Abstract
BACKGROUND
A staging/prognostic system has long been desired to better categorize pheochromocytoma (PC)/paraganglioma (PGL), which can be very aggressive in the setting of SDHB mutations.
METHODS
A retrospective analysis was conducted of clinical characteristics and outcomes including genetic testing results, tumor recurrence/metastasis, Ki67/MIB1% staining and tumor mitotic index (MI) in patients with PC/PGL.
RESULTS
Patients with SDHB mutation presented at younger age (33.0 vs. 49.6 years old, p<0.001), had increased local recurrence and distant metastases (47.6% vs. 9.1%, p<0.001, and 56.3% vs. 9.1%, p<0.001, respectively), and shorter median disease-free interval (DFI) (89.8 months, 95% CI: 36.0 – 96.4 vs. not reached, p<0.001). SDHB mutation, greatest tumor diameter, and open surgical resection were associated with higher local recurrence and distant metastases (p=0.006 and p<0.001, p<0.001 and p<0.001, p<0.001 and p<0.001, respectively). SDHB mutation and tumor diameter were independent risk factors for local recurrence (p=0.048, p=0.04) and metastases (p=0.004, p<0.001). Ki67% and MI were not associated with SDHB mutation (p=0.09, p=0.55), local recurrence (p=0.48, p=0.066), metastases (p=0.22, p=0.28) or DFI (p=0.69, p=0.19).
CONCLUSION
SDHB status and primary tumor size are more predictive of patient outcome than Ki67% or MI, and should be part of any clinically relevant prognostic scoring system.
Keywords: Adrenalectomy, Pheochromocytoma, Paraganglioma, SDHB, Ki67%, mitotic index
Introduction
Pheochromocytomas (PC) and paragangliomas (PGL) are rare neuroendocrine tumors arising from the adrenal medulla or paraganglia. When they recur or metastasize, they are considered to be malignant.1,2 These tumors can occur sporadically or in the setting of inherited cancer syndromes such as multiple endocrine neoplasia type 2, von Hippel-Lindau syndrome, and neurofibromatosis I, as well as myc-associated factor X and succinate dehydrogenase complex mutations (SDHx) in familial PC/PGL.3–6 In the setting of SDHB mutations, PC and abdominal/pelvic PGL can be very aggressive, with a high rate of local recurrence, distant metastases, and disease-specific mortality.7
Differentiating between benign and malignant PC/PGL is critical for optimized patient care. Surgery is the mainstay of treatment for primary tumors, and can be applied to locoregional disease or recurrences and may be palliative in patients with functioning distant metastases.8 There is no widely used staging or prognostic scoring system for PC/PGL. Studies on prognostic factors associated with PC/PGL have shown the presence of SDHB mutation, primary tumor size, and age at diagnosis to be significant prognostic factors.9 Previously described staging systems based on pathologic data, such as the pheochromocytoma of the adrenal gland scaled score (PASS), have been shown to be associated with patient outcome in small cohort studies but have not been validated.10–14 The grading system for adrenal PC and PGL (GAPP), utilizing pathology findings to create a scoring system, has also been proposed, as it was associated with patient outcome in one study.15 No current grading system includes genetic mutation status, despite the known higher risk of disease recurrence, metastases, and mortality, especially in patients with germline SDHB mutations. An improved staging/prognostic system has long been desired to better categorize PC/PGL, which could allow for better treatment selection and improve prognostication and follow up measures. To our knowledge, no previous study has analyzed germline mutation status, tumor type, size, functionality, and histologic features such as Ki67/MIB-1% and mitotic index simultaneously. In this study, we performed a comprehensive analysis of genetic, clinical, biochemical and histologic variables in patients with localized PC/PGL to test the hypothesis that there are prognostic factors in patients with localized PC/PGL.
