Abstract
Background
Results of randomized trials on the effects of prenatal docosahexaenoic acid (DHA) on infant cognition are mixed, but most trials have used global standardized outcomes, which may not be sensitive to effects of DHA on specific cognitive domains.
Methods
Women were randomized to 600 mg/d DHA or a placebo for the last two trimesters of pregnancy. Infants of these mothers were then followed on tests of visual habituation at 4, 6, and 9 months of age.
Results
DHA supplementation did not affect look duration or habituation parameters but infants of supplemented mothers maintained high levels of sustained attention (SA) across the first year; SA declined for the placebo group. The supplemented group also showed significantly reduced attrition on habituation tasks, especially at 6 and 9 months.
Conclusion
The findings support with the suggestion that prenatal DHA may positively affect infants’ attention and regulation of state.
Introduction
Long-chain polyunsaturated fatty acids (LCPUFAs) and, in particular, docosahexaenoic acid (DHA) are associated with a number of positive effects on maternal and infant health (1). Interest in prenatal exposure to DHA has been fueled by findings showing improved pregnancy outcomes (e.g., gestation duration, birthweight) in both observational studies and randomized clinical trials (2–6). However, it has been hypothesized that prenatal exposure to DHA may also affect later development through fetal programming of the central nervous system and various other physiologic pathways. This possibility is supported by a number of observational studies that associate DHA status during pregnancy with positive long-term effects on the offspring (7–13). Several randomized trials of maternal DHA supplementation during pregnancy and/or lactation have been conducted with mixed results; the larger trials have not reported advantages for DHA supplementation during the first 18 months (14–16), although one small study found positive effects on problem solving (17). Of studies that followed infants into the preschool period, two reported significant benefits on IQ and neurodevelopmental measures (18, 19) while another did not (20). Positive effects of prenatal or postnatal DHA supplementation or status on attention in infancy and early childhood has been documented in several (9, 21–23), but not all (24) studies. Many of the studies showing null effects of prenatal supplementation have used global standardized tests for evaluating developmental outcome; these tests may not be sensitive to the effects of such supplementation in specific cognitive domains (25).
We report here on the results of a Phase III, double-blind, placebo-controlled randomized clinical trial (RCT, registered at www.clinicaltrials.gov as NCT00266825) of infants born to a large sample of mothers prenatally supplemented with DHA. The results of the other primary aims of the study (i.e., compliance, safety evaluation, and pregnancy outcomes) are documented elsewhere; supplementation had many positive effects, including the reduction of high-risk prematurity and increased birth length and weight (2). This study addresses the hypothesis that maternal DHA supplementation can enhance development, in particular visual attention, as assessed in infancy.
Results
Behavioral Measures
Peak look duration yielded a significant main effect for Age, F(2, 167.218 = 11.624 , p < 0.001) as look duration during habituation declined from 4 to 9 mos, but significant main effects or interactions involving DHA Group emerged. The analysis of looks to habituation yielded a marginally significant effect of DHA Group, F(2,165.373) = 3.69, p = .056, with infants from supplemented mothers (M = 6.8 looks, SD = 2.83) showing slightly fewer looks to habituation (i.e., slightly faster attainment of habituation) than infants from mothers on placebo (M = 7.31 looks, SD = 3.35).
Heart Rate
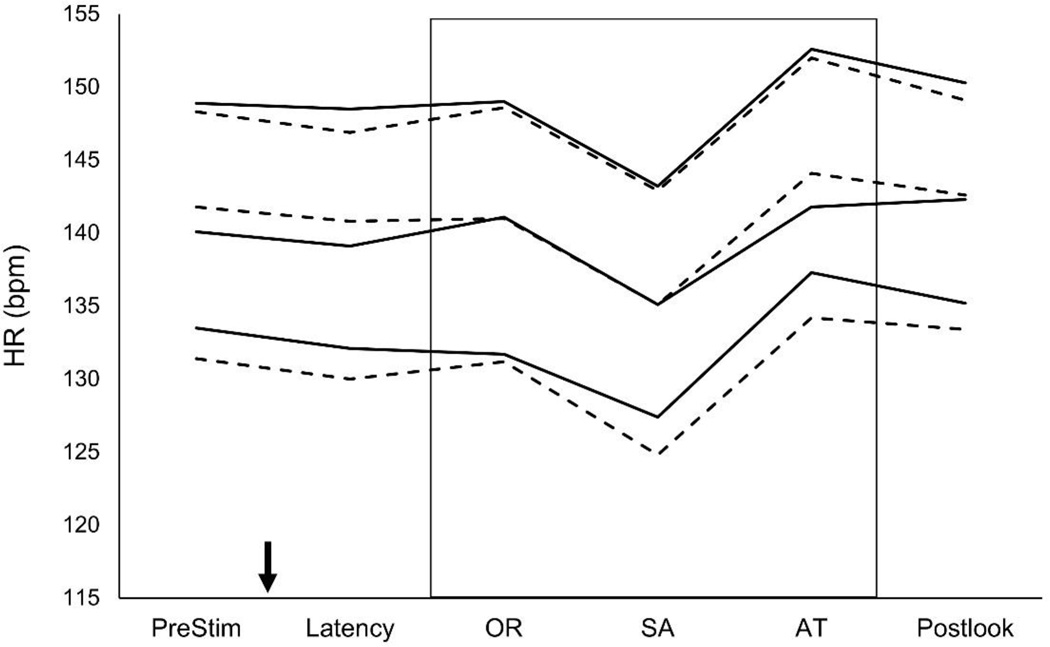
Heart rate (HR, expressed in beats/min) data were analyzed with mixed model methods on the prestimulus period (the 2-sec interval prior to stimulus onset), latency (the interval after stimulus onset but prior to the onset of looking), looking, and poststimulus (the 2-sec period after the stimulus has been withdrawn). Data for these phases for Placebo and Supplemented groups at all three ages, with Looking broken into HR-Defined phases of orienting (OR), sustained attention (SA), and attention termination (AT) are shown (see Figure 1; definitions and explanations of these three phases of attention are provided in the Method section under Measures Analyzed).
