Abstract
Cyanobactins are a diverse collection of natural products that originate from short peptides made on a ribosome. The amino acids are modified in a series of transformations catalyzed by multiple enzymes. The patellamide pathway is the most well studied and characterized example. Here we review the structures and mechanisms of the enzymes that cleave peptide bonds, macrocyclise peptides, heterocyclise cysteine (as well as threonine and serine) residues, oxidize five-membered heterocycles and attach prenyl groups. Some enzymes operate by novel mechanisms which is of interest and in addition the enzymes uncouple recognition from catalysis. The normally tight relationship between these factors hinders biotechnology. The cyanobactin pathway may be particularly suitable for exploitation with progress in vivo and in vitro approaches observed.
Introduction
Despite approximately 3000 human genes having been estimated to be involved in disease states, only a fraction (between 20 and 50 %) are thought to be potentially responsive to inhibition by traditional small molecule drugs [1]. Natural products (NPs) have long been successfully employed to bridge the gap between inhibitors and “undrugable” targets [2]. A particularly promising class of NPs is the ribosomally synthesized and post-translationally modified peptides (RiPPs). These NPs offer the practicality of genetically encoded compound libraries, which can be readily derivatized both enzymatically and chemically giving rise to very diverse molecules.
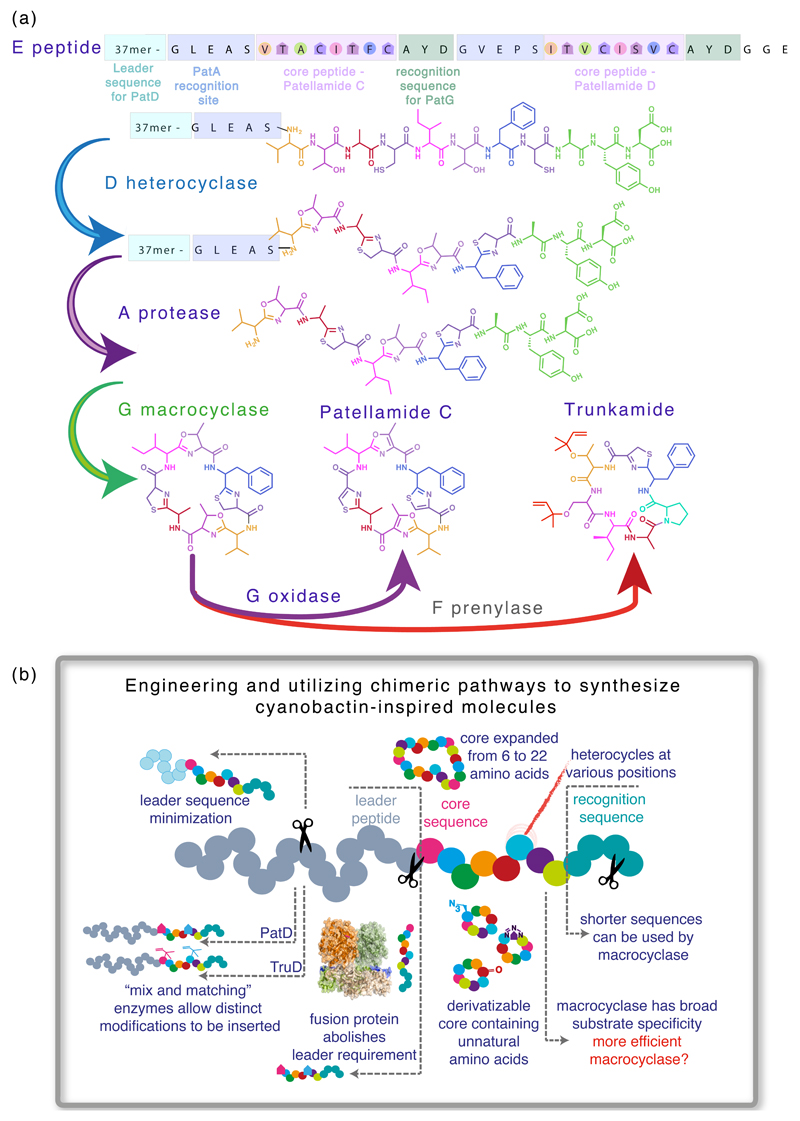
Cyanobactins are RiPPs which in general, although not always, contain a macrocyclic ring – forming a peptide bond between the N-terminal amine and another amino acid next to a recognition sequence. They can also contain heterocyclized (cyclodehydratized) serine, threonine and/or cysteine residues yielding (methyl)oxazoline and thiazoline rings or isoprenoid amino acids derivatives [3,4]. Cyanobactins are encoded as a precursor peptide (“E” on figure 1A) which possesses a leader sequence recognized by the cyclodehydratase proteins responsible for heterocycle formation (“D” on figure 1A). Once heterocyclized, the precursor peptide is cleaved by a serine protease (“A” on figure 1A) to contain solely the core natural product sequence followed by a short recognition sequence. The core peptide sequence is then macrocyclized by another serine protease working “in reverse”, i.e. catalysing peptide bond formation to yield the cyclic peptide (“G” on figure 1A). The cyclic peptide can then be further post-translationally modified by prenylation (“F” on figure 1A) and/or oxidation to include thiazoles and oxazoles (Oxidase domain of the “G” protein on figure 1A).
Figure 1. Exploring and improving the biosynthesis of cyanobactins.
(a) Natural products are synthesized as a precursor peptide (“E” on figure 1A), which is sequentially processed by the action of cyclodehydratase proteins responsible for heterocycle formation (“D” on figure 1A), a serine protease (“A” on figure 1A), a macrocyclase (“G” on figure 1A), and finally modified by prenylation (“F” on figure 1A) and/or oxidation (oxidase domain of the “G” protein on figure 1A).
(b) There is considerable potential for engineering cyanobactins.
