Abstract
The basal forebrain cholinergic projection system to the cortex mediates essential aspects of visual attention performance, including the detection of cues and the response to performance challenges (top-down control of attention). Higher levels of top-down control are mediated via elevated levels of cholinergic neuromodulation. The neuronal choline transporter (CHT) strongly influences the synthesis and release of acetylcholine (ACh). As the capacity of the CHT to import choline into the neuron is a major, presynaptic determinant of cholinergic neuromodulation, we hypothesize that genetically-imposed CHT capacity variation impacts the balance of bottom-up versus top-down control of visual attention. Following a brief review of the cognitive concepts relevant for this hypothesis, we describe the key results from our research in mice and humans that possess genetically-imposed changes in choline uptake capacity. CHT subcapacity variation is associated with poor top-down attentional control and attenuated (cholinergic) activation of right frontal regions. Conversely, mice overexpressing the CHT, and humans expressing a CHT variant hypothesized to enhance choline transporter function, are relatively resistant to challenges of visual attention performance. Genetic or environmental modulation of CHT expression and function may be associated with vulnerabilities for cognitive disorders.
Keywords: acetylcholine, attention, choline transporter, genetics, rodents, humans
1. Introduction
The basal forebrain cholinergic system plays a critical role in visual attention, as well as other functions including including attention-dependent motor performance and learning and memory (Bucci et al., 1998; Everitt and Robbins, 1997; Gill et al., 2000; Hasselmo and Sarter, 2011; Sarter et al., 2014a). Our research on the cholinergic system has focused especially on its contribution to detecting and responding to visual signals, both in relatively easy baseline conditions where signal detection can rely largely on bottom-up attention and in more challenging situations that require top-down control. Here we describe how genetic variation in the capacity of the high-affinity neuronal choline transporter (CHT; SLC5A7) affects visual attention, especially its top-down modulation.
Contrary to early views of cholinergic activity as a nonspecific neuromodulator involved primarily in general physiological states such as “arousal”, the findings over the last ten years indicate a great deal of both spatial and temporal specificity of cholinergic signaling. In terms of temporal specificity, there appear to be both phasic (transient) and tonic (neuromodulatory) modes of cholinergic signaling (Gritton et al., 2016; Hasselmo and Sarter, 2011; Howe et al., 2013; Parikh et al., 2007; Sarter, 2015; Sarter and Kim, 2015; Sarter et al., 2014b, 2016). Phasic or “transient” cholinergic signals causally mediate the detection of cues, that is, “the entirety of information concerning the presence of a signal into a system that allows the subject to report the existence of the signal (or cue) by an arbitrary response specified by the experimenter, and that provides feedback about the adequacy/accuracy of the response based on response outcome” (modified from Posner et al., 1980; for discussion of this definition see Sarter et al., 2016). Arguably the strongest demonstration of the function of cholinergic transients in cue detection involves the false detection of cues following optogenetic generation of such transients during non-cued trials, which normally do not result in phasic endogenous acetylcholine (ACh) release events (Gritton et al., 2016). In contrast, the neuromodulatory component of cholinergic activity, that varies on the scale of seconds to minutes, is significantly elevated when cue detection rates are impaired, such as when attentional performance is challenged by a distractor (St Peters et al., 2011b). These and related findings have supported the hypothesis that cholinergic neuromodulation mediates top-down functions which serve to stabilize or recover performance during and after performance challenges (Sarter et al., 2006).
The relationships between cholinergic neuromodulation and phasic cholinergic signaling are not well understood but we have hypothesized that the higher levels of cholinergic neuromodulation increases the likelihood and amplitude of cholinergic transients, thereby enhancing cue detection performance during or after distractor challenges (Howe et al., 2010; Parikh et al., 2010; Parikh and Sarter, 2008; Sarter, 2015). Furthermore, these two modes of cholinergic neurotransmission have been assumed to be mediated via separate populations of basal forebrain cholinergic neurons, in part because of evidence indicating that increases in cholinergic neuromodulation upregulates the amplitudes of cholinergic transients via stimulation of nicotinic acetylcholine receptors (nAChRs) that are expressed at glutamatergic terminals of neurons originating in the thalamus (Hasselmo and Sarter, 2011; Howe et al., 2010; Parikh et al., 2008, 2010; Sarter et al., 2014b, 2016; see also Lambe et al., 2003). In contrast to the generation of cortical cholinergic transients, that is largely based on cortical circuitry, levels of cholinergic neuromodulation are assumed to reflect primarily the influences of mesolimbic and telencephalic circuitry on basal forebrain neuronal activity. For example, we demonstrated increases in cholinergic neuromodulatory activity in rats performing an attention task and while performance was challenged by a distractor. These increases in neuromodulatory cholinergic activity appear to depend on mesolimbic output, reflecting the interactions between motivational and attentional functions (Neigh et al., 2004; St Peters et al., 2011b).
The role of muscarinic and nicotinic acetylcholine receptors (m/nAChR) in mediating the effects of cholinergic neuromodulatory activity and of cholinergic transients likewise remains largely unclear. While nAChR receptors are primarily located presynaptically, mAChR mediate effects of cholinergic activity on postsynaptic neurons and thus efferent cortical circuitry (Dani and Bertrand, 2007; Poorthuis et al., 2013; Thiele, 2013). Thus, the effects of mAChR antagonists on cognitive functions in rodents and humans have generally been thought to reflect attenuation of cortico-cortical and cortico-subcortical information transfer (Davidson et al., 1999; Klinkenberg and Blokland, 2010). Consistent with this view, we found that blocking M1 mAChR, but not nAChR, in the cortex attenuates the high frequency synchrony that supports the detection of cues (Howe et al., 2012). Research on the role of mAChR in cognition has been hampered by the complex and conflicting effects of mAChR antagonists such as scopolamine on cholinergic neurotransmission, and the absence of ligands to probe mAChR subtypes (Hasselmo and Sarter, 2011; Sarter, 2015).
In computational terms, we have proposed that higher levels of cholinergic neuromodulation reduce opportunity costs and therefore support task compliance, particularly in situations in which the costs for staying-on-task increase because of performance challenges such as long task sessions or the presentation of distractors. Trial-specific cholinergic transients reduce detection uncertainty, therefore fostering performance in cued trials specifically if such trials occur at a relatively low frequency or are interspersed within strings of non-cued trials (Sarter et al., 2014b). As higher levels of cholinergic neuromodulation foster the generation of transients, reduced opportunity costs and reduced detection uncertainty are coupled to stabilize, recover and enhance attentional performance. Put another way, the neuromodulatory component helps to maintain a stable representation of the task context, whereas transients appear to mediate activation of the specific response rule associated with detecting the signal.
Our general hypothesis is that variations of CHT capacity primarily constrain the capacity of cholinergic neurons to sustain elevated levels of cholinergic neuromodulation. Because higher levels of cholinergic neuromodulation in the cortex specifically mediate top-down attentional control functions, CHT capacity variations will be directly linked to attentional performance that is either dominated by bottom-up (CHT subcapacity variants) or by top-down mechanisms (CHT overcapacity variants). To evaluate this hypothesis, we will begin this review by clarifying the use of the terms bottom-up and top-down and describing the tasks used in rodents and humans to test these attentional biases. We will then briefly introduce the CHT and describe the evidence from our multi-species, translational research that has given rise to our main hypothesis and conclude by discussing implications of expressing CHT variants for psychopathology.