Figure 1.
Infant HR during the various phases of habituation trials analyzed with mixed models. For ease of exposition, values are collapsed across multiple looks during the habituation session. Although the graph shows the well-documented and highly robust changes in HR with age over the first year and the deceleration seen during infant looking while in SA, there are no differences between Supplemented (solid line) and Placebo (dashed line) groups at any point during the trial. The top pair of solid/dashed lines are data from 4 month-olds, the middle pair are from 6-month-olds, and the bottom pair are from 9-month-olds. The downward-pointing arrow represents the onset of the stimulus; the box represents encapsulates the period during which infants were looking at the stimulus. HR = heart rate, SA = sustained attention, PreStim = prestimulus period (before onset of stimulus), OR = orienting, AT = attention termination, Postlook = postlook period (after look is terminated but before withdrawal of stimulus). Data points represent successfully completed habituation sessions where HR data could be successfully coded: n=159 (n=72 and n=87 placebo and supplemented, respectively) at 4 months, n=172 (n=71 and n=101) at 6 months, and n=156 (n=68 and n=88) at 9 mo.
In each of the analyses for infant HR during Pre-stimulus, Latency, Looking, and Post-stimulus periods, data revealed expected significant main effects for Age (all ps < 0.001), which is due to the widely- and previously-reported decline in HR with infant age. No main effects or interaction involving DHA Group emerged at any point for HR analyses across looking during habituation.
HR Defined Phases of Attention
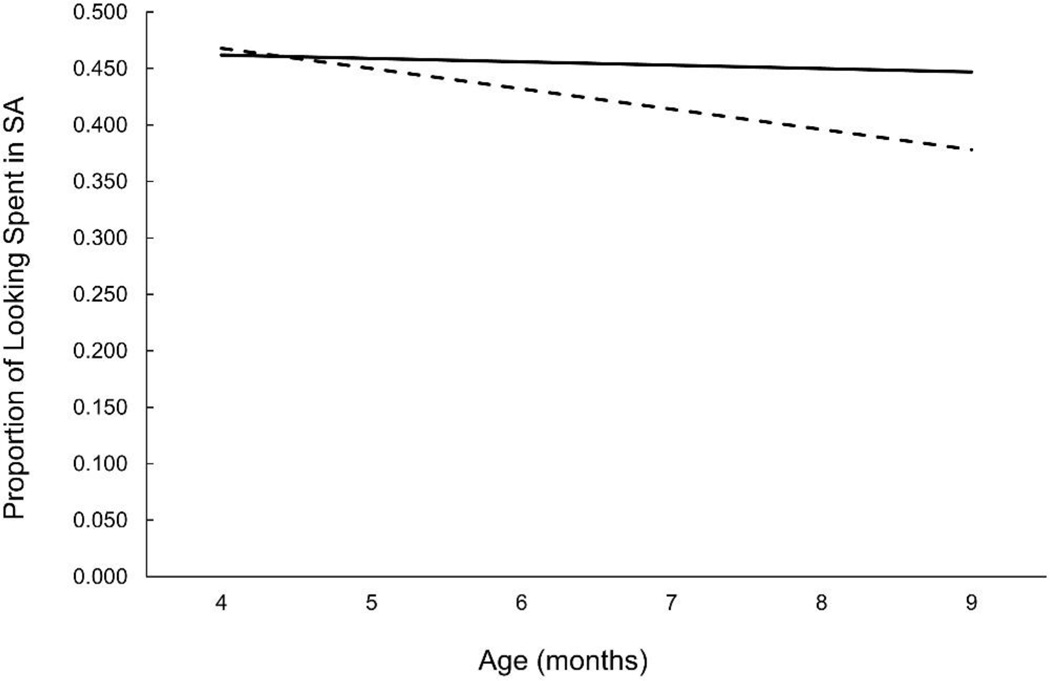
The proportion of time spent in OR increased significantly with age, F(2,176.512) = 4.58, p = 0.011), and the proportion of time spent in AT decreased with age, F(2, 180.826 = 3.88, p = 0.022). The main variable of interest, however, was proportion of time spent in SA, which reflects the relative amount of infants’ looking spent engaged with and actively processing the habituation stimulus. This analysis yielded a significant effect of Age, F(2, 179.027 = 3.31, p = 0.039) as SA decreased overall from 4 to 9 months, but this effect was moderated by a significant DHA Group X Age interaction, F(2, 179.027 = 3.51, p = 0.032). SA decreased significantly with age in the Placebo group, F(2, 75.172 = 3.91, p = .024) but not in the Supplemented group, F(2, 99.545 = 2.40, p = ns). Modeled data for SA from 4 to 9 months are shown (see Figure 2).
Figure 2.
Modeled data for proportion of time infants spent looking in SA as a function of randomized group assignment. Data are averaged across 4, 6, and 9 mo and reflect the significant Age X DHA Group interaction. Infants in the Placebo group (dashed line) showed a decrease in SA with age, infants in the Supplemented group (solid line) maintained levels of SA across the first year. Data shown are from completed habituation sessions where HR could be successfully coded: n=159 (n=72 and n=87 placebo and supplemented, respectively) at 4 months, n=172 (n=71 and n=101) at 6 months, and n=156 (n=68 and n=88) at 9 mo.
