicing somatic mutations, mutation rates were estimated for each tumor-normal combination.
To detect significant mutations, somatic variants detected by at least 2 SMDs or by MuTect, as well as high-quality indels, were annotated by Oncotator (45) and converted to a single MAF file. The MAF file was then processed by MutSigCV v1.4 (16) using the default settings and databases. Mutations detected as significant in only a single patient were excluded. Mutational signatures were assessed by assigning each SNV to one of seven classes as follows: T>C (A>G and T>C); C>T (G>A and C>T); C>G (G>C and C>G); T>G (A>C and T>G); T>A (A>T and T>A); C>A (G>T and C>A); and indels (insertions and deletions) (10). Mutational classes, based on their distribution between anatomic sites, were defined as follows: core mutations were shared by all four anatomic sites within a patient; shared mutations were present in two or three anatomic sites within a patient; and private mutations were detected in only a single anatomic site for a patient.
To assess germline mutation status of BRCA1/2 and other ovarian cancer susceptibility genes, Virmid and VarScan2 calls were utilized. Germline variants were filtered to remove potential false positives following the suggestions presented by the VarScan2 developers (41).
CNV Analysis
Refined alignments were scanned for copy number variations (CNVs) using VarScan2 (41) and custom scripts based on a previously published method (46). Due to the high overlap in segments between methods and less variability in the in-house method, we utilized the method introduced by Lonigro et al (46), which we term the “MI Method”. First, per-target (exon) coverage was estimated using GATK’s DepthOfCoverage tool (v3.4.0). Then, normalization was performed for each tumor-normal pair. Briefly, targets with coverage less than 10 in the matched normal sample were excluded. Then, per-target coverage in the tumor sample was divided by the per-target coverage in the matched normal sample resulting in coverage ratios for each target. Coverage ratios are then globally normalized by dividing each of them by the ratio of human mappings between the two samples (tumor/normal) and log2 transforming. Finally, the overall median value is subtracted, resulting in a set of log2-transformed coverage ratios with median zero for each tumor/normal matched pair. The normalized values were then segmented using the R/bioconductoR package ”copynumber” v1.6.0 (47). Segmented CNV calls were used to extract regions with log2R greater than 0.2 or less than 0.2, as performed with TCGA data, to extract the percentage of the genome that was amplified or deleted by anatomic site or patient. Copynumber segments were grouped by tissue (e.g., all STIC samples were run together) and used as inputs for GISTIC v2.0.22 (18).
Tumor Phylogenetics
Somatic mutations (both synonymous and non-synonymous) were combined with high-confidence LOH loci calls (>20× coverage, VarScan2) to generate a binary presence-absence matrix of genomic characters for each anatomic site within each patient. Inclusion of both LOH and SNV data lowers the likelihood of mutation reversion confounding phylogenetic analyses. Distance matrices were calculated using Jaccard-Tanimoto similarity coefficients with 100 bootstrap replicates and clustered using the unweighted pair group method with arithmetic mean (UPGMA) method to generate maximum likelihood dendrograms using DendroUPGMA (48). Cophenetic correlation coefficients were greater than 0.98 for all trees (49). Resulting dendrograms were plotted with the Interactive Tree of Life interface (50).
Immunohistochemistry
5 μm formalin-fixed, paraffin-embedded tissue sections were deparaffinized with xylene and rehydrated with a graded series of ethanol. Antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6) with 0.05% Tween 20 at 100oC. Samples were then incubated with 0.3% hydrogen peroxide for 20 mins at RT and blocked with 2.5% normal horse serum (Vector Laboratories) for 1 hr at RT. Primary antibodies (Stathmin, 1:200, Cell Signaling Technologies; Ki-67, 1:200, Thermo Scientific; PAX8, 1:200, Cell Signaling Technologies; β-catenin, 1:200, BD Biosciences; E-cadherin, 1:200, BD Biosciences; pan-p53, 1:200, Calbiochem) in 1.25% normal horse serum/PBS were incubated overnight at 4oC and visualized using the R.T.U VectaStain Kit and DAB (Vector Laboratories) and counterstained with hematoxylin.