Methods
A retrospective analysis was conducted in patients with germline testing confirming SDHB mutation status and PC or abdominal/pelvic PGL, treated at our institution from 1998 – 2015. All patients were enrolled in a clinical protocol after written informed consent and the studies were approval by the Office of Human Subject Research at the National Institutes of Health. All patients included in the study were tested for known germline mutations including SDHx, RET, VHL, NF1, and MAX. Of 256 patients with PC or abdominal/pelvic PGL, 49 carried SDHB mutations. Of 73 patients considered to be sporadic based on negative germline testing result and no family history, 35 were selected randomly as a comparison cohort. Clinical characteristics including age, gender, and mutation status, tumor characteristics including location, functional status, and size based on pathology were reviewed. Surgical data including operative approach were analyzed. The decision for a specific operative approach was multifactorial and based on mutation status, tumor type, and tumor size. A majority of patients with SDHB mutation, paraganglioma, larger tumors, and/or a contraindication to laparoscopic surgery had an open approach. Treatment outcomes including local recurrence, distant metastases, disease-free interval (DFI), and disease-specific death were also analyzed in all patients. DFI was defined as time following R0 resection with no evidence of local recurrence or distant metastasis on anatomic imaging (whole body CT/MRI), functional imaging (18F-FDG PET/CT, 18F-FDOPA PET/CT) and biochemical testing (serum and urine catecholamine and metanephrines, and serum chromogranin A). For patients who were never disease-free, DFI was defined as zero. Patients were followed from the date of their operation until the date of recurrence (local or distant), last follow-up or death. Pathologic data including greatest primary tumor size were analyzed in all patients.
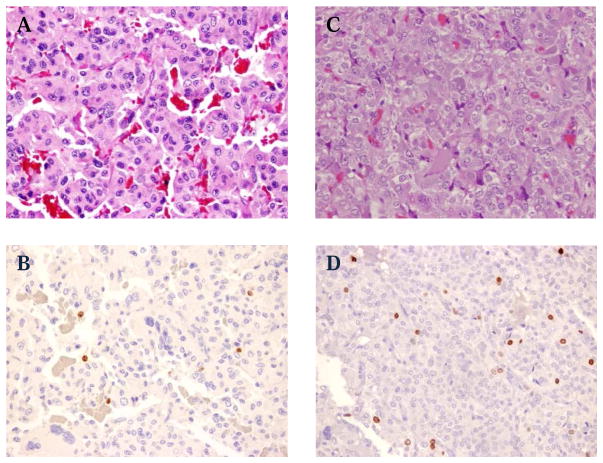
Primary tumor samples, when available, were stained and analyzed for Ki67/MIB-1% staining and mitotic index (MI). PC and PGL tumor specimens were re-examined by two pathologists, independently and blinded to the clinical follow-up data. Mitotic count was assessed based on examination of 50 high power fields (HPF). MI count was analyzed as 0 mitoses, 1 mitoses, or ≥ 2 mitoses per HPF. Immunohistochemical stain for MIB-1/Ki-67 [anti- human Ki-67 monoclonal mouse antibody at 1:200 dilution, (Dako M7240, Carpinteria, CA)] was performed on available formalin-fixed paraffin-embedded tissue (FFPE) sections, using an automated immunostainer following the manufacturer’s specifications. MIB-1 labeling index was based on the examination of two HPF (40X) demonstrating the highest staining, and the percent of positive tumor cells were scored as <1%, 1–3% and > 3% (Figure 1).
Figure 1.
Representative histologic images. (Hematoxylin and Eosin stain (A&C) and Ki67% stain (B&D) (X20). (A, B) Tumor sample from a patient with SDHB mutation and metastatic PGL, Ki67 1–3%. (C, D) Tumor from a patient with sporadic PC and no recurrence, Ki67% over 3%.
Associations between two dichotomous parameters were determined by Fisher’s exact test, while the association between an ordered categorical parameter and a dichotomous parameter was determined by an exact Cochran-Armitage test for trend. The association between a dichotomous parameter and a continuous parameter was determined by an exact Wilcoxon rank sum test. The Kaplan-Meier method was used to determine the probability of recurrence as a function of time. The statistical significance of the difference among curves was determined using a log-rank test. The factors which demonstrated association with time to recurrence in the univariate analyses were subsequently evaluated for their joint association with time to recurrence using a Cox proportional hazards model. All p-values are two-tailed and reported without adjustment for multiple comparisons.