After observing this improvement in the quality of attention in infants from supplemented mothers on the habituation task, we examined whether the effect persisted after adding various covariates into the analyses. We repeated this analysis, controlling for parental verbal ability (as measured on the Peabody Picture Vocabulary Test: PPVT), household income, maternal education, and additional DHA taken during pregnancy, and gestational age at enrollment. The DHA Group X Age interaction remained significant in each case.
Task Completion and Fussiness
An additional finding emerged from the analysis of infant habituation. From this task, there is some data loss due to fussiness or crying at each age. The proportion of loss varies widely across laboratories and across ages, although in this laboratory it tends to be between 10% and 20%. When we examined the distribution of infants whose data were unused due to behavioral state issues, we observed that these infants were significantly more likely to be from the Placebo group overall, especially at 6 and 9 mo. It is important to keep in mind that testers were blind to assignment group when these determinations were made. The number and percentage of infants excluded due to fussiness/crying as a function of randomized assignment are shown (see Table 1); the p values reported are from χ2 tests conducted on observed cell counts.
Table 1.
Subject loss (attrition) due to fussiness/crying on visual habituation tasks at 4, 6, and 9 months of age as a function of membership in the two randomized groups
| Age | Placebo | Supplemented | p | Effect Size (r) |
||
|---|---|---|---|---|---|---|
| Not Fussy | Fussy | Not Fussy | Fussy | |||
| 4 mo | 75 | 19 | 89 | 27 | ns | 0.04 |
| 6 mo | 72 | 23 | 107 | 6 | < 0.001 | 0.27 |
| 9 mo | 71 | 22 | 94 | 14 | < 0.05 | 0.14 |
| Overall | 218 | 64 | 290 | 47 | < 0.01 | 0.11 |
All numbers are counts of individual children (within ages) or total sessions (Overall). Differences at 4 months were not significant (20.2% vs. 23.3%), but fussiness was significantly reduced in the supplemented group relative to the placebo group at 6 months (24.20% vs. 5.3%), and at 9 months (23.5% vs.12.9%). Attrition due to fussiness over all ages was reduced by 41% (from 22.7% to 13.9%) with DHA supplementation. p values reported are from χ2 tests conducted on observed cell counts. Infants’ data at any particular age could also be excluded for reasons unrelated to fussiness (experimenter error, equipment failure, and parental interference); such additional exclusions totaled n=2 at 4 months, n=5 at 6 months, and n=4 at 9 mo.
Discussion
This project represents one of a very few follow-up studies on the effects of prenatal maternal supplementation on infant attention during the first year. At 4, 6, and 9 months, infants from mothers supplemented with prenatal DHA were not different from infants from mothers in the placebo group on purely behavioral or HR measures, although infants from supplemented mothers showed a marginal trend to habituate more quickly across all ages. More importantly, however, infants from supplemented mothers maintained a consistent level of SA (a higher-quality attentional state strongly associated with stimulus processing) from 4 to 9 months, while SA dropped off across the first year in infants from non-supplemented mothers. Although this outcome measure is a standardized index, and the interpretation of this pattern of change is not definitive, we think it important to note that this specific profile (i.e., the maintenance of consistent levels of SA across the first year), has been previously reported to be associated with higher preschool vocabulary and intelligence scores at 4 years (26). It is of interest that a behavioral measures of sustained attention was also the only neuropsychological domain assessed at 5 years to be enhanced by maternal DHA supplementation during lactation (22).
Prenatal DHA supplementation did not affect measures of look duration or HR. An unexpected finding to emerge from this trial was the observation that attrition from the visual habituation task attributable to fussiness (i.e., a presumed indicator of regulation of behavioral state) was significantly lower for infants of DHA supplemented mothers overall (and in particular at 6 and 9 months), suggesting another possible effect of early DHA status on infant development.
Lower HR has been reported in infants who are supplemented with DHA and arachidonic acid (ARA) and with fish oil (23, 27), however, we did not find an effect of prenatal supplementation with DHA on HR. All children in the study were receiving DHA and ARA at the time they were tested, either from infant formula or human milk feeding. Although no findings in this area are yet definitive, this pattern of results is consistent with effects attributable to the presence of LCPUFA or DHA in the individual’s diet, rather than to an early programming effect. Our group has shown previously that fetal HR variability is increased by prenatal DHA supplementation with 600 mg/d of DHA (28) and higher HR variability is linked to cognitive measures such as arousal and attention (29), but to our knowledge a link between fetal HR variability and cognitive function in infancy has not been investigated.
This trial has its limitations. Blood levels did show that the prenatal supplementation did affect DHA levels in both maternal and cord blood at delivery; however, we did not control for postnatal dietary intake, although we recorded it at regular intervals in the first 12 months of life. As noted above, all infants in the study received DHA and ARA from either human milk or modern infant formulas that include DHA and ARA. Despite postnatal consumption of DHA and ARA, the pattern of effects seen here following prenatal DHA supplementation (i.e., differences observed on early attention outcomes, but not on standardized developmental tests) echo those for a postnatal feeding trial of DHA and ARA supplementation that yielded strong effects on cognition and language when children were followed into the preschool period (23, 30). Our data suggest, therefore, that there are benefits to prenatal DHA supplementation in our US population over and above those of receiving DHA and ARA after birth. In addition, our decision not to invite children born <34 weeks to participate in follow-up could be criticized, however, we did not wish to conflate any longer-term direct effect of DHA on these children’s developmental outcome with the indirect effect of early preterm birth. A strength of the study is the relatively large size of the groups studied compared to most studies of infant development, which reduces the likelihood of a Type II error for some of the outcomes that were not affected by DHA supplementation.
