Abstract
Background
The impact of recent medical advances on disease presentation, extent of surgery, and disease specific survival (DSS) for patients with medullary thyroid cancer (MTC) is unclear.
Methods
We used the Surveillance, Epidemiology, and End Results registry to compare trends over three time periods, 1983–1992, 1993–2002, and 2003–2012.
Results
There were 2,940 patients diagnosed with MTC between 1983 and 2012. The incidence of MTC increased during this time period from 0.14 to 0.21 per 100,000 population, and mean age at diagnosis increased from 49.8 to 53.8 (p<0.001). The proportion of tumors ≤1cm also increased from 11.4% in 1983–1992, 19.6% in 1993–2002, to 25.1% in 2003–2012 (p<0.001), but stage at diagnosis remained constant (p=0.57). In addition, the proportion of patients undergoing a total thyroidectomy and lymph node dissection increased from 58.2% to 76.5% during the study period (p<0.001). In the most recent time interval, 5-year DSS improved from 86% to 89% in all patients (p<0.001), but especially for patients with regional (82% to 91%, p=0.003) and distant (40% to 51%, p=0.02) disease.
Conclusions
These data demonstrate that the extent of surgery is increasing for patients with MTC. DSS is also improving, primarily in patients with regional and distant disease.
Keywords: Medullary thyroid cancer, SEER, incidence, survival, thyroidectomy, lymph node dissection
Introduction
The incidence of thyroid cancer has steadily increased over the last 30 years (1, 2). In 2015, the American Cancer Society estimated that 62,450 patients would be diagnosed with thyroid malignancies (3). Although medullary thyroid cancer (MTC) accounts for only 5% of thyroid cancers, it is responsible for approximately 13% of all thyroid cancer related deaths (4, 5). Most cases of MTC are sporadic, however, up to 25% are associated with a hereditary mutation in the RET proto-oncogene (6, 7).
Also, over the past three decades, the diagnosis and treatment of MTC has advanced significantly. Genetic testing for RET proto-oncogene mutations is now available and recommended for anyone with a diagnosis of MTC (8). If a germline mutation is identified, relatives can be screened, and carriers can be treated prophylactically with a total thyroidectomy (9). In addition, tumor markers, such as calcitonin and carcinoembryonic antigen (CEA), help guide the initial extent of surgery and facilitate earlier detection of recurrence (8). For patients with metastatic disease, receptor tyrosine kinase inhibitors (RTKIs) are emerging systemic agents that improve progression free survival (10–12). Unfortunately, previous work suggests that these advances have not yet translated into improved long-term survival (13). In order to help clinicians keep up with the rapid advancements in the care of patients with MTC, several organizations have published and updated consensus guidelines, most recently in 2015 (14–16). These guidelines recommend that surgery for MTC should at least include a total thyroidectomy with bilateral central neck dissection (15). Adherence to these recommendations is associated with improved survival (17).
Although no national dataset captures the individual impact of these advancements on the care of patients with MTC, we hypothesized that taken together, these advances have changed the presentation, treatment, and outcomes of MTC. Therefore, the aim of this study was to compare the presentation and treatment of patients with MTC over the past three decades in a population level cohort. We also sought to determine whether long-term survival has improved over the same time interval.
Methods
We performed a population-based, retrospective cohort study of all patients with MTC in the Surveillance, Epidemiology, and End Results (SEER) registry diagnosed between 1983 and 2012 (18). All SEER regions were included. The study cohort was identified with the following ICD-O-3 codes: 8345- medullary carcinoma with amyloid stroma and 8510- medullary carcinoma, NOS of the thyroid (site code C739). Exclusion criteria included patients with a missing geographical region, diagnosis without biologic or microscopic confirmation, diagnosis at autopsy or death, and tumors size 0 mm or tumor in situ (Figure 1). Patients were grouped into three 10-year time intervals based on their year of diagnosis: 1983 – 1992, 1993 – 2002, and 2003 – 2012. The University of Wisconsin Institutional Review Board approved our protocol, and the National Cancer Institute provided access to the SEER registry - version 1973 – 2012 on April 27, 2015.
Figure 1. Study Cohort Inclusion and Exclusion Flowchart.
