Abstract
Background
Tuberculosis-diabetes co-morbidity (TB-DM) is characterized by increased inflammation with elevated circulating levels of inflammatory cytokines and other factors. Circulating angiogenic factors are intricately involved in the angiogenesis-inflammation nexus.
Methods
To study the association of angiogenic factors with TB-DM, we examined the systemic levels of VEGF-A, VEGF-C, VEGF-D, VEGF-R1, VEGF-R2, VEGF-R3 in individuals with either TB-DM (n=44) or TB alone (n=44).
Results
Circulating levels of VEGF-A, C, D, R1, R2 and R3 were significantly higher in TB-DM compared to TB individuals. Moreover, the levels of VEGF-A, C, R2 and/or R3 were significantly higher in TB-DM with bilateral or cavitary disease or with hemoptysis, suggesing an association with both disease severity and adverse clinical presentation. The levels of these factors also exhibited a significant positive relationship with bacterial burdens and HbA1c levels. In addition, VEGF-A, C and R2 levels were signifantly higher (at 2 months of treatment) in culture positive compared to culture negative TB-DM individuals. Finally, the circulating levels of VEGF-A, C, D, R1, R2 and R3 were significantly reduced following successful chemotherapy at 6 months.
Conclusion
Our data demonstrate that TB-DM is associated with heightened levels of circulating angiogenic factors, possibly reflecting both dysregulated angiogenesis and exaggerated inflammation.
Keywords: Tuberculosis, Diabetes, Angiogenesis, Biomarkers
INTRODUCTION
Diabetes mellitus (DM) increases the risk of pulmonary tuberculosis (TB) by 2 to 3 fold and is associated with greater odds of cavitary disease, positive sputum smear, delayed sputum conversion, treatment failure, relapse and death [1, 2]. TB-DM co-morbidity is now a common occurrence in most low and middle income countries endemic for TB and serves as the major impediment in the elimination of TB worldwide [3]. The pathogenesis of TB-DM co-morbidity is not completely understood, but chronic inflammation appears to be the central underlying pathogenic feature [4, 5]. In addition, dysregulated angiogenesis also appears to be a major characteristic of TB and DM independently [6, 7]. Angiogenesis is typically regulated by the vascular endothelial growth family members, which includes VEGF-A, VEGF-B, VEGF-C and VEGF-D [8]. These factors bind with differing specificities to three mostly endothelial receptors - VEGF-R1, VEGF-R2 and VEGF-R3 to stimulate angiogenic processes [9].
Previous studies have demonstrated that elevated levels of VEGF-A is a characteristic feature of TB and that VEGF-A serves as an important biomarker distinguishing active disease from latent infection [7, 10–14]. In addition, we have recently shown that VEGF-A, VEGF-C and VEGF-R2 are all accurate biomarkers of disease severity, bacterial burden and response to treatment in pulmonary TB [15]. It has also been reported that interference with angiogenic or lymphangiogenic pathways in experimental models of mycobacterial infection results in significantly improved treatment outcomes as well as diminished mycobacterial growth [16, 17]. In addition, angiogenesis plays a prominent role in the pathogenesis of DM and its complications [6]. A paradoxical feature of DM pathogenesis appears to be that vascular impairment (or diminished angiogenesis) and excessive angiogenesis can co-exist in different organs of the same host [18].
Since angiogenesis and inflammation are intricately linked and are independent characteristics of TB and DM, we postulated that TB-DM would also be associated with heightened levels of systemic angiogenic factors. To this end, we examined the circulating levels of these angiogenic factors in individuals with TB-DM in comparison to TB alone. Our data reveal a significant elevation of all circulating angiogenic factors in TB-DM and a significant association of VEGF-A, VEGF-C and VEGF-R2 with disease severity, adverse clinical presentation, bacterial burden and poor glycemic control. Our data also suggest that the factors mentioned above could serve as accurate biomarkers for monitoring therapeutic responses in TB-DM.
MATERIALS AND METHODS
Ethics statement
This study was approved by the Ethics Committees of the Prof. M. Viswanathan Diabetes Research Center and NIRT. Informed consent was obtained from all participants.
Study population
Plasma samples were collected from 44 individuals with active pulmonary TB and diabetes mellitus (TB-DM) and 44 individuals with pulmonary TB and no diabetes (TB). These individuals were a subset of individuals recruited for the "Effects of Diabetes on Tuberculosis Severity" study presently underway at the Prof. M. Viswanathan Diabetes Research Center and the National Institute for Research in Tuberculosis [19]. Consecutively enrolled individuals were recruited for this study. The baseline demographic characteristics of the study population are shown in Table 1. PTB was diagnosed on the basis of sputum smear and culture positivity. Chest X-rays were used to determine cavitary disease as well as unilateral versus bilateral involvement. Smear grades were used to determine bacterial burdens and classified as 1+, 2+ and 3+. At the time of enrollment, all active TB cases had no record of prior TB disease. DM was diagnosed on the basis of oral glucose tolerance test and/or glycated hemoglobin (HbA1c) levels (for known diabetics), according to the WHO criteria. The DM individuals were a combination of known DM (n=34) and newly diagnosed DM (n=10). All the individuals were HIV seronegative and anti-tuberculous treatment naïve. Anthropometric measurements, hematological and biochemical parameters were obtained using standardized techniques as detailed elsewhere. All individuals had pan-sensitive Mycobacterium tuberculosis on sputum culture at enrollment and all received standard tuberculosis treatment (Directly Observed Treatment Short Course - DOTS with isoniazid, rifampicin, pyrazinamide and ethambutol for 2 months, followed by isoniazid and rifampicin for 4 months). All individuals were smear and culture negative at the end of 6 months of therapy (Table 1). Blood samples were collected at baseline, 2 months and 6 months of anti-TB treatment (ATT). These PTB individuals are different from the individuals described in our previous study [15].
Table 1.