Results
Eighty-four patients treated at the National Institutes of Health clinical center for PC/PGL were included in the study; 49 patients had germline SDHB mutations, and 35 patients had sporadic disease. The clinical, biochemical and genetic characteristics and treatments used in the study cohort are summarized in Table 1. Sixty-five patients with isolated local disease underwent complete resection of their primary tumor with negative margins (R0). Thirteen patients with the primary surgical resection performed at outside institution underwent resection of locoregional recurrences and/or isolated distant metastases. Three patients underwent resection of their primary tumors with synchronous metastases also treated with resection or external beam radiation. Three patients presented with extensive metastatic disease and were treated with cisplatin, vincristine and doxorubicin. The follow up time for the sporadic group of patients was shorter than for patients with SDHB mutations, although this was not statistically significant (58.2 ± 8.5 vs. 95.9 ± 16.7 months, respectively, P=0.29). There were no perioperative deaths.
Table 1.
Clinical, Biochemical, Genetic and Histologic Characteristics of Study Cohort
| Sporadic (n=35) | SDHB (n=49) | P value | |
|---|---|---|---|
|
| |||
| Sex | |||
| Men | 18 | 34 | P=0.11 |
| Women | 17 | 15 | |
|
| |||
| Age (years ± SD) | 49.6 ± 2.1 | 33.0 ± 2.3 | P<0.001 |
|
| |||
| Mean Tumor Size § (n=71) | 4.5 ± 0.4 | 5.2 ± 0.6 | P=0.79 |
|
| |||
| Tumor Type (n=68)# | |||
| Pheochromocytoma | 34 | 6 | |
| Paraganglioma | 1 | 27 | P<0.001 |
|
| |||
| Functionality (n=64) | |||
| Nonfunctioning | 0 | 11 | |
| Metanephrine* | 4 | 0 | |
| Normetanephrine* | 11 | 14 | |
| Dopamine* | 0 | 6 | |
| Combination^ | 8 | 10 | P<0.001 |
|
| |||
| Approach (n=66) | |||
| Open | 8 | 24 | |
| Laparoscopic | 26 | 8 | P<0.001 |
|
| |||
| Follow up (months) | 58.2 ± 8.5 | 95.9 ± 16.7 | P = 0.29 |
|
| |||
| Locoregional recurrence | 3/33 (9.1%) | 20/42 (47.6%) | P<0.001 |
|
| |||
| Distant metastases | 3/33 (9.1%) | 27/48 (56.3%) | P<0.001 |
|
| |||
| Death | 2 | 5 | P=0.69 |
Data are presented as frequencies, mean ± SD, and percentages unless otherwise stated.
Mean tumor diameter in cm ± SD.
Number of patients with biochemical value above normal range.
Combination of biochemical secretion.
Based on histology and anatomic location at the time of the operation or on anatomic imaging studies.
Patients with SDHB mutations presented at a younger age compared to patients without SDHB mutation (33.0 ± 2.3 vs. 49.6 ± 2.1 years old, respectively, p<0.001). Patients with SDHB mutation were more likely to have nonfunctioning tumors and PGL (p=0.005 and p<0.001, respectively). Local recurrence and distant metastases were significantly more frequent in patients with SDHB mutation compared to patients without an SDHB mutation (47.6% vs. 9.1%, p<0.001, and 56.3% vs. 9.1%, p<0.001, respectively). Five of the 7 patient deaths were in patients with SDHB mutations (Table 1). Percent of tumor cell positive for Ki67 staining and MI were not significantly different between patients with and without SDHB mutation (p=0.12).
Requiring an open surgical approach was associated with higher locoregional recurrence (p<0.001). The tumor functional status was not associated with locoregional recurrence. The percent staining for Ki67 was also not associated with locoregional recurrence (p=0.48). There were no locoregional recurrences in any of the 7 patients with a Ki67% of 1–3%, nor the 5 patients who had Ki67% greater than 3%. MI also was not associated with locoregional recurrence (p=0.066). However, the mean MI was lower in patients without locoregional recurrence as compared to patients with locoregional recurrence (1.2 versus 4.7, respectively, p=0.04). Only 2 patients with a MI ≥ 2 had locoregional recurrence. Mean greatest tumor diameter for patients who did not have locoregional recurrence was 4.5 ± 0.5 cm, significantly smaller than the mean tumor diameter in patients who had locoregional recurrence, 7.6 ± 0.8 cm (p<0.001) (Table 2). Mean tumor diameter for patients without distant metastases was 3.7 ± 0.3 cm, and 7.7 ± 0.9 cm for those with distant metastases (p<0.001). PGL and requiring an open surgical approach were associated with distant metastases (p=0.004 and p<0.001, respectively). The tumor functional status was not associated with distant metastases. Ki67% and MI were not associated with distant metastases (p=0.22 and p=0.28, respectively) (Table 3).
