Abstract
Persistent infection with oncogenic Human Papillomavirus (HPV) is necessary but not sufficient for the development of cervical cancer. The factors promoting persistence as well those triggering carcinogenetic pathways are incompletely understood. Rapidly evolving evidence indicates that the vaginal microbiome (VM) may play a functional role (both protective and harmful) in the acquisition and persistence of HPV, and subsequent development of cervical cancer. The first studies examining the vaginal microbiome and the presence of an HPV infection using next generation sequencing techniques (NGS) identified higher microbial diversity in HPV-positive as opposed to HPV-negative women. Furthermore, there appears to be a temporal relationship between the VM and HPV infection in that specific community state types (CSTs) may be correlated with a higher chance of progression or regression of the infection. Studies describing the VM in women with pre-invasive disease (squamous intra-epithelial neoplasia – SIL) consistently demonstrate a dysbiosis in women with the more severe disease. Although it is plausible that the composition of the VM may influence the host innate immune response, susceptibility to infection and the development of cervical disease, the studies to date do not prove causality. Future studies should explore the causal link between the VM and the clinical outcome in longitudinal samples from existing biobanks.
Introduction
Although cervical cancer is largely preventable through detection and treatment of the pre-invasive precursor, high-grade squamous intra-epithelial lesions (HSIL), it remains the commonest female malignancy in virtually all low-resource countries and the seventh more frequent malignancy in females worldwide 1. It is estimated that, globally, almost 530,000 women develop cervical invasive disease annually and more than 265,000 die from the disease 2. The comparatively low incidence of cervical cancer in affluent societies is largely related to the presence of population–based screening programmes and education that led to a dramatic decrease in the incidence and mortality from the disease 3. Although the HPV vaccine has the potential to dramatically decrease these rates, the introduction into most developing countries has been slow so these changes are not expected to impact cervical cancer rates for at least one to two decades.
HPV and Cervical Carcinogenesis
There is strong evidence that infection with Human Papillomavirus (HPV) is a necessary, but not sufficient for the development of cervical pre-invasive and invasive disease. With more than 200 HPV subtypes recognised today, it is only a fraction of these that has been found to have a carcinogenic potential 4. Of these, subtypes HPV-16 and HPV-18 are most commonly associated with invasive cancers and are thought to cause approximately 65-75% of cases. It is now recognised that it is the persistence of infection by these, and a handful of other high-risk oncogenic HPV subtypes that leads to precancerous lesions.
One of the early observations in most epidemiology studies was the high frequency of HPV DNA detection. The more HPV types tested, not surprisingly the higher frequency of detection. In most studies, but not all, age influenced prevalence with young age being associated with higher rates—some as high as 45% in western societies 5. This vulnerability is thought to be due to immature or naïve immune responses as well as biologic vulnerability of the immature cervical epithelium inherent in adolescents 6. There is some evidence to suggest that the microbiome of epithelium predominantly covered by columnar epithelium as seen in immature cervixes differs to that in epithelium predominantly covered by squamous epithelium 7. It may be reasonable to ask “Does the microbiome of immature squamous epithelia contribute to the vulnerability to HPV infections? “.
Interestingly, lifetime risk of acquiring any HPV infection likely exceeds 80%. With more sensitive testing available, studies show that HPV infection is more commonly the rule, not the exception. HPV types associated with the alpha species predominate in the anogenital area but other HPV types such as the beta and gamma HPV types, once thought to be predominantly cutaneous only, can also be commonly detected 8-10. Since the lifetime risk of developing invasive cervical cancer is much lower at 0.6% 11, cervical cancer should be regarded as a rare complication of a very common infection by the human papillomavirus. The commonness of many of these HPV types, begs the question, “Should non-oncogenic HPV be considered commensal organisms and do they play a protective role against the oncogenic HPV types?”.
Epidemiology studies have shown that several risk factors have been correlated to heightened or reduced risk of cervical cancer. The most consistent factors are tobacco smoking, oral contraceptive use, and parity. All have biologic plausibility. Nicotine and its carcinogenic metabolites can be detected in cervical mucous 12 and smoking has been associated with a dampening of local immune markers 13. Both estrogen and progesterone increase cell proliferation and hence vulnerability to DNA damage 14,15. Higher parity may be associated with high levels of hormone exposure and/or repeated trauma 16. This raises many questions, including “Does smoking, hormonal contraceptives and parity negatively influence microbiota such that HPV carcinogenesis is promoted? “.
Observational data show that the estimated time from the infection to the development of invasive disease is approximately 15 years, although there may be a swift progression in rare cases (Figure 1). Cervical carcinogenesis normally has a lengthy precancerous phase that has been well defined through different grades of SIL, although the continuum of the carcinogenic process has been questioned in some cases 17. Despite these major advances in our current understanding of the disease, the exact factors that determine infection and / or disease that will persist, progress or, conversely, spontaneously resolve are incompletely understood.
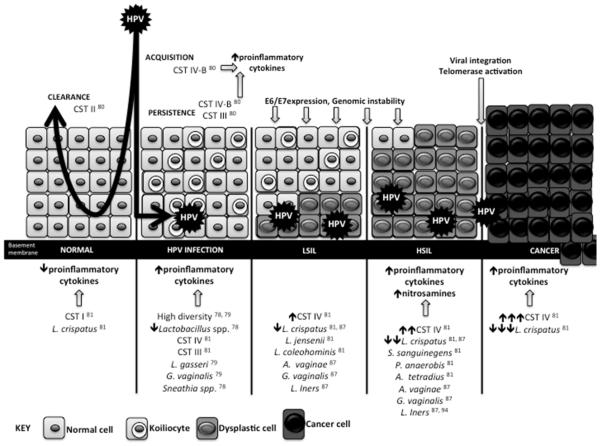
Figure 1. Interactions between the HPV, the vaginal microbiome and the host.
CST I and a high relative abundance of L.crispatus may be protective against HPV acquisition. Longitudinal studies have shown that transition states such as CST III and a BV-like state, CST IV-B, likely lead to pro-inflammatory states which cause tissue damage and promote E6/E7 expression, genomic instability and viral integration which ultimately promotes development of HSIL. Increasing lack of L.crispatus has also been associated with increasing SIL severity, and various other species have been associated with both presence of HPV infection and SIL disease states.
In general, HPV is a nonlytic infection, hence the inflammatory response to HPV is much more subtle than other mucosal infections, such as C. trachomatis. The initial immune response to acute HPV infections is likely mediated by the local innate immune system, probably involving mechanisms such as activation of toll-like receptors and natural killers cells to name a few 18. Persistent infections are likely cleared by the development of adaptive immune responses, which are dependent on antigen–presenting cells. HPV 16 is thought to down regulate both innate and adaptive immune responses. Recent data show that local microbial communities also play significant roles in regulating immune responses 19. Final pathways to cancer result in interference with telomerase activity and viral integration--although a percent of cancers are found to have episomal HPV DNA only 20. HPV E6 and E7 are known oncoproteins, which control fundamental carcinogenic events including proliferation, senescence, and apoptosis. Cellular targets include p53, E6AP, CBP, p300, Bak, hTERT, MAGUK, cIAP, survivin, p107, pRB, p130 21. One of the important questions is “Whether the microbiome can also manipulate these genes in addition to HPV’s influences or whether the observed changes are actually due to microbiome dysbiosis induced by HPV?”.
More recently, emerging data support the notion that the complex interactions of the host with the commensal bacteria in the vagina (vaginal microbiome, VM) may be involved in the natural history of the disease. This review aims to provide a comprehensive summary of the existing evidence describing the interplay between the host, HPV and the bacteria in the vaginal microbiome.
The vaginal microbiome
Much of the work to date on mucosal microbiota has been focused on the gut including its influence on immune function, behaviour as well as local and systemic inflammatory diseases 22-24. The Human Microbiome Project has extensively examined the vaginal microbiota although in contrast to the gut, less is understood about the role of the vaginal microbiome (VM) in human disease. A detailed description of the vaginal microbiome is outside the scope of this article and can be found in several review articles 25,26. In the following, we highlight certain aspects of the VM that are pertinent to our topic.
As any of the mucosal compartments, there are likely to be differences between specific anatomic sites within the vagina. There has been attempts at examining some of the vaginal compartments (cervix vs proximal vagina vs distal vagina), however, other sites remain not well characterized including ectocervical and endocervical or microbacteria associated with immature and mature cervical epithelium. Interpretation of these studies is also difficult because of the close proximity of these sites where contamination can occur during sampling. Defining “healthy” microbiota is challenging since community clustering seems to be a moving target with variability through the women’s menstrual cycle as well her reproductive age.