Immunofluorescence
Cells were fixed in 4% paraformaldehyde, permeabilized with 0.5% TritonX-100, and blocked with 10% goat serum. Primary antibodies (PAX8, 1:200, Cell Signaling Technologies; β-catenin, 1:200, BD Biosciences) were incubated overnight in 10% goat serum. Samples were probed with fluorescent secondary antibodies (Thermo Scientific) and Hoechst (Invitrogen) for one hour before washing and mounting with ProLong Gold. Images were collected on a Zeiss LSM 510 Meta Confocal microscope and images processed with ImageJ.
Cell culture
HeyA8 (Gordon Mills, MD Anderson, Houston, TX; received June 2006), TYK-nu (Gottfried Koneczny, University of California, Los Angeles; November 2014), and HT-29 (Marcus Peter, Northwestern University, Chicago, IL; May 2009) cells were grown under recommended culture conditions and genotyped to confirm their authenticity (IDEXX Bioresearch short tandem repeat marker profiling every three months; all cell lines last validated January–July 2016). All cell lines were mycoplasma-negative. Immortalized FT33-TAg FTEC cells were a gift from Dr. Ronnie Drapkin (University of Pennsylvania; December 2014) and were cultured in 2% Ultroser G (Crescent Chemical) in DMEM/F12 50/50 (Corning) (22).
Ex vivo metastasis assays
Normal fallopian tube, endometrial, and omental tissues were collected from patients with benign gynecological conditions at the time of surgery. Fimbriae or 0.5 cm3 endometrial or omental tissues were dissected and transferred into 500 μl of FTEC media, known to support the proliferation and survival of primary fallopian tube epithelial cells (22). To each well, 5×104 HGSOC cells (TYK-nu or HeyA8) were added. For some experiments, spheroids were labeled with CellTracker Green (Thermo Scientific, 1:1000 dilution). Following 72 hrs of co-culture, fimbriae were fixed in 4% formaldehyde, dehydrated through graded alcohols and xylenes, and embedded in paraffin blocks. 5 μm sections were cut for each tissue and processed for IHC and immunohistochemistry (p53, Ki-67, and stathmin).
Isolation of primary HGSOC spheroids
Ascites fluid from HGSOC patients was passed through a 40 μm nylon mesh and washed with PBS to remove single cells. Isolated spheroids were maintained in non-adherent plates (Corning).
Isolation of primary fallopian tube epithelial cells
Fallopian tubes were collected from patients with benign gynecological conditions. Primary fallopian tube secretory epithelial cells (FTECs) were extracted and cultured as previously described (22).
In vitro fallopian tube adhesion assay
96-well plates were coated with fibronectin (0.1 μg/ml). Immortalized FTECs (FT33-TAg cells) were plated at 1×104 cells per well and grown to confluency (48 hrs). Ovarian cancer cells were labeled with CellTracker Green (1:1000) and added to wells of plate (5×104 cells per well) and incubated for 15 mins (37oC, 5% CO2). For β1-inhibitor experiments, cells were treated in suspension with AIIB2 blocking antibody or antibody control (5 μg/ml) for 30 mins. Alternatively, 5×104 HGSOC cells (TYK-nu or HeyA8) were plated in a 96 well plate and allowed to form spheroids for 24 hrs before being labeled with CellTracker Green (1:1000) and transferred to FTEC plates as for single cell suspensions. Plates were washed once with PBS, fixed in 4% paraformaldehyde, and imaged with a Zeiss Axio Observer.A1 with AxioVision software (Rel 4.8). Cells were counted (single-cells) or total area covered by cells quantified (spheroids) to quantify adhesion with ImageJ.