In summary, prenatal maternal DHA supplementation conferred advantages for the infants on attentional tasks (SA and behavioral state) during the first year of life. The pattern of effects seen here parallels that found for a postnatal feeding trial with DHA and arachidonic acid that yielded strong effects on cognition and language when children were followed into the preschool period (23, 30), and suggests that benefits of prenatal DHA supplementation might persist into the preschool period despite the fact that all in the cohort were fed a source of DHA and arachidonic acid during the first year of life.
Methods
Subjects
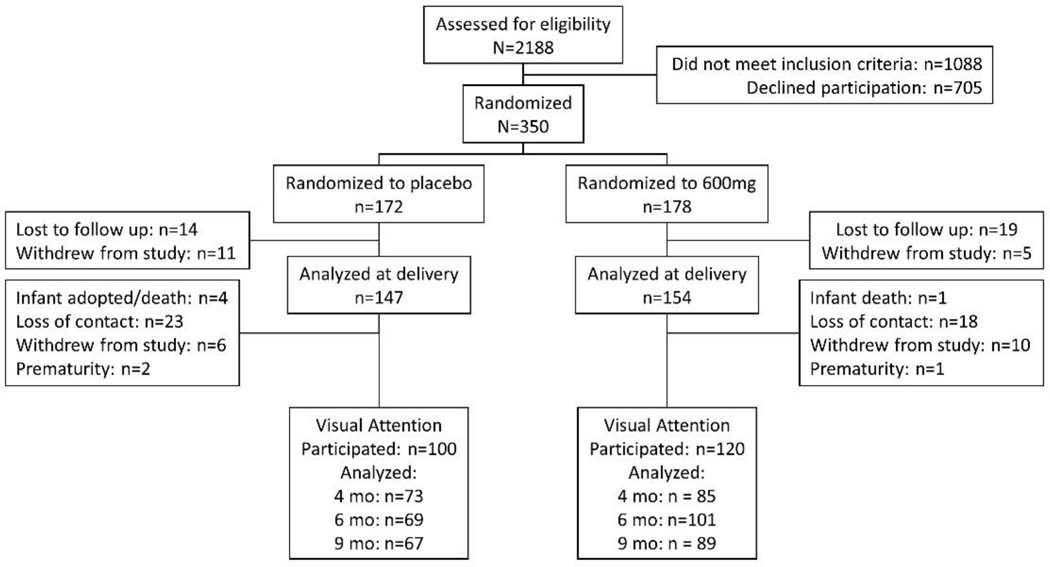
The Consolidated Standards of Reporting Trials (CONSORT) Diagram for the RCT is shown here (see Figure 3). Subjects were consented at enrollment during pregnancy for all follow-up measures. Details regarding the enrollment, randomization, blinding, Data Safety and Monitoring Board (DSMB) function, data checking and integrity, inclusion/exclusion criteria, compliance, and demographics of the sample are reported in the primary paper from this RCT (2). Informed consent was obtained from all participants and the study was approved by the University of Kansas Medical Center Human Subjects Committee. We invited all infants born to women in the Kansas University DHA Outcomes Study (KUDOS) pregnancy trial to participate in follow-up. In making those invitations, we made the strategic decision to exclude infants born <34 weeks gestation (n=8), because premature infants show impoverished performance on visual habituation tasks (31) and experience significant delays on standardized tests in toddlerhood (32) and because we predicted that this group would be differentially distributed between the placebo and supplemented groups. We reasoned that, by excluding early preterms, the follow-up would provide a more direct test of the effects of prenatal DHA supplementation on later infant development, rather than reveal effects that might be moderated by reductions in prematurity.
Figure 3.
CONSORT diagram for follow-up of RCT on DHA prenatal supplementation. The attrition from delivery to the first-year tasks was 31.9% for the placebo group and 22.1% for the supplemented group.
Subjects received either 3 capsules/d of an orange-flavored marine algae-oil source of DHA (200 mg DHA/capsule, DHASCO, from DSM Nutritional Products, Parsippany, NJ, USA; formerly Martek Biosciences) from enrollment at a mean 14.5 weeks gestation until birth (treatment), or 3 capsules containing half soybean and half corn oil (placebo, also orange-flavored). DSM Nutritional Products donated the capsules for the study but had no role in the study design, analysis, interpretation, or dissemination.
This clinical trial had two general aims. The first aim was to determine the effect of prenatal DHS supplementation on pregnancy outcomes. These outcomes (for which the study was powered) are reported in a previous publication (2) and the hypotheses were supported: supplementation increased gestation, birth weight, and birth length. In addition, DHA was observed to reduce the number of early preterm deliveries (<34 weeks gestation). The second primary aim of the trial was to determine the effects of prenatal DHA on development of infants born to these mothers. The current report focuses on visual habituation from 4 to 9 months of age. Per standard clinical trial methodology, testers remained blind to assignment group for all determinations, data coding, and analysis.
The demographic characteristics of the sample not followed up versus the characteristics of the sample that was followed after birth are shown (see Table 2). Compared to the children in the study not in the follow-up sample, those in the follow-up sample had mothers who were more compliant with capsule intake. However, the cohort included all major US racial/ethnic groups with a wide range of education and income. The demographic characteristics of the follow-up sample broken out by Placebo vs. Supplemented groups are also shown (see Table 3
Table 2.
