Abstract
The emergence of multidrug-resistant bacterial and fungal strains poses a threat to human health that requires the design and synthesis of new classes of antimicr obial agents. We evaluated bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones for their antibacterial and antifungal activities against panels of Gram-positive/Gram-negative bacteria as well as fungi. We investigated their potential to develop resistance against both bacteria and fungi by a multi-step, resistance-selection method, explored their potential to induce the production of reactive oxygen species, and assessed their toxicity. In summary, we found that these compounds exhibited broad-spectrum antibacterial and antifungal activities against most of the tested strains with minimum inhibitory concentration (MIC) values ranging from <0.5->500 μM against bacteria and 1.0->31.3 μg/mL against fungi; and in most cases, they exhibited either superior or similar antimicrobial activity compared to those of the standard drugs used in the clinic. We also observed minimal emergence of drug resistance to these newly synthesized compounds by bacteria and fungi even after 15 passages, and we found weak to moderate inhibition of the human Ether-à-go-go-related gene (hERG) channel with acceptable IC50 values ranging from 1.12-3.29 μM. Overall, these studies sh ow that bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones are potentially promising scaffolds for the discovery of novel antibacterial and antifungal agents.
Keywords: Bishydrazones, Candida albicans, Cytotoxicity, hERG channel, Resistance, Staphylococcus aureus
Graphical Abstract
1. Introduction
The emergence of multidrug-resistant bacteria and fungi as human pathogens warrants a continued focus on the development of new pharmacophores for the treatment of these devastating and often fatal infections. The rise of multidrug-resistant bacteria, such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE), adversely affects the efficacy of many known, standard-of-care, antibacterial agents.1 Evidence of the impact of these multidrug-resistant strains appears in a 2011 report from the Centers for Disease Control and Prevention (CDC) that estimates that the national incidence of invasive MRSA infections was 80,461 cases and 650 deaths. This mortality rate is among the highest recorded for bacterial infections.2 Likewise, listerosis, which is a common foodborne illness caused by Listeria monocytogenes, represents a serious illness afflicting elderly people, newborns, and those with impaired immune systems. Estimates are that 19% of deaths associated with the consumption of contaminated foods in the United States are due to L. monocytogenes.3
The incidence of invasive fungal infections is also on the rise due to an increasing population of critically ill patients as a result of the human immunodeficiency virus (HIV), systemic diseases such as cancer, and the increasing occurrence of organ transplantation.4 The National Healthcare Safety Network (NHSN) at the CDC reported that Candida spp. ranked fifth amongst hospital-acquired pathogens.5 Candida spp. are reported as the fourth most common causative pathogens of nosocomial and of fatal bloodstream infections.6 Eukaryotic C. albicans shares a close evolutionary relationship, as well as many cellular mechanisms, with their human hosts, and represents challenges for treatment of systemic fungal infections. There is a clear need for new antimicrobials that selectively inhibit these microorganisms without causing host toxicity.
Pentamidine represents an archetypical, biscationic antibiotic with a symmetrical structure containing two amidinium functional groups separated by a flexible 1,5-diphenoxypentane spacer.7 Developed initially as an antiprotozoal agent, pentamidine currently finds applications in both the treatment of protozoan diseases, such as Trypanosoma brucei gambiense
(West African trypanosomiasis), as well as systemic fungal infections caused by Pneumocystis jirovecii, often seen in patients with HIV. Related compounds include symmetrical bisamidines, (e.g., furimidazoline),8 developed principally as topoisomerase inhibitors for cancer treatment. In addition to these biscationic compounds, other hydrazone-containing and guanidine-containing molecules possess a range of promising biological activities, including antitubercular,9,10 anti-HIV,11,12 anticonvulsant,13 anticancer,14 anti-inflammatory,15,16 antimalarial,17,18 antibacterial,19,20 and antifungal activities.21,22 Recently, bis(N-amidinohydrazones) were reported to inhibit the calcium-dependent serine endoprotease, furin, which activates immature proteins to their functional, mature forms.23
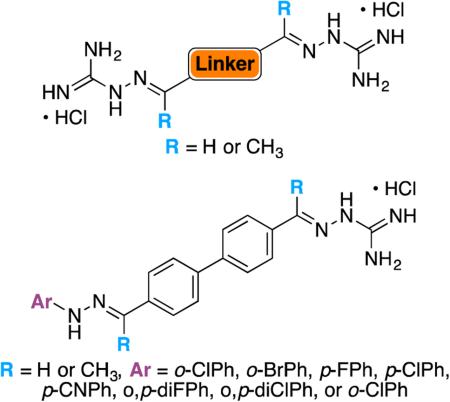
Inspired by the above successes, we were intrigued by the possibility that biscationic pharmacophores in either the bis(N-amidinohydrazones) or the N-(amidino)-N'-aryl-bishydrazone family with either a flexible or a rigid spacer would offer advantages in terms of antibacterial and antifungal activities not found in the flexible bisamidines, as well as in the hydrazone- and guanidine-containing molecules currently in the literature. To investigate the potential of such compounds, we synthesized nine symmetrical bis(N-amidinohydrazones) (3A-B and 4A,C-H) and eight asymmetrical N-(amidino)-N'-aryl-bishydrazones (7Aa-Ag and 8Aa) (Scheme 1). Further, we evaluated the antibacterial and antifungal activities of these novel compounds against panels of bacterial strains (four Gram-positive, six Gram-negative, and one mycobacterial) and seven Candida albicans strains. Because the development of resistance represents a crucial problem in antimicrobial drug development, we also established the potential for resistance development by bacteria and fungi against these compounds. Additionally, we measured the production of reactive oxygen species (ROS) of some of these compounds in yeast cells, determined their in vitro cytotoxicity and their affinity for the human Ether-à-go-go-related gene (hERG) potassium channel. The combination of testing new antimicrobial agents and early screening for resistance and toxicity represents an avenue that may produce pharmacophores of potential utility in the treatment of diseases.
Scheme 1.
Synthetic scheme for the preparation of A. the bis(N-amidinohydrazones) and B. N-(amidino)-N'-aryl-bishydrazones.