Demographics of the study groups and biochemical parameters in TB-DM and TB
| Study Demographics | |||
| TB-DM | TB | p Value | |
| No. of subjects recruited | 44 | 44 | - |
| Gender (Male / Female) | 33/11 | 37/7 | - |
| Median Age (Range) | 50 (34 – 70) | 41 (25 – 67) | - |
| Median Height, cm | 157 (135 – 169) | 161 (143 – 181) | - |
| Median Weight, kg | 52 (32 – 67) | 46 (30 – 90) | - |
| Smear Grade: 0/1+/2+/3+ | 0/16/14/14 | 0/18/16/10 | - |
|
Culture Results: Negative:0/1+/2+/3+ |
0/12/17/15 | 0/28/12/4 | - |
| Biochemical Parameters | TB-DM | TB | p Value |
| Fasting Blood Glucose, mg/dL | 181 (99–417) | 92 (62–112) | p<0.0001 |
| Post Prandial Glucose, mg/dL | 220 (180–448) | 119 (76–137) | p<0.0001 |
| Glycated hemoglobin level, % | 10.2 (6.6 – 15.6) | 5.6 (5.0 – 5.8) | p<0.0001 |
| Serum Triglycerides, mg/dL | 108 (33 – 178) | 74 (39 – 142) | p<0.0001 |
| Total Cholesterol, mg/dL | 172 (92 – 258) | 151 (86 – 192) | p=0.0092 |
| HDL Cholesterol, mg/dL | 37 (20 – 83) | 36 (19 – 69) | p=0.2428 |
| LDL Cholesterol, mg/dL | 94 (51 – 165) | 83 (49 – 107) | p=0.0243 |
| VLDL Cholesterol, mg/dL | 38 (6 – 76) | 28 (15 – 48) | p=0.0088 |
| Urea, mg/dL | 18 (2 – 32) | 16 (8 – 29) | p=0.1301 |
| Creatinine, mg/dL | 0.9 (0.5 – 1.8) | 0.9 (0.6 – 1.2) | p=0.4985 |
| Total Bilirubin, mg/dL | 0.65 (0.4 – 1.4) | 0.4 (0.1 – 0.9) | p<0.0001 |
| Total Protein, g/dL | 8.2 (6.2 – 9.6) | 8.2 (7.1 – 9.7) | p=0.8646 |
| Serum Albumin, g/dL | 4.1 (2.5 – 4.7) | 4 (2.7 – 5.1) | p=0.0501 |
| Serum Globulins, g/dL | 4 (2.7 – 12.3) | 4.4 (3 – 5.3) | p=0.0838 |
| SGOT, U/l | 17 (6 – 42) | 19 (10 – 35) | p=0.1095 |
| SGPT, U/l | 14 (5 – 40) | 12 (5 – 66) | p=0.1790 |
| Alkaline Phosphatase, U/l | 260 (94 – 499) | 221 (140 – 497) | p=0.0126 |
| Vitamin D3, ng/ml | 12 (3 – 30) | 17 (3 – 46) | p=0.0008 |
The values represent the geometric mean (and the 95% confidence intervals) except for age where the median (and the range) are depicted.
ELISA
Circulating levels of VEGF-A, VEGF-C, VEGF-R1, VEGF-R2 and VEGF-R3 were measured using the Duoset ELISA Development System (R&D Systems). Quantikine ELISA kit (R&D Systems) was used for measuring VEGF-D. The lowest detection limits were as follows: VEGF-A, 31.25 pg/ml; VEGF-C, 62.5 pg/ml; VEGF-D, 62.5 pg/ml; VEGF-R1, 125 pg/ml; VEGF-R2, 31.25 pg/ml; VEGF-R3, 156.25 pg/ml.
Statistical Analysis
Geometric means (GM) were used for measurements of central tendency. Statistically significant differences between the two groups were analyzed using the Mann-Whitney test with Holm's correction for multiple comparisons. Linear trend post-test was used to compare angiogenic factor concentrations with smear grades (reflecting bacterial burdens) and Spearman rank correlation was used to compare angiogenic factor concentrations with HbA1c levels. Wilcoxon signed rank test was used to compare angiogenic factor concentrations before, during and after ATT. Analyses were performed using GraphPad PRISM Version 5.01.
RESULTS
Study population characteristics
The baseline characteristics including demographic and biochemical features of the study population are shown in Table I. As can be seen, compared to subjects without diabetes (TB), those with diabetes and TB (TB-DM) had significantly higher levels of fasting and post-prandial glucose, glycated hemoglobin, serum cholesterol, VLDL, LDL, triglycerides, total bilirubin and alkaline phosphatase. No significant differences were observed in age, sex, BMI, smear or culture grades at baseline between the 2 groups (Table 1). In addition, the smear and culture grades at baseline and at 2 and 6 months of ATT; and the levels of HbA1c at baseline and 6 months of TB-DM individuals are shown in Table 2. No significant differences were observed in HbA1c levels before and after ATT in TB-DM individuals.
Table 2.
Smear and culture grades and HbA1c levels before, during and after ATT in TB-DM individuals
| Study Demographics | TB DM | ||
|---|---|---|---|
| 0 Month | 2nd Month | 6th Month | |
|
Smear Grade: 0/1+/2+/3+ |
0/16/14/14 | 30/11/0/3 | 44/0/0/0 |
|
Culture Results: 0/1+/2+/3+ |
0/12/17/15 | 29/14/1/0 | 44/0/0/0 |
|
Glycated hemoglobin level, % |
10.2 (6.6 – 15.6) | - | 9.85 (4.8 – 17.7) |
Heightened levels of circulating angiogenic factors in TB-DM
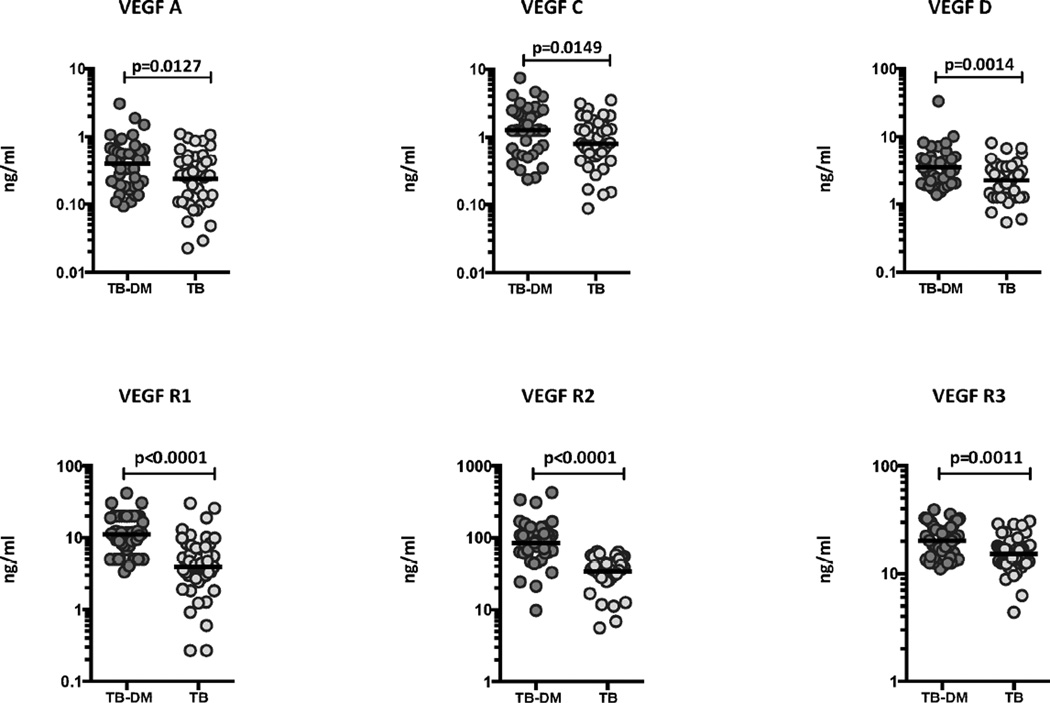
To determine the systemic levels of circulating angiogenic factors in TB-DM and TB, we measured the circulating levels of VEGF-A, C, D, R1, R2 and R3 in TB-DM and TB individuals (Figure 1). As shown, the systemic levels of VEGF-A (Geometric Mean of 0.39 ng/ml in TB-DM versus 0.23 ng/ml in TB), VEGF-C (GM of 1.3 ng/ml in TB-DM versus 0.80 ng/ml in TB) and VEGF-D (GM of 3.5 ng/ml in TB-DM versus 2.2 ng/ml in TB) were significantly higher in TB-DM compared to TB individuals. Similarly, the circulating levels of VEGF-R1 (GM of 11.11 ng/ml in TB-DM versus 3.92 ng/ml in TB), VEGF-R2 (GM of 84.8 ng/ml in TB-DM versus 33.9 ng/ml in TB) and VEGF-R3 (GM of 20.2 ng/ml in TB-DM versus 15.2 ng/ml in TB) were significantly higher in TB-DM compared to TB individuals. Thus, TB-DM is associated with elevated systemic levels of circulating angiogenic factors.