Participants in the RCT follow-up versus those who did not participate
| Variable | Not Followed Up (N=71) |
Followed Up (N=230) |
Effect sizea |
pb |
|---|---|---|---|---|
| Gestation at enrollment (d) | 15.2 ± 3.7c | 14.6 ± 3.5 | 0.18 | ns |
| Gestation at delivery (d) | 38.5 ± 3.3 | 39.4 ± 1.4 | 0.45 | <.05 |
| Birthweight (g) | 2997 ± 731 | 3357 ± 231 | 0.63 | <.001 |
| Birth length (cm) | 47.9 ± 4.1 | 49.8 ± 2.6 | 0.61 | <.001 |
| Birth Head Circumference (cm) | 32.8 ± 2.6 | 34.2 ±1.6 | 0.73 | <.001 |
| Pre-Pregnancy BMI | 24.7 ± 4.9 | 25.5 ± 4.9 | 0.16 | ns |
| Additional supplemental DHA during Pregnancy (%) |
3 | 17 | 1.04 | <.001 |
| Additional supplemental DHA during Pregnancy (mg/d) |
4.9 ± 29.3 | 36.5 ± 84.4 | 0.40 | <.001 |
| Iron Supplement during Pregnancy (%) |
27 | 21 | 0.18 | ns |
| Average capsules taken (per wk) | 11.2 ± 5.4 | 17.1 ± 4.4 | 1.08 | <.001 |
| History of smoking (%) | 39 | 44 | 0.11 | ns |
| History of smoking (pack-years)d | 1.1 ± 2.5 | 1.7 ± 3.5 | 0.18 | ns |
| Smoked during pregnancy (%) | 31 | 34 | 0.07 | ns |
| Smoking during Pregnancy (cigarettes/d) |
1.9 ± 3.4 | 2.0 ± 4.4 | 0.02 | ns |
| Alcohol use before Pregnancy (%) | 34 | 60 | 0.59 | <.001 |
| Alcohol used during Pregnancy (%) (no. drinks/d) |
0.04 ± 0.3 | 0.00 ± 0.0 | 0.23 | ns |
| Maternal Age at Enrollment (y) | 23.4 ± 4.3 | 26.0 ± 4.8 | 0.54 | <.001 |
| Maternal ethnicity (% Hispanic) | 13 | 6 | 0.47 | ns |
| Maternal race (% Black) | 64 | 31 | 0.75 | <.001 |
| Maternal PPVT | 96.8 ± 14.4 | 99.5 ± 15.2 | 0.18 | ns |
| Maternal Education (y) | 12.5 ± 2.1 | 14.11 ± 2.8 | 0.60 | <.001 |
| Income by Zip Code (US$) | 39,959 ± 20,619 | 46,377 ± 17,778 | 0.34 | <.05 |
Cohen’s d for continuous variables and logit d for binary variables. Cohen’s d effect sizes are typically characterized as small (0.2 to 0.5), medium (0.5 to 0.8) or large (0.8 and above).
CONSORT guidelines do not recommend significance testing for data such as these, but we have provided p values. We caution against drawing substantive conclusions from these values as they are presented strictly as summary statistics and not for statistical significance.
Mean ± SD (all such values); determined by using SPSS (IBM, Armonk, NY, USA);.
Years smoked × packs/d.
Table 3.
Comparison of infants followed whose mothers received prenatal DHA supplement versus those whose mothers received a placebo.
| Variable | Placebo (N=107) |
Supplement (N=123) |
Effect sizea |
p |
|---|---|---|---|---|
| GA at Enrollment (weeks) | 14.0 ± 3.5b | 15.0 ± 3.4 | 0.29 | <.05 |
| GA at Delivery (weeks) | 39.4 ± 1.1 | 39.4 ± 1.6 | 0.05 | ns |
| Birthweight (g) | 3306 ± 422 | 3400 ± 528 | 0.19 | ns |
| Birth Length (cm) | 49.7 ± 2.4 | 49.9 ± 2.7 | 0.06 | ns |
| Birth Head Circumference (cm) | 34.1 ± 1.2 | 34.4 ± 1.9 | 0.15 | ns |
| Pre-Pregnancy BMI | 25.0 ± 4.8 | 26.0 ± 5.0 | 0.20 | ns |
| Additional supplemental DHA during Pregnancy (%) |
22 | 12 | 0.40 | <.05 |
| Additional supplemental DHA during Pregnancy (mg/d) |
47.1 ± 92.5 | 27.5 ± 75.9 | 0.23 | ns |
| Iron Supplement during Pregnancy (%) |
21 | 22 | 0.03 | ns |
| Average capsules taken (per wk) | 17.0 ± 4.4 | 17.3 ± 4.4 | 0.07 | ns |
| History of smoking (%) | 48 | 40 | 0.17 | ns |
| History of smoking (pack-years)c | 2.0 ± 3.8 | 1.4 ± 3.2 | 0.16 | ns |
| Smoking during Pregnancy (%) | 40 | 29 | 0.27 | ns |
| Smoking during Pregnancy (cigarettes/d) |
2.4 ± 5.1 | 1.7 ± 3.8 | 0.16 | ns |
| Alcohol before Pregnancy (%) | 62 | 59 | 0.07 | ns |
| Alcohol before Pregnancy (no. drinks/d) |
0.3 ± 0.8 | 0.1 ± 0.4 | 0.20 | ns |
| Alcohol during Pregnancy (%) | 3 | 1 | 0.62 | ns |
| Alcohol during Pregnancy no. drinks/d) |
0.0 ± 0.00 | 0.0 ± 0.00 | 0.00 | ns |
| Maternal Age at Enrollment (y) | 26.0 ± 4.9 | 26.0 ± 4.8 | 0.00 | ns |
| Maternal ethnicity (% Hispanic) | 8 | 4 | 0.18 | ns |
| Maternal race (% Black) | 35 | 28 | 0.18 | ns |
| Maternal PPVT | 99.1 ± 15.8 | 99.7 ± 14.7 | 0.04 | ns |
| Maternal Education (y) | 13.9 ± 2.9 | 14.3 ± 2.7 | 0.15 | ns |
| Income by Zip Code (US$) | 44,625 ±17,409 | 47,898 ± 18,024 | 0.18 | ns |
Cohen’s d for continuous variables and logit d for binary variables. Cohen’s d effect sizes are typically characterized as small (0.2 to 0.5), medium (0.5 to 0.8) or large (0.8 and above).