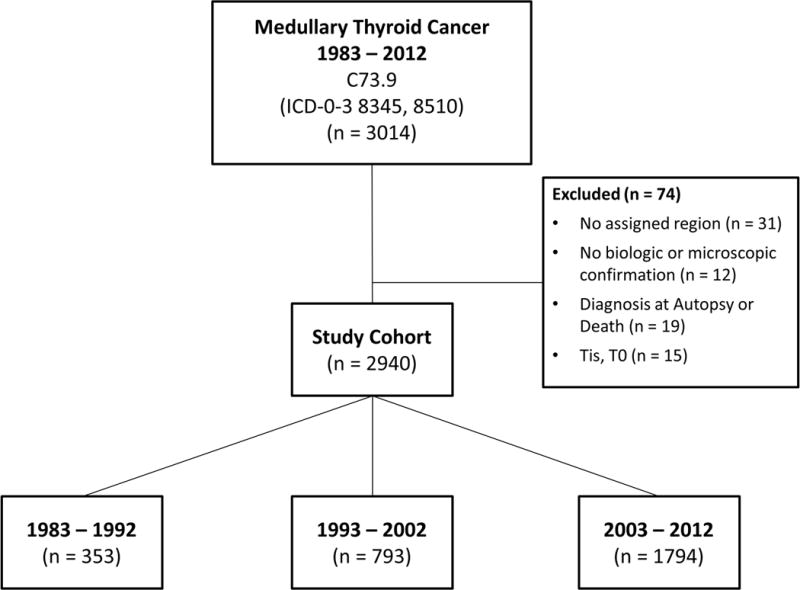
This diagram depicts how the final study cohort was defined.
Variable Definitions
Demographic variables included age, gender, and race. Tumor variables included size, multifocality, extrathyroidal extension, number of regional lymph nodes examined, number of positive regional lymph nodes, SEER historic stage, site-specific surgery, and radiation. Tumor size was recorded starting in 1988, halfway through the earliest decade in the study. Stage was classified according to SEER historic stage due to its consistency across the study period. The three SEER stages are local disease [confined to the thyroid], regional disease [tumors with extra-thyroidal extension or metastases to cervical lymph nodes], and distant disease [metastatic disease beyond the cervical lymph nodes]. Site-specific surgery codes for the thyroid began in 1983 and defined the type of operation being performed. We classified anything less than a total thyroidectomy as a partial thyroidectomy even if it was coded as a subtotal or near total thyroidectomy. Surgery NOS and Thyroidectomy NOS were classified together as unknown surgery. To capture all patients undergoing a lymph node dissection, any patients with lymph nodes examined were considered to have undergone a lymph node dissection. While this definition might have included patients with 1 or 2 nodes removed unintentionally, it most reliably identified patients who did not have a lymph node dissection.
Survival
Disease specific survival (DSS) was determined from the date of diagnosis to the date of death from MTC. Patients were censored at date of death from other causes or date of last follow-up. Overall survival was determined from date of diagnosis to date of death from any cause. Follow-up is updated annually in the SEER registry. Due to the study design, follow-up was the longest for those patients diagnosed in the earliest time interval. In order to address this difference, we reported survival at 5 and 10 years in addition to overall survival.
Statistical Analysis
Descriptive statistics were used to quantify variables across the three time periods and included count with percent for nominal variables and mean with standard deviation (SD) for continuous variables. Fisher’s exact tests compared categorical variables except when a P value could not be calculated. In these instances, a Chi-squared P value was reported. Analysis of variance examined differences in continuous variables. Age-adjusted incidence was calculated based on the 2000 U.S. census and reported per 100,000 people. Survival was estimated with the Kaplan-Meier method and compared with the log-rank test. P values less than 0.05 were considered statistically significant. Statistical analyses were performed using SAS 9.2 software (SAS Institute Inc.).
Results
Over the 30 year time period (1983 – 2012), 2,940 patients were diagnosed with MTC. Of these, 353 (12%) were diagnosed between 1983 and 1992, 793 (27%) between 1993 and 2002, and 1,794 (61%) between 2003 and 2012. The majority of patients (n = 1924 or 65.4%) were classified as medullary carcinoma, NOS (8510) while the remainder (n = 1016 or 34.6%) were considered to have medullary carcinoma with amyloid stroma (8345). Mean follow-up was 194.5 months in the 1983–1992 cohort, 126.2 months in the 1993–2002 cohort, and 46.6 months in the 2003–2012 cohort.
Incidence
The age-adjusted incidence of MTC increased over the three decades (Figure 2A). Between 1983 and 2012, the mean annual age-adjusted incidence of MTC rose significantly from 0.14 to 0.21 per 100,000 people (p < 0.001). When examined by stage, incidence increased for all stages, with the greatest change observed in patients with localized disease (p ≤ 0.004 for all) (Figure 2B).
Figure 2. Age-adjusted Incidence of Medullary Thyroid Cancer.