Figure 1.
Elevated systemic levels of circulating angiogenic factors in TB-DM individuals. The plasma levels of VEGF-A, VEGF-C, VEGF-D, VEGF-R1, VEGF-R2 and VEGF-R3 were measured in TB-DM (n=44) and TB (n=44) individuals. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons.
Circulating angiogenic factors are markers of disease severity in TB-DM
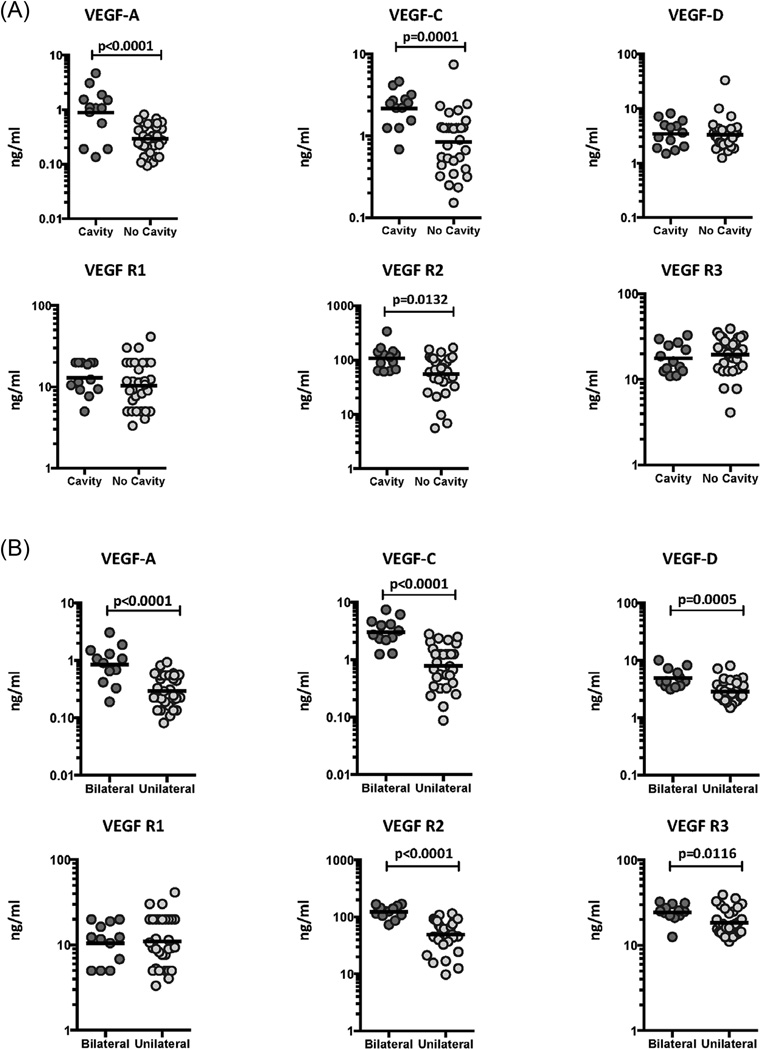
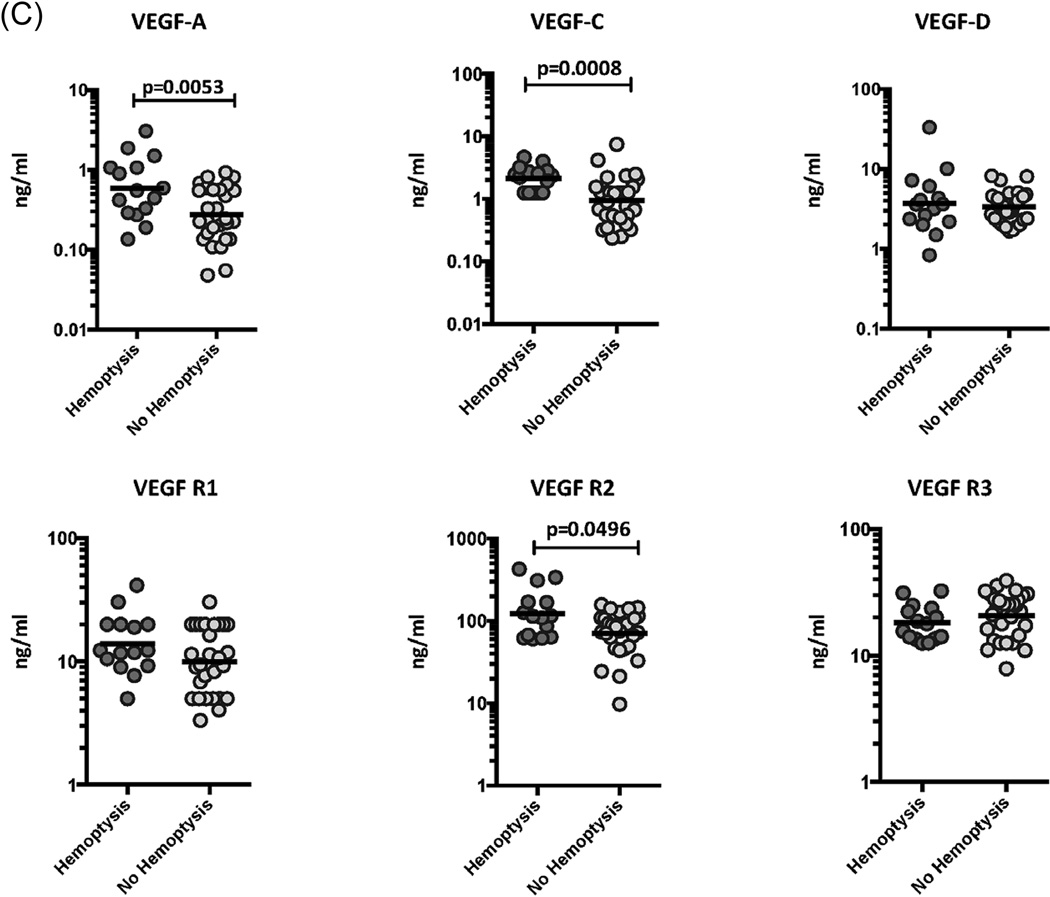
We have previously shown an association between circulating angiogenic factors and disease severity in TB individuals [15]. To determine the association between the systemic levels of circulating angiogenic factors and disease severity in TB-DM, we measured the circulating levels of VEGF-A, C, D, R1, R2 and R3 in TB-DM individuals with cavitary versus non-cavitary disease, unilateral versus bilateral disease and those with or without hemoptysis (Figure 2). As shown in Figure 2A, the systemic levels of VEGF-A (GM of 0.88 ng/ml in cavitary vs. 0.16 ng/ml in non-cavitary disease), VEGF-C (GM of 2.2 ng/ml vs. 0.84 ng/ml), VEGF-R2 (GM of 108.4 ng/ml vs. 55.4 ng/ml) and VEGF-R3 (GM of 14.6 ng/ml vs. 19.4 ng/ml) were significantly higher in TB-DM individuals with cavitary disease compared to those without. Similarly, as shown in Figure 2B, the circulating levels of VEGF-A (GM of 0.85 ng/ml in bilateral vs. 0.11 ng/ml in unilateral disease), VEGF-C (GM of 3.0 ng/ml vs. 0.77 ng/ml), VEGF-D (GM of 4.9 ng/ml vs. 2.6 ng/ml), VEGF-R2 (GM of 123.1 ng/ml vs. 43.7 ng/ml) and VEGF-R3 (GM of 24.3 ng/ml vs. 14.7 ng/ml) were significantly higher in TB-DM individuals with bilateral disease compared to those with unilateral disease. Finally, as shown in Figure 2C, the circulating levels of VEGF-A (GM of 0.43 ng/ml in TB-DM with hemoptysis vs. 0.14 ng/ml in TB-DM without hemoptysis), VEGF-C (GM of 2.1 ng/ml vs. 0.94 ng/ml) and VEGF-R2 (GM of 121.8 ng/ml vs. 70.3 ng/ml) were significantly higher in TB-DM individuals with hemoptysis compared to those without, suggesting a possible link between dysregulated angiogenesis and increased risk of hemorrhage from pulmonary TB lesions. Thus, disease severity and adverse clinical presentation in TB-DM is associated with elevated systemic levels of circulating angiogenic factors.
Figure 2.
Elevated systemic levels of VEGF-A, VEGF-C and VEGF-R2 in cavitary and bilateral disease and in TB-DM individuals with hemoptysis. (A) The plasma levels of VEGF-A, C, D, VEGF-R1, R2 and R3 were measured in TB-DM individuals with cavitary versus non-cavitary disease. (B) The plasma levels of VEGF-A, C, D, VEGF-R1, R2 and R3 were measured in TB-DM individuals with bilateral versus unilateral disease. (C) The plasma levels of VEGF-A, C, D, VEGF-R1, R2 and R3 were measured in TB-DM individuals with hemoptysis and compared to those without. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons.
Circulating angiogenic factors are markers of bacterial burdens and exhibit a positive relationship with HbA1c levels in TB-DM
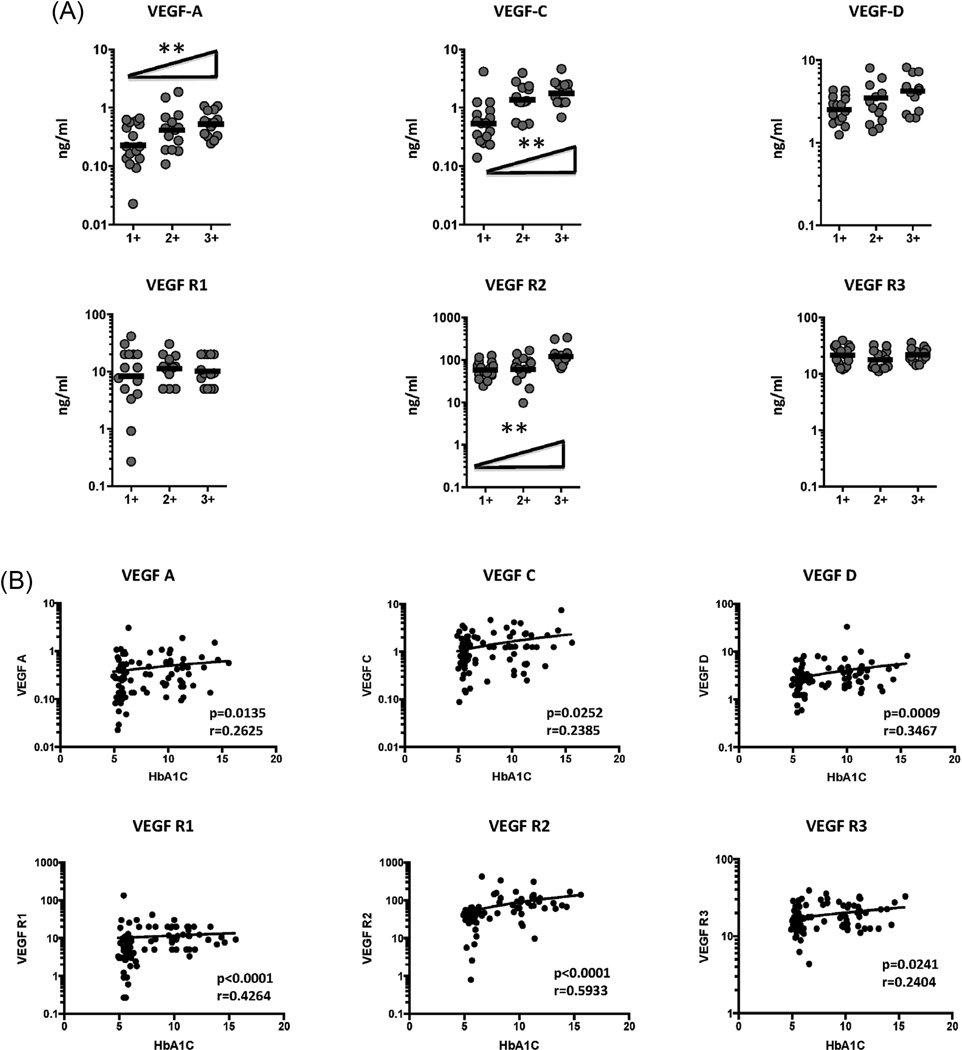
We have previously shown an association between circulating angiogenic factors and bacterial burdens in TB individuals [15]. To determine the association between the systemic levels of circulating angiogenic factors and bacterial burden in TB-DM, we correlated the circulating levels of VEGF-A, C, D, R1, R2 and R3 in TB-DM individuals with smear grade classified as 1+, 2+ and 3+ (Figure 3A). As shown, the systemic levels of VEGF-A, VEGF-C and VEGF-R2 exhibited a significant positive correlation with smear grades in TB-DM individuals, indicating a positive association of these factors with bacterial burdens.