In vitro fallopian tube epithelial clearance assay
The method was adapted from Iwancki et al (51). Glass bottom 8-chamber slides were coated with 0.1 μg/ml fibronectin. Primary FTECs were directly plated into each well (2×104 cells/well) at passage zero and grown to 100% confluency (72 hrs) before labeling with CellTracker Green (1:1000). In parallel, 5×103 TYK-nu cells were plated in a non-adherent 96-well plate (Corning) to form spheroids for 72 hrs. Spheroids were directly transferred from 96-well plate to the FTEC monolayer and incubated for 12 hrs before fixation and staining for actin (AlexaFluor 647 phalloidin, Thermo Scientific). Images were collected on a Zeiss LSM 510 Meta Confocal microscope and images processed with ImageJ.
Supplementary Material
Statement of Significance.
We find that that the putative precursor lesions for high grade serous ovarian cancers, serous tubal intraepithelial carcinomas, possess most of the genomic aberrations present in advanced cancers. In addition, a proportion of STIC represent intraepithelial metastases to the fallopian tube rather than the origin of HGSOC.
Acknowledgments
We thank the Harris Family Foundation for their generous support. We thank Drs. H.A. Kenny, M. Curtis, K. Watters, and A. Mukherjee from the University of Chicago ovarian cancer laboratory for helpful discussions. We are very thankful to Gail Isenberg, University of Chicago, for carefully editing the manuscript.
Financial Support: This work was supported by a Marsha Rivkin Foundation award (M. Eckert), a National Cancer Institute grant - CA111882 (E. Lengyel), a Harris Family Foundation award (S.D. Yamada), and a University of Chicago Comprehensive Cancer Center Team Science award (E. Lengyel and S. Volchenboum).
Footnotes
The authors disclose no potential conflicts of interest.
Authors’ Contributions
Conception and design: M.A. Eckert, E. Lengyel
Development of methodology: M.A. Eckert, K.M. Hernandez, J. Andrade, S.L. Volchenboum, P. Faber
Acquisition of data: M.A. Eckert, S. Pan, P. Faber, A. Montag, R. Lastra
Bioinformatics: K.M. Hernandez, S.L. Volchenboum, J. Andrade
Sample acquisition, pathology review: S.D. Yamada, E. Lengyel, R. Lastra, A. Montag
Writing of manuscript: M.A. Eckert, K.M. Hernandez, M.E. Peter, E. Lengyel
Editing of the manuscript: M.A. Eckert, S.L. Volchenboum, SD Yamada, E. Lengyel
Study supervision: M.A. Eckert, E. Lengyel
References
- 1.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, et al. Inherited Mutations in Women With Ovarian Carcinoma. JAMA oncology. 2015:1–9. doi: 10.1001/jamaoncol.2015.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 4.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, et al. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian cancer. Journal of Pathology. 2001;195(4):451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn E, Kurman RJ, Vang R, Sehdev AS, Han G, Soslow R, et al. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma-evidence supporting the clonal relationship of the two lesions. Journal of Pathology. 2012;226:421–6. doi: 10.1002/path.3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. Journal of Pathology. 2007;211:26–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 7.Perets R, Wyant GA, Muto KW, Bijron JG, Poole BB, Chin KT, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca; Tp53; Pten models. Cancer Cell. 2013;24:751–65. doi: 10.1016/j.ccr.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–67. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 9.The Cancer Genome Atlas N. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–33. doi: 10.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467(7319):1114–7. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDaniel AS, Stall JN, Hovelson DH, Cani AK, Liu CJ, Tomlins SA, et al. Next-Generation Sequencing of Tubal Intraepithelial Carcinomas. JAMA oncology. 2015;1(8):1128–32. doi: 10.1001/jamaoncol.2015.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarz RF, Ng CK, Cooke SL, Newman S, Temple J, Piskorz AM, et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: a phylogenetic analysis. PLoS Med. 2015;12(2):e1001789. doi: 10.1371/journal.pmed.1001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McPherson A, Roth A, Laks E, Masud T, Bashashati A, Zhang AW, et al. Divergent modes of clonal spread and intraperitoneal mixing in high-grade serous ovarian cancer. Nat Genet. 2016;48(7):758–67. doi: 10.1038/ng.3573. [DOI] [PubMed] [Google Scholar]