2. Results and discussion
2.1. Design and chemical synthesis of biscationic compounds with two chemically identical termini
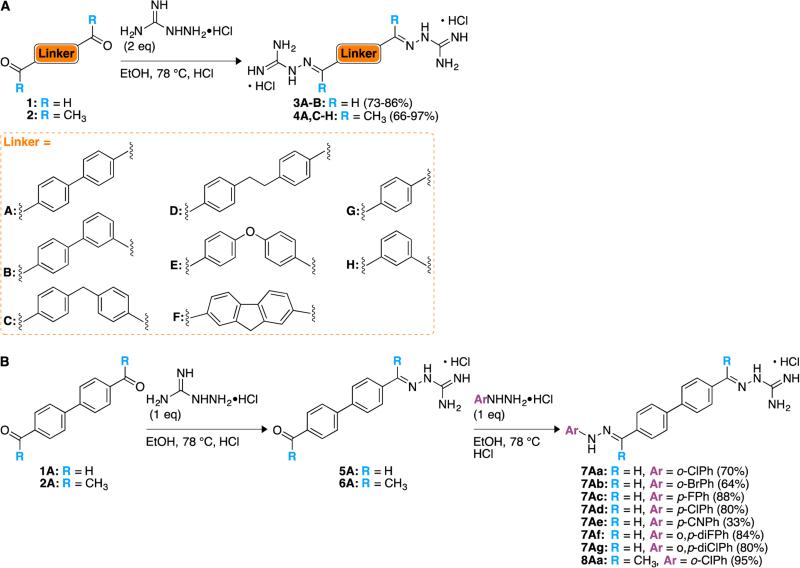
The reported syntheses of biscationic compounds containing two identical termini bearing guanidinium, amidinium, or pyridinium groups attached to aryl or alkyl spacers typically involved the modification of spacers with termini bearing primary halides, carbonyl, or nitrile groups.24-26 Alkylation of α,ω-dihaloalkanes, for example, with 2-aminopyridine led to bis(2-aminopyridinium) salts.24 Modification of the nitrile group in 5-(4-cyanophenyl)furan-2-carboxylic acid and completion of the spacer by linking the carboxylate groups led to bisamidinium agents.27 In the same fashion, we modified the carbonyl groups in either bisaldehyde 1 or bisketone 2, which in some cases were in regiochemically distinct positions in the spacer (e.g., 1B, 2B, 2H), with N-aminoguanidine hydrochloride to obtain the biscationic products 3 and 4, respectively (Scheme 1A).
2.2. Design and chemical synthesis of biscationic compounds with chemically non-identical termini
Synthetic approaches to bicationic agents with two chemically non-identical termini often involved the construction of the spacer as the ultimate step. For example, the coupling of an amidino-substituted naphthol with a guanidine-substituted benzoic acid secured biscationic esters with an amidinium group at one terminus and a guanidinium group at the other.28 Our approach differed from this strategy in that we utilized a stepwise, chemoselective modification of bisaldehydes 1 or bisketones 2 in order to arrive at biscationic systems with different cationic groups at each terminus. Condensations of one equivalent of N-aminoguanidine hydrochloride with 1A and 2A led to the efficient production of monosubstituted N-amidinohydrazones 5A and 6A, respectively. The hydrochloride salts of these monocatioinic products were readily crystallized free from starting materials. The subsequent treatment of 5A and 6A with N-arylhydrazines provided the desired, biscationic agents 7Aa-Ag and 8Aa, respectively, which we describe as N-(amidino)-N'-aryl-bishydrazones (Scheme 1B). Cavallini described the monocationic N-amidinohydrazones as antibacterial agents some years ago, but the range of organisms and MIC50 values were, in general, unimpressive.29
With these first generations of bis(N-amidinohydrazones) 3 and 4 and N-(amidino)-N'-aryl-bishydrazones 7 and 8 in hand, we aimed to answer the following eight apriori questions in terms of structure-activity-relationship (SAR). We included the numbers of the compounds used to answer these questions into parentheses following the questions. The eight questions are as follows: (i) are linkers comprised of only one aromatic ring sufficient to confer antimicrobial activity? (compounds 4G and 4H); (ii) is the presence of methyl groups on the carbons alpha to the linker beneficial for antimicrobial activity? (compounds 3A versus 4A, and 7Aa versus 8Aa); (iii) how does the substitution pattern of the biphenyl linker affect antimicrobial activity? (compounds 3A and 3B); (iv) how does the length of the flexible linear alkyl spacer between the two phenyl rings affect antimicrobial activity? (compounds 4A, 4C, and 4D); (v) is the introduction of rigidity in the linker beneficial for antimicrobial activity? (compound 4C versus 4F); (vi) is the presence of an oxygen atom in the linker beneficial for antimicrobial activity? (compound 4C versus 4E); (vii) how does the identity of the substituent, when located at the same position on the aryl ring of the side chain, affect antimicrobial activity? (compounds 7Aa versus 7Ab, 7Ac versus 7Ad versus 7Ae, and 7Af versus 7Ag); and finally, (viii) how does the substitution pattern of the aryl ring (ortho versus para-monosubstituted versus ortho,para-disubstituted) affect antimicrobial activity? (compounds 7Aa versus 7Ad versus 7Ag).
2.3. Antibacterial activity
To determine if our novel bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones displayed antibacterial activity, we first evaluated compounds 3A-8Aa against a panel of Gram-positive (4 strains), Gram-negative (6 strains), and one mycobacterial strain in a concentration range of 0.5-500 μM (Table 1). We used the aminoglycoside amikacin (AMK), the β-lactam ampicillin (AMP), and the fluoroquinolone ofloxacin (OFX) as positive controls. In general, the novel compounds displayed excellent (MIC values ≤0.5-7.8 μM), intermediate (also referred to as moderate) (15.6-31.3 μM), or low (also referred to as poor) (62.5-≥500 μM) antibacterial activity against the bacterial strains tested. It is important to note that these MIC value ranges are used from here on to qualitatively described all compounds tested, including the positive controls.
Table 1.
MIC values in μg/mL and (μM)a for compounds 3A,B, 4A,C-H, 7Aa-Ag, and 8Aa against various bacterial strains.