This pathway, albeit extremely promising in terms of generating RiPPs diversity, has several defects from a technology view that should be improved to make the production of diverse cyclic peptides in large scale routine. Several approaches have been used to trim the precursor peptide by eliminating the requirement of a long peptide leader (which is discarded during processing) [5**], to introduce unnatural amino acids, to process long sequences in the core peptide [6*,7**], to devise chimeric pathways utilizing enzymes from different pathways and organisms and to produce cyanobactins in vitro in a one-pot reaction [7**,8]. Figure 1B schematically illustrates the progress made so far and highlights targets for further improvement. Our understanding of the catalytic and chemical mechanisms of the enzymes involved in cyanobactin biosynthesis has greatly increased in the past few years. In this review article we discuss the major discoveries and current proposed mechanisms for the enzymes involved in cyanobactin biosynthesis.
Heterocyclase
Peptide heterocycles reduce polarity, add chemical diversity and conformational rigidity to peptides. They are common in both linear and macrocyclic bioactive natural products [9,10]. Many cyanobactins, such as patellamides [11–15], trunkamides [16,17] and microcyclamides [18–20] contain thiazol(in)e or oxazol(in)e rings within their cyclic backbones. Phylogenetic studies [21–23] showed that clusters capable of biosynthesizing cyanobactins that bear heterocycles encode a YcaO domain-containing heterocyclase (cyclodehydratase), denoted in each pathway the letter D preceded by the name of that pathway, such as PatD (“D” on figure 1A). An optional, FMN-dependent oxidase (dehydrogenase) – discussed below – may also be present, either as a domain of the protein G or as a separate gene product, and carry out the dehydrogenation of thiazoline and oxazoline moieties to thiazoles and oxazoles, respectively [23,24]. PatD-like heterocyclases were shown to use a range of natural and unnatural substrates, including those containing non-proteinogenic amino acids and non-amino acids [5**,6*,7**,21,25–29*]. The promiscuity of this class of enzymes make them desirable tools for the creation of libraries of heterocycle-containing compounds, either as part of a system leading to cyanobactin-like macrocycles [29*] or as stand-alone enzymes.
Heterocyclization by PatD-like heterocyclases is both ATP and magnesium dependent, as are other YcaO domain-containing enzymes such as the thiazole/oxazole-modified microcin (TOMM) heterocyclase BalhD from Bacillus sp. Al Hakam [30–32]. Heterocycles on RiPPs form through the reaction between the hydroxyl or sulfhydryl group from serine, threonine or cysteine residues and the amide bond N-terminal to it [4]. An electron pair from the heteroatom on the amino acid side chain attacks the carbonyl oxygen of the amide bond, leading to the formation of a hemiorthoamide (Figure 2F), which subsequently attacks ATP on one of the phosphate atoms, forming an adenylate, and a phosphoryl or pyrophosphoryl leaving group [33]. An unambiguous mechanism for the nucleophilic attack by the tetrahedral oxygen has yet to be established. ADP and inorganic phosphate (Pi) were observed as the product of ATP hydrolysis during microcin B17 biosynthesis and the BalhC/D systems, establishing a kinase mechanism (Figure 2F) [4,33]. Structural analyses of ATP-bound E. coli YcaO and LynD supported this showing the γ-phosphate exposed to attack and the α-phosphate shielded by the protein [5**,33]. However, AMP and pyrophosphate (PPi) have been detected during catalysis by TruD and LynD [5**,27]. Furthermore, E. coli YcaO, whose function is unknown, hydrolyzed ATP to AMP and PPi [34].
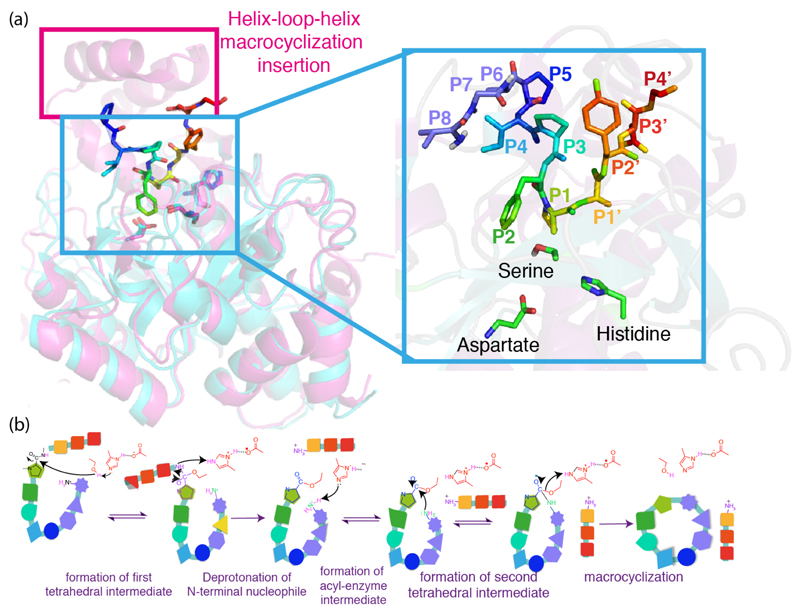
Figure 2. Structure based design of a constitutively active cyanobactin heterocyclase.
(a) The structure of LynD (4v1v) in complex with its substrate peptide PatE’. The two monomers are shown in orange and teal (chain A) and green (chain B), and the peptide is shown in blue. The boxed region is further illustrated in the rest of the figure 2B-E. (b) Activation of the enzyme by peptide binding. LynD is aligned with TruD (4bs9, black) which was crystallized as an apoenzyme. Residues 371-415 on LynD form an alpha helix that interacts with the peptide, whereas the corresponding residues on TruD are disordered. (c) The RiPP precursor peptide recognition element (RRE) proposed by Burkhart et al. [36**] comprising of three helices and three beta sheets, in the N-terminal domain of LynD (teal). Residues in the RRE interacts with residues on PatE’ and mediates its binding. (d) Model of substrate binding. PatE’ (blue) was partially ordered in the structure. It is postulated that from the interface between the N-terminal domain of chain A (teal) and the C-terminal domain of chain B (green), PatE’ extends to the nucleotide-biding active site of chain B, where the core peptide region (cyan) is catalysed. (e) Model of the fusion enzyme. Part of the leader peptide from PatE’ was fused to the N-terminus of LynD via a linker region (purple). The fused enzyme is thought to be constitutively active and able to bind and process leaderless peptides (cyan). (f) The kinase model of heterocyclization. For simplicity the substrate was shown as a pentapeptide with the sequence TFCAYD, the F and C highlighted in green.