- 15.Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502(7471):333–9. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawrence MS, Stojanov P, Polak P, Kryukov GV, Cibulskis K, Sivachenko A, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499(7457):214–8. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.George J, Alsop K, Etemadmoghadam D, Hondow H, Mikeska T, Dobrovic A, et al. Nonequivalent gene expression and copy number alterations in high-grade serous ovarian cancers with BRCA1 and BRCA2 mutations. Clin Cancer Res. 2013;19(13):3474–84. doi: 10.1158/1078-0432.CCR-13-0066. [DOI] [PubMed] [Google Scholar]
- 18.Mermel CH, Schumacher SE, Hill B, Meyerson ML, Beroukhim R, Getz G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011;12(4):R41. doi: 10.1186/gb-2011-12-4-r41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart CJ, Leung YC, Whitehouse A. Fallopian tube metastases of non-gynaecological origin: a series of 20 cases emphasizing patterns of involvement including intra-epithelial spread. Histopathology. 2012;60(6B):E106–14. doi: 10.1111/j.1365-2559.2012.04194.x. [DOI] [PubMed] [Google Scholar]
- 20.Rabban JT, Vohra P, Zaloudek CJ. Nongynecologic metastases to fallopian tube mucosa: a potential mimic of tubal high-grade serous carcinoma and benign tubal mucinous metaplasia or nonmucinous hyperplasia. Am J Surg Pathol. 2015;39(1):35–51. doi: 10.1097/PAS.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 21.Bijron JG, Seldenrijk CA, Zweemer RP, Lange JG, Verheijen RH, van Diest PJ. Fallopian tube intraluminal tumor spread from noninvasive precursor lesions: a novel metastatic route in early pelvic carcinogenesis. Am J Surg Pathol. 2013;37(8):1123–30. doi: 10.1097/PAS.0b013e318282da7f. [DOI] [PubMed] [Google Scholar]
- 22.Karst AM, Drapkin R. Primary culture and immortalization of human fallopian tube secretory epithelial cells. Nat Protoc. 2012;7:1755–64. doi: 10.1038/nprot.2012.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karst AM, Levanon K, Duraisamy S, Liu JF, Hirsch MS, Hecht JL, et al. Stathmin 1, a marker of PI3K pathway activation and regulator of microtubule dynamics, is expressed in early pelvic serous carcinomas. Gynecol Oncol. 2011;123(1):5–12. doi: 10.1016/j.ygyno.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmet AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. Journal of Pathology. 2010;221(1):49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiryu T, Hoshi H, Matsui E, Iwata H, Kokubo M, Shimokawa K, et al. Endotracheal/endobronchial metastases : clinicopathologic study with special reference to developmental modes. Chest. 2001;119(3):768–75. doi: 10.1378/chest.119.3.768. [DOI] [PubMed] [Google Scholar]
- 26.Nik NN, Vang R, Shih Ie M, Kurman RJ. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu Rev Pathol. 2014;9:27–45. doi: 10.1146/annurev-pathol-020712-163949. [DOI] [PubMed] [Google Scholar]
- 27.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118(4):1367–79. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nieman KM, Kenny HA, Penicka CV, Ladanyi A, Buell-Gutbrod R, Zillhardt M, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zinsser KR, Wheeler JE. Endosalpingiosis in the omentum: a study of autopsy and surgical material. Am J Surg Pathol. 1982;6(2):109–17. doi: 10.1097/00000478-198203000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Prentice L, Stewart A, Mohiuddin S, Johnson NP. What is endosalpingiosis? Fertil Steril. 2012;98(4):942–7. doi: 10.1016/j.fertnstert.2012.06.039. [DOI] [PubMed] [Google Scholar]