2. Bottom-up versus top down control of attention
2.1. Bottom-up vs. top-down
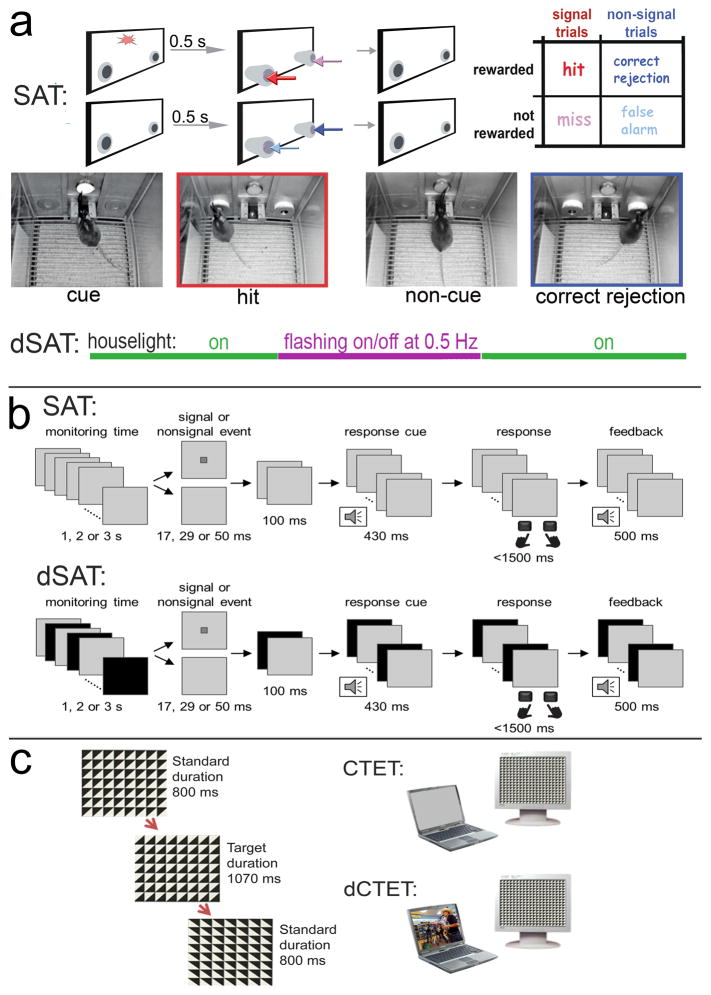
The selection of stimuli to control behavior may reflect the saliency of a stimulus (bottom-up) and it may be influenced by longer-term strategies and goals that bias our attention toward certain sources and types of stimuli (top-down; e.g., Buschman and Miller, 2007; Connor et al., 2004; Miller and D’Esposito, 2005; Sarter et al., 2001). The Sustained Attention Task (SAT) and its distractor/disruptor condition (dSAT; see below) can be used to assess both of these aspects of attentional function (see task diagrams in Figure 1). Bottom-up attention refers to the relatively automatic attraction to, or capture of, attention by salient environmental stimuli. The target signal in the SAT is a classic example of the type of salient stimulus that attracts attention bottom-up: It is a brief, sudden-onset change. Top-down processes are the internal representations and goals that influence how you respond: In the SAT, they guide whether you press the right key/lever or the left one, and that you respond with the keypress rather than touching the screen.
Figure 1. Tasks used in rodents and humans to assess bottom-up and top-down attention.
a: The rodent Sustained Attention Task (SAT) consists of a randomized sequence of cue and non-cue events, followed by extension of the two response ports (McGaughy et al., 1996; McGaughy and Sarter, 1995; St Peters et al., 2011a). Hits and correct rejections, but not misses and false alarms, are rewarded. Note the matching colors of the arrows pointing to the response ports for the four response categories and the arrows in the outcome matrix on the right, indicating that, in cued trials, hits (red arrows) are rewarded and in non-cued trials, correct rejections are rewarded (dark blue). Conversely, misses (pink arrows) and false alarms (light blue arrows) are not rewarded and trigger the intertrial interval. The photographs depict the mouse version of the task and show the onset of a cued (left) and non-cued trial (third from left), and a hit (red frame, matching arrow color) and a correct rejection (dark blue frame). dSAT: To test top-down control mechanisms, a disruptor (houselights flashing at 0.5 Hz; dSAT) impairs performance, followed by performance recovery. The disruptor is presented during a period in the middle of a regular session (see violet line), typically for 16 min of a 60 min test session. SAT performance is associated with significant increases in levels of cholinergic neuromodulation in the cortex, and dSAT performance further elevates cholinergic activity and this increase correlates positively with post-distractor recovery (St Peters et al., 2011b). b: The human SAT, similar to the rodent task, requires reporting the presence or absence of a cue, and in the dSAT the computer screen flashes continuously from gray to black at a rate of 10 Hz (Demeter et al., 2013; Demeter et al., 2008). Human subjects increase right frontal activity (BA9) while performing the dSAT, indicating their persistent reliance on top-down mechanisms (Demeter et al., 2011). c: The CTET (Continuous Temporal Expectancy Test; O’Connell et al., 2009) consists of a black and white grid made up of squares divided into triangles. The triangles rotate randomly (90°, 180°, or 270°) and consecutive rotations are separated by 800 ms. Participants press the spacebar when noting that a longer period (1070 ms) separated consecutive rotations. The distractor-CTET uses a laptop oriented 32° to the left of the main task computer. The laptop is silent and displays a gray screen during CTET, and it plays content-rich video clips with sound during dCTET.
2.2. Challenging top-down attention: Disruptors and distractors
Imagine that as you are reading in your office, the lights overhead begin to flicker, similar to the changing background in the dSAT (Fig. 1). Although you can still see the text, this disruptor taxes your ability to stay on task and absorb the information. Compare this to a situation where you have your email open in another window on the screen, and in your peripheral vision see when each new message arrives. That distractor imposes an additional challenge by directly competing for the focus of your attention.
It is important to acknowledge species-specific differences in the efficacy of irrelevant or non-target stimuli to challenge attentional performance and in the subject’s ability to deploy top-down mechanisms to resist such interference and maintain or recover performance. The relatively low top-down control of rodents makes them highly vulnerable to disruption, with cholinergic neuromodulatory activity-associated differences in top-down control being revealed primarily by differential post-disruption performance recovery. Humans’ generally greater top-down control as well as situational constraints (i.e., they are seated in front of the testing computer) renders their performance relatively immune to disruption. Thus, the additional demands imposed by competitive distraction are required to reveal performance differences between human genetic groups varying in cholinergic function, although there are significant group differences in the patterns of challenge-evoked right prefrontal activation (Berry et al., 2014a, 2015). While keeping this issue in mind, the SAT and its attentional challenge condition, the dSAT, allow the assessment of bottom-up and top-down control functions in both species.