| Gram-positive | Gram-negative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cpd | A (Bsu) | B (Lmo) | C (MRSA) | D (VRE) | E (Aba) | F (Ecl) | G (Eco) | H (Kpn) | I (Pae) | J (Sen) | K (Msm) |
| AMK | 3.0-12.2 (3.9-15.6) | 3.0 (3.9) | 24.5 (31.3) | 97.7 (125) | 24.5 (31.3) | 48.9 (62.5) | 24.5 (31.3) | 24.5 (31.3) | 48.9 (62.5) | 3.0-6.1 (3.9-7.8) | 12.2 (15.6) |
| AMP | >92.8 (>250) | 92.8 (250) | 92.8 (250) | 92.8 (250) | >92.8 (>250) | >92.8 (>250) | >92.8 (>250) | >92.8 (>250) | >92.8 (>250) | 92.8 (250) | >92.8 (>250) |
| OFX | ≤0.2 (≤0.5) | 0.4 (1.0) | 0.4 (1.0) | 2.8 (7.8) | ≤0.2 (≤0.5) | ≤0.2 (≤0.5) | 1.4-2.8 (3.9-7.8) | 5.6-11.3 (15.6-31.3) | 1.4 (3.9) | 5.6-11.3 (15.6-31.3) | 5.6 (15.6) |
| 3A | >198 (>500) | 0.4 (1.0) | 1.5 (3.9) | 0.8 (2.0) | >198 (>500) | >198 (>500) | >198 (>500) | >198 (>500) | >198 (>500) | >198 (>500) | >198 (>500) |
| 3B | >198 (>500) | 0.4 (1.0) | 1.5 (3.9) | 1.5 (3.9) | >198 (>500) | >198 (>500) | >198 (>500) | >198 (>500) | >198 (>500) | >198 (>500) | 0.4 (1.0) |
| 4A | >212 (>500) | >212 (>500) | >212 (>500) | >212 (>500) | >212 (>500) | 52.9 (125) | 26.5 (62.5) | 6.6 (15.6) | 0.8 (2.0) | >212 (>500) | >212 (>500) |
| 4C | >219 (>500) | 1.7 (3.9) | 1.7 (3.9) | 0.9 (2.0) | >219 (>500) | >219 (>500) | >219 (>500) | >219 (>500) | >219 (>500) | >219 (>500) | 0.9 (2.0) |
| 4D | >226 (>500) | 0.5 (1.0) | 0.5-0.9 (1.0-2.0) | 0.5 (1.0) | >226 (>500) | >226 (>500) | >226 (>500) | >226 (>500) | >226 (>500) | >226 (>500) | 0.5 (1.0) |
| 4E | 54.9 (125) | 27.5 (62.5) | 54.9 (125) | 1.7 (3.9) | 27.5 (62.5) | 110 (250) | 54.9 (125) | 27.5 (62.5) | 27.5 (62.5) | 54.9 (125) | 54.9-110 (125-250) |
| 4F | 3.4 (7.8) | <0.2 (<0.5) | 0.4-0.9 (1.0-2.0) | 0.4 (1.0) | 218 (500) | 218 (500) | 218 (500) | 13.6-27.2 (31.3-62.5) | 0.4-0.9 (1.0-2.0) | 1.7 (3.9) | 0.4 (1.0) |
| 4G | 86.8 (250) | 86.8 (250) | >173 (>500) | 43.4 (125) | 173 (500) | >173 (>500) | >173 (>500) | 173 (500) | 86.8 (250) | 86.8-173 (250-500) | >173 (>500) |
| 4H | 86.8 (250) | 86.8 (250) | >173 (>500) | 43.4 (125) | >173 (>500) | >173 (>500) | >173 (>500) | >173 (>500) | 173 (500) | 21.7-86.8 (62.5-250) | 5.4 (15.6) |
| 7Aa | >215 (>500) | 0.4-0.9 (1.0-2.0) | 0.9 (2.0) | 0.9 (2.0) | >215 (>500) | >215 (>500) | >215 (>500) | >215 (>500) | >215 (>500) | >215 (>500) | 1.7 (3.9) |
| 7Ab | >235 (>500) | 0.9 (2.0) | 0.9 (2.0) | 0.9 (2.0) | >235 (>500) | >235 (>500) | >235 (>500) | >235 (>500) | >235 (>500) | >235 (>500) | 1.8 (3.9) |
| 7Ac | >205 (>500) | 0.8 (2.0) | 3.2 (7.8) | 1.6 (3.9) | >205 (>500) | >205 (>500) | >205 (>500) | >205 (>500) | >205 (>500) | >205 (>500) | 1.6 (3.9) |
| 7Ad | >214 (>500) | 1.7 (3.9) | 3.3 (7.8) | 1.7 (3.9) | >214 (>500) | >214 (>500) | >214 (>500) | >214 (>500) | >214 (>500) | >214 (>500) | 1.7 (3.9) |
| 7Ae | 13.1 (31.3) | 3.3 (7.8) | 6.5 (15.6) | 0.8-1.6 (2.0-3.9) | 6.5-13.1 (15.6-31.3) | 209 (500) | 26.1-52.2 (62.5-125) | 6.5-13.1 (15.6-31.3) | 13.1-26.1 (31.3-62.5) | 6.5 (15.6) | 6.5-13.1 (15.6-31.3) |
| 7Af | >214 (>500) | 0.9-1.7 (2.0-3.9) | 1.7 (3.9) | 1.7 (3.9) | >214 (>500) | >214 (>500) | >214 (>500) | >214 (>500) | >214 (>500) | >214 (>500) | 0.9-1.7 (2.0-3.9) |
| 7Ag | >231 (>500) | 3.6 (7.8) | 3.6 (7.8) | 3.6 (7.8) | >231 (>500) | >231 (>500) | >231 (>500) | >231 (>500) | >231 (>500) | >231 (>500) | 1.8 (3.9) |
| 8Aa | 26.6-53.3 (62.5-125) | 26.6-53.3 (62.5-125) | 53.3 (125) | 1.7 (3.9) | 26.6 (62.5) | 213 (500) | 213 (500) | 26.6-53.3 (62.5-125) | 213 (500) | 26.3-53.3 (62.5-125) | 213 (500) |
These antibacterial MIC values were determined originally in μM using a range of 0.5 to 500 μM. These values are presented into parentheses. The values in μg/mL are presented for comparison with the antifungal MIC values, which were determined originally in μg/mL.
Gram-positive: A (Bsu) = B. subtilis 168, B (Lmo) = L. monocytogenes ATCC 19115, C = MRSA, D = VRE.
Gram-negative: E (Aba) = A. baumannii ATCC 19606, F (Ecl) = E. cloacae ATCC 13047, G (Eco) = E. coli MC1061, H (Kpn) = K. pneumoniae ATCC 27736, I (Pae) = P. aeruginosa ATCC 27853, J (Sen) = S. enterica ATCC 14028.
Mycobacterial: K (Msm) = M. smegmatis MC2-155. Note: All strains without an ATCC number are either clinical isolates or obtained from a different source than ATCC (source provided in supporting information).
Control antibiotics: AMK = amikacin, AMP = ampicillin, OFX = ofloxacin.
Overall, when analyzing the MIC data obtained against the Gram-positive bacterial strains (strains A-D), we note that most of the novel bis(N-amidinohydrazones) (3A,B, 4C,D, 4F) and N-(amidino)-N'-aryl-bishydrazones (7Aa-Ad, and 7Af-Ag) showed excellent antibacterial activity against Listeria monocytogenes ATCC 19115 (strain B, Lmo) (MIC <0.5-7.8 μM), methicillin-resistant Staphylococcus aureus (strain C, MRSA) (MIC = 1.0-7.8 μM), and vancomycin-resistant enterococcus (strain D, VRE) (MIC = 1.0-7.8 μM). All of the novel compounds displayed poor activity against Bacillus subtilis 168 (strain A, Bsu), with the exception of compound 4F, which displayed excellent activity (MIC = 7.8 μM) against this strain. Likewise, compound 7Ae exhibited excellent antibacterial activity against L. monocytogenes ATCC 19115 (strain B, Lmo; MIC = 7.8 μM) and VRE (strain D; MIC = 2.0-3.9 μM), but only moderate antibacterial activity against B. subtilis 168 (strain A, Bsu; MIC = 31.3 μM) and MRSA (strain C; MIC = 15.6 μM), respectively. In general, compounds 4A, 4E (except against strain D, VRE), 4G, 4H, and 8Aa (except against strain D) showed poor antibacterial activity (MIC = 62.5-≥500 μM) against all four Gram-positive bacterial strains tested. Overall, compound 4F was the most active against Gram-positive bacteria.