Substrate recognition by PatD-class of enzymes combines almost complete insensitivity to the residues flanking the transformed residue within core region with binding to a sequence in the N-terminal leader [5**,26,27]. The use of a leader sequence is also seen in the biosynthetic transformation of other RiPPs [3,35]. Despite the lack of sequence similarities between the leader peptides of different classes of RiPPs, their recognition proteins share a structural motif for peptide interaction, identified and named ‘RiPP precursor peptide recognition element (RRE)’ by a recent bioinformatics study [36**]. For instance, LynD (Figure 2A) and the lantibiotic nisin dehydratase NisB recognize the consensus sequences ‘LAELSEEAL’ and ‘FNDL’, respectively [5**,37]. However, both utilized a ‘peptide clump’ (Figure 2C) consisting of three alpha helices and three beta sheets (winged helix-turn helix motif) on the enzyme [5**,36**,37]. These interactions position the core region, which is C-terminal to the leader such that it reaches the active site of the enzyme for post-translational modification to take place [5**,37].
The leader peptide activates the cyanobactin heterocyclase whether added in trans or in cis [26]. The structure of PatE’-bound to LynD, revealed the peptide recognition residues interact simultaneously with the N-terminal domain of one enzyme monomer and the C-terminal catalytic domain of the other enzyme monomer in the enzyme dimer. The second interaction orders an active site loop proving a rationale for enzyme activation (Figure 2B) [5**]. The presence of the recognition sequence is essential for the processivity of the enzyme [5**,26,27]. Cognate leader peptides added in trans, have also been shown to enhance the activities of the lacticin 481 synthetase LctM, and the nisin synthetases NisB and NisC [38,39]. Both LctM and LynD have been engineered to include their cognate leader (or part) peptide and display increased activity [5**,40]. The engineered LynD (LynD fusion) is capable of processively processing peptides that have no sequence N-terminal to the core (Figure 2E) [5**]. This fusion protein is a useful tool in biotechnology as it allows the facile use of entirely synthetic substrates.
Protease
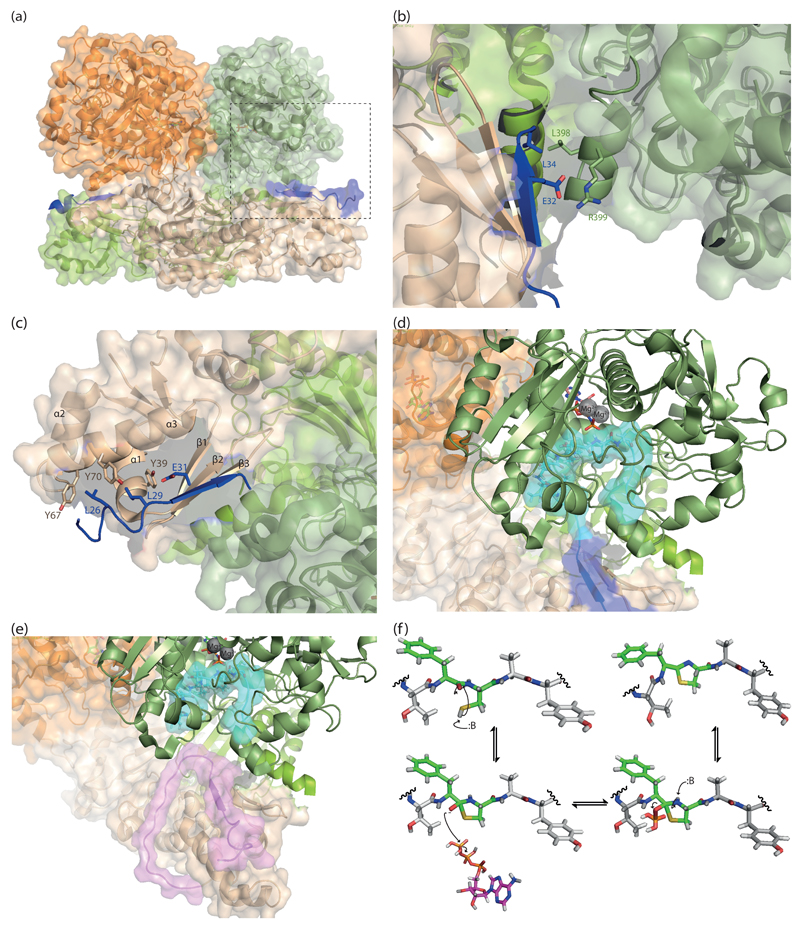
The crystal structures of proteases PatA and PagA [41,42] reveal the same catalytic triad – composed of serine, aspartate and histidine and typical of subtilisin-like serine proteases [43], the macrocyclase domain of PatG shares this fold (Figure 3a). The similarity was predicted on sequence grounds and mechanistically is unsurprising since both the protease and macrocyclase form an acyl enzyme intermediate; the difference is the fate of this intermediate. The current proposed catalytic mechanism for both A and G proteins is shown in Figure 3b. A similar trend in which very subtle differences in primary sequence set apart peptide bond formation and cleavage has been observed in evolutionarily distant macrocyclases from Amanita and Galerina mushrooms [44,45].
Figure 3. Making and breaking peptide bonds.