Table 2.
Clinical Characteristics and Relationship with Locoregional Recurrence in Patients undergoing R0 resection.
| No Recurrence | Recurrence | P value | |
|---|---|---|---|
|
| |||
| Mutation status (n=63) | |||
| Sporadic | 28 | 1 | |
| SDHB mutation | 24 | 10 | P=0.008 |
|
| |||
| Mean Tumor Size (cm) (n=53) | 4.5 ± 0.5 | 7.6 ± 0.8 | P<0.001 |
|
| |||
| Functionality (n=45) | |||
| Nonfunctioning | 5 | 1 | |
| Metanephrine* | 3 | 0 | |
| Normetanephrine* | 15 | 1 | |
| Dopamine* | 3 | 3 | |
| Combination^ | 10 | 4 | P=0.13 |
|
| |||
| Tumor type (n=52)# | |||
| Pheochromocytoma | 30 | 2 | |
| Paraganglioma | 17 | 3 | P=0.36 |
|
| |||
| Approach (n=52) | |||
| Open | 11 | 9 | |
| Laparoscopic | 32 | 0 | P<0.001 |
|
| |||
| Ki67% (n=40) | |||
| Under 1% | 25 | 3 | |
| 1 – 3% | 7 | 0 | |
| Over 3% | 5 | 0 | P=0.48 |
|
| |||
| Mitotic Index (n=38) | |||
| 0 | 16 | 0 | |
| 1 | 12 | 1 | |
| ≥2 | 7 | 2 | P=0.066 |
Data are presented as frequencies and percentages unless otherwise stated.
Mean tumor diameter in cm ± SD.
Number of patients with biochemical value above normal range.
Combination of biochemical secretion.
Based on histology and anatomic location at the time of the operation or on anatomic imaging studies.
Table 3.
Clinical Characteristics and Relationship with Distant Metastases in Patients undergoing R0 resection.
| No Metastases | Metastases | P value | |
|---|---|---|---|
|
| |||
| Mutation status (n=63) | |||
| Sporadic | 28 | 1 | |
| SDHB mutation | 17 | 17 | P<0.001 |
|
| |||
| Mean Tumor Size (cm) (n=53) | 3.7 ± 0.3 | 7.7 ± 0.9 | P<0.001 |
|
| |||
| Functionality (n=48) | |||
| Nonfunctioning | 5 | 3 | |
| Metanephrine* | 3 | 0 | |
| Normetanephrine* | 11 | 5 | |
| Dopamine* | 3 | 3 | |
| Combination^ | 10 | 5 | P=0.77 |
|
| |||
| Tumor type (n=52)# | |||
| Pheochromocytoma | 30 | 2 | |
| Paraganglioma | 12 | 8 | P=0.004 |
|
| |||
| Approach (n=52) | |||
| Open | 7 | 13 | |
| Laparoscopic | 31 | 1 | P<0.001 |
|
| |||
| Ki67% (n=40) | |||
| Under 1% | 25 | 3 | |
| 1 – 3% | 6 | 1 | |
| Over 3% | 3 | 2 | P=0.22 |
|
| |||
| Mitotic Index (n=38) | |||
| 0 | 15 | 1 | |
| 1 | 10 | 3 | |
| ≥2 | 7 | 2 | P=.28 |
Data are presented as frequencies and percentages unless otherwise stated.
Mean tumor diameter in cm ± SD.
Number of patients with biochemical value above normal range.
Combination of biochemical secretion.
Based on histology and anatomic location at the time of the operation or on anatomic imaging studies.