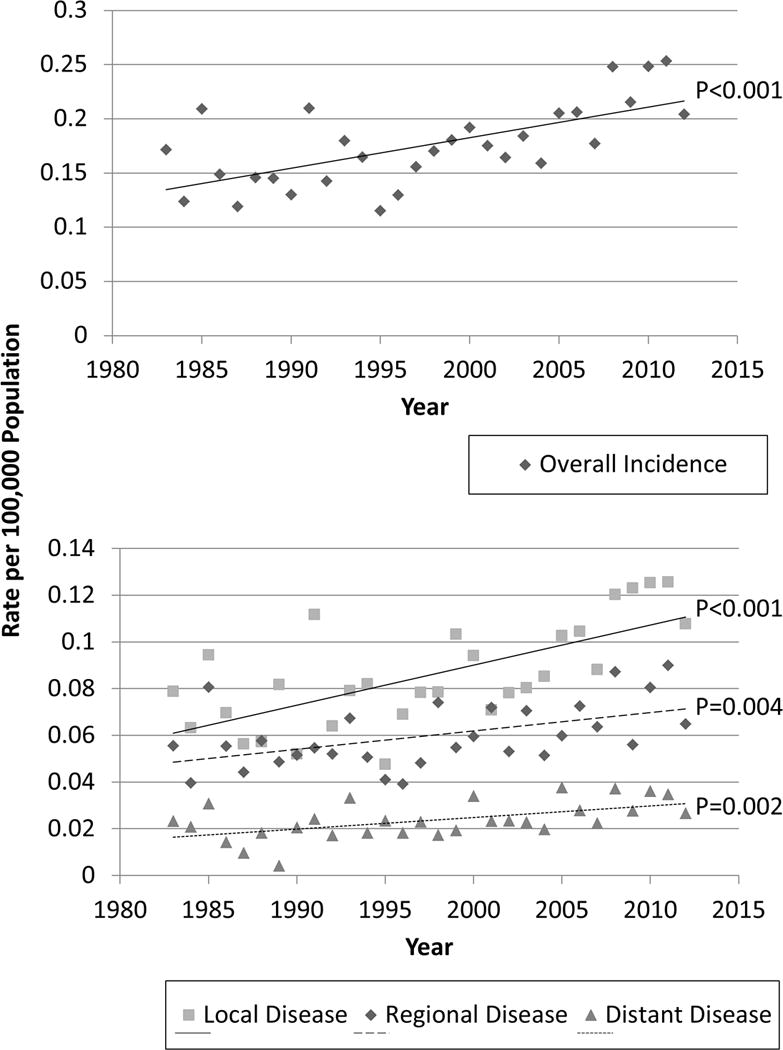
The incidence of Medullary Thyroid Cancer increased overall (top) and across all stages of disease (bottom).
Patient Demographics and Tumor Characteristics
The mean (SD) age at diagnosis was 52.6 (17.6) years for all patients and increased slightly over the study period from 49.8 (18.9) years to 53.8 (17.1) years (p < 0.001). Gender distribution remained constant (Table 1). Although the majority of patients were White, the proportion of Black patients increased from 3.7% to 6.8% to 8.5% from 1983 – 1992, 1993 – 2002, 2003 – 2012, respectively (p < 0.001). Tumor size did not change significantly during the three decades, but the proportion of medullary microcarcinomas, tumors ≤ 1 cm in diameter, increased steadily from 11.4% to 19.6% to 25.1% over the respective time intervals (p < 0.001). On the other hand, extrathyroidal extension decreased during the most recent time period, and was present in only 6.6% of cases from 2003 – 20012 (p < 0.001). The changes in disease presentation did not result in a shift towards diagnosis at an earlier stage as the proportion of patients with local, regional, and distant disease remained constant (p = 0.57).
Table 1.
Demographics and Tumor Characteristics of 2,940 patients with Medullary Thyroid Cancer based on Time Interval
| Variable | 1983 – 1992 (n = 353) | 1993 – 2002 (n = 793) | 2003 – 2012 (n = 1794) | P |
|---|---|---|---|---|
| Age, mean (SD) | 49.8 (18.9) | 51.2 (17.9) | 53.8 (17.1) | <0.001 |
| Gender, n (%) | 0.68 | |||
| Female | 214 (60.6) | 464 (58.5) | 1042 (58.1) | |
| Male | 139 (39.4) | 329 (41.5) | 752 (41.9) | |
| Race | <0.001 | |||
| White | 327 (92.6) | 681 (85.9) | 1515 (84.5) | |
| Black | 13 (3.7) | 54 (6.8) | 152 (8.5) | |
| Other | 13 (3.7) | 58 (7.3) | 127 (7.1) | |
| Tumor Size, mean (SD) mm | 28.1 (30.8) | 28.4 (21.6) | 26.3 (30.5) | 0.24 |
| Tumor Size, n (%) | <0.001 | |||
| ≤1cm | 16 (11.4) | 131 (19.6) | 412 (25.1) | |
| >1 and ≤2cm | 47 (33.3) | 151 (22.6) | 408 (24.8) | |
| >2 and ≤4cm | 63 (44.7) | 243 (36.4) | 518 (31.5) | |
| >4cm | 15 (10.6) | 142 (21.3) | 307 (18.7) | |
| Multifocal, n (%) | 21 (13.0) | 120 (17.6) | 266 (18.6) | 0.20 |
| Extrathyroidal Extension, n (%) | 26 (16.1) | 139 (20.4) | 94 (6.6) | <0.001 |
| SEER Historic Stage, n (%) | 0.57 | |||
| Local | 167 (50.3) | 380 (49.6) | 911 (51.9) | |
| Regional | 124 (37.4) | 273 (35.6) | 592 (33.7) | |
| Distant | 41 (12.4) | 113 (14.8) | 252 (14.4) |
SEER- Surveillance, Epidemiology and End Results
Extent of Surgery and Radiation
Over the three decade study period, the extent of surgery increased with each time interval. The proportion of patients undergoing a total thyroidectomy increased to 81.1% in the most recent decade (Table 2). Of those patients treated with surgery, only 10.7% received less than a total thyroidectomy in the last decade. The percentage of patients having a lymph node dissection also increased, from 53.2% in 1983 – 1992, 61.7% in 1993 – 2002, to 68.8% in 2003 – 2012 (p < 0.001). Additionally, the extent of lymph node dissections increased. The mean (SD) number of lymph nodes harvested per dissection doubled between the earliest and the most recent time intervals (11.4 (14.8) to 22.8 (23.2) nodes, p < 0.001). We also examined the percentage of total thyroidectomies performed with a concurrent lymph node dissection, and found that these concomitant procedures increased steadily with each subsequent decade (Figure 3). There was no change in the use of radioisotopes for MTC treatment during the study period and the rate ranged from 2.1% to 5.6%.