2.3. Cholinergic mediation of top-down control
As mentioned previously, the cholinergic system acts in different circuits and timescales to support different aspects of attention, and these are distinct both temporally and spatially. The response to the bottom-up saliency of the sudden-onset target signal involves phasic ACh release events that are generated in part in interaction with thalamocortical circuits (above; Gritton et al., 2016; Sarter et al., 2014b). In contrast, the top-down attention functions involved in increasing and maintaining attention to the task, such as in response to disruptors, are supported by longer-timescale (seconds to minutes) cholinergic stimulation of right fronto-parietal circuitry (Paolone et al., 2010; St Peters et al., 2011b).
In rodents, performance during the no-disruptor SAT increases (neuromodulatory) right PFC ACh levels above no-task baseline. However, the amount of ACh increase is poorly correlated with performance, reflecting that while some minimal degree of top-down control is needed to engage the overall task set of monitoring for and responding to the cue, beyond that detecting and responding to the sudden-onset cue used in the SAT is driven primarily by bottom-up attention (and may be more strongly related on a trial-by-trial basis to the transient response). In contrast, the attentional challenge condition (dSAT) robustly impairs performance while further elevating levels of cholinergic neuromodulation, and higher increases are correlated with better disruptor resistance and better performance recovery (St Peters et al., 2011b). As a result, behavioral differences between mice expressing different CHT capacity variants are seen largely in the attentional challenge condition, rather than in the no-disruptor SAT (below).
3. Choline transporter regulation and function
In the brain, the CHT is uniquely expressed in cholinergic neurons (Ferguson et al., 2003; Kus et al., 2003). The transport of choline into presynaptic terminals via the high-affinity, hemicholinium-3 (HC-3)-sensitive CHT represents the rate-limiting step for ACh synthesis and release (Fig. 2; Bazalakova and Blakely, 2006; Ferguson and Blakely, 2004; Sarter and Parikh, 2005). Extracellular choline plasma concentrations are relatively stable at low-μM levels (Parikh and Sarter, 2006), and therefore variations in CHT capacity arise instead from two main sources. First, genetically-imposed CHT protein variants have different choline transport capacities (Okuda and Haga, 2000; Okuda et al., 2000, 2002). Second, CHT capacity in neurons is regulated by the density of CHTs in synaptosomsal plasma membrane (Apparsundaram et al., 2005; Ferguson et al., 2004; Gates et al., 2004; Holmstrand et al., 2010; Parikh et al., 2013; Ribeiro et al., 2003; Sarter and Parikh, 2005).
Figure 2. Schematic illustration of the main steps of presynaptic cholinergic transmission.
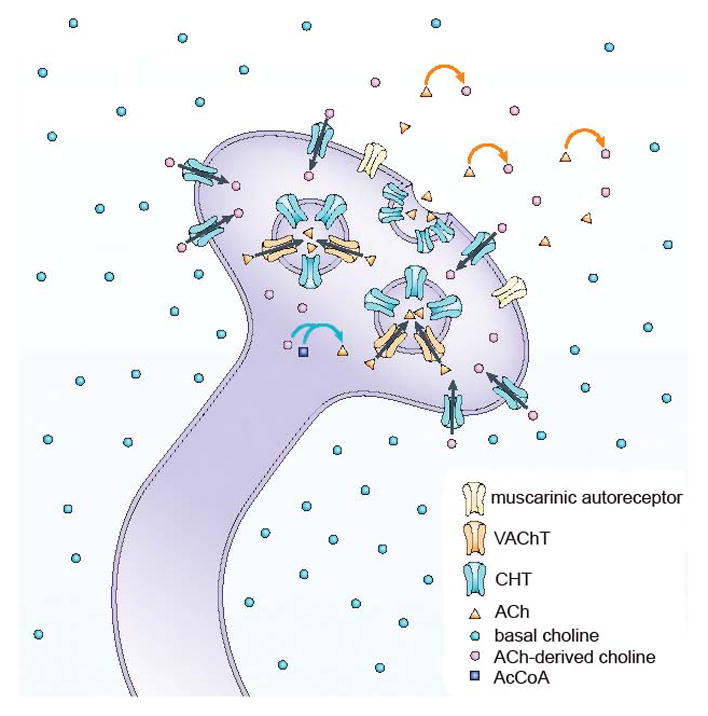
The cholinergic terminal is situated in the extracellular space, which contains low (micromolar) and relatively stable concentrations of basal choline. Hydrolysis of acetylcholine (ACh) (orange arrows) gives rise to ACh-derived choline, which is then effectively recycled into the presynaptic terminals by hemicholinium-3-sensitive, high-affinity choline uptake transporters (CHT). ACh-derived choline and acetyl-coenzyme A together give rise to ACh in the presynaptic terminal, catalysed by choline acetyltransferase (ChAT; blue arrows). The illustration is not intended to suggest that only choline molecules that are derived from ACh are transported back into the terminal; rather, it reflects evidence indicating that amounts proportional to ACh-derived choline are rapidly cleared from the extracellular space. The illustration does not depict the low-affinity transporter and the use of choline for other purposes, such as phospholipid metabolism (Lockman and Allen, 2002). Vesicular acetylcholine transporters pack newly synthesized ACh into vesicles. Because most CHTs are located on the vesicular membrane, changes in the activity of cholinergic terminals are predicted to alter the trafficking of CHTs between the plasma and vesicular membrane (Ferguson et al., 2004; Ferguson et al., 2003; Simon et al., 1976). (Figure and legend reproduced, in accordance with NPG Licence Policy, from Sarter and Parikh, 2005.)
3.1. Attentional performance upregulates CHT capacity
Pursuing mechanisms to support our earlier demonstration of SAT-associated increases in cortical cholinergic neuromodulation (Paolone et al., 2013; St Peters et al., 2011b), we found that synaptosomal, CHT-mediated (HC-3-dependent) choline uptake was enhanced in right frontal cortex in rats that completed a SAT session, but not in rats that performed a behavioral control procedure with reduced demands on attention. In separate groups of rats, SAT performance also increased the density of CHT in the synaptosomal plasma membrane (Apparsundaram et al., 2005). Subsequent experiments in mice confirmed that SAT performance upregulates CHT capacity exclusively in the right cortex (Parikh et al., 2013). The mechanisms underlying performance-associated outward mobilization of CHT remains essentially unclear, but likely involves depolarization-dependent as well as depolarizatIon-independent, vesicular membrane and early endosome-based CHT delivery to the plasma membrane at a rate that surpasses transporter endocytosis (Pinthong et al., 2008; Ribeiro et al., 2003, 2005, 2006).