- 31.McCoubrey A, Houghton O, McCallion K, McCluggage WG. Serous adenocarcinoma of the sigmoid mesentery arising in cystic endosalpingiosis. J Clin Pathol. 2005;58(11):1221–3. doi: 10.1136/jcp.2005.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perets R, Drapkin R. It’s Totally Tubular....Riding The New Wave of Ovarian Cancer Research. Cancer Res. 2016;76(1):10–7. doi: 10.1158/0008-5472.CAN-15-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falconer H, Yin L, Gronberg H, Altman D. Ovarian cancer risk after salpingectomy: a nationwide population-based study. J Natl Cancer Inst. 2015;107(2) doi: 10.1093/jnci/dju410. [DOI] [PubMed] [Google Scholar]
- 34.Daly MB, Dresher CW, Yates MS, Jeter JM, Karlan BY, Alberts DS, et al. Salpingectomy as a means to reduce ovarian cancer risk. Cancer Prev Res (Phila) 2015;8(5):342–8. doi: 10.1158/1940-6207.CAPR-14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Przybycin CG, Kurman RJ, Ronnett BM, Shih Ie M, Vang R. Are all pelvic (nonuterine) serous carcinomas of tubal origin? Am J Surg Pathol. 2010;34(10):1407–16. doi: 10.1097/PAS.0b013e3181ef7b16. [DOI] [PubMed] [Google Scholar]
- 36.Colon E, Carlson JW. Evaluation of the fallopian tubes after neoadjuvant chemotherapy: Persistence of serous tubal intraepithelial carcinoma. Int J Gynecol Pathol. 2014 doi: 10.1097/PGP.0b013e3182a142c2. [DOI] [PubMed] [Google Scholar]
- 37.Tarasov A, Vilella AJ, Cuppen E, Nijman IJ, Prins P. Sambamba: fast processing of NGS alignment formats. Bioinformatics. 2015;31(12):2032–4. doi: 10.1093/bioinformatics/btv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Auwera GA, Carneiro MO, Hartl C, Poplin R, Del Angel G, Levy-Moonshine A, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics. 2013;43:11 0 1–33. doi: 10.1002/0471250953.bi1110s43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31(3):213–9. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22(3):568–76. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim S, Jeong K, Bhutani K, Lee J, Patel A, Scott E, et al. Virmid: accurate detection of somatic mutations with sample impurity inference. Genome Biol. 2013;14(8):R90. doi: 10.1186/gb-2013-14-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao R, Hernandez K, Huang L, Kang W, Bartom E, Onel K, et al. ExScalibur: A High-Performance Cloud-Enabled Suite for Whole Exome Germline and Somatic Mutation Identification. PLoS One. 2015;10(8):e0135800. doi: 10.1371/journal.pone.0135800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos AH, Lichtenstein L, Gupta M, Lawrence MS, Pugh TJ, Saksena G, et al. Oncotator: cancer variant annotation tool. Hum Mutat. 2015;36(4):E2423–9. doi: 10.1002/humu.22771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lonigro RJ, Grasso CS, Robinson DR, Jing X, Wu YM, Cao X, et al. Detection of somatic copy number alterations in cancer using targeted exome capture sequencing. Neoplasia. 2011;13(11):1019–25. doi: 10.1593/neo.111252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nilsen G, Liestol K, Van Loo P, Moen Vollan HK, Eide MB, Rueda OM, et al. Copynumber: Efficient algorithms for single- and multi-track copy number segmentation. BMC Genomics. 2012;13:591. doi: 10.1186/1471-2164-13-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Vallve S, Palau J, Romeu A. Horizontal gene transfer in glycosyl hydrolases inferred from codon usage in Escherichia coli and Bacillus subtilis. Mol Biol Evol. 1999;16(9):1125–34. doi: 10.1093/oxfordjournals.molbev.a026203. [DOI] [PubMed] [Google Scholar]
- 49.Sokal RR, Rohlf FJ. The Comparison of Dendrograms by Objective Methods. Taxon. 1962;11(2):33–40. [Google Scholar]
- 50.Letunic I, Bork P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics. 2007;23(1):127–8. doi: 10.1093/bioinformatics/btl529. [DOI] [PubMed] [Google Scholar]
- 51.Iwanicki M, Davidowitz RA, Ng MR, Besser A, Muranen T, Merritt M, et al. Ovarian cancer spheroids use myosin-generated force to clear the mesothelium. Cancer Discov. 2011;1(7):144–57. doi: 10.1158/2159-8274.CD-11-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.