Table 2.
Treatment Patterns of Patients with Medullary Thyroid Cancer according to Time Interval
| Variable | 1983 – 1992 (n = 353) | 1993 – 2002 (n = 793) | 2003 – 2012 (n = 1794) | P |
|---|---|---|---|---|
| Lymph Node Dissection, n (%) | 99 (53.2) | 483 (61.7) | 1221 (68.8) | <0.001 |
| No. of Lymph Nodes Removed, mean (SD) | 11.4 (14.8) | 16.9 (18.9) | 22.8 (23.2) | <0.001 |
| Positive Lymph Nodes, n (%) | 63 (64.3) | 286 (59.5) | 668 (54.8) | 0.06 |
| No. of Positive Lymph Nodes, mean (SD) | 3.7 (6.5) | 4.7 (8.8) | 5.5 (9.6) | 0.07 |
| Lymph Node Ratio (positive/removed), mean (SD) | 0.35 (0.40) | 0.31 (0.36) | 0.24 (0.32) | <0.001 |
| Surgery of the Primary Tumor, n (%) | <0.001 | |||
| None | 7 (2.1) | 55 (7.1) | 147 (8.2) | |
| Unknown Surgery | 157 (47.2) | 17 (2.2) | 25 (1.4) | |
| Partial Thyroidectomy | 26 (7.8) | 106 (13.6) | 166 (9.3) | |
| Total Thyroidectomy | 143 (42.9) | 601 (77.2) | 1448 (81.1) | |
| Total Thyroidectomy with Any Lymph Node Dissection, n (%) | 82 (58.2) | 414 (69.2) | 1106 (76.5) | <0.001 |
| Radiation, n (%) | 0.06 | |||
| None | 289 (85.3) | 626 (81.8) | 1484 (84.7) | |
| External Beam | 43 (12.7) | 96 (12.6) | 192 (11.0) | |
| Radioisotope | 7 (2.1) | 43 (5.6) | 77 (4.4) |
Figure 3. Trend in Lymph Node Dissections at the time of Total Thyroidectomy.
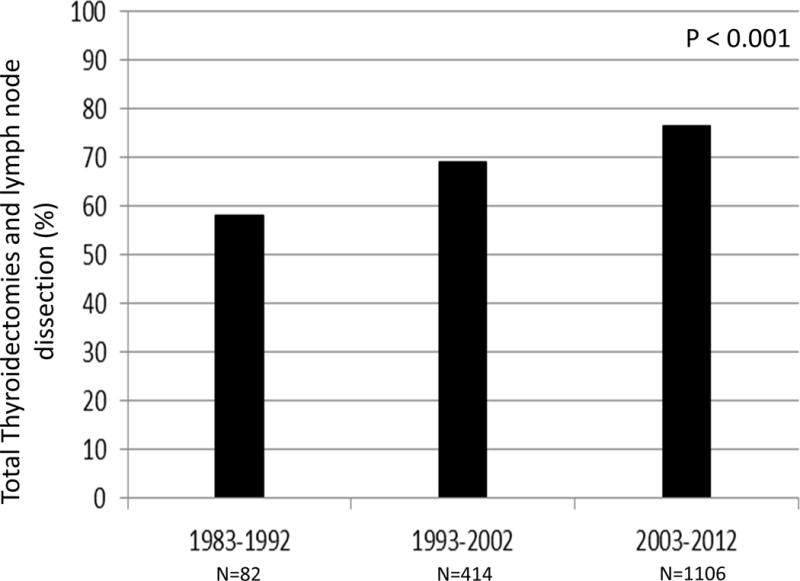
This histogram demonstrates that the proportion of patients who underwent a total thyroidectomy with a lymph node dissection increased from 58.2% to 69.2% to 76.5% in the last 30 years (p < 0.001).