4. CHT subcapacity and vulnerability to demands on top-down control
4.1. CHT+/− mice
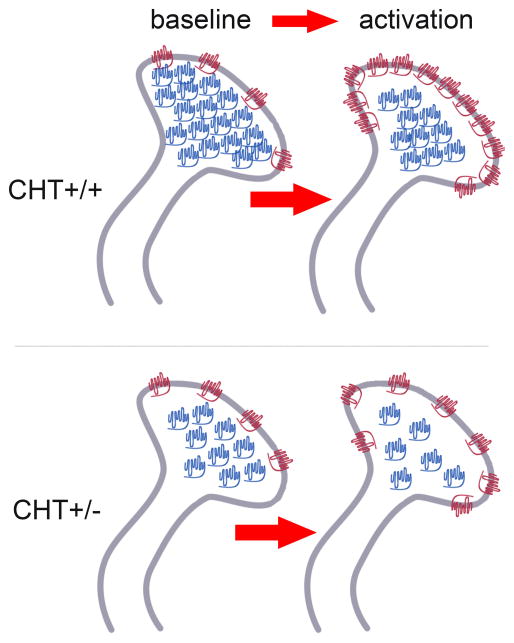
CHT heterozygous mice (CHT+/− mice) support HC-3 sensitive choline uptake and basal ACh release at WT levels (Ferguson et al., 2004), but are vulnerable to manipulations that tax cholinergic activity (Bazalakova et al., 2007). At baseline, the density of CHTs in cortical synaptosomal plasma membrane-enriched fractions from CHT+/− animals matches those observed in wild type (CHT+/+) mice, reflecting that CHT heterozygosity primarily reduces intracellular CHT density (Parikh et al., 2013). Thus, WT and CHT+/− mice support equivalent basal choline transport, ACh release and choline clearance. Instead, genetic variation In the CHT mediates functional (stimulation or task-related) differences in cholinergic capacity: CHT+/− mice show attenuated BF electrical stimulation (BF-ES) -induced increases in plasma membrane CHT density, ACh release, and choline clearance (Parikh et al., 2013). Likewise, CHT+/− mice have significantly lower right frontal synaptosomal plasma membrane (“surface”) CHT densities than WT mice after attentional performance. Because of their relatively low levels of cholinergic neuromodulation, CHT+/− mice are predicted to be severely vulnerable to the effects of disruptor, as indicated primarily by relatively poor post-disruptor attentional performance. This hypothesis remains to be tested, as in our initial studies relatively low SAT performance by both groups prevented a robust test of potential strain differences in response to the disruptor (Parikh et al., 2013).
Together, these results indicate that CHT+/− mice model a specific form of CHT subcapacity. The actual choline uptake capacity of the protein remains at WT levels whereas the lower intracellular CHT densities at baseline limit outward mobilization and thus the capacity of cholinergic neurons to sustain elevated levels of cholinergic neurotransmission (Fig. 3). Interestingly, the contribution made by CHT to cholinergic signaling, one linked more to imposed demand versus basal tone, is conserved across phyla, as demonstrated in the enhanced motor fatigue of nematodes lacking the transporter (CHO-1) in the context of normal basal locomotion (Matthies et al., 2006).
Figure 3. Symbolic Illustration of the effects of BF stimulation or attentional performance on the subcellular synaptosomal distribution of CHTs.
At baseline, comparable densities of synaptosomal plasma membrane CHTs (depicted in red) in CHT+/+ and CHT+/− mice support similar levels of basal ACh release and choline uptake. CHT heterozygosity primarily reduces the density of intracellular CHTs (in blue). Therefore, stimulation of cholinergic neurons or attentional performance reveal the impact of CHT heterozygosity, yielding attenuated levels of CHTs in plasma membrane and thus attenuated levels of cholinergic neuromodulation. (Adopted from Parikh et al., 2013, and reproduced in accordance with the J. Neurosci. Licence to Publish.)
4.2. I89V humans
Okuda and colleagues described a single nucleotide polymorphism (SNP) in the human CHT that reduced choline uptake capacity by 40–50% when expressed in a mammalian cell line (rs1013940 of SLC5A7; termed the I89V variant; Okuda et al., 2002). About a decade ago we began searching for I89V subjects to test the hypothesis that they are vulnerable to distractors and to study associated brain mechanisms. On the Poor Attentional Control scale (PAC; Huba et al., 1982), administered during saliva collection sessions, I89V subjects self-report higher distractibility and mind-wandering than WT (Berry et al., 2014a). The groups were equivalent on boredom, indicating that I89V were not simply interpreting the scales differently or showing a response bias compared to WT. Item analyses showed that questions related to external distraction (e.g., “I find it difficult to concentrate when the TV or radio is on”) best discriminated between the groups. These real-world situations require top-down attention to focus on the task at hand and resist salient external stimuli that attract bottom-up attention. In contrast, the dSAT presents a perceptual challenge and it is the target (a sudden-onset signal) that has considerable bottom-up salience. Thus, as would be expected, I89V subjects were not impaired performing the dSAT.
To more closely approximate the real-world conditions in which I89V subjects report attentional impairment, we added a video distractor (simulating “when the TV is on”) to a duration-discrimination task where the target has very low bottom-up salience and thus requires considerable top-down attention, making performance highly sensitive to lapses of attention and time-on-task declines (Continuous Temporal Expectancy Task, CTET, (O’Connell et al., 2009); see Fig. 1c). Using ungenotyped young adults, we first demonstrated that initial performance, time-on-task effects, and disruptor vulnerability could be independently assessed, and that vulnerability to the video distractor correlated with self-reported distractibility on the same scale used with I89V (Berry et al., 2014b). We next tested age- and gender-matched WT and I89V subjects in the video CTET. As expected, we found that I89V had a selective vulnerability to distraction, with equivalent initial performance and time-on-task declines (Berry et al., 2014a). To our knowledge, these results are the first to link the I89V polymorphism to a specific cognitive capacity.
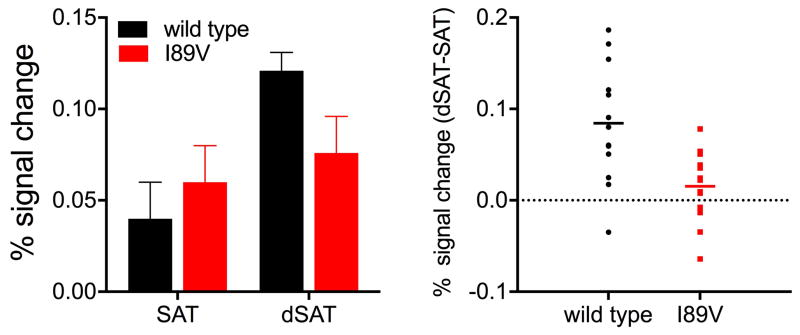
As described above, visuospatial attention processes are right-lateralized across species (Langner and Eickhoff, 2013), and in WT mice and rats, SAT-related increases in CHT surface density are limited to right PFC (Apparsundaram et al., 2005; Parikh et al., 2013). Likewise, human fMRI studies show right PFC activations during SAT performance, and right-lateralized frontoparietal activation increases in response to the dSAT challenge (Berry et al., 2014a; Demeter et al., 2011). As noted above, we found that CHT+/− mice do not show the same right PFC increases in CHT surface density after task performance (Parikh et al., 2013). Likewise, in humans, I89V subjects do not show the right PFC activation increases typically seen in response to the dSAT challenge (Fig. 4; Berry et al., 2015; c.f., Demeter et al., 2011). Furthermore, multivariate pattern analysis (MVPA) within an a priori region of interest within right PFC, based on our prior studies using the dSAT in ungenotyped subjects, successfully discriminated (84.6%; p=0.01) between WT and I89V subjects (Berry et al., 2015). Control analyses ruled out potential global differences in activation or hemodynamic response, and I89V and WT subjects had equivalent performance, ruling out performance confounds on activation. A whole-brain MVPA identifying those voxels that showed a differential response to the distractor for I89V subjects versus WT subjects suggested that alternative neurocognitive processes (increased reliance on bottom-up attention) supported the preserved performance of I89V subjects.