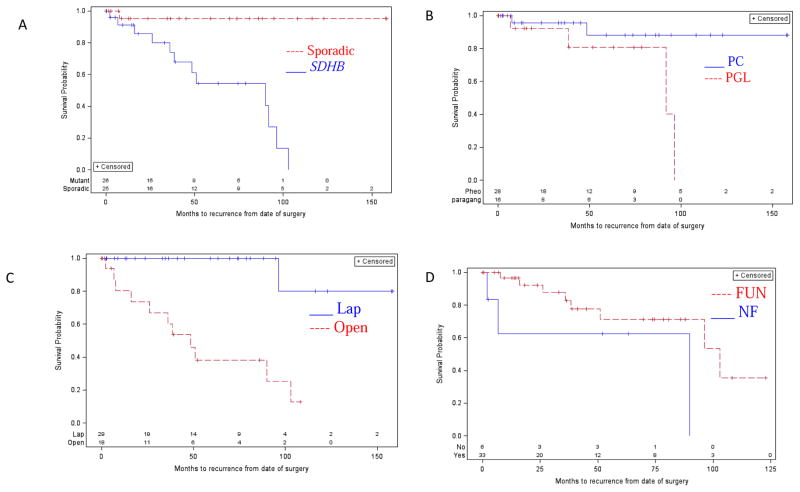
By logistic regression, only SDHB mutation status and tumor diameter were independent risk factors for locoregional recurrence (p=0.05 and p=0.04, respectively) and distant metastases (p=0.004 and p<0.001, respectively). Compared to patients with sporadic disease, patients with SDHB mutation and PC/PGL had a shorter DFI (89.8 months, 95% CI: 36.0 – 96.4 vs. median not reached, p<0.001). Patients with PGL had shorter DFI compared to patients with PC (91.8 months, 95% CI: 38.4 – 96.4 vs. median not reached, p=0.044). Patients who required an open rather than a laparoscopic surgical approach also had a shorter DFI (48.5 months, 95% CI: 16.0 – 103.0 vs. median not reached, p<0.001). There was no significant difference in median DFI between functional and nonfunctional tumors (89.8 months, 95% CI: 2 – 89.8, vs. 103.0 months, 95% CI: 51.0 – undefined, p=0.11) (Figure 2).
Figure 2.
Prognostic factors associated with disease-free interval (DFI). SDHB mutation status, PGL, and requiring an open surgical approach were significantly associated with decreased DFI. (A) SDHB mutation vs. no mutation. Median time of 89.8 months (95% CI: 36.0 – 96.4) vs. median not reached (p<0.001). (B) PGL vs. PC. Median not reached vs. 91.8 months (95% CI: 38.4 – 96.4, p=0.044). C) Open vs. laparoscopic (lap) surgical approach. Median time of 48.5 months (95% CI: 16.0 – 103.0) vs. median not reached (p<0.001). D) Nonfunctional (NF) vs. functional (FUN) tumor. Median time of 89.8 months (95% CI: 2 – 89.8) vs. 103.0 months (95% CI: 51.0 – undefined, p=0.11).
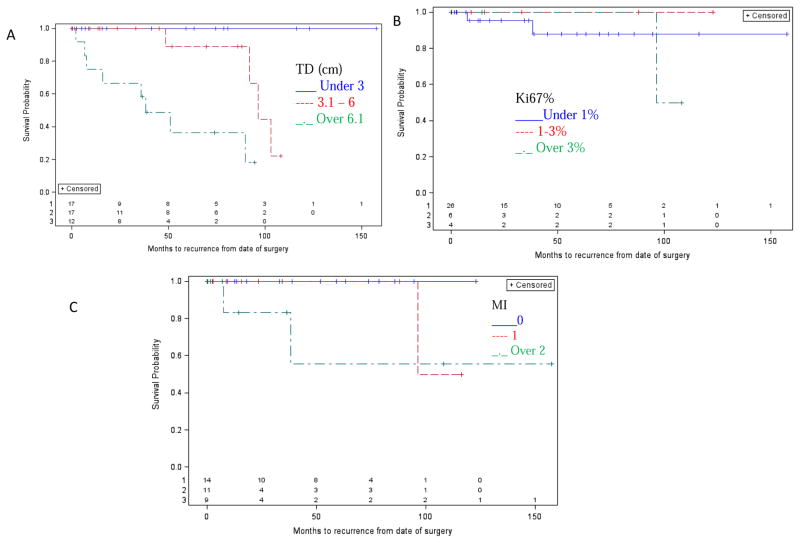
Larger tumor diameter was found to be associated with a shorter DFI (median not reached for < 3.0 cm vs. 96.4 months for 3.1–6.0 cm (95% CI: 48.5 – undefined) vs. 38.4 months for > 6.1 cm (95% CI: 6.8 – undefined, p<0.001). Ki67% and MI were not associated with DFI (p=0.69 and p=0.19, respectively) (Figure 3). In the Cox proportional hazards model analysis, SDHB mutation status and tumor diameter (<6.1 vs. >=6.1 cm) were found to be jointly associated with shorter DFI with a hazard ratio of 16.2 (95% CI: 1.9 – 138.5, p=0.011) and 15.4 (95% CI: 2.6 – 92.2, p=0.003), respectively.