(a) overlay of the crystal structures of PatA (PDB 4h6v) and PatGmac (macrocyclase domain, PDB 4akt), showing that the three-dimensional structure is largely conserved between these two proteins catalyzing opposite reactions. The pink box highlights a helix-loop-helix motif thought to be crucial for protecting the acyl-enzyme intermediate from a water nucleophile in the “G proteins”. The inset shows the active site of PatGmac, in which the site chain for the catalytic histidine was added (since the complex structure was determined with a histidine to alanine mutant) and the three initial residues form the core sequence, labeled P6, P7, and P8 were added according to a structure prediction carried out using PEPFOLD (http://bioserv.rpbs.univ-paris-diderot.fr/services/PEP-FOLD/). (b) The mechanism proposed for the macrocyclase enzyme, differs from serine proteases in that the formation of the second tetrahedral intermediate, the N-terminal amine of the peptide acts as a nucleophile not water.
Unfortunately, the crystal structure for PatGmac lacks electron density for the N-terminal amine of the core peptide sequence [42], which precludes any conclusions as to how substrate positioning is affecting catalysis. The hypothesis that positioning plays only a minor role in the reaction catalyzed by the macrocyclase enzyme is substantiated by empirical evidence as to the notable substrate promiscuity it possesses. Peptides with remarkable variability, containing from 6 to 22 amino acids in the core region and a combination of natural and unnatural amino acids have been successfully macrocyclized, as long as they possess the signature sequence proline/heterocycle)-AYD [29*]. Recently, peptide substrates containing up to three triazole groups were shown to be macrocyclized [46**], further expanding the substrate scope of the macrocyclase enzyme to include non-peptidic scaffolds.
Sortase A, another enzyme frequently used for peptide or protein ligation, requires the sequence LPXTG for substrate recognition, as well as at least one glycine in the nucleophilic sequence [47]. Despite the fact that a sortase with broader substrate specificity has been identified by directed evolution [48], because the sequence LPXTG (or similar) is retained after cyclization, sortase reactions efficiently produce peptides with fifteen amino acids or more [49].
The hydrolysis reaction catalyzed by PatA is several orders of magnitude slower than observed for other proteases [50] and turnover numbers in the vicinity of 1 day-1 for the macrocyclase enzyme have been reported [42,50,51]. The in vitro synthesis of patellamides requires a “lag” between the production of the precursor peptide “E” and cleavage of the leader, since the heterocyclase enzyme needs to convert serines/threonines/cysteines to their respective heterocyclic products prior to leader removal. The reason underlying PatGmac’s poor catalytic efficiency could lie on the fact that the macrocyclase enzyme seems to trade-off efficiency for a very simple recognition motif. Much faster macrocyclase enzymes Butelase 1, PCY1, (both from plant) and POPB, from mushroom [44,52,53*,54] have been reported but they require more than the simple heterocycle plus three residue motif of PatGmac. Alternatively the macrocyclase enzyme may have evolved to be bottleneck of the process regulating production of biologically active products [7**,41].
Oxidase
Following heterocyclization, thiazolines and oxazolines are in many cases oxidised by a flavin mononucleotide (FMN)-dependent dehydrogenase to yield thiazoles and oxazoles [4]. In the biosynthesis of microcin, a linear RiPP, the same order was observed for heterocyclization and oxidation starting on the C-terminus and proceeding towards the N-terminus of the peptide substrate. Oxidase enzymes from related organisms were successfully employed interchangeably but more distantly related (in sequence terms) enzymes failed to produce azole products [32,33,55,56**].
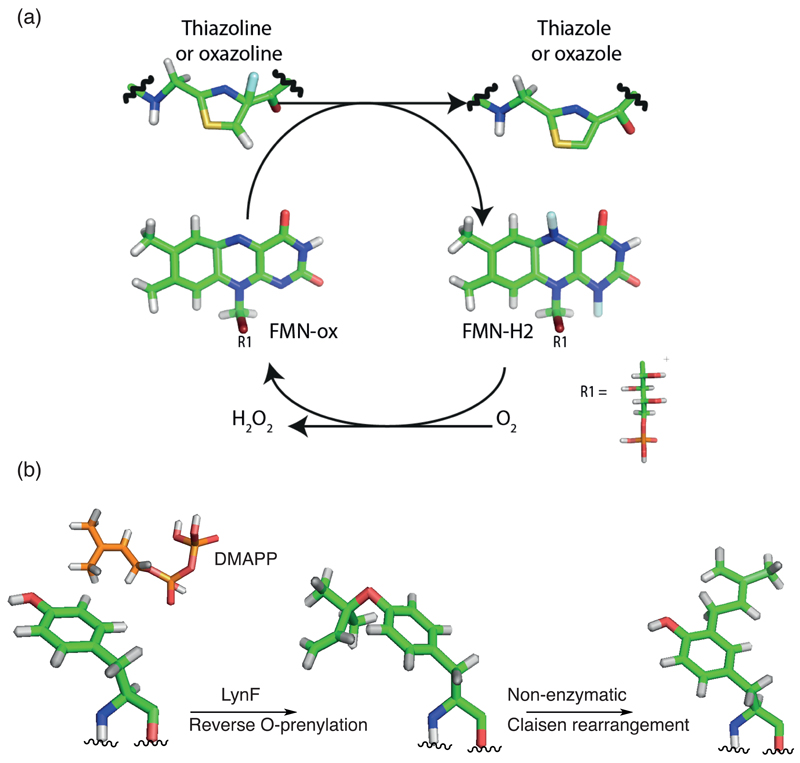
Despite substantial information available on the substrate preference in terms of specificity/promiscuity of enzymes catalyzing oxazoline/thiazoline oxidation, as well as order of reaction [8,56**,57], little information is available about the catalytic and chemical mechanism by which these reactions proceed. Primary sequence alignments with FMN-bound dehydrogenases from RiPPs and non-ribosomal peptide (NRPs) synthetases show little sequence homology. The only highly conserved residues apart from those known to be involved in FMN binding are a lysine-tyrosine motif (KY-motif) [56**]. Mutagenesis of the KY motif stops the production of microcin B17 in vitro, as well as the oxidation of a BalhA substrate [58]. BcerB from Bacillus cereus 172560W showed reduced activity when K185A mutant was tested, whereas both Y186A and the double mutant K185A/Y186A were inactive [56**]. Analogously, in the related enzyme from Sulfolobus acidocaldarius DSM 639, SaciB, mutation of either lysine or tyrosine resulted in complete loss of activity [56**]. A mechanism for oxidation has been proposed based on for EpoB and BlmIII, NRPs enzymes catalyzing thiazole formation in epothilone and bleomycin biosynthesis (Figure 4a) [59].