The healthy, premenopausal vaginal bacterial communities are usually populated by Lactobacillus spp. that are commonly regarded to ensure a low pH 27, which is thought to provide the first-line of defence against pathogenic agents. Additionally, these bacteria are able to produce numerous other protective peptides and metabolites capable of inhibiting bacterial growth, adhesion and through disruption of biofilms. All Lactobacillus, however, are not necessarily stable or “healthy”. L. iners is present in all women including those with “dysbiosis” whereas L. crispatus is mostly seen in ‘healthy” women. For example, in one study, a predominance of L. iners predicted the development of bacterial vaginosis (BV), one of the best-studied vaginal “dysbiosis” 28-32. In comparison, L. crispatus predominance appears protective against the development of BV 33.
Ravel et al. were the first to classify the VM according to structure, using more recent next generations sequencing platforms (NGS) 34. They studied 396 healthy women and identified 282 taxa in total, proving the VM is far more complicated that previously appreciated. Based on the presence of a particular Lactobacillus species, or their absence they assigned 5 different community state types (CSTs); CST I, II, III and V are dominated by Lactobacillus crispatus, L. gasseri, L. iners and L. jensenii respectively, and CST IV (bacterial vaginosis associated bacteria) conversely, is a heterogeneous group typified by depletion of Lactobacillus spp. with presence of strictly anaerobic species such as Gardnerella, Megasphera, Sneathia and Prevotella. Longitudinal studies show that within-subject variation is lower over time than those between-subjects in the vagina, which also exhibits greater longitudinal stability that most other body compartments 35. The majority of women have a relatively stable VM with a relatively low diversity in comparison to other mucosal sites, and it is those with the highest diversity VM in whom we observe the greatest instability (i.e moving from one state to another) 36.
Bacterial vaginosis (CST IV) is an enigmatic disorder characterized by increased species diversity. Prevalence of the condition is high ranging from 12% in Australian women 37, up to 50% in sub-Saharan African women 38, making it the most prevalent vaginal disorder of women of reproductive-age. This has important public health implications, because BV has been associated with serious and significant reproductive morbidity including pelvic inflammatory disease 39, miscarriage 40 and may increase the risk of pre-term birth between 2 and 4-fold 40. Furthermore, BV is associated with increased rates of bacterial sexually-transmitted infections (STIs) 41 and human immunodeficiency virus (HIV) transmission 42, which demonstrates how important it is to understand the interplay between the microbes and potential pathogens.
Interestingly the gut does appear to be a reservoir for many of the VM species (both healthy and pathogenic) but clearly the vagina is protected from colonization of the majority of gut species. As mentioned, the overall diversity of the VM is several-fold less than the gut. In contrast the diversity of Lactobacillus spp. is much higher in the vagina. Certain bacterial species are also shared with the mouth such as G. vaginalis 43. Many of the BV associated bacteria can be found in the rectum. In one study, presence of Megasphaera and Sneathia spp. in the rectum was predictive of clinical BV in the women during follow-up 44.
Longitudinal studies of the VM indicate that bacterial community structure is dynamic and hormonally influenced with a propensity to become less stable during menstruation 36 and conversely more stable and less diverse during normal pregnancy 45,46. The vaginal microbiota are also likely affected by numerous exogenous factors, although most of these have not been well studied specifically in longitudinal samples. Of particular interest are hormonal contraceptives and cigarette smoking since both have been associated with the development of cervical cancer 47,48. Combined oral contraceptive use is associated with increased level of inflammatory cytokines in the cervix 49. The source of these cytokines is unknown however it is plausible that the microbiota influence this inflammatory environment as is found in the gut 50. Acute inflammation may be protective against acquisition of STIs including HPV, however, chronic exposure to inflammation is toxic to cells resulting in DNA damage and potentially carcinogenic changes 51.
Although many studies found conflicting data, in general, oral contraception (OC) use is associated with decreased risk of BV 52. The few studies available using NGS continue to show a protective effect in that hormonal contraceptives are associated with Lactobacillus species dominated microbiomes 53-55. Most of these studies were conducted over short periods of time, so the long-term effect of OCs on the VM and immune cells remains unknown. Studies on other methods such as the intrauterine device (IUD) and medroxyprogesterone acetate (MPA) are rare and show no major impact 52. One study by Mitchell et al. 56 showed that MPA users had a decline in vaginal H2O2 producing lactobacilli but did not have an increased risk of developing BV.
Tobacco use and VM has also not been well studied. Certainly, tobacco smoking has been associated with altered diversity in the gut, oral cavity and respiratory tract 57,58. Bradshaw et al. 59 showed that BV (using Nugent Score) was increased in women who smoked more than 30 cigarettes per week. Brotman et al. 60 have further used NGS to show that in a small cross-sectional cohort of 20 smokers and 20 non-smokers, smokers had a significantly higher prevalence of CST IV (50% smokers vs 15% non-smokers), and identified Peptostreptococcus and Veillonella as genera most significantly associated with smoking. Further studies are required to ascertain whether smoking does indeed have a causal relationship with VM alteration and dysbiosis.
Vaginal microbiome, HPV and immune response
There are no good direct data that show how altered VM influences local immune function. However, it is plausible that the stability and composition of the vaginal microbiome may play an important role in determining host innate immune response and susceptibility to infection as well as playing a role further downstream regarding the development of cervical disease.
Several studies have shown that BV and BV-associated bacteria effect immune parameters within the vagina including cytokines/chemokines, antimicrobial proteins and immune cell populations 61. BV, as defined by Nugent’s has shown relatively consistently higher levels of IL-8 and IL-2 beta 62. In vitro studies of individual bacteria show that several are capable of inducing proinflammatory responses. For example, Atopobium vaginae, a BV associated bacteria activates the proinflammatory transcription factor nuclear factor (NF)-κB, tumor necrosis factor (TNF) alpha, interleukin (IL)-6 and IL-8, macrophage inflammatory protein (MIP) 3 alpha and Regulated on Activation, Normal T Expressed and Secreted (RANTES) 63,64. Similarly, the BV associated bacteria G. vaginalis, P. bivia, M. mulieris, S. amnii and S. sanguinegens have also been shown to induce similar in vitro cytokine and chemokine profiles 65-67. Clinical studies also show that microbiomes with a predominance of BV-associated bacteria and increased diversity have similar proinflammatory profiles with elevated IL-8, IL-1α, IL-1β, INF gamma, TNF-α and granulocyte-macrophage colony-stimulating factor (GM-CSF) compared to women normal flora 66,67. Clinical studies have also demonstrated immunosuppressive effects with lower levels of INF gamma-induced protein 10 and soluble leukocyte protease inhibitor (SLPI) in women with BV 68,69. In comparison, certain bacteria, primarily a predominance of specific Lactobacillus species, such as L. crispatus, L. jensenii, and L. gasseri, is associated with a relatively non-inflammatory state in the cervicovaginal environment. These pro-inflammatory states result in tissue damage possibly enhancing HPV’s oncogenic potential. Despite DNA damage, expression of E6 and E7 result in inhibition of apoptosis and enhance cellular proliferation increasing aneuploidy and chromatin abnormalities leading to the development of cervical dysplasia and cancer. As mentioned, Lactobacillus spp. provides many protective substances of which many may be relevant to HPV. In vitro studies show that Lactobacillus spp. exerts cytotoxic effects on cervical tumor cells 70. Human normal fibroblast-like cervical (HNCF) and HeLa cervical cancer cell lines were treated with L.crispatus and L.gasseri, which inhibited cell proliferation and induced cell death to a significantly greater degree in the cancer cell line. This indicates probable interactions amongst cervical cells, the microbiota and metabolites 70.
BV has also been shown to effect immune cell populations within the vaginal mucosa. Findings are not consistent with evidence of suppression as well as enhancement of leukocytes (reviewed in 61. Conflicting data is likely due to the fact that most of these studies used the Nugent’s criteria rather than NGS for defining “dysbiosis”.
Of course, there are likely genetic and hormonal factors that play a role in host response to vaginal pathogens. These include cytokine and chemokine polymorphisms and endogenous and exogenous synthetic hormones making these interactions complex and poorly understood 71,72. In addition, cervical epithelial type (columnar, metaplastic and squamous) also influence immune response. In one study women with large areas of cervical columnar epithelium typical of pubertal cervixes, had much higher levels of proinflammmatory cytokines 73.
HPV infection and the vaginal microbiome
Several pieces of evidence suggest that HPV is affected by the vaginal microbiome. In meta-analyses of mostly cross-sectional studies, the presence of BV was associated with higher rates of HPV infection (12 studies; odds ratio (OR) 1.43, 95%CI 1.11 to 1.84) 74 suggesting that a diverse, Lactobacillus-depleted microbiome may contribute to HPV persistence. There is also evidence to suggest that persistence is more likely in those with altered microbiome. In one study, women with persistent high risk (hr) HPV had a prevalence of BV of 11% compared to only 5% in those women who cleared their hrHPV 75. Similarly, King et al. 76 found that women diagnosed with BV had delayed clearance of HPV (adjusted Hazard ratio (aHR)=0.84, 95%CI: 0.72, 0.97). The major limitation of these studies was that all used the crude measure of BV using Nugent’s criteria, which is highly subjective 77.