Survival Analyses
To determine whether or not patient outcomes also changed during the past 30 years, we investigated survival. We found that DSS improved for all patients (p = 0.01, Figure 4). When examined by SEER stage, DSS increased significantly for those with regional disease (p = 0.003) and distant metastases (p = 0.02), but not for those with localized disease (Figure 4). To account for the difference in follow-up between the three time intervals, we also examined 5 and 10-year DSS, which revealed similar results. Five and 10-year DSS improved for the entire cohort and specifically for those with regional and distant disease (Table 3). Although no statistically significant improvement was noted in patients with localized MTC, DSS was excellent with 99% of patients alive at 5 years after diagnosis in the most recent decade. In addition to DSS, we analyzed overall survival and found no differences between the time periods. Overall survival at 5 years was 81% for 1983 – 1992, 80% for 1993 – 2002, and 83% for 2003 – 2012 (p = 0.11). Overall survival at 10 years was 71%, 71%, and 72% for 1983 – 1992, 1993 – 2002, and 2003 – 2012, respectively (p = 0.20).
Figure 4. Disease Specific Survival for patients with Medullary Thyroid Cancer based on Time Interval.
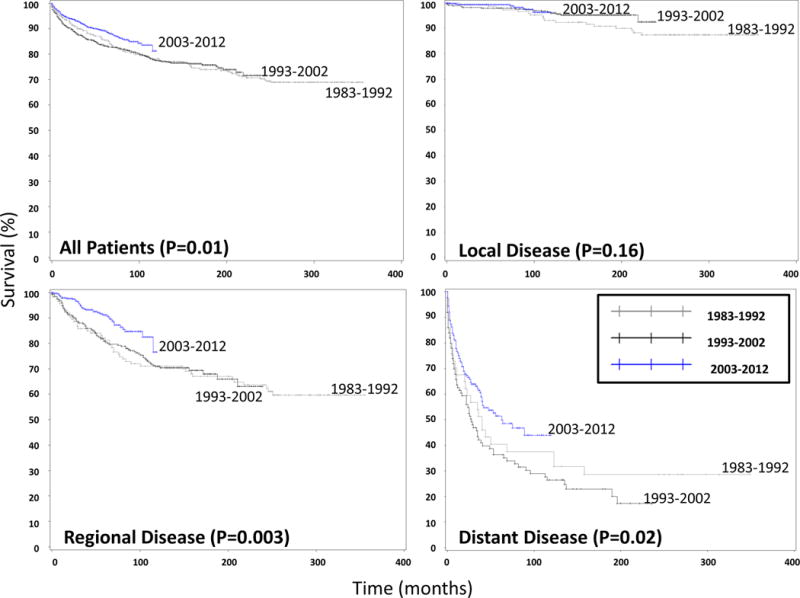
The four Kaplan-Meier survival curves demonstrate improved disease specific survival (DSS) for all patients (top left) and for those patients with regional (bottom left) and distant (bottom right) disease, but not with localized disease (top right).
Table 3.
Disease Specific Survival of Patients with Medullary Thyroid Cancer by Time Interval
| 1983–1992 | 1993–2002 | 2003–2012 | P | |
|---|---|---|---|---|
| All patients | ||||
| 5- year | 86% | 83% | 89% | <0.001 |
| 10- year | 78% | 77% | 81% | 0.009 |
| Local Disease | ||||
| 5- year | 98% | 98% | 99% | 0.22 |
| 10- year | 93% | 96% | 96% | 0.28 |
| Regional Disease | ||||
| 5- year | 82% | 81% | 91% | <0.001 |
| 10- year | 71% | 71% | 77% | 0.003 |
| Distant Metastases | ||||
| 5- year | 40% | 36% | 51% | 0.02 |
| 10- year | 37% | 26% | 44% | 0.02 |
Discussion
Over the past few decades, medical advances have changed the environment in which MTC is managed. Whether or not the disease presentation, treatment, and survival of patients with MTC have changed alongside these advances is unclear. Therefore, we undertook the current study to determine the trends in MTC over the past 30 years. The data demonstrate that the incidence of MTC is rising, which mirrors changes seen in well-differentiated thyroid cancers, but the extent of disease at diagnosis remains similar. Additionally, to the best of our knowledge, this study is the first to demonstrate a statistically significant improvement in DSS over time in patients with MTC. This improvement is most notable in patients with regional and distant disease.