Figure 3.
Positive relationship between systemic levels of angiogenic factors and smear grades as well as HbA1c levels in TB-DM individuals. (A) The relationship between the plasma levels of VEGF-A, C, D, VEGF-R1, R2 and R3 and smear grades as estimated by sputum smears was examined in TB-DM individuals. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using linear trend post-test. (B) The relationship between the plasma levels of VEGF-A, C, D, VEGF-R1, R2 and R3 and HbA1c levels was examined in TB-DM individuals. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Spearman Rank Correlation.
To determine the association between systemic levels of circulating angiogenic factors and glycemic control in TB-DM, we correlated the circulating levels of VEGF-A, C, D, R1, R2 and R3 in TB-DM individuals with HbA1c levels at baseline (Figure 3B). As shown, the systemic levels of VEGF-A, VEGF-C, VEGF-D, VEGF-R1, VEGF-R2 and VEGF-R3 exhibited a significant positive relationship with HbA1c levels in TB-DM individuals, indicating a positive association of these factors with poor glycemic control.
Elevated levels of circulating angiogenic factors are associated with delayed sputum culture conversion in TB-DM
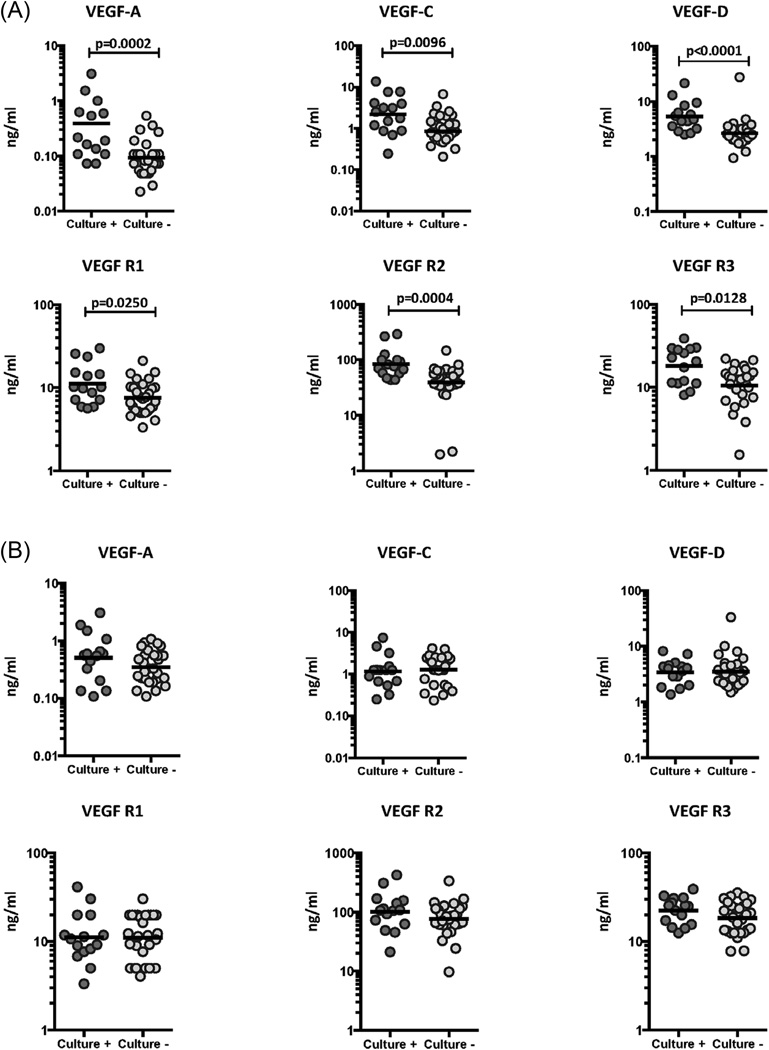
To determine the association of systemic levels of circulating angiogenic factors with the presence of bacteria in sputum cultures, we measured the systemic levels of VEGF-A, C, D, R1, R2 and R3 in TB-DM individuals at 2 months of ATT and compared levels in culture positive versus culture negative individuals (Figure 4). As shown in Figure 4A, the systemic levels of VEGF-A (GM of 0.39 ng/ml in culture positive vs. 0.09 ng/ml in culture negative), VEGF-C (GM of 2.2 ng/ml vs. 0.85 ng/ml) and VEGF-D (GM of 5.4 ng/ml vs. 2.6 ng/ml) were significantly higher in culture positive TB-DM compared to culture negative TB-DM individuals. Similarly, the circulating levels of VEGF-R1 (GM of 11.2 ng/ml vs. 7.6 ng/ml), VEGF-R2 (GM of 84.5 ng/ml vs. 39.2 ng/ml) and VEGF-R3 (GM of 18.16 ng/ml vs. 10.58 ng/ml) were significantly higher in culture positive TB-DM compared to culture negative TB-DM individuals. We also performed analysis to determine whether baseline VEGF values are significantly different in TB-DM individuals, who would remain culture positive at 2 months compared to those who would turn culture negative. As shown in Figure 4B, no significant differences in VEGF-A, C, D, R1, R2 and R3 was observed between the two groups of TB-DM individuals at baseline, indicating that these factors are not useful predictors of delayed sputum culture conversion in TB-DM. Therefore, continued culture positivity in TB-DM is associated with elevated systemic levels of circulating angiogenic factors.
Figure 4.
Elevated systemic levels of circulating angiogenic factors in culture positive TB-DM individuals at 2 months of ATT. (A) The plasma levels of VEGF-A, C, D, VEGF-R1, R2 and R3 were measured in culture positive (n=15) and culture negative (n=29) TB-DM individuals at 2 months of ATT. (B) The baseline plasma levels of VEGF-A, C, D, VEGF-R1, R2 and R3 were compared in TB-DM individuals who would remain culture positive (n=15) or turn culture negative (n=29) at 2 months of ATT. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm's correction for multiple comparisons.