One of the first studies to examine the vaginal microbiome and HPV using NGS was conducted in Korea and used cervico-vaginal samples collected as part of the Healthy Twin Study within Korean Genome Epidemiology Study of 912 women 78. A total of 68 women were analysed of which 23 were HPV-positive and 45 HPV-negative. The analysis of 45 premenopausal women with or without HPV infection found that Fusobacteria, particularly Sneathia spp. could be used as microbiological markers of HPV infection. HPV-positive women exhibited higher microbial diversity with a lower proportion of Lactobacillus spp. than HPV-negative women, while there was reduced abundance of L. iners in HPV infection positive versus negative women amongst 9 pairs of HPV-discordant monozygotic twins (P = 0.03) (Table 1, Figure 1).
Table 1.
Characteristics of studies exploring the association of HPV infection and cervical pre-invasive and invasive cervical disease to the vaginal microbiome using next generation sequence techniques.
| Study | Population | Cervical Sample | NGS technique | Key Findings |
|---|---|---|---|---|
| HPV infection | ||||
| Gao 2013 | 70 healthy women with normal cervical cytology -32 HPV-negative - 38 HPV-positive, infected with a single high-risk HPV subtype |
Sterile swab from near the vaginal fornix and Cervix; HPV DNA test: primers MY09/MY11 and GP5+/GP6+ and Hybrid Capture |
Nested PCR of 16S rRNA and PCR-denaturing gradient gel electrophoresis; plasmid cloning and sequence identification via NCBI BLAST database |
-Increased diversity in HPV-positive (mean=1.64; range=0-3.09) compared to HPV-negative women (mean=0.93; range=0-2.62) (p <0.001) - Lactobacillus: the most predominant genus, detected in all women -L. gasseri & G. vaginalis: isolated more in HPV- positive than HPV-negative women (p=0.005 and p=0.031, respectively) |
| Lee 2013 | 68 HPV-infected or uninfected female twins and their families - 9 HPV infection- discordant MZ twin pairs without CIN (N = 18) - pre-menopausal women with or without HPV infection (N=45) |
Cervical liquid-based cytology samples: ThinPre and Surepath™); HPV DNA test: primers MY09/MY11 and GP5+/GP6+, PCR amplicons of 450 and 150 bp and HPV typing (high vs. low risk); QIIME |
Genomic DNA extraction: DNA/RNA kit (Chemagen, Baesweiler, Germany); V2 and V3 regions of the 16S rRNA genes; 454 Life Sciences FLX Titanium machine (Roche, Indianapolis, IN, USA) |
-HPV-positive women higher microbial diversity with a lower proportion of Lactobacillus spp. than HPV-negative women -Fusobacteria, including Sneathia spp., possible microbiological marker associated with HPV infection -HPV-discordant MZ twins: reduced abundance of L. iners in HPV infection positive vs negative (P = 0.03) |
| Brotman 2014 | 32 women of reproductive age over 16 weeks twice weekly samples (937 microbiome samples - 930 HPV result) - 5 HPV negative throughout - 2 positive for 1 HPV subtype - 25 were positive for 2 or more HPV subtypes |
Cervical samples: mid- vagina self-samples; HPV DNA test: Roche Linear Array HPV Genotyping Test (6, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, or 68) |
V1-V2 hypervariable regions of 16S rRNA genes using primers barcoded 27F and 338R |
-CST was associated with remission of HPV (P=.008), but not with new detection of HPV (P=.10) -Low Lactobacillus CST IV-A higher transition to HPV positivity compared to CST I (aTRR: 1.86, 95%CI .52–6.74) -L. gasseri (CST II) had the fastest HPV remission and low Lactobacillus community with high proportions of the genera Atopobium (CST IV-B) had the slowest rate compared to L. crispatus (CSTI) (aTRR: 4.43, 95%CI 1.11–17.7; aTRR: 0.33, 95%CI .12–1.19, respectively) |
| SIL and ICC | ||||
| Mitra 2015 | 169 women: - normal (n=20) |
Sample posterior fornix: BBLTM CultureSwabTM containing liquid Amies (Becton Dickinson, Oxford, UK); HPV DNA test: Abbott RealTime HR HPV assay (Abbott M2000 platform) |
Whole-Genomic bacterial DNA extraction: QiAmp Mini DNA kit (Qiagen, Venlo, Netherlands); V1-V2 hypervariable regions of 16S rRNA genes; llumina MiSeq SOP Pipeline |
- Higher rates of CST IV (L.depleted, high diversity) with increasing disease severity (Normal=10%; LSIL=21%; HSIL=27%; ICC=40%). - Lower rates of CST I (L. crispatus-dominant) with increasing disease severity (Normal=50%; LSIL=42%; HSIL=40%; ICC=20%) - HSIL vs LSIL: higher levels Sneathia sanguinegens (P < 0.01), Anaerococcus tetradius (P < 0.05), Peptostreptococcus anaerobius (P<0.05) - lower levels L. jensenii (P<0.01). |
| Oh 2015 | Cases with CIN: -CIN 1 (n=55) -CIN 2 or 3 (n=15) Controls: -Normal cytology (n=25) -ASCUS (n=25) |
Cervical Sampler Brush (Digene Co. Gaithersburg, MD, USA); HPV DNA detection: Digene Hybrid capture II DNA Test (Qiagen, Gaithersburg, MD, USA) |
DNA extraction: Fast DNA SPIN extraction kits (MP Biomedicals, Santa Ana, CA, USA); V1–V3 regions of the 16S rRNA gene; a Roche/454 GS Junior system (Roche, Branford, CT, USA); excluded low- quality reads: average quality score <25 or a read length <300 bp; available in the EMBL SRA database |
The CIN risk was higher for the higher vs the lower tertile of: - predominance of A. vaginae, Gardnerella vaginalis, L. iners with a minority of L. crispatus: OR 5.80, 95%CI 1.73‒19.4) -A. vaginae: OR 6.63, 95%CI 1.61–27.2). -Risky microbial pattern and oncogenic HPV:OR 34.1, 95% CI 4.95–284.5 (synergistic effect). |
| Piyathilake 2016 | Cases: - CIN2 (n=208) - CIN3 (n=132) Non-cases: - CIN1 (n=90) All were HR-HPV positive |
Cervical mucus samples: Merocel ophthalmic sponges (Medtronic Xomed, Inc., Jacksonville, FL); HPV DNA test 13 subtypes: Roche Diagnostics Linear Array; QIIME suite and RDP database |
DNA extraction: Fecal DNA isolation kit from Zymo Research; V4 segment of the 16S rDNA gene; Illumina MiSeq; |
-CIN2+ higher in community types dominated by L. iners and unclassified Lactobacillus spp. vs those diverse taxa unclassified L., L. iners, Bifidobacteriaceae, Clostridiales, Allobaculum (OR=3.48, 95% CI: 1.27-9.55). -Women with CIN2+ enriched Lactobacillaceae, Lactobacillus, L. reuteri and several sub-genus level Lactobacillus OTUs (effect size>2.0; p< 0.05) - DNA oxidative damage does not mediate the effect of VM on natural history of HPV. |
aTRR: adjusted transition rate ratio; A. vaginae: Atopobium vaginae; CI: confidence interval; CIN: cervical intra-epithelial neoplasia; HPV: human papillomavirus; HR-HPV: high-risk HPV; HSIL: high-grade squamous intraepithelial lesion; ICC: invasive cervical cancer; L: Lactobacillus; LSIL: low-grade squamous intraepithelial lesion; MZ: monozygotic twins; NSG: next generation sequencing; OR: odds ratio; OTUs: operational taxonomic units; SIL: squamous intraepithelial lesion; VM: vaginal microbiome
Using culture independent PCR-denaturing gradient gel electrophoresis, Gao et al. 79 examined 70 healthy women (32 HPV negative and 38 HPV positive) with normal cervical cytology. Their group found that HPV positive women had greater biological diversity using the Shannon-Weiner diversity index. When they examined specific species, L. gasseri and G. vaginalis were significantly higher in HPV infected women.