One of the advances in MTC treatment is the ability to test for RET mutations which allows us to perform prophylactic thyroidectomy. The performance of prophylactic surgery may play a role in our observation that patients are being diagnosed at an older age. Yet despite the ability to treat a subset of patients before disease develops, the incidence of MTC continues to rise. This observed increase in incidence is likely multifactorial. First, improved ultrasound quality as well as cytology might result in earlier diagnosis where disease is still localized. In addition, finer sectioning of pathologic specimens may result in detection of more micro-MTCs as is shown in our results. While the incidence is increasing across all stages, the greatest increase is in those with localized disease, which supports the proposed impact of enhanced imaging techniques and more thorough examination of thyroid specimens. However, these observations are unlikely to entirely account for the observed increase in incidence given the greater incidence of regional and distant disease as well. Cramer and colleagues (1) made a similar observation for patients with papillary thyroid cancer and suggested that, since the incidence of tumors of all sizes were increasing, earlier detection alone could not account for the increase in incidence (1).
Despite the possibility of earlier detection, we did not see clear evidence of diagnosis at an earlier stage. This observation is not only because the proportion of patients with localized disease did not change significantly over the study period, but also because the mean tumor size remained constant. Our finding is in agreement with those of Kebebew and colleagues (13) who also did not appreciate a change in tumor size over time (13). We did, however, observe less extrathyroidal extension and an increased proportion of patients diagnosed with tumors 1 cm or less. Kazaure and colleagues (19) similarly reported that the proportion of these micro-MTCs was increasing relative to MTCs larger than 1 cm, but they also reported that even though these tumors were small, they were associated with a significant rate of poor prognostic characteristics, including lymph node metastases (19). Their results indicate that detecting smaller tumors does not always equate to diagnosis at an earlier stage. While less extrathyroidal extension and more micro-MTCs in the more recent time periods suggest a trend toward earlier detection, this finding did not translate into diagnosis at an earlier stage.
Another advance in the care of patients with MTC during the study period was the addition of guidelines for MTC treatment recommending increased extent of surgery (14, 15). Previous work has shown that compliance to the American Thyroid Association guidelines correlated with improved survival (17). Although we did not repeat that analysis here, it seems plausible that as the extent of surgery increases to reflect the standard of care, an improvement in survival will follow. The significant increase in the proportion of patients receiving a total thyroidectomy was initially apparent 10 years ago when Kebebew and colleagues (13) examined SEER data, and our analysis shows that this trend has continued (13). We also demonstrate that surgery has gradually become more extensive, which predates the improvement in survival. Kebebew and colleagues also saw a non-significant increase in the number of patients receiving a lymph node dissection (13). Our data indicate that this trend has also continued and now has become statistically significant. This increased extent of surgery may be partially attributable to the influence of the consensus guidelines. However, improvement in operative planning with the use of preoperative cervical ultrasound, calcitonin, CEA, and at times, RET testing has likely played a role as well. As it stands, 76.5% of the MTC patients in our study received the appropriate extent of surgery in the most recent decade, a total thyroidectomy with a lymph node dissection. The reason that nearly one quarter of patients still does not receive a total thyroidectomy with a nodal dissection is not entirely clear. While this observation might indicate that guidelines have not permeated all practices, the difficulty in diagnosing MTC with fine needle aspiration may mean that some patients are only being diagnosed after final pathologic review. In fact, after reviewing the cytology results from fine needle aspiration biopsies in patients with MTC, Essig and colleagues (20) reported that only 46% of the biopsy results indicated MTC (20). In addition, some of these diagnoses may have been incidental; however, the SEER database does not record the surgical indication. In some of these situations, a less than total thyroidectomy may have been appropriate. Furthermore, a high rate of incidentally identified MTCs might account for some of the observed increase in micro-MTCs as well as the excellent DSS noted for patients with localized disease.
The increase in the extent of surgery is not limited to a rise in total thyroidectomy or total thyroidectomy with lymph node dissection. Our results indicate that lymph node dissections are also more extensive as evidenced by an increase in the number of lymph nodes harvested. Interestingly, the proportion of patients with positive nodes did not increase in our study and trended in the opposite direction suggesting that the greater extent of lymph node dissections was not in response to clinically apparent disease. Rather this increase is likely due to compliance with recommendations to perform a prophylactic central neck dissection in all patients with MTC and to consider a prophylactic lateral neck dissection on the basis of the calcitonin level (15). Because the lymph node yield in our study increased while the number of positive nodes did not, the ratio of positive to examined lymph nodes decreased significantly over the study period. Leggett and colleagues (21) demonstrated that a low ratio predicted better survival for patients with MTC, which is supported by others (21–23). Similarly, it is reasonable to expect that the improvement in survival in the current study might be linked to the increasing extent of surgery given that the lymph node ratio decreased with each subsequent time interval, but causality cannot be assumed.
The increased number and extent of lymph node dissections also raises the concern that stage migration might account for the observed improvement in survival. While this theory is a possibility, the proportions of patients with local and regional disease have not shifted. Additionally, while stage migration might contribute to the observed improvement in DSS for patients with regional disease, it should not have an effect on those with distant disease.