On the other hand, the majority of the novel compounds remained inactive against most of the Gram-negative bacterial strains that we tested with MIC values ranging from 62.5->500 μM. As one of several exceptions, compound 4A exhibited excellent activity against P. aeruginosa ATCC 27853 (strain I, Pae; MIC = 2.0 μM) and moderate activity against Klebsiella pneumoniae ATCC 27736 (strain H, Kpn; MIC = 15.6 μM). Similarly, compound 4F also displayed excellent antibacterial activities against Pae strain I (MIC = 1.0-2.0 μM) and S. enterica ATCC 14028 (strain J, Sen; MIC = 3.9 μM), as well as moderate to low activity against Kpn strain H (MIC = 31.3-62.5 μM). Additionally, 7Ae showed only moderate antibacterial activities against Kpn strain H (MIC = 15.6-31.3 μM), strain I (MIC = 31.3-62.5 μM), and Sen strain J (MIC = 15.6 μM). Compounds 3B, 4C-D, 4F, 7Aa-Ad, and 7Af-Ag exhibited excellent antibacterial activity (MIC = 1.0-3.9 μM) against M. smegmatis MC2-155 (strain K, Msm), whereas compounds 4H and 7Ae showed only moderate activity (MIC = 15.6-31.3 μM) and compounds 3A, 4A, 4E, 4G, and 8Aa showed low activity (MIC = 125->500 μM) against this mycobacterial strain. It is noteworthy that when compared to clinically relevant antibacterial drugs, such as AMK (MIC = 3.9-125 μM), AMP (MIC ≥250 μM), and OFX (MIC ≤0.5-31.3 μM), the novel compounds showed either superior or comparable antibacterial activity against all bacterial strains tested.
2.4. Antifungal activity
Having established the antibacterial activity profile of the novel bis(N-amidinohydrazones) and N-(amidino)-N'-arylbishydrazones, we next determined their antifungal activity against a panel of seven Candida albicans strains using a concentration range of 0.5-31.3 μg/mL (Table 2). We used the common antifungal agent amphotericin B (AmB) as a positive control testing in the same concentration range of 0.5-31.3 μg/mL. For antifungal activity, we use the following descriptors: excellent (MIC = 0.5-3.9 μg/mL), moderate (MIC = 7.8-15.6 μg/mL), and poor (MIC = 31.3 μg/mL). We found that compounds 3B, 4F, 7Aa, 7Ab, and 7Af exhibited excellent antifungal activities against all fungal strains tested (MIC = 1.0-3.9 μg/mL), with 4F being overall the most active. The only exception to the aforementioned statement was 7Af, which displayed only moderate activity against C. albicans ATCC 90819 (MIC = 7.8 μg/mL). Similarly, compound 4A also displayed excellent antifungal activities (MIC = 2.0-3.9 μg/mL) against most strains, except against C. albicans ATCC 1003 (MIC = 7.8 μg/mL) and C. albicans ATCC 1237 (MIC = 31.3 μg/mL). Compounds 7Ad, 7Ae, 7Ag, and 8Aa showed only moderate fungal growth inhibition (MIC = 7.8-15.6 μg/mL) against the majority of the fungal strains, with the exception of C. albicans ATCC 64124 (MIC = 31.3 μg/mL for 7Ae, 7Ag, and 8Aa), C. albicans ATCC 90819 (MIC = 31.3 μg/mL for 7Ag), C. albicans ATCC 1003 (MIC = 3.9 μg/mL for 7Ad) and C. albicans ATCC 2310 (MIC = 3.9 μg/mL for 7Ad). Compounds 7Ac also exhibited excellent growth inhibition against most of the fungal strains tested, except against C. albicans ATCC 10231 (moderate activity, MIC = 7.8 μg/mL) and C. albicans ATCC 64124 (poor activity, MIC = 31.3 μg/mL). Just as observed against all bacterial strains, compounds 4G and 4H displayed no activity (MIC ≥31.3 μg/mL) against all fungal strains tested. It is important to emphasize that most of our bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones displayed either superior or comparable antifungal activities against the majority of the fungal strains tested, when compared to the clinically-relevant antifungal agent, AmB (MIC = 2.0-3.9 μg/mL).
Table 2.
MIC values in μg/mLa and (μM) for compounds 3A,B, 4A,C-H, 7Aa-Ag, and 8Aa against various C. albicans strains.