Both of these studies are cross-sectional and unable to determine whether HPV induces a change in the VM or that the VM influences persistence of HPV. Brotman et al. 80 examined the temporal relationship between the vaginal microbiome and HPV infection. Thirty-two women were to collect serial two-weekly self-samples of vaginal secretions over the course of 16 weeks. The authors identified a significant association of the CST to the chance of remission (P=.008), but this did not seem to affect detection of new HPV infections (P = .10). They were also able to examine the impact of CST on transition between HPV-negative and – positive states using the adjusted transition rate ratio (aTRR), which is equivalent to hazard rate ratio, and adjusted for normalized menstrual cycle time. Lactobacillus depleted CST IV-A when compared to L. crispatus-dominant CST I had higher transition rates to HPV positivity state, although the differences were not significant (aTRR: 1.86, 95% confidence interval (CI) 0.52–6.74). When compared to CST I, L. gasseri-dominant CST II was correlated with the fastest HPV regression, while CST IV-B was associated with the slowest (aTRR, 4.43, 95% CI, 1.11–17.70 and aTRR, 0.33, 95% CI, .12–1.19, respectively) (Table 1, Figure 1).
Pre-invasive and Invasive Cervical Cancer and the vagina microbiome
More recently, we published the first study describing the VM in 169 women with biopsy-proven cervical pre-invasive and invasive disease and compared them to healthy HPV negative controls 81. We found that the rate of Lactobacillus-depleted high diversity microbiome (CST IV) was increased two-fold in women with low-grade squamous intraepithelial neoplasia (LSIL), three-fold in women with high-grade squamous intraepithelial neoplasia (HSIL) and four-fold in women with invasive disease (normal = 2/20, 10%; LSIL = 11/52, 21%; HSIL = 25/92, 27%; cancer=2/5, 40%, p=0.06). There was a trend association between Lactobacillus crispatus-dominant VM (CST I) and increasing disease severity (normal=10/20, 50%; LSIL=22/52, 42%; HSIL=37/92, 40%; cancer=1/5, 20%, p=0.30) suggesting that this may be a microbiome community protective against the development of precancerous and cancerous lesions. Furthermore, Peptostreptococcus anaerobius and Anaerococcus tetradius were found to be more common in women with HSIL opposed to LSIL disease. That was also noted for Fusobacteria-primarily Sneathia sanguinegens (P < 0.01). These could be used as microbiological markers of clinically significant disease (Table 1, Figure 1). Although this was the first study to describe the VM in women with cervical disease, one of the limitations was the lack of adjustment for risk factors and possible confounders amongst the compared groups. Given the previously inconsistent evidence surrounding an association between BV and SIL/cervical cancer, there is a paucity of studies examining the potential bacterial-induced mechanisms of neoplasia in the cervix. Dysbiosis has been implicated in the carcinogenic pathways in the colorectal mucosa, and members of the Peptostreptococcus and Fusobacteria genera have been implicated not only in the pathogenesis of such a cancer 82,83, but the latter also associated with poorer prognosis 84. Aberrant WNT pathway signaling is implicated in oncogenesis, and Fusobacterium nucleatum expresses a cell surface virulence factor; FadA that is capable of activating the WNT pathway in one of the steps in colorectal carcinogenesis 85. Furthermore F.nucleatum appears capable of downregulating the T-cell-mediating anti-tumor response, with CD3-cell density inversely proportional to F.nucleatum DNA levels in human colon tissues 86.
Another report published subsequently by Oh et al. 87 included women with LSIL or HSIL on cytology vs normal controls (defined as normal or Atypical Squamous Cells of Undetermined significance (ASCUS) cytology). The results suggested that microbiome patterns determined by paucity of L. crispatus and occupied predominantly by A. vaginae and secondarily by G. vaginalis and L. iners were associated by an almost 6-fold increase in the risk of cervical LSIL/HSIL disease (higher vs lower tertile, Odds Ratio (OR) 5.80, 95%CI 1.73–19.4), and thus the authors defined this as a ‘risky microbial pattern’, and women were subsequently defined as being in the high, medium or low tertile according to relative abundance of the aforementioned species. The risk of SIL of women that were HPV positive and had a high-risk microbial pattern was significantly higher than HPV negative women with a low risk microbial score (OR 34.1, 95% CI 4.95–284.5). The findings were limited as there was no adjustment for risk factors, while the defined comparison groups merged different grades of disease severity. The authors compared women with both low- and high-grade disease to controls that also included the presence of ASCUS cytology, women that may harbor underlying high-grade disease 88 (Table 1, Figure 1). In spite of this, the study highlights the likely protective qualities of certain Lactobacillus species and the probable disease-driving potential of some CST IV-associated species. There are several proposed mechanisms through which Lactobacillus may afford protection beyond simple inhibition of CST IV-associated anaerobic growth. CST IV is associated with higher levels of amine production compared to the Lactobacillus dominant CST’s 89, and these biological amines are not only responsible for the characteristic malodourous discharge 90, but also result in nitrosamine production 91. These nitrosamines, also produced by tobacco smoking, are known carcinogens 92, and most interestingly, certain species of Lactobacillus are known to neutralize these carcinogens in vivo 93. It is plausible that Lactobacillus spp. not only prevent colonization of high amine-producing bacterial species, but they may also mop up these potentially carcinogenic amine derivatives, providing an additional layer of anti-carcinogenic protection. Most notably, this study by Oh et al. 87 demonstrates that L. crispatus appears most protective, whereas L.iners in contrast seems most commonly associated with disease, rather than health. This finding underscores other studies that show not all Lactobacillus species are equally protective. The differences in genetic and metabolic properties of the different Lactobacilli requires further investigation.
A more recent report by Piyathilake et al. 94 compared well-defined cytological groups of women with HSIL (n=340) versus LSIL (n=90); all women were high-risk HPV positive. The authors did not used the previously described CST’s to classify patients according to VM structure, but used the Dirichlet multinomial mixture model to partition samples into 4 different metacommunities (Partition 1-4). Bacterial communities of predominantly L. iners and unclassified Lactobacillus spp. (Partition 3) had higher HSIL+ levels as compared to those with diverse taxa unclassified Lactobacillus, L. iners, Bifidobacteriaceae, Clostridiales, Allobaculum (Partition 1) (OR=3.48, 95% CI: 1.27-9.55) when adjusted for other risk factors for HSIL. The samples of women with HSIL were particularly enriched with Lactobacillaceae, Lactobacillus, L. reuteri and several sub-genus level Lactobacillus operational taxanomic units (OTUs) (effect size>2.0; p< 0.05) (Table 1, Figure 1). These observations in women with HSIL in particular, are somewhat contradictory to what has been shown by the two aforementioned studies, as Partition 4 (CST IV/BV-like VM) was not associated with HSIL or worse. We suggest this may arise as a result of the ethnic differences between the cohorts, as ethnicity is a significant differentiating factor in microbiome composition 34. However, the observation that L.iners is associated with HSIL is interesting. As previously discussed, L.iners dominant VM’s are most likely to transition to CST IV, which Mitra et al. 81 associated with HSIL. Additionally L.iners has been implicated in depletion of reduced glutathione, unlike L.crispatus and L.jensenii, which are both seen to be associated with increased levels of reduced glutathione 95, indicating L.iners colonisation results in higher levels of oxidative stress, which again fits with the former hypothesis of Piyathilake et al. The authors also explored whether oxidative DNA damage associated with different microbiota influences the natural history of HPV persistence and cervical carcinogenesis. 8-hydroxy-2' - deoxyguanosine (8-OHdG) is a well characterized biomarker of oxidative stress-induced DNA damage, which has previously been shown to be elevated in SIL compared to healthy controls 96. Whilst oxidative stress has been implicated in the carcinogenic mechanisms of microbial-induced gastric cancers 97, the authors of this study did not find a significant correlation between 8-OHdG levels, SIL status, and the VM. BV-associated oxidative stress 98 may result in the generation of reactive oxygen species (ROS) that then create double-stranded DNA breaks in the host genome, as well as the HPV episome, facilitating HPV integration and ultimately neoplastic transformation, a mechanism also employed by the HPV E6 oncoprotein 99. It is noted the viral integration results in loss of E1 and E2 genes, which control E6 and E7 transcription. Consequently, transcription of these oncoproteins goes unchecked after viral integration leading to increased cellular proliferation, and decrease apoptosis 100.
Barriers and Limitations of the existing literature
Rapidly evolving evidence suggests the importance of the interactions between the human host, the innate immunity, the microbiome and virome in the genital tract with health and disease. Despite variations amongst the studies, the results consistently demonstrate differences in the microbiota noted in women with cervical disease that appear to correlate with the severity of the disease. Lactobacilli produce hydrogen peroxide and preserve an acid protective environment in the vagina with low pH. L. crispatus produces more lactic acid as compared to L.iners and L.iners is associated with increased risk of transiting from normal to abnormal CSTs 33. Studies consistently demonstrated lower rates of CSTI (L.crispatus) in women cervical disease and higher rates of L.iners 87 or dysbiosis 81. The reported differences across studies may be attributed to different sample collection and analysis techniques, different ethnicity, diet and genetic factors together with temporal shifts throughout the menstrual cycle and use of contraception (Table 1).