Figure 4. Oxidation and prenylation.
(a) Simplified reaction scheme for the reaction catalyzed by the thiazoline and/or oxazoline oxidase protein. During the course of the reactions, oxidized flavine mononucleotide (FMN-ox) receives either one hydride and one proton or 2 single electrons and 2 protons from the thiazoline or oxazoline, generating reduced flavine mononucleotide (FMN-red). The exact mechanism remains to be determined. The catalytic competent FMN-ox is produced by reaction with oxygen to release hydrogen peroxide. (b) Tyrosine O- and C-prenylation as examples of prenylation in cyanobactins. The hydroxyl group on tyrosine is reverse O-prenylated by LynF using DMAPP (Dimethylallyl pyrophosphate). The product can subsequently undergo non-enzymatic Claisen rearrangement to give C-prenylated tyrosine.
Prenylase
O-prenylation of serine, threonine and tyrosine residues are found in prenylagaramides, aestuaramides and trunkamides [3,21,60–64] and is catalyzed by a prenylase enzyme, exemplified by LynF [65]. The dimethylallylpyrophosphate (DMAPP) group on tyrosine has been shown to undergo non-enzymatic Claisen rearrangement to give C-prenylated products (Figure 4b) [62,65,66]. Although LynF-like enzyme only exhibited activity on cyclic substrates, distantly related small, linear cyanobactins such as viridisamides undergo prenylation on the N-terminus [22], indicating another mode of prenylation in the cyanobactin family. Moreover, C-3-prenylation of tryptophan residues was observed in Kawaguchipeptins from Mycrocystis aeruginosa NIES-88 [67*]. In vitro expression of the biosynthetic operon showed that both linear and cyclic substrates are accepted by the putative prenyltransferase, KgpF, (related in sequence to other cyanobactin prenylases [67*]). Interestingly the patellamide pathway has a prenylase, PatF whose structure is known [68], but the pathway does not make prenylated products and the enzyme is inactive [68].
Future Directions
Figure 1b highlights the progress made so far towards understanding biosynthetic enzymes and substrates involved in cyanobactins and RiPPs. Significant improvements in in vivo production of patellin, trunkamide and patellamide C have been reported allowing large sale efficient production of desired material [69**]. The production of highly diverse peptide libraries for high throughput screening would be best served by combining enzymes in vitro, this in turn may require further engineering The identification of new gene clusters will provide opportunities to add new enzyme activities to such an in vitro tool kit.
It remains an open question whether enzymes in the patellamide pathway (for example) form protein-protein interactions (PPIs) which could allow intermediates and substrates to be more efficiently or rapidly “funneled” through the pathway. Domains of unknown function (DUF) could acts as scaffolds facilitating these PPis. A key challenge is to combine the simple substrate specificity of PatGMac with the turnover rates of other macrocyclases; this will be achieved either by making PatGmac faster or the redesigning the recognition elements of the faster enzymes. In either approach detailed structural and mechanistic information will be essential.
Acknowledgements
This work was supported by the European Research Council (339367), UK Biotechnology and Biological Sciences Research Council (K015508/1). J.H.N. is a Royal Society Wolfson Merit Award Holder and 1000 talent scholar at Sichuan University.
References
- 1.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM. Natural Products as Sources of New Drugs from 1981 to 2014. Journal of Natural Products. 2016 doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 3.Arnison PG, Bibb MJ, Bierbaum G, Bowers AA, Bugni TS, Bulaj G, Camarero JA, Campopiano DJ, Challis GL, Clardy J, et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30:108–160. doi: 10.1039/c2np20085f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li YM, Milne JC, Madison LL, Kolter R, Walsh CT. From peptide precursors to oxazole and thiazole-containing peptide antibiotics: microcin B17 synthase. Science. 1996;274:1188–1193. doi: 10.1126/science.274.5290.1188. [DOI] [PubMed] [Google Scholar]
- **5.Koehnke J, Mann G, Bent AF, Ludewig H, Shirran S, Botting C, Lebl T, Houssen WE, Jaspars M, Naismith JH. Structural analysis of leader peptide binding enables leader-free cyanobactin processing. Nat Chem Biol. 2015;11:558–563. doi: 10.1038/nchembio.1841. [This work represents a significant improvement in the in vitro production of cyanobactin-like peptides, since the size of the recognition sequence was greatly diminished.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- *6.Oueis E, Adamson C, Mann G, Ludewig H, Redpath P, Migaud M, Westwood NJ, Naismith JH. Derivatisable Cyanobactin Analogues: A Semisynthetic Approach. Chembiochem. 2015;16:2646–2650. doi: 10.1002/cbic.201500494. [Cyclic peptides with orthogonal reactive groups were introduced into the patellamide scaffold, allowing labelling with fluorescent labels.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- **7.Sardar D, Lin ZJ, Schmidt EW. Modularity of RiPP Enzymes Enables Designed Synthesis of Decorated Peptides. Chemistry & Biology. 2015;22:907–916. doi: 10.1016/j.chembiol.2015.06.014. [In this work, extremely long sequences in the core peptide were employed, and a chimeric pathway, utilizing enzymes from different pathways, and organisms was assembled to produce cyanobactins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houssen WE, Bent AF, McEwan AR, Pieiller N, Tabudravu J, Koehnke J, Mann G, Adaba RI, Thomas L, Hawas UW, et al. An efficient method for the in vitro production of azol(in)e-based cyclic peptides. Angew Chem Int Ed Engl. 2014;53:14171–14174. doi: 10.1002/anie.201408082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Z. Muscarine, imidazole, oxazole, and thiazole alkaloids. Nat Prod Rep. 2011;28:1143–1191. doi: 10.1039/c0np00074d. [DOI] [PubMed] [Google Scholar]