Figure 4. I89V subjects fail to activate right PFC in response to challenge.
Controls, but not I89V humans increase right PFC activation in response to attentional challenge. Percent signal change in the bar graphs (left) is reported relative to fixation baseline. Individual participant data (right) is plotted as percent signal change for the index dSAT-SAT. A significant group by disruptor interaction indicated controls increased activation in right PFC but I89V did not (for details see Berry et al., 2015).
5. CHT overcapacity variants and resistance to disruptors/distractors
5.1. CHT-OXP
Using bacterial artificial chromosome (BAC)-mediated transgenic methods, we generated mice with additional, integrated additional copies of the mouse Slc5a7 gene (Holmstrand et al., 2014). CHT overexpressors (CHT-OXP) are viable, develop normally and breed at WT rates. Immunohistochemistry revealed markedly elevated CHT expression in the cell bodies of cholinergic neurons and axons projecting to regions known to receive cholinergic innervation. Biochemical studies indicated a 2- to 3-fold elevation in total CHT protein levels in the CNS. CHT densities in CHT-OXP animals are markedly higher in both synaptic plasma membrane and intracellular fractions, paralleled by significant increases in [3H]HC-3 binding and synaptosomal and lymphocyte choline transport (Holmstrand et al., 2014). Furthermore, and consistent with their CHT overcapacity, we recently reported that negative modulation of CHT function in vivo, by administering the negative CHT modulator ML352 (Ennis et al., 2015), rapidly attenuated presynaptic ACh release in WT but not CHT-OXP (Valuskova et al., 2015). Elevation of ACh levels in CHT-OXP are not associated with compensatory changes in the activity of either ChAT or AChE (Holmstrand et al., 2014), suggesting that compensations at the level of synthesis or metabolic capacity do not arise to offset the functional impact of CHT overexpression. In the dSAT, CHT-OXP mice recovered more rapidly and completely than WT from the effects of the disruptor (Sarter et al., 2015). This finding suggests that the heightened capacity of CHT-OXP mice for cholinergic modulation of PFC allows them to more quickly reinstate top-down control and the task-set representation guiding correct performance.
5.2. 3′UTR-T humans
Neumann et al. found that a G to T substitution at the CHT 3′UTR (rs333229 of SLC5A7) was associated with differences in cholinergically-mediated autonomic nervous system function (enhanced parasympathetic function, reduced sympathetic function), increased heart rate variability (which in turn is associated with enhanced autonomic and emotional-cognitive control), reduced depressive symptoms, and reduced corticolimbic reactivity to negative emotional salience (Neumann et al., 2006; Neumann et al., 2005; Neumann et al., 2012). They noted that this is consistent with the idea that the 3′/UTR-T variant is associated with enhanced CHT function, although the molecular and neuronal mechanisms of this potential enhancement have not yet been explored. In a retrospective analysis of our data re-coding for this SNP, we found that I89WT subjects with a 3′UTR T allele have lower PAC Boredom scores (t(298)=1.99, P=0.05, d=0.23) than other groups (Isaacs et al., 2015). Furthermore, using the CTET (see Fig. 1), we found that I89WT/3′UTR-T humans are not vulnerable to the CTET’s video disruptor (Isaacs et al., 2015).
6. CHT variants: cognitive “costs” of variations in cholinergic capacity
Results from our converging research in mice and humans expressing CHT variants support the following general hypotheses: Low CHT capacity subjects, owing to their bottom-up biases, will not be impaired performing the basic target-driven SAT (and other tasks that rely on bottom-up attention). Instead, they will show specific impairments in situations requiring increased top-down control. Conversely, high CHT capacity subjects will not show performance advantages when target detection can rely on bottom-up salience. Instead, their advantage will be selective to situations requiring top-down activation of internal task representations guiding the response to environmental inputs.
We have emphasized the detriments of low cholinergic function and the advantages of high cholinergic activity. However, there are likely advantages and disadvantages to both. While the stable representations associated with high cholinergic activity permit efficient performance of the current task, the complementary cost may be less efficient processing of environmental stimuli that unexpectedly become relevant later on or signal a need to switch tasks, and suboptimal engagement with new environments that require the utilization of, and switching between, multiple cues (Steelman et al., 2013; Walther and Fei-Fei, 2007). Most of us have experienced the phenomenon of being deeply engrossed in a task (high cholinergic activity) and failing to notice that someone else has walked into the room until they startle us by speaking or tapping our shoulder. While this may be startling for us, it may be even more detrimental for an animal so intent on digging for food or preparing a nest that it fails to notice an approaching predator.
This interpretation receives support form findings indicating that although I89V subjects are more vulnerable to external distractors, they also have better memory on a subsequent surprise quiz for the content of those distractors (Berry et al., 2014). Furthermore, evidence indicating better performance by I89V subjects on divergent-thinking creativity tasks also supports a more open and exploratory approach to cognition overall (Berry, Craig, & Lustig, unpublished results). Similar patterns are seen in other groups with reduced top-down control and likely reduced cholinergic function (e.g., older adults and individuals with attention deficit disorder – the latter of which is associated with genetic variation in the CHT; English et al., 2009), and also intra-individually as a function of circadian cycle (e.g., Biss et al., 2013; Healey et al., 2008; Lustig and Jantz, 2015; White and Shah, 2006). In short, high cholinergic activity supports a processing style that is stable, efficient, and narrow, whereas low cholinergic activity may be associated with processing that is labile, exploratory, and expansive. This characterization is likewise consistent with the cholinergic system’s suggested role in memory, with high cholinergic activity thought to suppress excitatory feedback connections to allow distinctive encoding of target stimuli, whereas low cholinergic activity supports consolidation and associative processing (Hasselmo, 1999).
7. Conclusions: Genetically-imposed CHT abnormalities as models of CHT capacity variation in the general population and associated disease risks
The human CHT I89V variant has been associated with ADHD and depression severity (English et al., 2009; Hahn et al., 2008). Thus, exploring the neuronal and cognitive mechanisms associated with CHT variants may establish new avenues for treating the attentional dysfunctions associated with these disorders. In particular, such treatments may have an important impact on the ability to sustain top-down control in the face of distractors and other challenges.
Moreover, the study of genetically-imposed CHT capacity variations may provide an extreme example of the impact of CHT regulatory variations observed in outbred populations. For example, we found that outbred rats with a propensity for addiction-like behavior (”sign trackers”) fail to sustain elevated levels of cortical cholinergic neuromodulation to support attentional performance (Paolone et al., 2013). CHT-mediated choline uptake of sign-trackers fails to increase in response to stimulation and instead regresses below pre-stimulation baseline (Kucinski et al., 2015). Although the cellular underpinnings of such “CHT regression” remain unknown, they may involve changes in the plasma membrane mobilization and recycling of CHT as well as the functional status of cell surface CHT (e.g., Gates et al., 2004). Moreover, these findings suggest that human CHT regulatory variation will have a functional impact on the signaling capacity of cholinergic neurons, and thus underlie dimensional differences in major psychological traits which can serve as endophenotypes of mental disorders.