Mean ± SD.(all such values); determined by using SPSS (IBM);
Years smoked × packs/d
Longitudinal Measures
We chose postnatal measures based on the extant literature showing DHA affecting behavioral measures of visual attention (9). Visual habituation was administered at multiple time points to provide data on developmental trajectories to ensure assessment at points of maximum developmental sensitivity (33).
Visual Habituation and Heart Rate
Infants were evaluated at 4, 6, and 9 months of age (corrected for gestational age) on a visual habituation protocol that was augmented with simultaneous measurement of HR. This outcome is well-suited to the first year but less appropriate beyond 12 months, when infants become increasingly mobile (34). Visual habituation is a well-known measure of nonassociative visual learning, in which the infant’s visual and cardiac responses are assessed to repeated stimulus presentations. In this procedure, the infant is seated in a darkened room facing a screen on which visual stimuli are shown. The stimulus is shown repeatedly, and observers code infants’ looking to the stimuli over the repetitive presentations and HR is simultaneously collected during the session. Look duration decreases over the course of these repetitions. The decline in looking (habituation) reflects the infant’s learning and memory for the presented stimulus, and HR reflects the quality of the infant’s attention during looking; HR deceleration during looking is associated with engagement and active processing of the stimulus shown. The presentations continue until the infant’s looking declines (habituates) to a predetermined criterion. Details of the testing situation and recording of infant looking are reported elsewhere and the protocol was identical to that used in an RCT on postnatal feeding (23, 30).
The stimuli used were two-dimensional faces of adults showing neutral expressions; the same set was used in a previous RCT involving zinc and iron (35). Along with allowing for the calculation of infant HR during the session, this protocol also allows for the derivation of different types or phases of attention during looking (36); most notable among those phases is sustained attention (SA), which reflects active processing of the stimulus. As in previous reports (23, 30), the primary measures of interest were look duration during habituation, which reflects how quickly the stimulus is learned (34); and the proportion of time looking spent in SA, which indicates the proportion of time spent engaged and processing the stimulus (37, 38).
Statistical Analysis
Analyses
Given that longitudinal data were available at 4, 6, and 9 months of age, we conducted mixed-model analyses (which use all available data) with Subjects as a random factor, Age as a within-subject factor, and DHA group as a between-subject factor (preliminary analyses involving infant gender did not yield significant effects or interactions). Covariance was left unstructured as a conservative default. After initial tests were performed, appropriate demographic covariates were entered into analyses in order to rule out alternative plausible explanations for significant outcomes.
Analyses of look duration variables from visual habituation were conducted only on data from sessions that were complete and judged (by blinded observers) to have yielded usable data; analyses of HR and HR-defined phases from visual habituation were conducted on complete and usable habituation sessions but further required HR data from sessions judged (again, by blinded HR coders) to be usable. Infants’ data were also excluded for reasons unrelated to fussiness (experimenter error, equipment failure, and parental interference). The number of sessions analyzed are presented in the CONSORT diagram.
Measures Analyzed
The measures analyzed from the visual habituation paradigm were derived from three basic categories. The first category included behavioral measures of peak look duration and number of looks to habituation; look duration has been reported to be affected by DHA status in one study of prenatal maternal supplementation (9) but not in a subsequent clinical trial of postnatal feeding (23). The second category was infants’ heart rate (HR) during the various points of the habituation protocol, which has been shown to be affected by postnatal supplementation (23, 28). The third category reflected a coupling of behavior and HR (39, 40), and included the proportion of time spent in HR-defined phases of attention. During periods of looking in the habituation procedure, infants typically show robust and sustained HR decelerations. Considerable evidence suggests that active engagement and processing of the visual stimulus occurs when the infant’s HR is decelerated (38). The use of HR during infant looking allows attention to be parsed into separate phases of SA (the period of HR deceleration seen during infant looking), OR (the phase of looking prior to the occurrence of deceleration), and AT (the phase during which the infant remains looking after SA but after HR has returned to baseline levels). Details on the computation of these variables are available in numerous previously-published reports (23, 30).
Acknowledgments
We are grateful to the numerous Psychology and Dietetics and Nutrition graduate students who assisted with data collection and entry and to the families who have allowed us to follow their children. The authors’ responsibilities are as follows: JC, KMG, BJG, and SEC designed the study; JC conducted statistical analyses; JC and SEC wrote the manuscript and had primary responsibility for the final content; EHK, DJS, JMT, TD and CCB set up and coordinated the study and had the primary responsibility for the day-to-day management of the study, including recruiting subjects and data collection, entry and management, and supervision of students. All authors read and approved the final manuscript.