- 10.Roy RS, Gehring AM, Milne JC, Belshaw PJ, Walsh CT. Thiazole and oxazole peptides: biosynthesis and molecular machinery. Nat Prod Rep. 1999;16:249–263. doi: 10.1039/a806930a. [DOI] [PubMed] [Google Scholar]
- 11.Degnan BM, Hawkins CJ, Lavin MF, McCaffrey EJ, Parry DL, van den Brenk AL, Watters DJ. New cyclic peptides with cytotoxic activity from the ascidian Lissoclinum patella. J Med Chem. 1989;32:1349–1354. doi: 10.1021/jm00126a034. [DOI] [PubMed] [Google Scholar]
- 12.Fu X, Do T, Schmitz FJ, Andrusevich V, Engel MH. New cyclic peptides from the ascidian Lissoclinum patella. J Nat Prod. 1998;61:1547–1551. doi: 10.1021/np9802872. [DOI] [PubMed] [Google Scholar]
- 13.In Y, Doi M, Inoue M, Ishida T, Hamada Y, Shioiri T. Molecular conformation of patellamide A, a cytotoxic cyclic peptide from the ascidian Lissoclinum patella, by X-ray crystal analysis. Chem Pharm Bull (Tokyo) 1993;41:1686–1690. doi: 10.1248/cpb.41.1686. [DOI] [PubMed] [Google Scholar]
- 14.McDonald LA, Ireland CM. Patellamide E: a new cyclic peptide from the ascidian Lissoclinum patella. J Nat Prod. 1992;55:376–379. doi: 10.1021/np50081a016. [DOI] [PubMed] [Google Scholar]
- 15.Rashid MA, Gustafson KR, Cardellina JH, 2nd, Boyd MR. Patellamide F, A new cytotoxic cyclic peptide from the colonial ascidian Lissoclinum patella. J Nat Prod. 1995;58:594–597. doi: 10.1021/np50118a020. [DOI] [PubMed] [Google Scholar]
- 16.Salvatella X, Caba JM, Albericio F, Giralt E. Solution structure of the antitumor candidate trunkamide A by 2D NMR and restrained simulated annealing methods. J Org Chem. 2003;68:211–215. doi: 10.1021/jo026464s. [DOI] [PubMed] [Google Scholar]
- 17.Wipf P, Uto Y. Total synthesis and revision of stereochemistry of the marine metabolite trunkamide A. J Org Chem. 2000;65:1037–1049. doi: 10.1021/jo9914566. [DOI] [PubMed] [Google Scholar]
- 18.Ziemert N, Ishida K, Quillardet P, Bouchier C, Hertweck C, de Marsac NT, Dittmann E. Microcyclamide biosynthesis in two strains of Microcystis aeruginosa: from structure to genes and vice versa. Appl Environ Microbiol. 2008;74:1791–1797. doi: 10.1128/AEM.02392-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portmann C, Blom JF, Kaiser M, Brun R, Juttner F, Gademann K. Isolation of aerucyclamides C and D and structure revision of microcyclamide 7806A: heterocyclic ribosomal peptides from Microcystis aeruginosa PCC 7806 and their antiparasite evaluation. J Nat Prod. 2008;71:1891–1896. doi: 10.1021/np800409z. [DOI] [PubMed] [Google Scholar]
- 20.Ishida K, Nakagawa H, Murakami M. Microcyclamide, a cytotoxic cyclic hexapeptide from the cyanobacterium Microcystis aeruginosa. J Nat Prod. 2000;63:1315–1317. doi: 10.1021/np000159p. [DOI] [PubMed] [Google Scholar]
- 21.Donia MS, Ravel J, Schmidt EW. A global assembly line for cyanobactins. Nat Chem Biol. 2008;4:341–343. doi: 10.1038/nchembio.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leikoski N, Liu L, Jokela J, Wahlsten M, Gugger M, Calteau A, Permi P, Kerfeld CA, Sivonen K, Fewer DP. Genome mining expands the chemical diversity of the cyanobactin family to include highly modified linear peptides. Chem Biol. 2013;20:1033–1043. doi: 10.1016/j.chembiol.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 23.Martins J, Leao PN, Ramos V, Vasconcelos V. N-terminal protease gene phylogeny reveals the potential for novel cyanobactin diversity in cyanobacteria. Mar Drugs. 2013;11:4902–4916. doi: 10.3390/md11124902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koehnke J, Bent AF, Houssen WE, Mann G, Jaspars M, Naismith JH. The structural biology of patellamide biosynthesis. Curr Opin Struct Biol. 2014;29:112–121. doi: 10.1016/j.sbi.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donia MS, Hathaway BJ, Sudek S, Haygood MG, Rosovitz MJ, Ravel J, Schmidt EW. Natural combinatorial peptide libraries in cyanobacterial symbionts of marine ascidians. Nat Chem Biol. 2006;2:729–735. doi: 10.1038/nchembio829. [DOI] [PubMed] [Google Scholar]
- 26.Goto Y, Ito Y, Kato Y, Tsunoda S, Suga H. One-pot synthesis of azoline-containing peptides in a cell-free translation system integrated with a posttranslational cyclodehydratase. Chem Biol. 2014;21:766–774. doi: 10.1016/j.chembiol.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Koehnke J, Bent AF, Zollman D, Smith K, Houssen WE, Zhu X, Mann G, Lebl T, Scharff R, Shirran S, et al. The cyanobactin heterocyclase enzyme: a processive adenylase that operates with a defined order of reaction. Angew Chem Int Ed Engl. 2013;52:13991–13996. doi: 10.1002/anie.201306302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koehnke J, Morawitz F, Bent AF, Houssen WE, Shirran SL, Fuszard MA, Smellie IA, Botting CH, Smith MC, Jaspars M, et al. An enzymatic route to selenazolines. Chembiochem. 2013;14:564–567. doi: 10.1002/cbic.201300037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *29.Ruffner DE, Schmidt EW, Heemstra JR. Assessing the combinatorial potential of the RiPP cyanobactin tru pathway. ACS Synth Biol. 2015;4:482–492. doi: 10.1021/sb500267d. [The authors examined the sequence selectivity of the trunkamide pathway analyzing more than 300 compounds.