Circulating angiogenic factors are significantly diminished following ATT
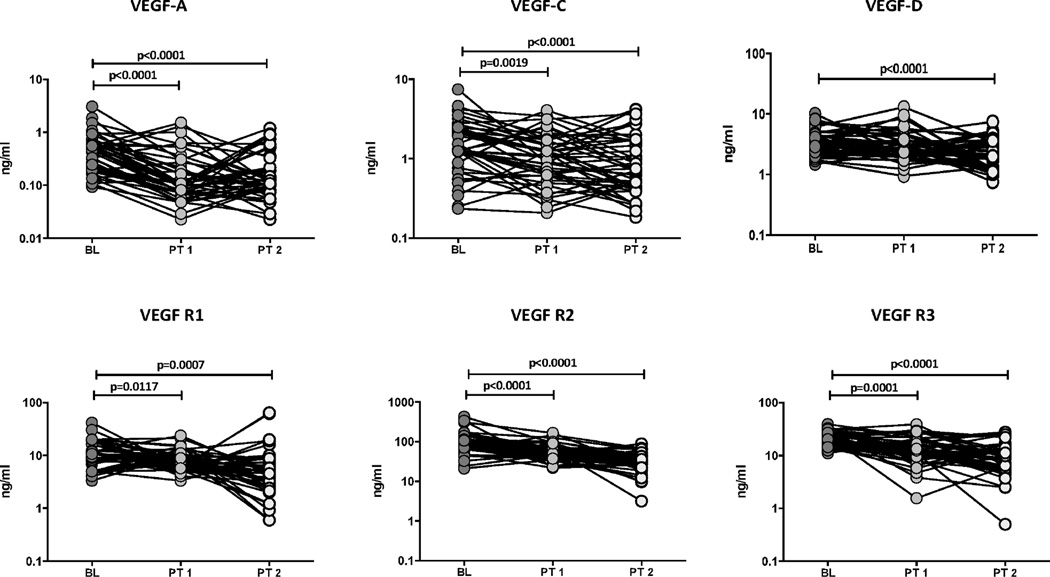
To determine whether the elevated levels of circulating angiogenic factors are directly associated with TB disease, we determined the levels of these factors in TB-DM individuals at baseline (BL), at 2 months (PT1) and at the end of 6 months of ATT (PT2). As shown in Figure 5, at the end of 2 and 6 months of ATT, the circulating levels of VEGF-A (GM of 0.39 ng/ml at BL vs. 0.13 ng/ml at PT1 and 0.13 ng/ml at PT2), VEGF-C (GM of 1.4 ng/ml at BL vs. 0.99 ng/ml at PT1 and 0.81 ng/ml at PT2) and VEGF-D (GM of 3.38 ng/ml at BL vs. 2.11 ng/ml at PT2) were all significantly lower in TB-DM individuals. Similarly, the levels of VEGF-R1 (GM of 11.1 ng/ml at BL vs. 7.9 ng/ml at PT1 and 5.1 ng/ml at PT2), VEGF-R2 (GM of 88.4 ng/ml at BL vs. 55.4 ng/ml at PT1 and 31.2 ng/ml at PT2) and VEGF-R3 (GM of 20.2 ng/ml at BL vs. 12.7 ng/ml at PT1 and 8.4 ng/ml at PT2) were also significantly lower at 2 and 6 months of ATT compared to baseline. Thus, successful treatment of TB-DM is associated with significantly diminished levels of circulating angiogenic factors in PTB.
Figure 5.
Diminished systemic levels of angiogenic factors at 2 and 6 months of ATT in TB-DM individuals. The plasma levels of VEGF-A, C, D, VEGF-R1, R2 and R3 were measured in TB-DM individuals at baseline (BL), at 2 months (PT1) and at 6 months (PT2) of ATT. The data are represented as line graphs with each line representing a single individual. P values were calculated using the Wilcoxon signed rank test.
DISCUSSION
TB-DM is characterized by the presence of an exaggerated inflammatory response with augmented levels of circulating inflammatory cytokines and other factors. Thus, individuals with TB-DM exhibit elevated systemic levels of Type 1 (IFNγ. TNF-α, IL-2), Type 17 (IL-17A) and other pro-inflammatory (IL-1β, IL-6 and IL-18) cytokines [20, 21]. This is associated with an expansion of pathogen-specific Th1 and Th17 response as well as an increased repertoire of cytokine-producing and cytotoxic molecule expression CD8+ T cells and NK cells [22, 23]. Similarly, TB-DM is also associated with increased frequencies of central memory CD4+ and CD8+ T cells [24]. In addition, plasma levels of inflammatory factors, including heme oxygenase-1, tissue inhibitor of matrix metalloproteinase-4, leptin, visfatin-1 and platelet activating factor-1 but not acute phase proteins are higher in TB-DM compared to TB alone individuals [25, 26], indicating the presence of a pronounced inflammatory milieu.
Vascular dysfunction is a major contributor to the pathogenesis of DM and dysregulated angiogenesis and endothelial activation are often the main features of diabetic complications. Persistent, uncontrolled angiogenesis is a key pathological characteristic of the microvascular complications of DM [6]. Moreover, in DM, there are two tissue specific paradoxical events in the vasculature with excessive formation of premature blood vessels afflicting the retina, while deficiency of small blood vessels in the skin, contributes to impaired wound healing [6]. Hence, the use of angiogenic inhibitors is a common treatment modality for diabetic retinopathy [27, 28]. Similarly, abnormal angiogenesis and vascular dysfunction are also prominent features of active TB and utilization of host-directed therapies aimed at inhibition of these pathways has been proposed as an adjunct measure for TB treatment [16, 17]. In addition, the chronic inflammation engendered in active TB disease has been posited to influence angiogenic and lymphangiogenic activity in different models of TB infection [16, 17, 29]. However, the role of circulating angiogenic factors has never been explored in detail in TB-DM co-morbidity.
Our study reveals three salient features in terms of the association of TB-DM and circulating angiogenic factors. First, TB-DM is characterized by elevated levels of VEGF-A, C, D and their endogenous receptors. Since, we have previously shown that TB per se is characterized by enhanced levels of VEGF-A, C and R2 compared to latently infected or not infected individuals [15], this result implies that TB-DM is actually associated with super-enhanced levels of VEGF family of growth factors. VEGF-A is a known major player in angiogenesis and lymphangiogenesis and is induced in response to tissue inflammation, hypoxia and pro-inflammatory cytokines [30, 31]. VEGF-C and VEGF-D are known to modulate lymphangiogenesis via VEGF-R3 signaling [32] and are known to be present at elevated levels in diseases associated with pathological lymphangiogenesis. Among the VEGF receptors, only two (VEGF-R2 and VEGF-R3) drive angiogenesis, while VEGF-R1 mostly acts to restrict the angiogenic response [33]. Although the VEGF receptors are predominantly expressed on endothelial cells, soluble forms of VEGF receptors are known to be induced in inflammation [9]. Thus, elevated concentrations of VEGF-R2 and R3 reflect underlying angiogenesis or lymphangiogenesis, while elevated levels of VEGF-R1 reflect suppressed angiogenesis [9]. Our study clearly reveals an important association of TB-DM with enhanced levels of all of the above factors. While we have not explored the direct implications of this enhancement in terms of actual pathological angiogenesis or lymphangiogenesis, the potential inference from these findings is that TB-DM presents with a dysregulated angiogenic milieu that possibly reflects both underlying inflammatory processes and vascular/endothelial dysfunction.