Financial Support: This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (R01 HD047315), the Office of Dietary Supplements, and the Kansas Intellectual and Developmental Disabilities Research Center (P30 HD002528). DSM Nutritional Products (Parsippany, NJ, US) donated the DHA and placebo capsules for the study.
Footnotes
Category of Study: Clinical
Disclosures: None of the authors declare a potential conflict of interest.
References
- 1.Koletzko B, Lien E, Agostoni C, et al. The roles of long-chain polyunsaturated fatty acids in pregnancy, lactation and infancy: review of current knowledge and consensus recommendations. J Perinat Med. 2008;36:5–14. doi: 10.1515/JPM.2008.001. [DOI] [PubMed] [Google Scholar]
- 2.Carlson SE, Colombo J, Gajewski BJ, et al. DHA supplementation and pregnancy outcomes. Am J Clin Nutr. 2013;97:808–815. doi: 10.3945/ajcn.112.050021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larqué E, Gil-Sánchez A, Prieto-Sánchez MT, Koletzko B. Omega 3 fatty acids, gestation and pregnancy outcomes. Br J Nutr. 2012;107:S77–S84. doi: 10.1017/S0007114512001481. [DOI] [PubMed] [Google Scholar]
- 4.Makrides M, Duley L, Olsen SF. Marine oil, other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre-eclampsia or intrauterine growth restriction. Cochrane Database of Systematic Reviews. 2006:75. doi: 10.1002/14651858.CD003402.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Makrides M, Gibson RA, McPhee AJ, et al. Effect of DHA Supplementation During Pregnancy on Maternal Depression and Neurodevelopment of Young Children A Randomized Controlled Trial. J Am Med Assoc. 2010;304:1675–1683. doi: 10.1001/jama.2010.1507. [DOI] [PubMed] [Google Scholar]
- 6.Ramakrishnan U, Stein AD, Parra-Cabrera S, et al. Effects of docosahexaenoic acid supplementation during pregnancy on gestational age and size at birth: Randomized, double-blind, placebo-controlled trial in Mexico. Food Nutr Bull. 2010;31:108–116. doi: 10.1177/15648265100312S203. [DOI] [PubMed] [Google Scholar]
- 7.Bakker EC, van Houwelingen AC, Hornstra G. Early nutrition, essential fatty acid status and visual acuity of term infants at 7 months of age. Eur J Clin Nutr. 1999;53:872–879. doi: 10.1038/sj.ejcn.1600868. [DOI] [PubMed] [Google Scholar]
- 8.Cheruku SR, Montgomery-Downs HE, Farkas SL, Thoman EB, Lammi-Keefe CJ. Higher maternal plasma docosahexaenoic acid during pregnancy is associated with more mature neonatal sleep-state patterning. Am J Clin Nutr. 2002;76:608–613. doi: 10.1093/ajcn/76.3.608. [DOI] [PubMed] [Google Scholar]
- 9.Colombo J, Kannass KN, Shaddy DJ, et al. Maternal DHA and the development of attention in infancy and toddlerhood. Child Dev. 2004;75:1254–1267. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 10.Innis SM, Friesen RW. Essential n-3 fatty acids in pregnant women and early visual acuity maturation in term infants. Am J Clin Nutr. 2008;87:548–557. doi: 10.1093/ajcn/87.3.548. [DOI] [PubMed] [Google Scholar]
- 11.Malcolm CA, McCulloch DL, Montgomery C, Shepherd A, Weaver LT. Maternal docosahexaenoic acid supplementation during pregnancy and visual evoked potential development in term infants: a double blind, prospective, randomised trial. Arch Dis Child. 2003;88:F383–F390. doi: 10.1136/fn.88.5.F383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Willatts P, Forsyth JS, Agostoni C, Bissenden J, Casaear P, Boehm G. Long-chain polyunsaturated fatty acid supplementation in infancy and cognitive function in later childhood. J Reprod Infant Psychol. 2003;21:257–258. doi: 10.1136/bmj.326.7396.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Goor SA, Dijck-Brouwer DA, Doornbos B, et al. Supplementation of DHA but not DHA with arachidonic acid during pregnancy and lactation influences general movement quality in 12-week-old term infants. Br J Nutr. 2010;103:235–242. doi: 10.1017/S0007114509991528. [DOI] [PubMed] [Google Scholar]
- 14.Helland IB, Saugstad OD, Smith L, et al. Similar effects on infants of n-3 and n-6 fatty acids supplementation to pregnant and lactating women. Pediatr. 2001;108 doi: 10.1542/peds.108.5.e82. art. no.-e82. [DOI] [PubMed] [Google Scholar]
- 15.Jensen CL, Llorente AM, Voigt RG, et al. Effects of maternal docosahexaenoic acid (DHA) supplementation on visual and neurodevelopmental function of breast-fed infants of maternal depression and cognitive interference. Pediatr Res. 1999;45:1675. [Google Scholar]
- 16.Gibson RA, Neumann MA, Makrides M. Effect of increasing breast milk docosahexaenoic acid on plasma and erythrocyte phospholipid fatty acids and neural indices of exclusively breast fed infants. Eur J Clin Nutr. 1997;51:578–584. doi: 10.1038/sj.ejcn.1600446. [DOI] [PubMed] [Google Scholar]
- 17.Judge MP, Harel O, Lammi-Keefe CJ. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. Am J Clin Nutr. 2007;85:1572–1577. doi: 10.1093/ajcn/85.6.1572. [DOI] [PubMed] [Google Scholar]
- 18.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatr. 2003:111. doi: 10.1542/peds.111.1.e39. [DOI] [PubMed] [Google Scholar]