The published studies are also limited in interpretation since samples examined are in the vast majority obtained from the vagina and not the cervix. The cervical microbiota have been shown to be similar to vaginal but with lower bacterial loads 101. Interestingly, cervicitis was associated with changes in the microbiota at the cervical level only whereas in BV, bacterial changes are seen in both cervix and vagina. This finding may explain why BV is associated with premature delivery, which is likely an effect on the cervical integrity 102. Also, gut studies have shown that examining the microbiota from washes are quite different than those from biopsy samples. Washes or swabs often used in VM studies may not get at biofilms, which are very adherent to cells. One hypothesis regarding the development of dysbiosis is that certain bacteria create biofilms (ie. cohesive bacteria). In doing so, they create a scaffolding for other bacteria. When bacteria are dispersed (ie. not cohesive), there appears to be overall a lower bacterial load 103,104. It may be also that viruses may also co-exist favorably in these biofilms, which inhibit penetration of natural antimicrobial compounds, and possibly anti-viral compounds 105. In addition, most studies do not observe the 3 dimensional relationships of the microbiome, which are likely representative of functional communities which one bacteria or virus may live off of others, making these relationships even more complex 106.
Approximately one third of the high-grade premalignant lesions go on to develop invasive cervical disease, if not treated. It is plausible that women with Lactobacillus-depleted CST IV or Lactobacillus iners-dominant microbiomes are those whose lesions are likely to persist and to progress to clinically significant pre-invasive or invasive lesions. However, it is important to note that the findings of the studies to date only demonstrate a possible association between cervical precancer, persistent HPV infection and the synthesis of the vaginal microbiota; they do not prove causality. It may be that the presence of an ‘unhealthy’ Lactobacillus spp.-depleted microbiome renders some women more susceptible to HPV persistence and the development of CIN and cancer. This idea is also supported by studies suggesting that women with BV have much higher rates of sexually transmitted disease, including HPV 41,74. Conversely, it may be that HPV infection has an impact on the host’s immune defences and the mucosal metabolism with an adverse effect on the community structure of the vaginal microbiome. The infection of the basal membrane of the mucosal surfaces by HPV initiates a cascade of mediated mechanism related to inflammation, activation of the mucosal immunity with pro-inflammatory cytokines, interferons, activation of macrophages and NK cells and the integration of the viral DNA. All these inflammatory processes and changes in the immune and mucosal environment may in turn impact on the vaginal microbiome 107-111. Interestingly, this similar increase in diversity and low lactobacilli has also been associated with HIV acquisition 112. Two studies found that incident HPV was associated with HIV seroconversion 113,114. It is plausible that the mechanism may be that HPV results in a microbiota change vulnerable to HIV acquisition.
Future directions
There appears to be a complex relationship between the host and the VM and composition of the VM may play a role in host susceptibility to HPV infection, its persistence and subsequent development of dysplastic and ultimately neoplastic lesions. The evaluation of all these interactions can be challenging, as the VM shows significant intra-individual variability, while different HPV subtypes that may be present at a given time may follow a different independent course, and these shifts in variability can be rapid. The clinical correlation of these periods of variability remain unknown, therefore it remains essential to include functional assays of pathogenesis such as proteomics, metabolomics and peptidomes. Cancer conversely, is a slow-growing disease and thus future longitudinal studies must be very precisely designed in order to determine a causal link, and this is reviewed by Thomas et al. 115. Further mechanistic studies are now required to gain a deeper understanding of this relationship with a view to development of future therapeutic strategies. HPV infection is cleared from the body by a predominantly innate immune response, which remains incompletely understood 18. It is entirely plausible that the VM is able to signal through the cells of the innate immune system, which reside in the cervix, altering this immune response rendering an individual more susceptible to HPV infection, and negatively affecting a subsequent response to clear the viral infection--this interaction requires closer investigation. Furthermore, the cervical epithelial surfaces are known to secrete small antimicrobial peptides, which are often referred to as the ‘natural antibiotics’, and several of these have potent antiviral activity 116. Presence or absence of particular bacterial species are certainly capable of modulating host cell activity, and thus expression, production and activity of these proteins could also be affected by VM composition. The impact of the VM on host cell function likely arise due to changes in the metabolic environment, due to the production and utilization of numerous compounds by the VM, which can result in suppression or activation of cell signaling and metabolic pathways. Metabonomic studies using analytical chemistry techniques such as nuclear magnetic resonance (NMR) and mass spectroscopy (MS) are ideal methods for identification and quantification of the changes in the metabolic milieu to study the association of VM composition and health and disease states 117.
Many studies of the VM focus on the gross structure of the VM, however it is possible that particular species are more heavily involved in disease initiation and progression than others as suggested by both Mitra et al. 81 and Oh et al. 87. It is widely accepted that not all strains of a particular species are pathogenic, and Escherichia coli is a well-established example of this, with strains such as O157:H7 causing enterohaemorrhagic colitis due to the toxin-coding genes in their genome 118, whereas other strains do not contain these genes, and do not cause significant disease. Therefore in the case of the VM, it may be the case that only certain strains of L.iners predispose to HPV acquisition and persistence, or conversely only certain L.crispatus strains that are protective. Metagenomics is an exciting new field that can be used to study the impact of strains, and identify particular bacterial genes that are associated with cervical pathogenesis.
To add another dimension to the picture, we must also consider the impact that the virome may play in this interaction. Although we are well aware that HPV causes these dysplastic and cancerous lesions of the cervix, whole genome shotgun sequencing has been used to show that there are many other types of virus in the human vagina 119. It is conceivable that these viruses also play a role in the dynamics of the cervicovaginal environment and should also be incorporated into longitudinal studies..
Taking this information forward to the bedside, there are several avenues that could be pursued for translational application. Firstly, if a particular bacterial species or strain is found to be implicated in disease it may be possible to develop rapid bedside tests, using either microchip array or metabonomic technologies for identification of patients at highest risk, which could be used to triage those patients in need of more intense observation or treatment. Secondly, it is possible to manipulate the vaginal microbiome using probiotics. This has been successfully demonstrated as a way of both treating BV, and reducing recurrence 120. There are currently numerous commercially available probiotics, however further studies are required in order to determine exactly which preparation is the most effective and protective with regards to clearing HPV and preventing cervical dysplasia and neoplastic transformation. At present there are a lack of medical therapies for treatment of HPV infection and SIL and the current gold standard treatment is surgical ablation or excision of the dysplastic area of the cervix, which although very effective for reducing the risk of future invasive cancer, is associated with significant obstetric morbidity 121,122 as well as neonatal morbidity and mortality 123. Such a development would be a major breakthrough in the field of gynaecological oncology, particularly in developing countries where the HPV vaccine is infrequently available and gynaecologic procedures unavailable, and even in the numerous developed countries where vaccine uptake is poor.
Conclusion
There is a wealth of emerging evidence to suggest that the cervico-vaginal bacterial population plays a substantial role in the persistence of the virus and the presence of subsequent cervical pre-invasive disease. The role of the microbiome in other HPV-related cancers such as vulvar, anal and oropharyngeal cancers has not yet been explored but likely play important roles. Future studies assessing the impact of the vaginal microbiome in cervical carcinogenesis should explore the causal link in longitudinal samples from existing biobanks that will allow correlation of the microbiome to the clinical outcome and in particular progression or regression of HPV infection and cervical disease. These studies should seek also incorporate measures of microbiome byproducts (i.e. metabolomics), which may be associated with immune dysfunction and cell dysregulation.
Acknowledgements
Funding source: British Society of Colposcopy Cervical Pathology Jordan/Singer Award (P47773)(MK); Imperial College Healthcare Charity (MK, AM)(P47907); Genesis Research Trust (MK)(P55549); Imperial Healthcare NHS Trust NIHR Biomedical Research Centre (MK)(P45272); NIHR Academic Clinical Fellowship programme (AM); National Institutes of Health: National Cancer Institute (#R37CA51323)(ABM).
Abbreviations
- 8-OHdG
8-hydroxy-2' -deoxyguanosine
- ASCUS
Atypical squamous cells of uncertain significance
- BV
Bacterial vaginosis
- CST
Community state type
- HNCF
Human normal fibroblast-like cervical
- HPV
Human Papillomavirus
- HSIL
High-grade squamous intraepithelial lesion
- IUD
intrauterine device
- LSIL
Low-grade squamous intraepithelial lesion
- MPA
Medroxyprogesterone acetate
- NGS
Next-generation sequencing
- OC
Oral contraceptive
- OTU
Operational taxonomic unit
- SIL
Squamous intraepithelial lesion
- STI
sexually-transmitted infection
- VM
Vaginal Microbiome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have read the journal's policy on conflicts of interest and declare that they do not have any conflict of interest to declare.