| C. albicans ATCC strain # | |||||||
|---|---|---|---|---|---|---|---|
| Cpd | 1003a | 1237b | 2310c | 2876c | 10231b | 64124b | 90819b |
| AmB | 3.9 (4.2) | 3.9 (4.2) | 3.9 (4.2) | 3.9 (4.2) | 3.9 (4.2) | 2.0-3.9 (2.1-4.2) | 2.0 (2.1) |
| 3A | 7.8 (19.7) | 15.6 (39.5) | >31.3 (>79.2) | 7.8 (19.7) | 15.6 (39.5) | >31.3 (>79.2) | >31.3 (>79.2) |
| 3B | 3.9 (9.9) | 3.9 (9.9) | 3.9 (9.9) | 3.9 (9.9) | 3.9 (9.9) | 3.9 (9.9) | 3.9 (9.9) |
| 4A | 7.8 (18.4) | >31.3 (>73.9) | 3.9 (9.2) | 2.0 (4.7) | 2.0-3.9 (4.7-9.2) | 2.0 (4.7) | 3.9 (9.2) |
| 4C | 7.8 (17.8) | 7.8 (17.8) | 7.8 (17.8) | 3.9 (8.9) | 7.8 (17.8) | 7.8 (17.8) | 7.8 (17.8) |
| 4D | 15.6 (34.6) | 15.6 (34.6) | 31.3 (69.3) | 3.9 (8.6) | 7.8 (17.3) | >31.3 (>69.3) | 7.8 (17.3) |
| 4E | 7.8 (17.8) | 7.8 (17.8) | 7.8 (17.8) | 3.9 (8.9) | 7.8 (17.8) | 7.8 (17.8) | 7.8 (17.8) |
| 4F | 1.0 (2.3) | 2.0 (4.6) | 1.0 (2.3) | 1.0 (2.3) | 1.0-2.0 (2.3-4.6) | 1.0 (2.3) | 2.0 (4.6) |
| 4G | >31.3 (>90.1) | >31.3 (>90.1) | >31.3 (>90.1) | >31.3 (>90.1) | >31.3 (>90.1) | 31.3 (90.1) | >31.3 (>90.1) |
| 4H | >31.3 (>90.1) | 31.3 (90.1) | 31.3 (90.1) | 7.8 (22.5) | >31.3 (>90.1) | >31.3 (>90.1) | >31.3 (>90.1) |
| 7Aa | 2.0 (4.6) | 3.9 (9.2) | 2.0 (4.6) | 2.0 (4.6) | 2.0 (4.6) | 3.9 (9.2) | 2.0 (4.6) |
| 7Ab | 2.0 (4.1) | 3.9 (8.3) | 3.9 (8.3) | 3.9 (8.3) | 2.0 (4.1) | 3.9 (8.3) | 3.9 (8.3) |
| 7Ac | 2.0 (4.7) | 7.8 (19.0) | 3.9 (9.5) | 3.9 (9.5) | 7.8 (19.0) | 31.3 (76.2) | 3.9 (9.5) |
| 7Ad | 3.9 (9.1) | 15.6 (36.5) | 3.9 (9.1) | 7.8 (18.3) | 7.8 (18.3) | 15.6 (36.5) | 7.8 (18.3) |
| 7Ae | 7.8 (18.7) | 15.6 (37.3) | 15.6 (37.3) | 15.6 (37.3) | 15.6 (37.3) | 31.3 (74.9) | 15.6 (37.3) |
| 7Af | 2.0 (4.5) | 3.9 (9.1) | 3.9 (9.1) | 3.9 (9.1) | 1.0 (2.3) | 3.9 (9.1) | 7.8 (18.2) |
| 7Ag | 7.8 (16.8) | 15.6 (33.8) | 7.8 (16.8) | 15.6 (33.8) | 7.8 (16.8) | 31.3 (67.8) | 31.3 (67.8) |
| 8Aa | 15.6 (34.3) | 15.6 (34.3) | 15.6 (34.3) | 15.6 (34.3) | 15.6 (34.3) | 31.3 (68.7) | 15.6 (34.3) |
These antifungal MIC values were determined originally in μg/mL using a range of 0.5 to 31.3 μg/mL. The values in μM into parentheses are presented for comparison with the antibacterial MIC values, which were determined originally in μM.
Indicates strains that are resistant to FLC, ITC, and VOR according to ATCC.
Indicates strains that are susceptible to FLC, ITC, and VOR according to ATCC.
Control antifungal agent: AmB = amphotericin B.
2.5. SAR analysis
Using the antibacterial and antifungal results presented above (Tables 1 and 2), we addressed the eight apriori questions posed:
-
(i)
Are linkers comprised of only one aromatic ring sufficient to confer antimicrobial activity? We concluded that bis(N-amidinohydrazones) with linkers comprised of a single phenyl ring, regardless of its substitution pattern (para or meta, as in compounds 4G and 4H, respectively), displayed insufficient activity as antimicrobial agents to warrant further study of their antibacterial or antifungal activity.
-
(ii)
Is the presence of methyl groups on the carbons alpha to the linker beneficial for antimicrobial activity? Comparing the MIC values of compounds 3A and 4A as well as 7Aa and 8Aa, which only differ by the absence or presence of a methyl group on the carbons alpha to the phenyl rings, led us to conclude that the methyl group was detrimental to the antibacterial activity of these compounds. However, the presence of the methyl group appeared beneficial in terms of antifungal activity. Compound 4A was overall a much more active antifungal agent than compound 3A.
-
(iii)
How does the substitution pattern of the biphenyl linker affect antimicrobial activity? When evaluating compounds 3A and 3B, the antibacterial MIC values were similar in all cases, except against strain K where the MIC value for compound 3B (MIC = 1 μM) was 500 times lower than that for compound 3A (MIC >500 μM). From these data, we deduced that the substitution pattern of the biphenyl linker had minimal effect on antibacterial activity. However, we found the substitution pattern of the biphenyl linker to have a substantial effect on antifungal activity. For example, compound 3B was a much more active antifungal agent than compound 3A.
-
(iv)
How does the length of the flexible linear alkyl spacer between the two phenyl rings affect antimicrobial activity? When comparing the MIC values of compounds 4A, 4C, and 4D, again we observed opposing trends in antibacterial and antifungal activities. Longer flexible alkyl linkers between the two phenyl rings correlated with a decrease in antibacterial MIC values, but correlated with an increase in antifungal MIC values.
-
(v)
Is the introduction of rigidity in the linker beneficial for antimicrobial activity? By contrasting the MIC value profile of compound 4C containing a flexible linker to that of compound 4F containing a rigid linker, we concluded that introducing rigidity in the linker was overall beneficial for antimicrobial activity.
-
(vi)
Is the presence of an oxygen atom in the linker beneficial for antimicrobial activity? A comparison of the MIC value profiles of compounds 4C and 4E indicated that replacing the methylene bridging the two phenyl groups of the linker by an oxygen atom had no effect on antifungal activity, but was detrimental to antibacterial activity.
-
(vii)
How does the identity of the substituent, when located at the same position on the aryl ring of the side chain, affect antimicrobial activity? By comparing compounds 7Aa-7Ag, we observed that the nature of the substituent in the ortho position of the mono-substituted aryl group of compounds 7Aa and 7Ab did not affect antibacterial or antifungal activity, whereas the nature of the substituent in the para position of the mono-substituted aryl group of compounds 7Ac-7Ae had opposing effects on antibacterial (CN > halogen) and antifungal (halogen > CN) activity. We also observed that the o,p-difluorinated and o,p-dichlorinated aryl moieties of compounds 7Af and 7Ag had similar overall antibacterial activity, but different antifungal activity, in which the o,p-difluorinated group possessed improved antifungal activity.
-
(viii)
How does the substitution pattern of the aryl ring (ortho versus para-monosubstituted versus ortho,para-disubstituted) affect antimicrobial activity? Finally, comparison of the activity resulting from different chlorination patterns in compounds 7Aa (ortho), 7Ad (para), and 7Ag (o,p-diCl), revealed minimal effects on antibacterial activity, but substantial effects on antifungal activity. The o-Cl N-(amidino)-N'-aryl-bishydrazone was superior to the p-Cl analogue, which in turn was much better than o,p-diCl analogue. In summary, the similarity and differences in the trends observed in this promising, preliminary SAR will be used to lay the foundation for the future discovery of compounds capable of selectively targeting either bacteria or fungi.