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McIntosh JA, Donia MS, Schmidt EW. Insights into heterocyclization from two highly similar enzymes. J Am Chem Soc. 2010;132:4089–4091. doi: 10.1021/ja9107116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McIntosh JA, Schmidt EW. Marine molecular machines: heterocyclization in cyanobactin biosynthesis. Chembiochem. 2010;11:1413–1421. doi: 10.1002/cbic.201000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melby JO, Dunbar KL, Trinh NQ, Mitchell DA. Selectivity, directionality, and promiscuity in peptide processing from a Bacillus sp. Al Hakam cyclodehydratase. J Am Chem Soc. 2012;134:5309–5316. doi: 10.1021/ja211675n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dunbar KL, Melby JO, Mitchell DA. YcaO domains use ATP to activate amide backbones during peptide cyclodehydrations. Nat Chem Biol. 2012;8:569–575. doi: 10.1038/nchembio.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunbar KL, Chekan JR, Cox CL, Burkhart BJ, Nair SK, Mitchell DA. Discovery of a new ATP-binding motif involved in peptidic azoline biosynthesis. Nat Chem Biol. 2014;10:823–829. doi: 10.1038/nchembio.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oman TJ, van der Donk WA. Follow the leader: the use of leader peptides to guide natural product biosynthesis. Nat Chem Biol. 2010;6:9–18. doi: 10.1038/nchembio.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **36.Burkhart BJ, Hudson GA, Dunbar KL, Mitchell DA. A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat Chem Biol. 2015;11:564–570. doi: 10.1038/nchembio.1856. [This recent bioinformatics study identified that the recognition proteins which bind leader peptides of different classes of RiPPs, share a structural motif for peptide interaction, identified and named ‘RiPP precursor peptide recognition element (RRE).] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ortega MA, Hao Y, Zhang Q, Walker MC, van der Donk WA, Nair SK. Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature. 2015;517:509–512. doi: 10.1038/nature13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khusainov R, Kuipers OP. When the leader gets loose: in vivo biosynthesis of a leaderless prenisin is stimulated by a trans-acting leader peptide. Chembiochem. 2012;13:2433–2438. doi: 10.1002/cbic.201200437. [DOI] [PubMed] [Google Scholar]
- 39.Patton GC, Paul M, Cooper LE, Chatterjee C, van der Donk WA. The importance of the leader sequence for directing lanthionine formation in lacticin 481. Biochemistry. 2008;47:7342–7351. doi: 10.1021/bi800277d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oman TJ, Knerr PJ, Bindman NA, Velasquez JE, van der Donk WA. An engineered lantibiotic synthetase that does not require a leader peptide on its substrate. J Am Chem Soc. 2012;134:6952–6955. doi: 10.1021/ja3017297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agarwal V, Pierce E, McIntosh J, Schmidt EW, Nair SK. Structures of cyanobactin maturation enzymes define a family of transamidating proteases. Chem Biol. 2012;19:1411–1422. doi: 10.1016/j.chembiol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koehnke J, Bent A, Houssen WE, Zollman D, Morawitz F, Shirran S, Vendome J, Nneoyiegbe AF, Trembleau L, Botting CH, et al. The mechanism of patellamide macrocyclization revealed by the characterization of the PatG macrocyclase domain. Nat Struct Mol Biol. 2012;19:767–772. doi: 10.1038/nsmb.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siezen RJ, Leunissen JA. Subtilases: the superfamily of subtilisin-like serine proteases. Protein Sci. 1997;6:501–523. doi: 10.1002/pro.5560060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo H, Hong SY, Sgambelluri RM, Angelos E, Li X, Walton JD. Peptide macrocyclization catalyzed by a prolyl oligopeptidase involved in alpha-amanitin biosynthesis. Chem Biol. 2014;21:1610–1617. doi: 10.1016/j.chembiol.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo H, Hallen-Adams HE, Walton JD. Processing of the phalloidin proprotein by prolyl oligopeptidase from the mushroom Conocybe albipes. J Biol Chem. 2009;284:18070–18077. doi: 10.1074/jbc.M109.006460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **46.Oueis E, Jaspars M, Westwood NJ, Naismith JH. Enzymatic Macrocyclization of 1,2,3-Triazole Peptide Mimetics. Angew Chem Int Ed Engl. 2016 doi: 10.1002/anie.201601564. [Peptide substrates containing up to three triazole groups were shown to be macrocyclized, further expanding the substrate scope of the macrocyclase enzyme to include non-peptidic scaffolds.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Proft T. Sortase-mediated protein ligation: an emerging biotechnology tool for protein modification and immobilisation. Biotechnol Lett. 2010;32:1–10. doi: 10.1007/s10529-009-0116-0. [DOI] [PubMed] [Google Scholar]
- 48.Piotukh K, Geltinger B, Heinrich N, Gerth F, Beyermann M, Freund C, Schwarzer D. Directed evolution of sortase A mutants with altered substrate selectivity profiles. J Am Chem Soc. 2011;133:17536–17539. doi: 10.1021/ja205630g. [DOI] [PubMed] [Google Scholar]