Figure 3.
DFI and clinical and pathologic variables. Tumor diameter was significantly associated with decreased DFI, but not Ki67% or Mitotic Index. (A) Tumor diameter (TD) < 3.0 cm, 3.1 – 6.0, over 6.1 cm. Median not reached, vs. 95.4 months (95% CI: 48.5 – undefined), vs. 38.4 months (95% CI: 6.8 – undefined, p<0.001). (B) Ki67% was not associated with decreased DFI (p=0.69). (C) Mitotic index (MI) was not associated with decreased DFI (p=0.19).
Discussion
In this study, we performed a comprehensive analysis of clinical, biochemical, genetic, treatment, and histologic factors associated with patient outcome. We found that the presence of SDHB and primary tumor size were the only two independent factors jointly associated with shorter DFI. We found other variables were also associated with DFI, and locoregional and distant disease by univariate analysis. Our study findings suggest that a prognostic/staging system for PC and PGL should include the SDHB mutation status and primary tumor size.
Our results demonstrating that SDHB mutation status is associated with younger age at presentation, PGL, local recurrence, distant metastases, and decreased survival are consistent with previous studies.7,16 The association of the primary tumor size with locoregional recurrence and distant metastases is also consistent with other studies.9,17 Our finding that SDHB mutation status and primary tumor size are more predictive of patient outcomes than Ki67% or MI suggests that histologic-based grading systems may not be as useful. We found that Ki67% and MI are not associated with locoregional recurrence or distant metastases, nor were they associated with SDHB mutation status and shorter DFI.
We found an association between SDHB mutation, primary tumor size, requiring an open surgical approach, PGL and shorter DFI. Requiring an open surgical approach is most likely reflective of large, extra-adrenal, and or locally invasive tumors, and was not significant in a multivariate analysis. Although an association between Ki67% staining and metastatic disease has been reported, only a limited number of cases were analyzed.18 The GAPP score which includes Ki67% was based on a series of patients in which only 25% had metastatic disease, and 8% of patients had an SDHB mutations. One limitation of this study is a selection bias inherent in being a referral center for familial and aggressive cases of PC and PGL, which accounts for the large proportion of SDHB mutations, who may have closer postoperative surveillance as compared to patients with sporadic disease. The advantage to this referral pattern was the opportunity to examine a significant number of samples from patients with SDHB mutations and aggressive cancers. Our sample size did not allow for analysis of overall survival and the clinical and pathologic factors or subgroups of PC/PGL such as sporadic PC, SDHB PC, and SDHB PGL. Therefore, we cannot rule out the possibility within one or more of these subgroups there is a significant association between Ki67% or MI and patient outcomes.