2.6. Development of bacterial and fungal resistance studies
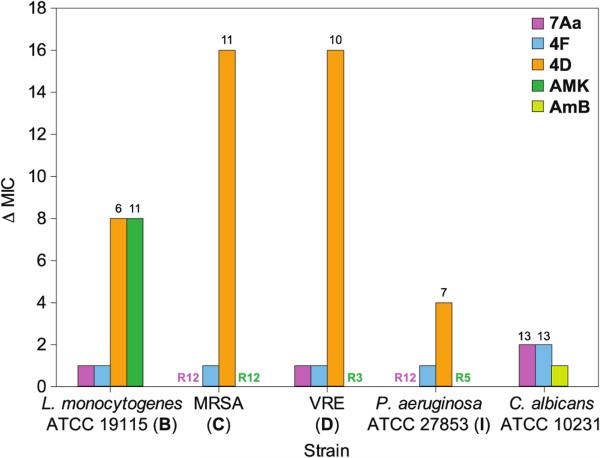
The emergence of antibiotic resistance by microbes is an inevitable process; however, the frequency at which resistance develops varies from one antibiotic to another. It is critical to assess the ability of new antimicrobials to evade, as long as possible, the development of bacterial and fungal resistance early in the drug discovery process. To establish if the bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones evade resistance in bacteria, we first performed a multi-step resistance selection experiment with compounds 4D, 4F, and 7Aa as well as with AMK as a reference drug against four bacterial strains (Figure 1). L. monocytogenes ATCC 19115 (strain B, Lmo), MRSA (strain C), VRE (strain D), and P. aeruginosa ATCC 27853 (strain I, Pae) were exposed to sub-inhibitory concentrations of compounds 4D, 4F, 7Aa, and AMK, and were sub-cultured for 15 serial passages to determine if an increase in MIC values occurred for each compound against the strains tested. L. monocytogenes ATCC 19115 (strain B, Lmo), MRSA (strain C), VRE (strain D), and P. aeruginosa ATCC 27853 (strain I, Pae) did not develop resistance to compound 4F,as demonstrated by the one-fold increase in MIC values after 15 serial passages. Likewise, strains B (Lmo) and D (VRE) did not develop resistance to compound 7Aa, but strains C (MRSA) and I (Pae) developed resistance to this compound after twelve serial passages. In contrast, we observed the rapid development of resistance to compound 4D in strains C (MRSA) and D (VRE)with a 16-fold increase in MIC values after fifteen serial passages. This development of resistance against compound 4D was observed also in strains B (Lmo) and I (Pae), where increases in relative MIC values by 8- and 4-folds, respectively, were observed after 15 passages. Interestingly, in strains D (VRE) and I (Pae), resistance to the control antibiotic, AMK, was observed also after 3 and 5 passages, respectively; whereas in strains C (MRSA) and B (Lmo), resistance developed after 12 and 15 passages, respectively. Overall, these results indicate that there is a low probability of emergence of resistance to these promising novel biscationic compounds 4D, 4F, and 7Aa.
Figure 1.
Changes in MIC values of L. monocytogenes ATCC 19115 (strain B), MRSA (strain C), VRE (strain D), and P. aeruginosa ATCC 27853 (strain I) treated with AMK (green), 7Aa (purple), 4F (blue), 4D (orange), as well as C. albicans ATCC 10231 treated with AmB (yellow), 7Aa (purple), and 4F (blue) over 15 cycles. Numbers above the bars represent the passage when either bacterial or fungal cells developed resistance.
We next investigated the development of drug resistance in C. albicans ATCC 10231 to our best antifungal compounds 7Aa and 4F, and we found that C. albicans ATCC 10231 did not to develop drug resistance to either 7Aa or 4F, despite repeated treatments with sub-MIC drug concentrations. Only a slight 2-fold shift in MIC values was observed after 13 passages. These results also suggest the low probability of onset of drug resistance by fungi to these novel compounds.
2.7. Measurement of ROS induction in fungal cells
Various antifungal drugs, such as AmB and miconazole, as well as novel antifungal agents, were shown to mediate their inhibitory effect by inducing intracellular ROS production.30-32 Universally, eukaryotic cells produce basal amount of ROS in mitochondria as a byproduct of cellular metabolism. In response, the cellular enzymatic antioxidants, including superoxide dismutase and glutathione peroxidase, scavenge ROS in cells. However, overproduction of deleterious ROS perturbs the delicate, intracellular equilibrium between ROS production and scavenging, and the result is cellular damage.
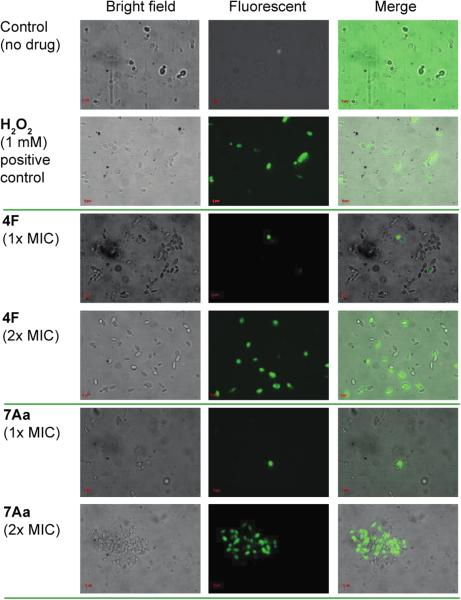
In order to investigate the ability of compounds 4F and 7Aa to alter ROS production in C. albicans ATCC 10231 (strain A), we performed a fluorescent-based assay using a 2',7'-dichlorodihydrofluorescein diacetate (DCFH-DA) dye.33 We found that treatment of C. albicans ATCC 10231 (strain A) with compounds 4F and 7Aa at their 1× and 2× MIC values increased intracellular ROS production in this fungal strain (Figure 2). As expected, the H2O2 positive control (at 1 mM) also induced ROS production in yeast cell, whereas no ROS induction was observed with untreated yeast cells (negative control). Although it remains to be determined if ROS production is critical for growth inhibition and death of yeast cells, these results suggest one direction for future mechanistic exploration, which is outside of the scope of the current proof-of-principle exploration for the first generations of bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones as antimicrobials.
Figure 2.
Effect of compounds 4F and 7Aa on intracellular ROS production by C. albicans ATCC 10231. Yeast cells were treated in the absence of drug (negative control), 1 mM of H2O2 (positive control), or 4F and 7Aa, at 1× and 2× respective MIC values for 1 h at 35 °C. After staining with DCFH-DA (20 μg/mL), the samples were analyzed using a Zeiss Axovert 200M fluorescence microscope.