The paper conforms to the relevant ethical guidelines for human and animal research.
Authorship agreement: The authors have read the journal's authorship agreement. The manuscript has been reviewed by and approved by all named authors.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer Journal international du cancer. 2015;136(5):E359–86. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cervical Cancer . International Agency for Research on Cancer. World Health Organization: 2012. Estimated Incidence, Mortality and Prevalence Worldwide in 2012. GLOBOCAN 2012. [Google Scholar]
- 3.Peto J, Gilham C, Fletcher O, et al. The cervical cancer epidemic that screening has prevented in the U. Lancet. 2004;364(9430):249–56. doi: 10.1016/S0140-6736(04)16674-9. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. The Journal of pathology. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Dunne EF, Unger ER, Sternberg M, et al. Prevalence of HPV infection among females in the United States. JAMA. 2007;297(8):813–9. doi: 10.1001/jama.297.8.813. [DOI] [PubMed] [Google Scholar]
- 6.Moscicki AB, Palefsky J, Gonzales J, et al. Human papillomavirus infection in sexually active adolescent females: prevalence and risk factors. Pediatr Res. 1990;28(5):507–13. doi: 10.1203/00006450-199011000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Edelman SM, Lehti TA, Kainulainen V, et al. Identification of a high-molecular-mass Lactobacillus epithelium adhesin (LEA) of Lactobacillus crispatus ST1 that binds to stratified squamous epithelium. Microbiology. 2012;158:1713–22. doi: 10.1099/mic.0.057216-0. Pt 7. [DOI] [PubMed] [Google Scholar]
- 8.Hampras SS, Giuliano AR, Lin HY, et al. Natural history of cutaneous human papillomavirus (HPV) infection in men: the HIM study. PLoS One. 2014;9(9):e104843. doi: 10.1371/journal.pone.0104843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dona MG, Gheit T, Latini A, et al. Alpha, beta and gamma Human Papillomaviruses in the anal canal of HIV-infected and uninfected men who have sex with men. J Infect. 2015;71(1):74–84. doi: 10.1016/j.jinf.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Bottalico D, Chen Z, Dunne A, et al. The oral cavity contains abundant known and novel human papillomaviruses from the Betapapillomavirus and Gammapapillomavirus genera. J Infect Dis. 2011;204(5):787–92. doi: 10.1093/infdis/jir383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Cancer Institute SEER Stat Fact Sheets: Cervix Uteri Cancer 2012. [Available from: http://seer.cancer.gov/statfacts/html/cervix.html.
- 12.McCann MF, Irwin DE, Walton LA, et al. Nicotine and cotinine in the cervical mucus of smokers, passive smokers, and nonsmokers. Cancer Epidemiol Biomarkers Prev. 1992;1(2):125–9. [PubMed] [Google Scholar]
- 13.Barton SE, Maddox PH, Jenkins D, et al. Effect of cigarette smoking on cervical epithelial immunity: a mechanism for neoplastic change? Lancet. 1988;2(8612):652–4. doi: 10.1016/s0140-6736(88)90469-2. [DOI] [PubMed] [Google Scholar]
- 14.Remoue F, Jacobs N, Miot V, et al. High intraepithelial expression of estrogen and progesterone receptors in the transformation zone of the uterine cervix. Am J Obstet Gynecol. 2003;189(6):1660–5. doi: 10.1016/s0002-9378(03)00852-4. [DOI] [PubMed] [Google Scholar]
- 15.Hwang LY, Ma Y, Benningfield SM, et al. Factors that influence the rate of epithelial maturation in the cervix in healthy young women. J Adolesc Health. 2009;44(2):103–10. doi: 10.1016/j.jadohealth.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen KE, Schmiedel S, Norrild B, et al. Parity as a cofactor for high-grade cervical disease among women with persistent human papillomavirus infection: a 13-year follow-up. Br J Cancer. 2013;108(1):234–9. doi: 10.1038/bjc.2012.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moscicki AB, Schiffman M, Burchell A, et al. Updating the natural history of human papillomavirus and anogenital cancers. Vaccine. 2012;30(Suppl 5):F24–33. doi: 10.1016/j.vaccine.2012.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanley MA. Epithelial cell responses to infection with human papillomavirus. Clin Microbiol Rev. 2012;25(2):215–22. doi: 10.1128/CMR.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose WA, 2nd, McGowin CL, Spagnuolo RA, et al. Commensal bacteria modulate innae immune responses of vaginal epithelial cell multilayer cultures. PLoS One. 2012;7(3):e32728. doi: 10.1371/journal.pone.0032728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110(5):525–41. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- 21.Wise-Draper TM, Wells SI. Papillomavirus E6 and E7 proteins and their cellular targets. Front Biosci. 2008;13:1003–17. doi: 10.2741/2739. [DOI] [PubMed] [Google Scholar]
- 22.Bercik P, Denou E, Collins J, et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology. 2011;141(2):599–609. doi: 10.1053/j.gastro.2011.04.052. 09 e1-3. [DOI] [PubMed] [Google Scholar]
- 23.Ohland CL, Kish L, Bell H, et al. Effects of Lactobacillus helveticus on murine behavior are dependent on diet and genotype and correlate with alterations in the gut microbiome. Psychoneuroendocrinology. 2013;38(9):1738–47. doi: 10.1016/j.psyneuen.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 24.McDonald D, Hornig M, Lozupone C, et al. Towards large-cohort comparative studies to define the factors influencing the gut microbial community structure of ASD patients. Microb Ecol Health Dis. 2015;26:26555. doi: 10.3402/mehd.v26.26555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van de Wijgert JH, Borgdorff H, Verhelst R, et al. The vaginal microbiota: what have we learned after a decade of molecular characterization? PLoS One. 2014;9(8):e105998. doi: 10.1371/journal.pone.0105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brotman RM. Vaginal microbiome and sexually transmitted infections: an epidemiologic perspective. J Clin Invest. 2011;121(12):4610–7. doi: 10.1172/JCI57172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boskey ER, Cone RA, Whaley KJ, et al. Origins of vaginal acidity: high D/L lactate ratio is consistent with bacteria being the primary source. Hum Reprod. 2001;16(9):1809–13. doi: 10.1093/humrep/16.9.1809. [DOI] [PubMed] [Google Scholar]
- 28.McMillan A, Dell M, Zellar MP, et al. Disruption of urogenital biofilms by lactobacilli. Colloids Surf B Biointerfaces. 2011;86(1):58–64. doi: 10.1016/j.colsurfb.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Boris S, Barbes C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2(5):543–6. doi: 10.1016/s1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 30.Aroutcheva A, Gariti D, Simon M, et al. Defense factors of vaginal lactobacilli. Am J Obstet Gynecol. 2001;185(2):375–9. doi: 10.1067/mob.2001.115867. [DOI] [PubMed] [Google Scholar]
- 31.Reid G, Heinemann C, Velraeds M, et al. Biosurfactants produced by Lactobacillus. Methods Enzymol. 1999;310:426–33. doi: 10.1016/s0076-6879(99)10033-8. [DOI] [PubMed] [Google Scholar]
- 32.Ocana VS, Pesce De Ruiz Holgado AA, Nader-Macias ME. Characterization of a bacteriocin-like substance produced by a vaginal Lactobacillus salivarius strain. Appl Environ Microbiol. 1999;65(12):5631–5. doi: 10.1128/aem.65.12.5631-5635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verstraelen H, Verhelst R, Claeys G, et al. Longitudinal analysis of the vaginal microflora in pregnancy suggests that L. crispatus promotes the stability of the normal vaginal microflora and that L. gasseri and/or L. iners are more conducive to the occurrence of abnormal vaginal microflora. BMC Microbiol. 2009;9:116. doi: 10.1186/1471-2180-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ravel J, Gajer P, Abdo Z, et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4680–7. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gajer P, Brotman RM, Bai G, et al. Temporal dynamics of the human vaginal microbiota. Sci Transl Med. 2012;4(132):132ra52. doi: 10.1126/scitranslmed.3003605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradshaw CS, Walker J, Fairley CK, et al. Prevalent and incident bacterial vaginosis are associated with sexual and contraceptive behaviours in young Australian women. PLoS One. 2013;8(3):e57688. doi: 10.1371/journal.pone.0057688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chico RM, Mayaud P, Ariti C, et al. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA. 2012;307(19):2079–86. doi: 10.1001/jama.2012.3428. [DOI] [PubMed] [Google Scholar]
- 39.Ness RB, Kip KE, Hillier SL, et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am J Epidemiol. 2005;162(6):585–90. doi: 10.1093/aje/kwi243. [DOI] [PubMed] [Google Scholar]