Finally, short of studying mice that express the human CHT sub- and over-capacity variants, tests of the hypothesis that cholinergic mechanisms mediate the cognitive and brain activation effects of the human CHT variants remains a major challenge. Parallel rodent-human testing in the SAT and dSAT provides an anchor for our converging-evidence approach to close the “neuroscience gap” in the behavioral genetics of cholinergic systems (Demeter et al., 2011; Demeter et al., 2008; St Peters et al., 2011b).
Highlights.
The neuronal choline transporter influences levels of cholinergic neuromodulation.
Cholinergic neuromodulation mediates top-down attentional control.
Reduced CHT activity is associated with vulnerability to attentional distraction.
Elevations in CHT activity may bestow resistance to distraction.
Acknowledgments
The authors’ research was supported by PHS grants MH086530, DA031656, and NS091856 (MS, CL) and MH073159 (RDB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apparsundaram S, Martinez V, Parikh V, Kozak R, Sarter M. Increased capacity and density of choline transporters situated in synaptic membranes of the right medial prefrontal cortex of attentional task-performing rats. J Neurosci. 2005;25(15):3851–3856. doi: 10.1523/JNEUROSCI.0205-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazalakova MH, Blakely RD. The high-affinity choline transporter: a critical protein for sustaining cholinergic signaling as revealed in studies of genetically altered mice. Handb Exp Pharmacol. 2006;(175):525–544. doi: 10.1007/3-540-29784-7_21. [DOI] [PubMed] [Google Scholar]
- Bazalakova MH, Wright J, Schneble EJ, McDonald MP, Heilman CJ, Levey AI, Blakely RD. Deficits in acetylcholine homeostasis, receptors and behaviors in choline transporter heterozygous mice. Genes Brain Behav. 2007;6(5):411–424. doi: 10.1111/j.1601-183X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- Berry AS, Blakely RD, Sarter M, Lustig C. Cholinergic capacity mediates prefrontal engagement during challenges to attention: evidence from imaging genetics. Neuroimage. 2015;108:386–395. doi: 10.1016/j.neuroimage.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Demeter E, Sabhapathy S, English BA, Blakely RD, Sarter M, Lustig C. Disposed to distraction: genetic variation in the cholinergic system influences distractibility but not time-on-task effects. J Cogn Neurosci. 2014a;26(9):1981–1991. doi: 10.1162/jocn_a_00607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry AS, Li X, Lin Z, Lustig C. Shared and distinct factors driving attention and temporal processing across modalities. Acta Psychol (Amst) 2014b;147:42–50. doi: 10.1016/j.actpsy.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biss RK, Ngo KWJ, Hasher L, Campbell KL, Rowe G. Distraction Can Reduce Age-Related Forgetting. Psychological Science. 2013;24(4):448–455. doi: 10.1177/0956797612457386. [DOI] [PubMed] [Google Scholar]
- Bucci DJ, Holland PC, Gallagher M. Removal of cholinergic input to rat posterior parietal cortex disrupts incremental processing of conditioned stimuli. J Neurosci. 1998;18(19):8038–8046. doi: 10.1523/JNEUROSCI.18-19-08038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Connor CE, Egeth HE, Yantis S. Visual attention: bottom-up versus top-down. Curr Biol. 2004;14(19):R850–852. doi: 10.1016/j.cub.2004.09.041. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davidson MC, Cutrell EB, Marrocco RT. Scopolamine slows the orienting of attention in primates to cued visual targets. Psychopharmacology (Berl) 1999;142(1):1–8. doi: 10.1007/s002130050855. [DOI] [PubMed] [Google Scholar]
- Demeter E, Guthrie SK, Taylor SF, Sarter M, Lustig C. Increased distractor vulnerability but preserved vigilance in patients with schizophrenia: evidence from a translational Sustained Attention Task. Schizophr Res. 2013;144(1–3):136–141. doi: 10.1016/j.schres.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: a continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. Neuroimage. 2011;54(2):1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008;22(6):787–799. doi: 10.1037/a0013712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English BA, Hahn MK, Gizer IR, Mazei-Robison M, Steele A, Kurnik DM, Stein MA, Waldman ID, Blakely RD. Choline transporter gene variation is associated with attention-deficit hyperactivity disorder. J Neurodev Disord. 2009;1(4):252–263. doi: 10.1007/s11689-009-9033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis EA, Wright J, Retzlaff CL, McManus OB, Lin Z, Huang X, Wu M, Li M, Daniels JS, Lindsley CW, Hopkins CR, Blakely RD. Identification and characterization of ML352: a novel, noncompetitive inhibitor of the presynaptic choline transporter. ACS Chem Neurosci. 2015;6(3):417–427. doi: 10.1021/cn5001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Bazalakova M, Savchenko V, Tapia JC, Wright J, Blakely RD. Lethal impairment of cholinergic neurotransmission in hemicholinium-3-sensitive choline transporter knockout mice. Proc Natl Acad Sci USA. 2004;101(23):8762–8767. doi: 10.1073/pnas.0401667101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Blakely RD. The choline transporter resurfaces: new roles for synaptic vesicles? Mol Interv. 2004;4(1):22–37. doi: 10.1124/mi.4.1.22. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Savchenko V, Apparsundaram S, Zwick M, Wright J, Heilman CJ, Yi H, Levey AI, Blakely RD. Vesicular localization and activity-dependent trafficking of presynaptic choline transporters. J Neurosci. 2003;23(30):9697–9709. doi: 10.1523/JNEUROSCI.23-30-09697.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates J, Jr, Ferguson SM, Blakely RD, Apparsundaram S. Regulation of choline transporter surface expression and phosphorylation by protein kinase C and protein phosphatase 1/2A. J Pharmacol Exp Ther. 2004;310(2):536–545. doi: 10.1124/jpet.104.066795. [DOI] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20(12):4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritton HJ, Howe WM, Mallory CS, Hetrick VL, Berke JD, Sarter M. Cortical cholinergic signaling controls the detection of cues. Proc Natl Acad Sci U S A. 2016;113(8):E1089–1097. doi: 10.1073/pnas.1516134113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MK, Blackford JU, Haman K, Mazei-Robison M, English BA, Prasad HC, Steele A, Hazelwood L, Fentress HM, Myers R, Blakely RD, Sanders-Bush E, Shelton R. Multivariate permutation analysis associates multiple polymorphisms with subphenotypes of major depression. Genes Brain Behav. 2008;7(4):487–495. doi: 10.1111/j.1601-183X.2007.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation: acetylcholine and memory consolidation. Trends in Cognitive Sciences. 1999;3(9):351–359. doi: 10.1016/s1364-6613(99)01365-0. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36(1):52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey MK, Campbell KL, Hasher L. Cognitive aging and increased distractibility: costs and potential benefits. In: Sossin WS, Lacaille JC, Castellucci VF, Belleville S, editors. Essence of Memory. 2008. pp. 353–363. [DOI] [PubMed] [Google Scholar]