- 19.Jensen CL, Voigt RG, Prager TC, et al. Effects of maternal docosahexaenoic acid intake on visual function and neurodevelopment in breastfed term infants. Am J Clin Nutr. 2005;82:125–132. doi: 10.1093/ajcn.82.1.125. [DOI] [PubMed] [Google Scholar]
- 20.Makrides M, Gould JF, Gawlik NR, et al. Four-year follow-up of children born to women in a randomized trial of prenatal dha supplementation. J Am Med Assoc. 2014;311:1802–1804. doi: 10.1001/jama.2014.2194. [DOI] [PubMed] [Google Scholar]
- 21.Kannass KN, Colombo J, Carlson SE. Maternal DHA levels and toddler free-play attention. Dev Neuropsychol. 2009;34:159–174. doi: 10.1080/87565640802646734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen CL, Voigt RG, Llorente AM, et al. Effects of Early Maternal Docosahexaenoic Acid Intake on Neuropsychological Status and Visual Acuity at Five Years of Age of Breast-Fed Term Infants. J Pediatr. 2010;157:900–905. doi: 10.1016/j.jpeds.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res. 2011;70:406–410. doi: 10.1203/PDR.0b013e31822a59f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould JF, Makrides M, Colombo J, Smithers LG. Randomized controlled trial of maternal omega-3 long-chain PUFA supplementation during pregnancy and early childhood development of attention, working memory, and inhibitory control. Am J Clin Nutr. 2014;12:12. doi: 10.3945/ajcn.113.069203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo J, Carlson SE. Is the measure the message: the BSID and nutritional interventions. Pediatr. 2012;129:1166–1167. doi: 10.1542/peds.2012-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colombo J, Shaddy DJ, Blaga OM, Anderson CJ, Kannass KN, Richman WA. Early attentional predictors of vocabulary in childhood. In: Colombo J, McCardle P, Freund L, editors. Infant pathways to language: Methods, models, and research directions. New York, NY, US: Psychology Press; 2009. pp. 143–167. [Google Scholar]
- 27.Lauritzen L, Christensen JH, Damsgaard CT, Michaelsen KF. The effect of fish oil supplementation on heart rate in healthy Danish infants. Pediatr Res. 2008;64:610–614. doi: 10.1203/PDR.0b013e318186e5c5. [DOI] [PubMed] [Google Scholar]
- 28.Gustafson KM, Carlson SE, Colombo J, et al. Effects of docosahexaenoic acid supplementation during pregnancy on fetal heart rate and variability: a randomized clinical trial. Prostaglandins Leukot Essent Fatty Acids. 2013;88:331–338. doi: 10.1016/j.plefa.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafson KM, Colombo J, Carlson SE. Docosahexaenoic acid and cognitive function: Is the link mediated by the autonomic nervous system? Prostaglandins Leukot Essent Fatty Acids. 2008;79:135–140. doi: 10.1016/j.plefa.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colombo J, Carlson SE, Cheatham CL, et al. Long-term effects of LCPUFA supplementation on childhood cognitive outcomes. Am J Clin Nutr. 2013;98:403–412. doi: 10.3945/ajcn.112.040766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kavsek M, Bornstein MH. Visual habituation and dishabituation in preterm infants: A review and meta-analysis. Res Dev Disab. 2010;31:951–975. doi: 10.1016/j.ridd.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spencer-Smith MM, Spittle AJ, Lee KJ, Doyle LW, Anderson PJ. Bayley-III Cognitive and Language Scales in Preterm Children. Pediatr. 2015;135:e1258–e1265. doi: 10.1542/peds.2014-3039. [DOI] [PubMed] [Google Scholar]
- 33.Colombo J. Recent advances in infant cognition: Implications for long-chain polyunsaturated fatty acid supplementation studies. Lipids. 2001;36:919–926. doi: 10.1007/s11745-001-0802-9. [DOI] [PubMed] [Google Scholar]
- 34.Colombo J, Mitchell DW. Infant visual habituation. Neurobiol Learn Mem. 2009;92:225–234. doi: 10.1016/j.nlm.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colombo J, Zavaleta N, Kannass KN, et al. Zinc Supplementation Sustained Normative Neurodevelopment in a Randomized, Controlled Trial of Peruvian Infants Aged 6–18 Months. J Nutr. 2014;144:1298–1305. doi: 10.3945/jn.113.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards JE. The development of sustained visual-attention in infants from 14 to 26 weeks of age. Psychophysiol. 1985;22:409–416. doi: 10.1111/j.1469-8986.1985.tb01625.x. [DOI] [PubMed] [Google Scholar]
- 37.Colombo J, Richman WA, Shaddy DJ, Greenhoot AF, Maikranz JM. Heart rate-defined phases of attention, look duration, and infant performance in the paired-comparison paradigm. Child Devel. 2001;72:1605–1616. doi: 10.1111/1467-8624.00368. [DOI] [PubMed] [Google Scholar]
- 38.Richards JE, Casey BJ. Development of sustained visual attention in the human infant. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research Hillsdale. NJ: Lawrence Erlbaum Associates, Inc.; 1992. pp. 30–60. [Google Scholar]
- 39.Richards JE. Development and stability in visual sustained attention in 14, 20, and 26 week old infants. Psychophysiol. 1989;26:422–430. doi: 10.1111/j.1469-8986.1989.tb01944.x. [DOI] [PubMed] [Google Scholar]
- 40.Richards JE. Sustained visual-attention in 8-week-old infants. Infant Beh Devel. 1989;12:425–436. [Google Scholar]