The second major finding from our study is the interesting relationship of circulating angiogenic factors with disease severity and bacterial burden in TB and with poor glycemic control in DM. Thus, it appears that (similar to our previous finding in TB) VEGF-A, C, R2 and R3 are important biomarkers of TB disease severity in the context of TB-DM as well [15]. Interestingly, while all the VEGF family members examined exhibited differences between TB-DM and TB, only the above mentioned factors were observed to correlate with disease severity. This could possibly indicate that all VEGF factors are not necessarily markers of TB disease severity and could potentially be related to the presence of DM as well. Moreover, the heightened levels of these angiogenic factors appear to be directly associated with bacterial burdens. This conclusion was further confirmed by our 2 month post-treatment data, where in culture positive TB-DM individuals had significantly elevated levels of angiogenic factors compared to culture negative individuals, indicating that delayed sputum culture conversion observed in a subset of TB-DM individuals is also associated with elevated circulating angiogenic factors. Thus, these circulating angiogenic factors have the potential to be reliable biomarkers of treatment success at the completion of therapy. However, systemic levels of angiogenic factors at baseline were not predictive of delayed sputum culture conversion, since TB-DM individuals who culture converted and those who remained positive did not exhibit any significant differences in these levels at baseline. Nevertheless, this result also provides evidence for an important association of differential VEGF family expression with bacterial burdens, since differences in VEGF family levels become manifest only upon reduction (and/or elimination) of bacterial burdens. Finally and of interest in the context of DM, it was also observed that poor glycemic control (in terms of HbA1c levels) also exhibited a positive correlation with enhanced systemic levels of angiogenic factors. Since we did not test for the presence of DM complications (specifically retinopathy), we cannot exclude a confounding effect of microvascular complications imposed by DM alone or any effect of treatment of complications (specifically anti-VEGF-A therapy) on these findings. Nevertheless, our data clearly suggest that the markers that characterize disease severity in TB are also marker of disease severity in DM and reinforce the negative role played by the co-morbidity on host physiological processes. In addition, our data also suggest that while diabetic complications are associated with pathological angiogenesis, the occurrence of active TB in DM individuals could potentially exacerbate microvascular complications.
The third and final salient finding from our study is the reversibility of the enhanced levels of circulating angiogenic factors. These results clearly delineate the association of active bacterial burdens with the increased levels of these angiogenic factors. Also, since accumulating evidence indicates that inflammation-associated angiogenesis is actively involved in pathophysiology of various inflammatory diseases [34], our data clearly implicate inflammation-associated angiogenesis as potential factor driving pathogenesis of TB-DM. This is further corroborated by our data on the association of hemoptysis with elevated levels of VEGF-A, C and R2 in TB-DM, since hemoptysis typically is more likely to occur in TB-DM than TB individuals [35–37]. Our results indicate that elevated levels of angiogenic factors could potentially reflect neovascularization in lung lesions and tissue-destructive immune pathology in TB-DM, leading to increased risk of hemoptysis [38]. Also, of interest, we were not able to discern any significant differences in the systemic levels of circulating angiogenic factors between known and newly diagnosed DM amongst the TB-DM (data not shown), although this might have been influenced by the small number of newly diagnosed DM (n=10) in our study. There were no differcnes in the glycemic control between the two groups at baseline or end of treatment. While our study is purely descriptive in nature and does not prove any cause and effect, it nevertheless provides important evidence of association and therefore, the need to explore the role of these factors in pathogenesis further.
Our study is the first to systemically explore the association of circulating angiogenic factors in TB-DM. Moreover, our findings reinforce the logic of using anti-angiogenic agents as host directed therapeutics in TB-DM and suggest that this could benefit the host by limiting both bacterial burden and lung pathology [39]. Our previous study [15] along with the data in this study demonstrates an important association of TB disease severity with angiogenic factors and suggests that TB-DM individuals might be a particularly good target population for anti-angiogenic host-directed therapies. In addition, these agents could prove beneficial as host adjuncts to reduce the duration of chemotherapy as well as agents reducing the incidence of relapse or recurrence. Finally, our study illustrates the complex network interlinking the pathogenesis of TB-DM in terms of host physiological disruption.
Highlights.
Angiogenic factors are associated with disease severity and adverse clinical presentation in TB-DM
The systemic increase in angiogenic factor levels is driven by dysglycemia and bacterial burdens
Acknowledgments
We thank the staff of Department of Clinical Research and the Department of Bacteriology, NIRT for valuable assistance in bacterial cultures and radiology and the Staff of MVDRC for valuable assistance in recruiting the patients for this study, R. Anuradha, Prabha Chandran and Gokul Raj of the NIH-NIRT-ICER for technical assistance. This work was jointly sponsored by the Indian Department of Biotechnology; the Indian Council of Medical Research; and the National Institute for Allergy and Infectious Diseases, National Institutes of Health, and administered by CRDF Global [grant USB1-31149-XX-13]. This work was also funded in part by the Division of Intramural Research, NIAID, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: None reported.