- 40.Leitich H, Bodner-Adler B, Brunbauer M, et al. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. Am J Obstet Gynecol. 2003;189(1):139–47. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 41.Brotman RM, Klebanoff MA, Nansel TR, et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J Infect Dis. 2010;202(12):1907–15. doi: 10.1086/657320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atashili J, Poole C, Ndumbe PM, et al. Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. AIDS. 2008;22(12):1493–501. doi: 10.1097/QAD.0b013e3283021a37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–17. doi: 10.1128/JB.00542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Srinivasan S, Hoffman NG, Morgan MT, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MacIntyre DA, Chandiramani M, Lee YS, et al. The vaginal microbiome during pregnancy and the postpartum period in a European population. Scientific reports. 2015;5:8988. doi: 10.1038/srep08988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romero R, Hassan SS, Gajer P, et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome. 2014;2(1):4. doi: 10.1186/2049-2618-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plummer M, Herrero R, Franceschi S, et al. Smoking and cervical cancer: pooled analysis of the IARC multi-centric case--control study. Cancer Causes Control. 2003;14(9):805–14. doi: 10.1023/b:caco.0000003811.98261.3e. [DOI] [PubMed] [Google Scholar]
- 48.Moreno V, Bosch FX, Munoz N, et al. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric case-control study. Lancet. 2002;359(9312):1085–92. doi: 10.1016/S0140-6736(02)08150-3. [DOI] [PubMed] [Google Scholar]
- 49.Fichorova RN, Chen PL, Morrison CS, et al. The Contribution of Cervicovaginal Infections to the Immunomodulatory Effects of Hormonal Contraception. MBio. 2015;6(5):e00221–15. doi: 10.1128/mBio.00221-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 52.Achilles SL, Hillier SL. The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS. 2013;27(Suppl 1):S5–15. doi: 10.1097/QAD.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van de Wijgert JH, Verwijs MC, Turner AN, et al. Hormonal contraception decreases bacterial vaginosis but oral contraception may increase candidiasis: implications for HIV transmission. AIDS. 2013;27(13):2141–53. doi: 10.1097/QAD.0b013e32836290b6. [DOI] [PubMed] [Google Scholar]
- 54.Vodstrcil LA, Hocking JS, Law M, et al. Hormonal contraception is associated with a reduced risk of bacterial vaginosis: a systematic review and meta-analysis. PLoS One. 2013;8(9):e73055. doi: 10.1371/journal.pone.0073055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borgdorff H, Gautam R, Armstrong SD, et al. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.86. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell CM, McLemore L, Westerberg K, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. J Infect Dis. 2014;210(4):651–5. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogtmann E, Flores R, Yu G, et al. Association between tobacco use and the upper gastrointestinal microbiome among Chinese men. Cancer Causes Control. 2015;26(4):581–8. doi: 10.1007/s10552-015-0535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahn J, Chen CY, Hayes RB. Oral microbiome and oral and gastrointestinal cancer risk. Cancer Causes Control. 2012;23(3):399–404. doi: 10.1007/s10552-011-9892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bradshaw CS, Walker SM, Vodstrcil LA, et al. The influence of behaviors and relationships on the vaginal microbiota of women and their female partners: the WOW Health Study. J Infect Dis. 2014;209(10):1562–72. doi: 10.1093/infdis/jit664. [DOI] [PubMed] [Google Scholar]
- 60.Brotman RM, He X, Gajer P, et al. Association between cigarette smoking and the vaginal microbiota: a pilot study. BMC Infect Dis. 2014;14:471. doi: 10.1186/1471-2334-14-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onderdonk AB, Delaney ML, Fichorova RN. The Human Microbiome during Bacterial Vaginosis. Clin Microbiol Rev. 2016;29(2):223–38. doi: 10.1128/CMR.00075-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitchell C, Marrazzo J. Bacterial vaginosis and the cervicovaginal immune response. Am J Reprod Immunol. 2014;71(6):555–63. doi: 10.1111/aji.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Libby EK, Pascal KE, Mordechai E, et al. Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infect. 2008;10(4):439–46. doi: 10.1016/j.micinf.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Doerflinger SY, Throop AL, Herbst-Kralovetz MM. Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J Infect Dis. 2014;209(12):1989–99. doi: 10.1093/infdis/jiu004. [DOI] [PubMed] [Google Scholar]
- 65.Anahtar MN, Byrne EH, Doherty KE, et al. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015;42(5):965–76. doi: 10.1016/j.immuni.2015.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson BL, Cu-Uvin S, Raker CA, et al. Subtle perturbations of genital microflora alter mucosal immunity among low-risk pregnant women. Acta Obstet Gynecol Scand. 2011;90(5):510–5. doi: 10.1111/j.1600-0412.2011.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hedges SR, Barrientes F, Desmond RA, et al. Local and systemic cytokine levels in relation to changes in vaginal flora. J Infect Dis. 2006;193(4):556–62. doi: 10.1086/499824. [DOI] [PubMed] [Google Scholar]
- 68.Novak RM, Donoval BA, Graham PJ, et al. Cervicovaginal levels of lactoferrin, secretory leukocyte protease inhibitor, and RANTES and the effects of coexisting vaginoses in human immunodeficiency virus (HIV)-seronegative women with a high risk of heterosexual acquisition of HIV infection. Clin Vaccine Immunol. 2007;14(9):1102–7. doi: 10.1128/CVI.00386-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Valore EV, Wiley DJ, Ganz T. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun. 2006;74(10):5693–702. doi: 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Motevaseli E, Shirzad M, Akrami SM, et al. Normal and tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J Med Microbiol. 2013;62:1065–72. doi: 10.1099/jmm.0.057521-0. Pt 7. [DOI] [PubMed] [Google Scholar]
- 71.Gomez LM, Sammel MD, Appleby DH, et al. Evidence of a gene-environment interaction that predisposes to spontaneous preterm birth: a role for asymptomatic bacterial vaginosis and DNA variants in genes that control the inflammatory response. Am J Obstet Gynecol. 2010;202(4):386 e1–6. doi: 10.1016/j.ajog.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 72.Scott ME, Ma Y, Farhat S, et al. Covariates of cervical cytokine mRNA expression by real-time PCR in adolescents and young women: effects of Chlamydia trachomatis infection, hormonal contraception, and smoking. J Clin Immunol. 2006;26(3):222–32. doi: 10.1007/s10875-006-9010-x. [DOI] [PubMed] [Google Scholar]
- 73.Hwang LY, Scott ME, Ma Y, et al. Higher levels of cervicovaginal inflammatory and regulatory cytokines and chemokines in healthy young women with immature cervical epithelium. J Reprod Immunol. 2011;88(1):66–71. doi: 10.1016/j.jri.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gillet E, Meys JF, Verstraelen H, et al. Bacterial vaginosis is associated with uterine cervical human papillomavirus infection: a meta-analysis. BMC Infect Dis. 2011;11:10. doi: 10.1186/1471-2334-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guo Y, You K, Qiao J, et al. Bacterial vaginosis is conducive to the persistence of HPV infection. Int J STD AIDS. 2012;23:581–4. doi: 10.1258/ijsa.2012.011342. [DOI] [PubMed] [Google Scholar]
- 76.King CC, Jamieson DJ, Wiener J, et al. Bacterial vaginosis and the natural history of human papillomavirus. Infect Dis Obstet Gynecol. 2011;2011:319460. doi: 10.1155/2011/319460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sha BE, Chen HY, Wang QJ, et al. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol. 2005;43(9):4607–12. doi: 10.1128/JCM.43.9.4607-4612.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JE, Lee S, Lee H, et al. Association of the vaginal microbiota with human papillomavirus infection in a Korean twin cohort. PLoS One. 2013;8(5):e63514. doi: 10.1371/journal.pone.0063514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao W, Weng J, Gao Y, et al. Comparison of the vaginal microbiota diversity of women with and without human papillomavirus infection: a cross-sectional study. BMC Infect Dis. 2013;13:271. doi: 10.1186/1471-2334-13-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brotman RM, Shardell MD, Gajer P, et al. Interplay Between the Temporal Dynamics of the Vaginal Microbiota and Human Papillomavirus Detection. J Infect Dis. 2014 doi: 10.1093/infdis/jiu330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mitra A, MacIntyre DA, Lee YS, et al. Cervical intraepithelial neoplasia disease progression is associated with increased vaginal microbiome diversity. Sci Rep. 2015;5:16865. doi: 10.1038/srep16865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu J, Feng Q, Wong SH, et al. Metagenomic analysis of faecal microbiome as a tool towards targeted non-invasive biomarkers for colorectal cancer. Gut. 2015 doi: 10.1136/gutjnl-2015-309800. [DOI] [PubMed] [Google Scholar]