- Holmstrand EC, Asafu-Adjei J, Sampson AR, Blakely RD, Sesack SR. Ultrastructural localization of high-affinity choline transporter in the rat anteroventral thalamus and ventral tegmental area: differences in axon morphology and transporter distribution. J Comp Neurol. 2010;518(11):1908–1924. doi: 10.1002/cne.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrand EC, Lund D, Cherian AK, Wright J, Martin RF, Ennis EA, Stanwood GD, Sarter M, Blakely RD. Transgenic overexpression of the presynaptic choline transporter elevates acetylcholine levels and augments motor endurance. Neurochem Int. 2014;73:217–228. doi: 10.1016/j.neuint.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Berry AS, Francois J, Gilmour G, Carp JM, Tricklebank M, Lustig C, Sarter M. Prefrontal cholinergic mechanisms instigating shifts from monitoring for cues to cue-guided performance: converging electrochemical and fMRI evidence from rats and humans. J Neurosci. 2013;33(20):8742–8752. doi: 10.1523/JNEUROSCI.5809-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe WM, Gritton H, Lusk N, Berke JD, Sarter M. Distinct behavioral and neurophysiological correlates of prefrontal acetylcholine and glutamate transients during attentional task performance. Society for Neuroscience Abstracts, abstract # 913.906.2012. [Google Scholar]
- Howe WM, Ji J, Parikh V, Williams S, Mocaër E, Trocmé-Thibierge C, Sarter M. Enhancement of attentional performance by selective stimulation of alpha4beta2* nAChRs: underlying cholinergic mechanisms. Neuropsychopharmacology. 2010;35(6):1391–1401. doi: 10.1038/npp.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huba GJ, Singer JL, Aneshensel CS, Antrobus JS. Short imaginal processes inventory manual. Research Psychologists Press; Post Huron, MI: 1982. [Google Scholar]
- Isaacs Y, Lin Z, Deldin P, Blakely R, Sarter M, Lustig C. The “good” choline transporter gene variant? Resilience against distractibility and depression. Society for Neuroscience Annual Meeting; Chicago, IL. 2015. [Google Scholar]
- Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34(8):1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Kucinski A, Koshy Cherian A, Valuskova P, Yegla B, Parikh V, Robinson T, Sarter M. Prone to addiction as well as to falls: Poor attention in sign-tracking rats extends to complex movement control and is associated with regression of choline transporter capacity. Society for Neuroscience Annual Meeting; Chicago, IL. 2015. [Google Scholar]
- Kus L, Borys E, Ping Chu Y, Ferguson SM, Blakely RD, Emborg ME, Kordower JH, Levey AI, Mufson EJ. Distribution of high affinity choline transporter immunoreactivity in the primate central nervous system. J Comp Neurol. 2003;463(3):341–357. doi: 10.1002/cne.10759. [DOI] [PubMed] [Google Scholar]
- Lambe EK, Picciotto MR, Aghajanian GK. Nicotine induces glutamate release from thalamocortical terminals in prefrontal cortex. Neuropsychopharmacology. 2003;28(2):216–225. doi: 10.1038/sj.npp.1300032. [DOI] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139(4):870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman PR, Allen DD. The transport of choline. Drug Dev Ind Pharm. 2002;28(7):749–771. doi: 10.1081/ddc-120005622. [DOI] [PubMed] [Google Scholar]
- Lustig C, Jantz T. Questions of age differences in interference control: When and how, not if? Brain Res. 2015;1612:59–69. doi: 10.1016/j.brainres.2014.10.024. [DOI] [PubMed] [Google Scholar]
- Matthies DS, Fleming PA, Wilkes DM, Blakely RD. The Caenorhabditis elegans choline transporter CHO-1 sustains acetylcholine synthesis and motor function in an activity-dependent manner. J Neurosci. 2006;26(23):6200–6212. doi: 10.1523/JNEUROSCI.5036-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110(2):247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology (Berl) 1995;117(3):340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- Miller BT, D’Esposito M. Searching for “the top” in top-down control. Neuron. 2005;48(4):535–538. doi: 10.1016/j.neuron.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Arnold HM, Rabenstein RL, Sarter M, Bruno JP. Neuronal activity in the nucleus accumbens is necessary for performance-related increases in cortical acetylcholine release. Neuroscience. 2004;123(3):635–645. doi: 10.1016/j.neuroscience.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, Hariri AR. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biol Psychiatry. 2006;60(10):1155–1162. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- Neumann SA, Lawrence EC, Jennings JR, Ferrell RE, Manuck SB. Heart rate variability is associated with polymorphic variation in the choline transporter gene. Psychosom Med. 2005;67(2):168–171. doi: 10.1097/01.psy.0000155671.90861.c2. [DOI] [PubMed] [Google Scholar]
- Neumann SA, Linder KJ, Muldoon MF, Sutton-Tyrrell K, Kline C, Shrader CJ, Lawrence EC, Ferrell RE, Manuck SB. Polymorphic variation in choline transporter gene (CHT1) is associated with early, subclinical measures of carotid atherosclerosis in humans. Int J Cardiovasc Imaging. 2012;28(2):243–250. doi: 10.1007/s10554-011-9831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell RG, Dockree PM, Robertson IH, Bellgrove MA, Foxe JJ, Kelly SP. Uncovering the neural signature of lapsing attention: electrophysiological signals predict errors up to 20 s before they occur. J Neurosci. 2009;29(26):8604–8611. doi: 10.1523/JNEUROSCI.5967-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Haga T. Functional characterization of the human high-affinity choline transporter. FEBS Lett. 2000;484(2):92–97. doi: 10.1016/s0014-5793(00)02134-7. [DOI] [PubMed] [Google Scholar]
- Okuda T, Haga T, Kanai Y, Endou H, Ishihara T, Katsura I. Identification and characterization of the high-affinity choline transporter. Nat Neurosci. 2000;3(2):120–125. doi: 10.1038/72059. [DOI] [PubMed] [Google Scholar]
- Okuda T, Okamura M, Kaitsuka C, Haga T, Gurwitz D. Single nucleotide polymorphism of the human high affinity choline transporter alters transport rate. J Biol Chem. 2002;277(47):45315–45322. doi: 10.1074/jbc.M207742200. [DOI] [PubMed] [Google Scholar]
- Paolone G, Angelakos CC, Meyer PJ, Robinson TE, Sarter M. Cholinergic control over attention in rats prone to attribute incentive salience to reward cues. J Neurosci. 2013;33(19):8321–8335. doi: 10.1523/JNEUROSCI.0709-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolone G, Howe WM, Gopalakrishnan M, Decker MW, Sarter M. Regulation and function of the tonic component of cortical acetylcholine release. In: Westerink B, Clinckers R, Smolders S, Sarre S, Michotte Y, editors. Monitoring molecules in neuroscience. Vrije Universiteit Brussels; Brussels: 2010. pp. 363–365. [Google Scholar]