- 49.Wu Z, Guo X, Guo Z. Sortase A-catalyzed peptide cyclization for the synthesis of macrocyclic peptides and glycopeptides. Chem Commun (Camb) 2011;47:9218–9220. doi: 10.1039/c1cc13322e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee J, McIntosh J, Hathaway BJ, Schmidt EW. Using marine natural products to discover a protease that catalyzes peptide macrocyclization of diverse substrates. J Am Chem Soc. 2009;131:2122–2124. doi: 10.1021/ja8092168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McIntosh JA, Robertson CR, Agarwal V, Nair SK, Bulaj GW, Schmidt EW. Circular logic: nonribosomal peptide-like macrocyclization with a ribosomal peptide catalyst. J Am Chem Soc. 2010;132:15499–15501. doi: 10.1021/ja1067806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barber CJ, Pujara PT, Reed DW, Chiwocha S, Zhang H, Covello PS. The two-step biosynthesis of cyclic peptides from linear precursors in a member of the plant family Caryophyllaceae involves cyclization by a serine protease-like enzyme. J Biol Chem. 2013;288:12500–12510. doi: 10.1074/jbc.M112.437947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *53.Nguyen GK, Kam A, Loo S, Jansson AE, Pan LX, Tam JP. Butelase 1: A Versatile Ligase for Peptide and Protein Macrocyclization. J Am Chem Soc. 2015;137:15398–15401. doi: 10.1021/jacs.5b11014. [Butelase 1 is a promising new macrocyclase with ample potential for applications in biotechnology and production of cyclic peptides, as well as protein conjugation.] [DOI] [PubMed] [Google Scholar]
- 54.Nguyen GK, Wang S, Qiu Y, Hemu X, Lian Y, Tam JP. Butelase 1 is an Asx-specific ligase enabling peptide macrocyclization and synthesis. Nat Chem Biol. 2014;10:732–738. doi: 10.1038/nchembio.1586. [DOI] [PubMed] [Google Scholar]
- 55.Gonzalez DJ, Lee SW, Hensler ME, Markley AL, Dahesh S, Mitchell DA, Bandeira N, Nizet V, Dixon JE, Dorrestein PC. Clostridiolysin S, a post-translationally modified biotoxin from Clostridium botulinum. J Biol Chem. 2010;285:28220–28228. doi: 10.1074/jbc.M110.118554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **56.Melby JO, Li X, Mitchell DA. Orchestration of enzymatic processing by thiazole/oxazole-modified microcin dehydrogenases. Biochemistry. 2014;53:413–422. doi: 10.1021/bi401529y. [The first and detail functional study of the properties of the dehydrogenase enzyme to form thiazoles and oxazoles.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Melby JO, Dunbar KL, Trinh NQ, Mitchell DA. Selectivity, Directionality, and Promiscuity in Peptide Processing from a Bacillus sp Al Hakam Cyclodehydratase. Journal of the American Chemical Society. 2012;134:5309–5316. doi: 10.1021/ja211675n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunbar KL, Mitchell DA. Insights into the mechanism of peptide cyclodehydrations achieved through the chemoenzymatic generation of amide derivatives. J Am Chem Soc. 2013;135:8692–8701. doi: 10.1021/ja4029507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schneider TL, Shen B, Walsh CT. Oxidase domains in epothilone and bleomycin biosynthesis: Thiazoline to thiazole oxidation during chain elongation. Biochemistry. 2003;42:9722–9730. doi: 10.1021/bi034792w. [DOI] [PubMed] [Google Scholar]
- 60.Donia MS, Fricke WF, Ravel J, Schmidt EW. Variation in tropical reef symbiont metagenomes defined by secondary metabolism. PLoS One. 2011;6:e17897. doi: 10.1371/journal.pone.0017897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Donia MS, Schmidt EW. Linking chemistry and genetics in the growing cyanobactin natural products family. Chem Biol. 2011;18:508–519. doi: 10.1016/j.chembiol.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Majmudar JD, Gibbs RA. Pericyclic prenylation: peptide modification through a Claisen rearrangement. Chembiochem. 2011;12:2723–2726. doi: 10.1002/cbic.201100612. [DOI] [PubMed] [Google Scholar]
- 63.McIntosh JA, Lin Z, Tianero MD, Schmidt EW. Aestuaramides, a natural library of cyanobactin cyclic peptides resulting from isoprene-derived Claisen rearrangements. ACS Chem Biol. 2013;8:877–883. doi: 10.1021/cb300614c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sivonen K, Leikoski N, Fewer DP, Jokela J. Cyanobactins-ribosomal cyclic peptides produced by cyanobacteria. Appl Microbiol Biotechnol. 2010;86:1213–1225. doi: 10.1007/s00253-010-2482-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McIntosh JA, Donia MS, Nair SK, Schmidt EW. Enzymatic basis of ribosomal peptide prenylation in cyanobacteria. J Am Chem Soc. 2011;133:13698–13705. doi: 10.1021/ja205458h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Osuna S, Kim S, Bollot G, Houk KN. Aromatic Claisen Rearrangements of O-prenylated tyrosine and model prenyl aryl ethers: Computational study of the role of water on acceleration of Claisen rearrangements. European J Org Chem. 2013:2013. doi: 10.1002/ejoc.201201738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Parajuli A, Kwak DH, Dalponte L, Leikoski N, Galica T, Umeobika U, Trembleau L, Bent A, Sivonen K, Wahlsten M, et al. A Unique Tryptophan C-Prenyltransferase from the Kawaguchipeptin Biosynthetic Pathway. Angew Chem Int Ed Engl. 2016;55:3596–3599. doi: 10.1002/anie.201509920. [This report described a distinct mode of post-translational modification, C-3 prenylation of tryptophan residues, found in cyanobactin natural products.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bent AF, Koehnke J, Houssen WE, Smith MC, Jaspars M, Naismith JH. Structure of PatF from Prochloron didemni. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2013;69:618–623. doi: 10.1107/S1744309113012931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **69.Tianero MD, Pierce E, Raghuraman S, Sardar D, McIntosh JA, Heemstra JR, Schonrock Z, Covington BC, Maschek JA, Cox JE, et al. Metabolic model for diversity-generating biosynthesis. Proc Natl Acad Sci U S A. 2016;113:1772–1777. doi: 10.1073/pnas.1525438113. [Dramatic improvements were observed in the in vivo production of patellin, trunkamide and patellamide C when metabolites such as mevalonate and cysteine were added to the growth media. This paper revolutionizes in vivo production of patellamides.] [DOI] [PMC free article] [PubMed] [Google Scholar]