- 84.Mima K, Nishihara R, Qian ZR, et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut. 2015 doi: 10.1136/gutjnl-2015-310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubinstein MR, Wang X, Liu W, et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14(2):195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mima K, Sukawa Y, Nishihara R, et al. Fusobacterium nucleatum and T Cells in Colorectal Carcinoma. JAMA Oncol. 2015;1(5):653–61. doi: 10.1001/jamaoncol.2015.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oh HY, Kim BS, Seo SS, et al. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin Microbiol Infect. 2015;21(7):674 e1–9. doi: 10.1016/j.cmi.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 88.Kinney WK, Manos MM, Hurley LB, et al. Where's the high-grade cervical neoplasia? The importance of minimally abnormal Papanicolaou diagnoses. Obstet Gynecol. 1998;91(6):973–6. doi: 10.1016/s0029-7844(98)00080-5. [DOI] [PubMed] [Google Scholar]
- 89.Nelson TM, Borgogna JL, Brotman RM, et al. Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front Physiol. 2015;6:253. doi: 10.3389/fphys.2015.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen KC, Forsyth PS, Buchanan TM, et al. Amine content of vaginal fluid from untreated and treated patients with nonspecific vaginitis. J Clin Invest. 1979;63(5):828–35. doi: 10.1172/JCI109382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pavic N. Is there a local production of nitrosamines by the vaginal microflora in anaerobic vaginosis/trichomoniasis? Med Hypotheses. 1984;15(4):433–6. doi: 10.1016/0306-9877(84)90159-2. [DOI] [PubMed] [Google Scholar]
- 92.Bartsch H, Montesano R. Relevance of nitrosamines to human cancer. Carcinogenesis. 1984;5(11):1381–93. doi: 10.1093/carcin/5.11.1381. [DOI] [PubMed] [Google Scholar]
- 93.Lidbeck A, Nord CE, Gustafsson JA, et al. Lactobacilli, anticarcinogenic activities and human intestinal microflora. Eur J Cancer Prev. 1992;1(5):341–53. doi: 10.1097/00008469-199208000-00002. [DOI] [PubMed] [Google Scholar]
- 94.Piyathilake CJ, Ollberding NJ, Kumar R, et al. Cervical Microbiota Associated with Risk of Higher Grade Cervical Intraepithelial Neoplasia in Women Infected with High-Risk Human Papillomaviruses. Cancer Prev Res (Phila) 2016 doi: 10.1158/1940-6207.CAPR-15-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Srinivasan S, Morgan MT, Fiedler TL, et al. Metabolic signatures of bacterial vaginosis. MBio. 2015;6(2) doi: 10.1128/mBio.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Romano G, Sgambato A, Mancini R, et al. 8-hydroxy-2'-deoxyguanosine in cervical cells: correlation with grade of dysplasia and human papillomavirus infection. Carcinogenesis. 2000;21(6):1143–7. [PubMed] [Google Scholar]
- 97.Arabski M, Klupinska G, Chojnacki J, et al. DNA damage and repair in Helicobacter pylori-infected gastric mucosa cells. Mutat Res. 2005;570(1):129–35. doi: 10.1016/j.mrfmmm.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 98.Holmes KK, Chen KC, Lipinski CM, et al. Vaginal redox potential in bacterial vaginosis (nonspecific vaginitis) J Infect Dis. 1985;152(2):379–82. doi: 10.1093/infdis/152.2.379. [DOI] [PubMed] [Google Scholar]
- 99.Williams VM, Filippova M, Filippov V, et al. Human papillomavirus type 16 E6* induces oxidative stress and DNA damage. J Virol. 2014;88(12):6751–61. doi: 10.1128/JVI.03355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schmitt A, Harry JB, Rapp B, et al. Comparison of the properties of the E6 and E7 genes of low- and high-risk cutaneous papillomaviruses reveals strongly transforming and high Rb-binding activity for the E7 protein of the low-risk human papillomavirus type 1. J Virol. 1994;68(11):7051–9. doi: 10.1128/jvi.68.11.7051-7059.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ling Z, Liu X, Chen X, et al. Diversity of cervicovaginal microbiota associated with female lower genital tract infections. Microb Ecol. 2011;61(3):704–14. doi: 10.1007/s00248-011-9813-z. [DOI] [PubMed] [Google Scholar]
- 102.Hyman RW, Fukushima M, Jiang H, et al. Diversity of the vaginal microbiome correlates with preterm birth. Reprod Sci. 2014;21(1):32–40. doi: 10.1177/1933719113488838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Swidsinski A, Doerffel Y, Loening-Baucke V, et al. Gardnerella biofilm involves females and males and is transmitted sexually. Gynecol Obstet Invest. 2010;70(4):256–63. doi: 10.1159/000314015. [DOI] [PubMed] [Google Scholar]
- 104.Patterson JL, Stull-Lane A, Girerd PH, et al. Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes. Microbiology. 2010;156:392–9. doi: 10.1099/mic.0.034280-0. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Verstraelen H, Swidsinski A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 2013;26(1):86–9. doi: 10.1097/QCO.0b013e32835c20cd. [DOI] [PubMed] [Google Scholar]
- 106.Falsetta ML, Klein MI, Colonne PM, et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82(5):1968–81. doi: 10.1128/IAI.00087-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hillier SL, Lau RJ. Vaginal microflora in postmenopausal women who have not received estrogen replacement therapy. Clin Infect Dis. 1997;25(Suppl 2):S123–6. doi: 10.1086/516221. [DOI] [PubMed] [Google Scholar]
- 108.Woodworth CD. HPV innate immunity. Front Biosci. 2002;7:d2058–71. doi: 10.2741/A898. [DOI] [PubMed] [Google Scholar]
- 109.Garcea G, Dennison AR, Steward WP, et al. Role of inflammation in pancreatic carcinogenesis and the implications for future therapy. Pancreatology. 2005;5(6):514–29. doi: 10.1159/000087493. [DOI] [PubMed] [Google Scholar]
- 110.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scott M, Stites DP, Moscicki AB. Th1 cytokine patterns in cervical human papillomavirus infection. Clinical and diagnostic laboratory immunology. 1999;6(5):751–5. doi: 10.1128/cdli.6.5.751-755.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Borgdorff H, Tsivtsivadze E, Verhelst R, et al. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014;8(9):1781–93. doi: 10.1038/ismej.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Smith-McCune KK, Shiboski S, Chirenje MZ, et al. Type-specific cervico-vaginal human papillomavirus infection increases risk of HIV acquisition independent of other sexually transmitted infections. PLoS One. 2010;5(4):e10094. doi: 10.1371/journal.pone.0010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gallagher KE, Baisley K, Grosskurth H, et al. The association between cervical human papillomavirus infection and subsequent HIV acquisition in Tanzanian and Ugandan women: a nested case-control study. J Infect Dis. 2016 doi: 10.1093/infdis/jiw094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thomas RM, Jobin C. The Microbiome and Cancer: Is the 'Oncobiome' Mirage Real? Trends Cancer. 2015;1(1):24–35. doi: 10.1016/j.trecan.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yarbrough VL, Winkle S, Herbst-Kralovetz MM. Antimicrobial peptides in the female reproductive tract: a critical component of the mucosal immune barrier with physiological and clinical implications. Hum Reprod Update. 2015;21(3):353–77. doi: 10.1093/humupd/dmu065. [DOI] [PubMed] [Google Scholar]
- 117.Nicholson JK, Holmes E, Kinross JM, et al. Metabolic phenotyping in clinical and surgical environments. Nature. 2012;491(7424):384–92. doi: 10.1038/nature11708. [DOI] [PubMed] [Google Scholar]
- 118.Griffin PM, Tauxe RV. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol Rev. 1991;13:60–98. doi: 10.1093/oxfordjournals.epirev.a036079. [DOI] [PubMed] [Google Scholar]
- 119.Wylie KM, Mihindukulasuriya KA, Zhou Y, et al. Metagenomic analysis of double-stranded DNA viruses in healthy adults. BMC Biol. 2014;12:71. doi: 10.1186/s12915-014-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vujic G, Jajac Knez A, Despot Stefanovic V, et al. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. Eur J Obstet Gynecol Reprod Biol. 2013;168(1):75–9. doi: 10.1016/j.ejogrb.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 121.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, et al. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet. 2006;367(9509):489–98. doi: 10.1016/S0140-6736(06)68181-6. [DOI] [PubMed] [Google Scholar]
- 122.Kyrgiou M, Mitra A, Arbyn M, et al. Fertility and early pregnancy outcomes after treatment for cervical intraepithelial neoplasia: systematic review and meta-analysis. BMJ. 2014;349:g6192. doi: 10.1136/bmj.g6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arbyn M, Kyrgiou M, Simoens C, et al. Perinatal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: meta-analysis. BMJ. 2008;337:a1284. doi: 10.1136/bmj.a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]