In our study, the risk for an SDHB mutation or tumor over 5 cm in diameter to have local recurrence and distant metastasis was extremely high. Given the association of an SDHB mutation and tumor size with significantly higher rates of local recurrence and distant metastasis, any scoring system that does not take these factors into consideration may not accurately predict outcomes and identify high-risk patients who require closer surveillance or adjuvant therapy after surgical treatment for PC/PGL.19
Historically approximately 10% of PCs/PGLs were thought to be familial, but discovery of SDHx, MAX, and other mutations has increased this estimate to as high as 40%, with apparently sporadic cases also bearing somatic mutations when subjected to whole exome or next generation sequencing.20 As more is understood about the genetic basis of PC/PGL, the importance of mutation status on the natural history of PC/PGL, and what diagnostic and localizing methods should be used in such cases of PC/PGL has become clearer.20 For example, tumors in patients with SDHB mutations have unique metabolic and expression profiles.21 Further, diagnostic modalities appropriate for sporadic PC/PGL may not be effective in SDHB PC/PGL.22 Clinical therapies directed at altered pathways in SDHB mutations can also be better applied with knowledge of mutation status and high likelihood of recurrence and metastases.21,23–25 Therefore, the importance of including mutation status as part of a grading/staging system is useful for prognosis as well as guiding therapy.
In summary, our study results support that patients with SDHB mutations and large tumors are at very high risk for recurrence and distant metastases, and these patients should have close postoperative surveillance. Germline mutation status should be tested in all patients with PGL, as well as any young patient presenting with PC/PGL. Knowledge of SDHB status may be necessary to accurately predict outcomes and improve the care of patients with PC and abdominal PGL, and should be part of any clinically relevant prognostic scoring system. Combining SDHB status and primary tumor size may be the most accurate way to create a grading system that can be widely adopted. Future studies are needed to determine if the incorporation of certain pathologic features such as Ki67%, differentiation, or necrosis may further augment the prognostic accuracy of genetic status and primary size. It is possible Ki67% may augment a grading system that includes mutation status and primary tumor size, but histologic data in isolation is not sufficient for a valuable grading/staging system, and in the setting of SDHB mutations, may not be predictive of prognosis.
Acknowledgments
Grant Support: This research was supported by the intramural research programs of the Center for Cancer Research, National Cancer Institute, and Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zarnegar R, Kebebew E, Duh QY, Clark OH. Malignant pheochromocytoma. Surg Oncol Clin N Am. 2006;15:555–71. doi: 10.1016/j.soc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Kebebew E, Duh QY. Benign and malignant pheochromocytoma: diagnosis, treatment, and follow-Up. Surg Oncol Clin N Am. 1998;7:765–89. [PubMed] [Google Scholar]
- 3.Baysal BE, Willett-Brozick JE, Lawrence EC, et al. Prevalence of SDHB, SDHC, and SDHD germline mutations in clinic patients with head and neck paragangliomas. J Med Genet. 2002;39:178–83. doi: 10.1136/jmg.39.3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez JM, Balsalobre M, Ponce JL, et al. Pheochromocytoma in MEN 2A syndrome. Study of 54 patients. World J Surg. 2008;32:2520–6. doi: 10.1007/s00268-008-9734-2. [DOI] [PubMed] [Google Scholar]
- 5.Friedrich CA. Von Hippel-Lindau syndrome. A pleomorphic condition. Cancer. 1999;86:2478–82. [PubMed] [Google Scholar]
- 6.Comino-Mendez I, Gracia-Aznarez FJ, Schiavi F, et al. Exome sequencing identifies MAX mutations as a cause of hereditary pheochromocytoma. Nat Genet. 2011;43:663–7. doi: 10.1038/ng.861. [DOI] [PubMed] [Google Scholar]
- 7.Amar L, Baudin E, Burnichon N, et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J Clin Endocrinol Metab. 2007;92:3822–8. doi: 10.1210/jc.2007-0709. [DOI] [PubMed] [Google Scholar]