One of the major findings of the current study is that, DSS improved for all patients with MTC and especially for those with regional or distant disease. Currently, the 5 and 10-year DSS for all MTC patients is 89% and 81%, respectively. Previous studies have failed to demonstrate this improvement likely because the duration of follow-up was too short and/or the cohort size was too small. For instance, Kebebew and colleagues (13) used the same registry 10 years ago and were unable to demonstrate a survival improvement over time. They suggested that it was too early to see the recent advances in the care of patients with MTC result in improved survival (13).
While the increased extent of surgery may account for the improvement in survival of patients with regional disease, it seems unlikely that more extensive surgery is responsible for the improved survival of patients with metastases. Esfandiari and colleagues (22) reported that total thyroidectomy with regional lymph node dissection predicted improved survival for patients with distant metastases, but this finding may simply be a marker of more comprehensive care (22). On the other hand, RTKIs were first used for patients with metastatic MTC during the most recent time interval in this study and are associated with an improvement in progression free survival (11, 12). In an international, randomized controlled trial published at the end of the most recent time period, cabozantinib demonstrated 40% greater progression free survival than placebo at 1 year (11). Vandetanib also demonstrated improved progression free survival in patients with locally advanced or metastatic MTC treated between 2006 and 2007 in a study that was published around the same time (12). These more novel therapies may account for the observed increase in DSS, especially because the greatest survival increase was in the most recent time interval.
Although the current study represents a large population based cohort of patients with MTC and is the first, to the authors’ knowledge, to demonstrate improved survival over time for patients with MTC, there are several limitations. First, despite the large size of the cohort, we still may have been underpowered to detect small, but clinically important differences. Another limitation is missing or vaguely coded data. For instance, while site-specific surgery codes were available during the entire study, a significant portion of the earliest time interval was coded as having “thyroidectomy, NOS.” Therefore, when examining the type of procedure performed (for example, partial versus total thyroidectomy), only the two most recent time periods have reliable data. This coding inconsistency limited our conclusions. Additionally, SEER does not record variables such as incidental identification of MTC in the thyroid or lymph nodes, cytology results, surgical indications, hereditary cases, RET mutational status, prophylactic surgery, chemotherapy type, recurrence, or hospital level factors, all of which would be valuable in the analysis of patients with MTC.
Other limitations involved recoding SEER data and limitations inherent to the study design. The American Joint Committee on Cancer staging for MTC changed during the study period and the variables in SEER are not sufficient to accurately reconstruct the most recent staging system. To avoid errors, we chose to only examine SEER historic stage which is categorized as local, regional, or distant disease. To capture all lymph node dissections, we considered any patient with lymph nodes excised to have undergone a nodal dissection. This definition may artificially inflate the number of nodal dissections reported, but should do so similarly across all time intervals. As mentioned earlier, the study design resulted in different follow-up for the three time intervals. That being said, the most recent time interval, there were 222 deaths out of 1533 at risk at 10 years of follow-up and 77 patients were followed to at least 9 years from the date of diagnosis. While this may have implications in survival estimations, we reported DSS at 5 years in addition to 10 years and overall in order to minimize the effect of the different follow-up.
Nonetheless, over the past 30 years, the incidence of MTC has increased. The disease presentation has largely remained the same except that patients are slightly older at initial diagnosis and a higher proportion has tumors 1 cm or less in diameter. Of significance, the extent of surgery has increased as has DSS for the entire cohort of patients with MTC. However, when examined by stage, only patients with regional and distant disease had a statistically significant improvement in DSS. While surgery alone is unlikely to account for all of the observed improvements in survival, our findings indicate that the medical advances in the management of MTC as a whole are likely impacting the treatment and outcomes of these patients in the U.S.