Author disclosure: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
REFERENCES
- 1.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harries AD, Lin Y, Satyanarayana S, et al. The looming epidemic of diabetes-associated tuberculosis: learning lessons from HIV-associated tuberculosis. Int J Tuberc Lung Dis. 2011;15:1436–1444. doi: 10.5588/ijtld.11.0503. i. [DOI] [PubMed] [Google Scholar]
- 4.Martinez N, Kornfeld H. Diabetes and immunity to tuberculosis. Eur J Immunol. 2014;44:617–626. doi: 10.1002/eji.201344301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Restrepo BI, Schlesinger LS. Host-pathogen interactions in tuberculosis patients with type 2 diabetes mellitus. Tuberculosis (Edinb) 2013;93(Suppl):S10–S14. doi: 10.1016/S1472-9792(13)70004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng R, Ma JX. Angiogenesis in diabetes and obesity. Rev Endocr Metab Disord. 2015;16:67–75. doi: 10.1007/s11154-015-9310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuyama W, Hashiguchi T, Matsumuro K, et al. Increased serum level of vascular endothelial growth factor in pulmonary tuberculosis. Am J Respir Crit Care Med. 2000;162:1120–1122. doi: 10.1164/ajrccm.162.3.9911010. [DOI] [PubMed] [Google Scholar]
- 8.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21:154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Jeltsch M, Leppanen VM, Saharinen P, Alitalo K. Receptor tyrosine kinase-mediated angiogenesis. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a009183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe Y, Nakamura M, Oshika Y, et al. Serum levels of vascular endothelial growth factor and cavity formation in active pulmonary tuberculosis. Respiration. 2001;68:496–500. doi: 10.1159/000050557. [DOI] [PubMed] [Google Scholar]
- 11.Alatas F, Alatas O, Metintas M, Ozarslan A, Erginel S, Yildirim H. Vascular endothelial growth factor levels in active pulmonary tuberculosis. Chest. 2004;125:2156–2159. doi: 10.1378/chest.125.6.2156. [DOI] [PubMed] [Google Scholar]
- 12.Mihret A, Bekele Y, Bobosha K, et al. Plasma cytokines and chemokines differentiate between active disease and non-active tuberculosis infection. J Infect. 2013;66:357–365. doi: 10.1016/j.jinf.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Ota MO, Mendy JF, Donkor S, et al. Rapid diagnosis of tuberculosis using ex vivo host biomarkers in sputum. Eur Respir J. 2014;44:254–257. doi: 10.1183/09031936.00209913. [DOI] [PubMed] [Google Scholar]
- 14.Riou C, Perez Peixoto B, Roberts L, et al. Effect of standard tuberculosis treatment on plasma cytokine levels in patients with active pulmonary tuberculosis. PLoS One. 2012;7:e36886. doi: 10.1371/journal.pone.0036886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar NP, Banurekha VV, Nair D, Babu S. Circulating Angiogenic Factors as Biomarkers of Disease Severity and Bacterial Burden in Pulmonary Tuberculosis. PLoS One. 2016;11:e0146318. doi: 10.1371/journal.pone.0146318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Datta M, Via LE, Kamoun WS, et al. Anti-vascular endothelial growth factor treatment normalizes tuberculosis granuloma vasculature and improves small molecule delivery. Proc Natl Acad Sci U S A. 2015;112:1827–1832. doi: 10.1073/pnas.1424563112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oehlers SH, Cronan MR, Scott NR, et al. Interception of host angiogenic signalling limits mycobacterial growth. Nature. 2015;517:612–615. doi: 10.1038/nature13967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolluru GK, Bir SC, Kevil CG. Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing. Int J Vasc Med. 2011;2012:918267. doi: 10.1155/2012/918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornfeld H, West K, Kane K, et al. High Prevalence and Heterogeneity of Diabetes in TB Patients from South India: A Report from the Effects of Diabetes on Tuberculosis Severity (EDOTS) Study. Chest. 2016 doi: 10.1016/j.chest.2016.02.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar NP, Sridhar R, Banurekha VV, et al. Type 2 diabetes mellitus coincident with pulmonary tuberculosis is associated with heightened systemic type 1, type 17, and other proinflammatory cytokines. Ann Am Thorac Soc. 2013;10:441–449. doi: 10.1513/AnnalsATS.201305-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Restrepo BI, Fisher-Hoch SP, Pino PA, et al. Tuberculosis in poorly controlled type 2 diabetes: altered cytokine expression in peripheral white blood cells. Clin Infect Dis. 2008;47:634–641. doi: 10.1086/590565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar NP, Sridhar R, Banurekha VV, Jawahar MS, Nutman TB, Babu S. Expansion of pathogen-specific T-helper 1 and T-helper 17 cells in pulmonary tuberculosis with coincident type 2 diabetes mellitus. J Infect Dis. 2013;208:739–748. doi: 10.1093/infdis/jit241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar NP, Sridhar R, Nair D, Banurekha VV, Nutman TB, Babu S. Type 2 diabetes mellitus is associated with altered CD8(+) T and natural killer cell function in pulmonary tuberculosis. Immunology. 2015;144:677–686. doi: 10.1111/imm.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar NP, Moideen K, Viswanathan V, Kornfeld H, Babu S. Effect of standard tuberculosis treatment on naive, memory and regulatory T cell homeostasis in tuberculosis-diabetes co-morbidity. Immunology. 2016 doi: 10.1111/imm.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrade BB, Pavan Kumar N, Sridhar R, et al. Heightened plasma levels of heme oxygenase-1 and tissue inhibitor of metalloproteinase-4 as well as elevated peripheral neutrophil counts are associated with TB-diabetes comorbidity. Chest. 2014;145:1244–1254. doi: 10.1378/chest.13-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pavan Kumar N, Nair D, Banurekha VV, et al. Type 2 diabetes mellitus coincident with pulmonary or latent tuberculosis results in modulation of adipocytokines. Cytokine. 2016;79:74–81. doi: 10.1016/j.cyto.2015.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A, Bade G, Trivedi A, Jyotsna VP, Talwar A. Postural variation of pulmonary diffusing capacity as a marker of lung microangiopathy in Indian patients with type 2 diabetes mellitus. Indian J Endocrinol Metab. 2016;20:238–244. doi: 10.4103/2230-8210.176343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Virgili G, Parravano M, Menchini F, Evans JR. Anti-vascular endothelial growth factor for diabetic macular oedema. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD007419.pub4. CD007419. [DOI] [PubMed] [Google Scholar]
- 29.Harding J, Ritter A, Rayasam A, Fabry Z, Sandor M. Lymphangiogenesis is induced by mycobacterial granulomas via vascular endothelial growth factor receptor-3 and supports systemic T-cell responses against mycobacterial antigen. Am J Pathol. 2015;185:432–445. doi: 10.1016/j.ajpath.2014.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 31.Koch S, Tugues S, Li X, Gualandi L, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J. 2011;437:169–183. doi: 10.1042/BJ20110301. [DOI] [PubMed] [Google Scholar]
- 32.Coso S, Bovay E, Petrova TV. Pressing the right buttons: signaling in lymphangiogenesis. Blood. 2014;123:2614–2624. doi: 10.1182/blood-2013-12-297317. [DOI] [PubMed] [Google Scholar]
- 33.Ho VC, Duan LJ, Cronin C, Liang BT, Fong GH. Elevated vascular endothelial growth factor receptor-2 abundance contributes to increased angiogenesis in vascular endothelial growth factor receptor-1-deficient mice. Circulation. 2012;126:741–752. doi: 10.1161/CIRCULATIONAHA.112.091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H, Kataru RP, Koh GY. Inflammation-associated lymphangiogenesis: a double-edged sword? J Clin Invest. 2014;124:936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chiang CY, Bai KJ, Lin HH, et al. The influence of diabetes, glycemic control, and diabetes-related comorbidities on pulmonary tuberculosis. PLoS One. 2015;10:e0121698. doi: 10.1371/journal.pone.0121698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gil-Santana L, Almeida-Junior JL, Oliveira CA, et al. Diabetes Is Associated with Worse Clinical Presentation in Tuberculosis Patients from Brazil: A Retrospective Cohort Study. PLoS One. 2016;11:e0146876. doi: 10.1371/journal.pone.0146876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Restrepo BI, Fisher-Hoch SP, Crespo JG, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135:483–491. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah M, Reed C. Complications of tuberculosis. Curr Opin Infect Dis. 2014;27:403–410. doi: 10.1097/QCO.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 39.Matty MA, Roca FJ, Cronan MR, Tobin DM. Adventures within the speckled band: heterogeneity, angiogenesis, and balanced inflammation in the tuberculous granuloma. Immunol Rev. 2015;264:276–287. doi: 10.1111/imr.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]