- 8.Ellis RJ, Patel D, Prodanov T, et al. Response after surgical resection of metastatic pheochromocytoma and paraganglioma: can postoperative biochemical remission be predicted? Journal of the American College of Surgeons. 2013;217:489–96. doi: 10.1016/j.jamcollsurg.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schovanek J, Martucci V, Wesley R, et al. The size of the primary tumor and age at initial diagnosis are independent predictors of the metastatic behavior and survival of patients with SDHB-related pheochromocytoma and paraganglioma: a retrospective cohort study. BMC Cancer. 2014;14:523. doi: 10.1186/1471-2407-14-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: a clinicopathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551–66. doi: 10.1097/00000478-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Strong VE, Kennedy T, Al-Ahmadie H, et al. Prognostic indicators of malignancy in adrenal pheochromocytomas: clinical, histopathologic, and cell cycle/apoptosis gene expression analysis. Surgery. 2008;143:759–68. doi: 10.1016/j.surg.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Ocal I, Avci A, Cakalagaoglu F, Can H. Lack of correlations among histopathological parameters, Ki-67 proliferation index and prognosis in pheochromocytoma patients. Asian Pac J Cancer Prev. 2014;15:1751–5. doi: 10.7314/apjcp.2014.15.4.1751. [DOI] [PubMed] [Google Scholar]
- 13.Jovanovic R, Kostadinova-Kunovska S, Bogoeva B, Spasevska L, Petrusevska G. Histological features, Ki-67 and Bcl-2 immunohistochemical expression and their correlation with the aggressiveness of pheochromocytomas. Prilozi. 2012;33:23–40. [PubMed] [Google Scholar]
- 14.Carlsen E, Abdullah Z, Kazmi SM, Kousparos G. Pheochromocytomas, PASS, and immunohistochemistry. Horm Metab Res. 2009;41:715–9. doi: 10.1055/s-0029-1238274. [DOI] [PubMed] [Google Scholar]
- 15.Kimura N, Takayanagi R, Takizawa N, et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocrine-related cancer. 2014;21:405–14. doi: 10.1530/ERC-13-0494. [DOI] [PubMed] [Google Scholar]
- 16.Ezzat Abdel-Aziz T, Prete F, Conway G, et al. Phaeochromocytomas and Paragangliomas: A difference in disease behaviour and clinical outcomes. J Surg Oncol. 2015;112:486–91. doi: 10.1002/jso.24030. [DOI] [PubMed] [Google Scholar]
- 17.Press D, Akyuz M, Dural C, et al. Predictors of recurrence in pheochromocytoma. Surgery. 2014;156:1523–7. doi: 10.1016/j.surg.2014.08.044. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 18.Tavangar SM, Shojaee A, Moradi Tabriz H, et al. Immunohistochemical expression of Ki67, c-erbB-2, and c-kit antigens in benign and malignant pheochromocytoma. Pathol Res Pract. 2010;206:305–9. doi: 10.1016/j.prp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Andersen KF, Altaf R, Krarup-Hansen A, et al. Malignant pheochromocytomas and paragangliomas - the importance of a multidisciplinary approach. Cancer Treat Rev. 2011;37:111–9. doi: 10.1016/j.ctrv.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Curras-Freixes M, Inglada-Perez L, Mancikova V, et al. Recommendations for somatic and germline genetic testing of single pheochromocytoma and paraganglioma based on findings from a series of 329 patients. J Med Genet. 2015;52:647–56. doi: 10.1136/jmedgenet-2015-103218. [DOI] [PubMed] [Google Scholar]
- 21.Rao JU, Engelke UF, Sweep FC, et al. Genotype-specific differences in the tumor metabolite profile of pheochromocytoma and paraganglioma using untargeted and targeted metabolomics. J Clin Endocrinol Metab. 2015;100:E214–22. doi: 10.1210/jc.2014-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fonte JS, Robles JF, Chen CC, et al. False-negative (1)(2)(3)I-MIBG SPECT is most commonly found in SDHB-related pheochromocytoma or paraganglioma with high frequency to develop metastatic disease. Endocrine-related cancer. 2012;19:83–93. doi: 10.1530/ERC-11-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matro J, Giubellino A, Pacak K. Current and future therapeutic approaches for metastatic pheochromocytoma and paraganglioma: focus on SDHB tumors. Horm Metab Res. 2013;45:147–53. doi: 10.1055/s-0032-1331211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fliedner SM, Yang C, Thompson E, et al. Potential therapeutic target for malignant paragangliomas: ATP synthase on the surface of paraganglioma cells. Am J Cancer Res. 2015;5:1558–70. [PMC free article] [PubMed] [Google Scholar]
- 25.Janssen I, Blanchet EM, Adams K, et al. Superiority of [68Ga]-DOTATATE PET/CT to Other Functional Imaging Modalities in the Localization of SDHB-Associated Metastatic Pheochromocytoma and Paraganglioma. Clin Cancer Res. 2015;21:3888–95. doi: 10.1158/1078-0432.CCR-14-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]