Footnotes
Disclosure Information: Nothing to Disclose
To be Presented as a podium presentation at the annual meeting for the American Association of Endocrine Surgeons, April 2016 in Baltimore, MD
References
- 1.Cramer JD, Fu P, Harth KC, Margevicius S, Wilhelm SM. Analysis of the rising incidence of thyroid cancer using the Surveillance, Epidemiology and End Results national cancer data registry. Surgery. 2010;148(6):1147–52. doi: 10.1016/j.surg.2010.10.016. discussion 52-3. [DOI] [PubMed] [Google Scholar]
- 2.Morris LG, Sikora AG, Tosteson TD, Davies L. The increasing incidence of thyroid cancer: the influence of access to care. Thyroid: official journal of the American Thyroid Association. 2013;23(7):885–91. doi: 10.1089/thy.2013.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The American Cancer Society. Cancer Facts and Figures. 2015 www.cancer.org; 2015 [cited 2016 January 14]
- 4.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88(5):1139–48. doi: 10.1002/(sici)1097-0142(20000301)88:5<1139::aid-cncr26>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Gilliland FD, Hunt WC, Morris DM, Key CR. Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the Surveillance, Epidemiology and End Results (SEER) program 1973–1991. Cancer. 1997;79(3):564–73. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 6.Pitt SC, Moley JF. Medullary, anaplastic, and metastatic cancers of the thyroid. Seminars in oncology. 2010;37(6):567–79. doi: 10.1053/j.seminoncol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Fialkowski EA, Moley JF. Current approaches to medullary thyroid carcinoma, sporadic and familial. Journal of surgical oncology. 2006;94(8):737–47. doi: 10.1002/jso.20690. [DOI] [PubMed] [Google Scholar]
- 8.Davies L, Morris LG, Haymart M, Chen AY, Goldenberg D, Morris J, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: The Increasing Incidence of Thyroid Cancer. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2015;21(6):686–96. doi: 10.4158/EP14466.DSCR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komminoth P, Kunz EK, Matias-Guiu X, Hiort O, Christiansen G, Colomer A, et al. Analysis of RET protooncogene point mutations distinguishes heritable from nonheritable medullary thyroid carcinomas. Cancer. 1995;76(3):479–89. doi: 10.1002/1097-0142(19950801)76:3<479::aid-cncr2820760319>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 10.Deshpande HA, Sheth K, Sosa JA, Roman S. Efficacy and tolerability of pharmacotherapy options for the treatment of medullary thyroid cancer. Clinical Medicine Insights Oncology. 2012;6:355–62. doi: 10.4137/CMO.S8305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elisei R, Schlumberger MJ, Muller SP, Schoffski P, Brose MS, Shah MH, et al. Cabozantinib in progressive medullary thyroid cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(29):3639–46. doi: 10.1200/JCO.2012.48.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells SA, Jr, Robinson BG, Gagel RF, Dralle H, Fagin JA, Santoro M, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(2):134–41. doi: 10.1200/JCO.2011.35.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kebebew E, Greenspan FS, Clark OH, Woeber KA, Grunwell J. Extent of disease and practice patterns for medullary thyroid cancer. Journal of the American College of Surgeons. 2005;200(6):890–6. doi: 10.1016/j.jamcollsurg.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 14.American Thyroid Association Guidelines Task F. Kloos RT, Eng C, Evans DB, Francis GL, Gagel RF, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid: official journal of the American Thyroid Association. 2009;19(6):565–612. doi: 10.1089/thy.2008.0403. [DOI] [PubMed] [Google Scholar]
- 15.Wells SA, Jr, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid: official journal of the American Thyroid Association. 2015;25(6):567–610. doi: 10.1089/thy.2014.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Sippel RS, O’Dorisio MS, Vinik AI, Lloyd RV, Pacak K, et al. The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010;39(6):775–83. doi: 10.1097/MPA.0b013e3181ebb4f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panigrahi B, Roman SA, Sosa JA. Medullary thyroid cancer: are practice patterns in the United States discordant from American Thyroid Association guidelines? Annals of surgical oncology. 2010;17(6):1490–8. doi: 10.1245/s10434-010-1017-0. [DOI] [PubMed] [Google Scholar]
- 18.Surveillance, Epidemiology and End Results (SEER) National Cancer Institute; Bethesda Md: 2015. [Internet] [Google Scholar]
- 19.Kazaure HS, Roman SA, Sosa JA. Medullary thyroid microcarcinoma: a population-level analysis of 310 patients. Cancer. 2012;118(3):620–7. doi: 10.1002/cncr.26283. [DOI] [PubMed] [Google Scholar]
- 20.Essig GF, Jr, Porter K, Schneider D, Debora A, Lindsey SC, Busonero G, et al. Fine needle aspiration and medullary thyroid carcinoma: the risk of inadequate preoperative evaluation and initial surgery when relying upon FNAB cytology alone. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2013;19(6):920–7. doi: 10.4158/EP13143.OR. [DOI] [PubMed] [Google Scholar]
- 21.Leggett MD, Chen SL, Schneider PD, Martinez SR. Prognostic value of lymph node yield and metastatic lymph node ratio in medullary thyroid carcinoma. Annals of surgical oncology. 2008;15(9):2493–9. doi: 10.1245/s10434-008-0022-z. [DOI] [PubMed] [Google Scholar]
- 22.Esfandiari NH, Hughes DT, Yin H, Banerjee M, Haymart MR. The effect of extent of surgery and number of lymph node metastases on overall survival in patients with medullary thyroid cancer. J Clin Endocrinol Metab. 2014;99(2):448–54. doi: 10.1210/jc.2013-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kandil E, Gilson MM, Alabbas HH, Tufaro AP, Dackiw A, Tufano RP. Survival implications of cervical lymphadenectomy in patients with medullary thyroid cancer. Annals of surgical oncology. 2011;18(4):1028–34. doi: 10.1245/s10434-010-1363-y. [DOI] [PubMed] [Google Scholar]