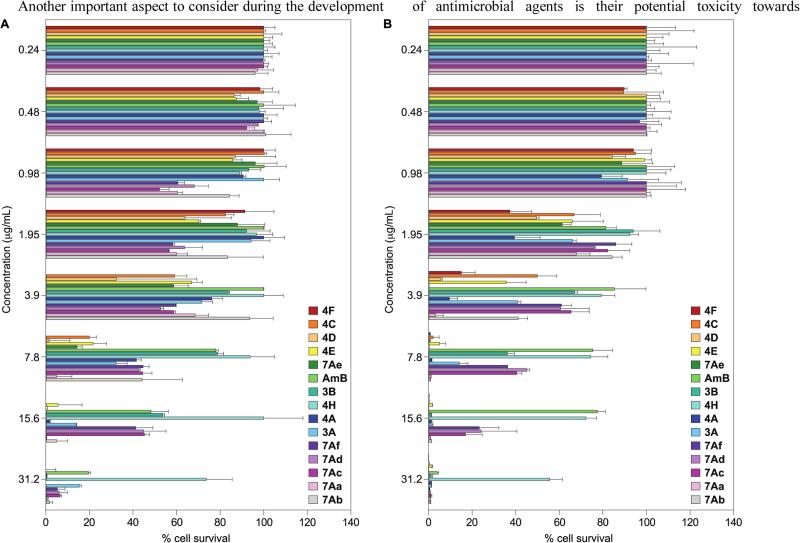
2.8. Cytotoxicity
Another important aspect to consider during the development of antimicrobial agents is their potential toxicity towards mammalian cells. Having established the potent antimicrobial activities of the novel bis(N-amidinohydrazones) and N-(amidinohydrazones)-N'-aryl-bishydrazones against bacteria and fungi, we determined the toxicity profile of these compounds against two mammalian cell lines (A549 and BEAS-2B; Figure 3) and measure the IC50 values for these compounds (Table 3). The majority of the novel compounds showed concentration-dependent toxicity with IC50 values of 1.7-6.7 μg/mL. Compounds 3B, 7Ac, 7Ad, and 7Af, with excellent antibacterial MIC values of <0.4-3.3 μg/mL, displayed IC50 values of 4.4-13.9 μg/mL against both mammalian cell lines. It is important to note that compounds 7Ac and 7Af also displayed excellent antifungal MIC values, indicating that these compounds are important leads for future evaluation.
Figure 3.
Mammalian cell cytotoxicity of selected bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones, as well as AmB (as a control) against A. A549 cell line and B. BEAS-2B cell line.
Table 3.
The cytotoxicity (IC50, μg/mL) of selected bis(N-amidinohydrazones) and N-(amidino)-N′-aryl-bishydrazones against A549 and BEAS-2B cell lines. Values are presented as mean ± SDEV.
| Mammalian cell line | ||
|---|---|---|
| Cpd | A549 | BEAS-2B |
| AmB | 18.2 ± 4.5 | 17.7 ± 6.4 |
| 3A | 6.6 ± 1.7 | 3.0 ± 0.7 |
| 3B | 13.9 ± 4.1 | 5.7 ± 1.8 |
| 4A | 6.3 ± 2.2 | 1.7 ± 0.5 |
| 4C | 4.3 ± 1.3 | 2.9 ± 0.9 |
| 4D | 2.9 ± 0.9 | 1.8 ± 0.6 |
| 4E | 3.9 ± 0.9 | 2.9 ± 0.9 |
| 4F | 2.5 ± 1.4 | 1.8 ± 0.6 |
| 4H | 31.2 ± 7.2 | 33.0 ± 4.6 |
| 7Aa | 2.8 ± 0.9 | 2.3 ± 1.0 |
| 7Ab | 6.7 ± 2.3 | 3.4 ± 1.3 |
| 7Ac | 4.4 ± 1.4 | 6.0 ± 1.2 |
| 7Ad | 4.9 ± 1.1 | 6.0 ± 1.1 |
| 7Ae | 4.2 ± 1.4 | 1.9 ± 0.8 |
| 7Af | 4.8 ± 1.3 | 5.8 ± 1.2 |
2.9. hERG inhibition assay
The hERG encodes a voltage gated potassium channel that plays an essential role in regulating hearth rhythm.34 Inhibition of the hERG channel disrupts the heart rhythm and may lead to cardiac death. Recently, drugs (e.g., terfendadine and cisapride) were withdrawn from the market due to their interaction with the hERG channel.35 Currently, the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) require evaluation at hERG for drug candidates.
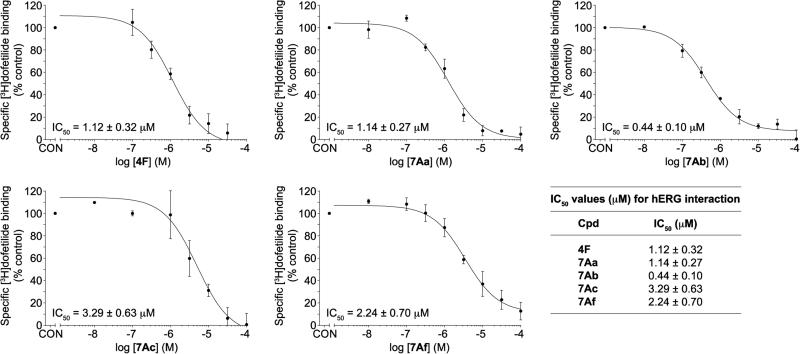
We performed a [3H]-dofetilide competition binding assay using HEK-293 cell membranes stably expressing the hERG channel to evaluate the activity of our most potent bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones at hERG (Figure 4). Compounds exhibiting IC50 values of >10 μM are considered to have low affinity for the hERG channel. Compounds displaying IC50 values in the range of 1-10 μM are considered to have moderate affinity, whereas compounds exhibiting IC50 values of <1 μM are considered to have high affinity for the hERG channel.36 The majority of the novel compounds displayed IC50 values within the acceptable range of 1-10 μM (Figure 4). Although compound 7Ab displayed excellent antifungal activities (2-3.9 μg/mL), it exhibited in vitro mammalian cell toxicity and was found to potently interact with the hERG channel (IC50 = 0.44 ± 0.10 μM), suggesting that the o-bromophenyl substituent is an unfavorable structural feature. However, the p-fluorophenyl and the 2,4-difluorophenyl groups in compounds 7Ac and 7Af displayed only moderate interaction with hERG (IC50 = 3.29 ± 0.63 μM and 2.24 ± 0.70 μM, respectively). Strikingly, both of these compounds displayed excellent antifungal activities and also exerted low mammalian cell toxicity. Similarly, compounds 4F and 7Aa displayed moderate affinity for hERG (IC50 = 1.12 ± 0.32 μM and 1.14 ± 0.27 μM, respectively); however, they displayed some toxicity against mammalian cells. Therefore, we concluded that compounds 7Ac and 7Af are the most promising N-(amidino)-N'-aryl-bishydrazone compounds.
Figure 4.
IC50 curves for hERG interaction by representative bis(N-amidinohydrazones) and N-(amidino)-N'-aryl-bishydrazones along with a table (bottom right) summarizing these IC50 values.