- Parikh V, Ji J, Decker MW, Sarter M. Prefrontal beta2 subunit-containing and alpha7 nicotinic acetylcholine receptors differentially control glutamatergic and cholinergic signaling. J Neurosci. 2010;30(9):3518–3530. doi: 10.1523/JNEUROSCI.5712-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple timescales. Neuron. 2007;56(1):141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nicotinic acetylcholine receptor agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28(14):3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cortical choline transporter function measured in vivo using choline-sensitive microelectrodes: clearance of endogenous and exogenous choline and effects of removal of cholinergic terminals. J Neurochem. 2006;97(2):488–503. doi: 10.1111/j.1471-4159.2006.03766.x. [DOI] [PubMed] [Google Scholar]
- Parikh V, Sarter M. Cholinergic mediation of attention: contributions of phasic and tonic increases in prefrontal cholinergic activity. Ann N Y Acad Sci. 2008;1129:225–235. doi: 10.1196/annals.1417.021. [DOI] [PubMed] [Google Scholar]
- Parikh V, St Peters M, Blakely RD, Sarter M. The presynaptic choline transporter imposes limits on sustained cortical acetylcholine release and attention. J Neurosci. 2013;33(6):2326–2337. doi: 10.1523/JNEUROSCI.4993-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinthong M, Black SAG, Ribeiro FM, Pholpramool C, Ferguson SSG, Rylett RJ. Activity and subcellular trafficking of the sodium-coupled choline transporter CHT is regulated acutely by peroxynitrite. Mol Pharmacol. 2008;73(3):801–812. doi: 10.1124/mol.107.040881. [DOI] [PubMed] [Google Scholar]
- Poorthuis RB, Bloem B, Schak B, Wester J, de Kock CP, Mansvelder HD. Layer-specific modulation of the prefrontal cortex by nicotinic acetylcholine receptors. Cereb Cortex. 2013;23(1):148–161. doi: 10.1093/cercor/bhr390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI, Snyder CRR, Davidson BJ. Attention and the detection of signals. Journal of Experimental Psychology: General. 1980;109(2):160–174. [PubMed] [Google Scholar]
- Ribeiro FM, Alves-Silva J, Volknandt W, Martins-Silva C, Mahmud H, Wilhelm A, Gomez MV, Rylett RJ, Ferguson SSG, Prado VF, Prado MAM. The hemicholinium-3 sensitive high affinity choline transporter is internalized by clathrin-mediated endocytosis and is present in endosomes and synaptic vesicles. J Neurochem. 2003;87(1):136–146. doi: 10.1046/j.1471-4159.2003.01974.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Black SA, Prado VF, Rylett RJ, Ferguson SS, Prado MA. The “ins” and “outs” of the high-affinity choline transporter CHT1. J Neurochem. 2006;97(1):1–12. doi: 10.1111/j.1471-4159.2006.03695.x. [DOI] [PubMed] [Google Scholar]
- Ribeiro FM, Black SAG, Cregan SP, Prado VF, Prado MAM, Rylett RJ, Ferguson SSG. Constitutive high-affinity choline transporter endocytosis is determined by a carboxyl-terminal tail dileucine motif. J Neurochem. 2005;94(1):86–96. doi: 10.1111/j.1471-4159.2005.03171.x. [DOI] [PubMed] [Google Scholar]
- Sarter M. Behavioral-cognitive targets for cholinergic enhancement. Curr Opinion Behav Sci. 2015;4:22–26. doi: 10.1016/j.cobeha.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Albin RL, Kucinski A, Lustig C. Where attention falls: Increased risk of falls from the converging impact of cortical cholinergic and midbrain dopamine loss on striatal function. Exp Neurol. 2014a;257:120–129. doi: 10.1016/j.expneurol.2014.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: The neurobiology of attentional effort. Brain Res Rev. 2006;51:155–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Rev. 2001;35(2):146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Kim Y. Interpreting chemical neurotransmission in vivo: techniques, time scales, and theories. ACS Chem Neurosci. 2015;6(1):8–10. doi: 10.1021/cn500319m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Berry AS, Gritton H, Howe WM, Parikh V. What do phasic cholinergic signals do? Neurobiol Learn Memory. 2016;130:135–141. doi: 10.1016/j.nlm.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Lustig C, Blakely RD, Koshy Cherian A, Valuskova P, Parikh V, Kim Y, Tronson NC, Ennis EA. Super-cholinergic mice and humans: cholinergic-cognitive-affective resiliencies. ACNP Annual Meeting; Hollywood, FL. 2015. [Google Scholar]
- Sarter M, Lustig C, Howe WM, Gritton H, Berry AS. Deterministic functions of cortical acetylcholine. Eur J Neurosci. 2014b;39(11):1912–1920. doi: 10.1111/ejn.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Parikh V. Choline transporters, cholinergic transmission and cognition. Nat Rev Neurosci. 2005;6(1):48–56. doi: 10.1038/nrn1588. [DOI] [PubMed] [Google Scholar]
- Simon JR, Atweh S, Kuhar MJ. Sodium-dependent high affinity choline uptake: a regulatory step in the synthesis of acetylcholine. J Neurochem. 1976;26(5):909–922. doi: 10.1111/j.1471-4159.1976.tb06472.x. [DOI] [PubMed] [Google Scholar]
- St Peters M, Cherian AK, Bradshaw M, Sarter M. Sustained attention in mice: Expanding the translational utility of the SAT by incorporating the Michigan Controlled Access Response Port (MICARP) Behav Brain Res. 2011a;225(2):574–583. doi: 10.1016/j.bbr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Peters M, Demeter E, Lustig C, Bruno JP, Sarter M. Enhanced control of attention by stimulating mesolimbic-corticopetal cholinergic circuitry. J Neurosci. 2011b;31(26):9760–9771. doi: 10.1523/JNEUROSCI.1902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman KS, McCarley JS, Wickens CD. Great expectations: top-down attention modulates the costs of clutter and eccentricity. J Exp Psychol Appl. 2013;19(4):403–419. doi: 10.1037/a0034546. [DOI] [PubMed] [Google Scholar]
- Thiele A. Muscarinic signaling in the brain. Annu Rev Neurosci. 2013;36(1):271–294. doi: 10.1146/annurev-neuro-062012-170433. [DOI] [PubMed] [Google Scholar]
- Valuskova P, Koshy Cherian A, Pitchers K, Kim Y, Lindsley C, Ennis E, Blakely RR, Sarter M. Negative modulation of choline transporter (CHT) function reveals superior cholinergic capacity of CHT-overexpressors. Society for Neuroscience Annual Meeting; Chicago IL. 2015. [Google Scholar]
- Walther DB, Fei-Fei L. Task-set switching with natural scenes: measuring the cost of deploying top-down attention. J Vis. 2007;7(11):9, 1–12. doi: 10.1167/7.11.9. [DOI] [PubMed] [Google Scholar]
- White HA, Shah P. Uninhibited imaginations: creativity in adults with Attention-Deficit/Hyperactivity Disorder. Personality and Individual Differences. 2006;40(6):1121–1131. [Google Scholar]