3. Conclusions
In summary, we discovered nine bis(N-amidinohydrazones) and eight N-(amidino)-N'-aryl-bishydrazones as first-generation antibacterial and antifungal agents. These compounds displayed low potential for the development of resistance and were found to induce ROS production in a C. albicans strain. They also were found to display reasonable toxicity profiles against two mammalian cell lines as well as acceptable levels of interaction with hERG channels. However, as these compounds display both antibacterial and antifungal activity, it is possible that they aim at a target shared by prokaryotes and eukaryotes and potentially display lower selectivity. However, in light of our SAR study, we conclude that compounds 4F, 7Ac, and 7Af, which showed the most promise, warrant further optimization. Such studies are currently underway in our laboratories.
4. Experimental section / Supplementary material
The supporting information includes experimental procedures, characterization data (melting point, 1H and 13C NMR analysis along with the spectra, and high-resolution mass spectrometry and elemental analyses) of all compounds synthesized and studied. Experimental protocols for all biological experiments also are provided. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was supported by startup funds from the College of Pharmacy (to S.G.-T.) and by the Office of the Dean of the College of Medicine (to D.S.W.) at the University of Kentucky. It was also supported by NIH grant AI090048 (to S.G.-T.); and NIH grants U01 DA013519, UL1TR000117 and T32 DA016176 (to L.P.D. and J.R.N.). D.S.W. was also supported in part through collaborations involving NIH grants P20 RR020171 (to L. Hersh), CA172379 (to C. Liu), and CA187273 (to V. Rangnekar). The manuscript's contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, NIAID, or NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention . Office of Infectious Disease Antibiotic Resistance Threats in the United States; 2013. [Google Scholar]
- 2.Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, Lessa FC, Lynfield R, Nadle J, Petit S, Ray SM, Schaffner W, Townes J, Fridkin S. M.S.I. Emerging Infections Program-Active Bacterial Core Surveillance, National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern. Med. 2013;173:1970–1978. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. Foodborne illness acquired in the United States - major pathogens. Emerg. Infect. Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fridkin SK, Jarvis WR. Epidemiology of nosocomial fungal infections. Clin. Microbiol. Rev. 1996;9:499–511. doi: 10.1128/cmr.9.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S, T. National Healthcare Safety Network, N.F. Participating Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect. Control Hosp. Epidemiol. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 6.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 7.Nguewa PA, Fuertes MA, Cepeda V, Iborra S, Carrion J, Valladares B, Alonso C, Perez JM. Pentamidine is an antiparasitic and apoptotic drug that selectively modifies ubiquitin. Chem. Biodivers. 2005;2:1387–1400. doi: 10.1002/cbdv.200590111. [DOI] [PubMed] [Google Scholar]
- 8.Bilik P, Tanious F, Kumar A, Wilson WD, Boykin DW, Colson P, Houssier C, Facompre M, Tardy C, Bailly C. Novel dications with unfused aromatic systems: trithiophene and trifuran derivatives of furimidazoline. ChemBioChem. 2001;2:559–569. doi: 10.1002/1439-7633(20010803)2:7/8<559::AID-CBIC559>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 9.Rollas S, Kucukguzel SG. Biological activities of hydrazone derivatives. Molecules. 2007;12:1910–1939. doi: 10.3390/12081910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedia KK, Elcin O, Seda U, Fatma K, Nathaly S, Sevim R, Dimoglo A. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity. Eur. J. Med. Chem. 2006;41:1253–1261. doi: 10.1016/j.ejmech.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Kucukguzel G, Kocatepe A, De Clercq E, Sahin F, Gulluce M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006;41:353–359. doi: 10.1016/j.ejmech.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Chang L, Whittaker NF, Bewley CA. Crambescidin 826 and dehydrocrambine A: new polycyclic guanidine alkaloids from the marine sponge Monanchora sp. that inhibit HIV-1 fusion. J. Nat. Prod. 2003;66:1490–1494. doi: 10.1021/np030256t. [DOI] [PubMed] [Google Scholar]
- 13.Kucukguzel SG, Mazi A, Sahin F, Ozturk S, Stables J. Synthesis and biological activities of diflunisal hydrazide-hydrazones. Eur. J. Med. Chem. 2003;38:1005–1013. doi: 10.1016/j.ejmech.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Sidoryk K, Switalska M, Jaromin A, Cmoch P, Bujak I, Kaczmarska M, Wietrzyk J, Dominguez EG, Zarnowski R, Andes DR, Bankowski K, Cybulski M, Kaczmarek L. The synthesis of indolo[2,3-b]quinoline derivatives with a guanidine group: highly selective cytotoxic agents. Eur. J. Med. Chem. 2015;105:208–219. doi: 10.1016/j.ejmech.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Todeschini AR, de Miranda ALP, da Silva KCM, Parrini SC, Barreiro EJ. Synthesis and evaluation of analgesic, antiinflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives. Eur. J. Med. Chem. 1998;33:189–199. [Google Scholar]
- 16.Said M, Badshah A, Shah NA, Khan H, Murtaza G, Vabre B, Zargarian D, Khan MR. Antitumor, antioxidant and antimicrobial studies of substituted pyridylguanidines. Molecules. 2013;18:10378–10396. doi: 10.3390/molecules180910378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Melnyk P, Leroux V, Sergheraert C, Grellier P. Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg. Med. Chem. Lett. 2006;16:31–35. doi: 10.1016/j.bmcl.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 18.Laville R, Thomas OP, Berrue F, Marquez D, Vacelet J, Amade P. Bioactive guanidine alkaloids from two Caribbean marine sponges. J. Nat. Prod. 2009;72:1589–1594. doi: 10.1021/np900244g. [DOI] [PubMed] [Google Scholar]
- 19.Fair RJ, Hensler ME, Thienphrapa W, Dam QN, Nizet V, Tor Y. Selectively guanidinylated aminoglycosides as antibiotics. ChemMedChem. 2012;7:1237–1244. doi: 10.1002/cmdc.201200150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gürsoy A, Terzioglu N, Ötük G. Synthesis of some new hydrazidehydrazones, thiosemicarbazides and thiazolidinones as possible antimicrobials. Eur. J. Med. Chem. 1997;32:653–757. [Google Scholar]
- 21.Hua H-M, Peng J, Dunbar DC, Schinazi RF, de Castro Andrews AG, Cuevas C, Garcia-Fernandez LF, Kelly M, Hamann MT. Batzelladine alkaloids from the caribbean sponge Monanchora unguifera and the significant activities against HIV-1 and AIDS opportunistic infectious pathogens. Tetrahedron. 2007;63:11179–11188. [Google Scholar]
- 22.Özdemir A, Turan-Zitouni G, Kaplancikli ZA, Demirci F, Iscan G. Studies on hydrazone derivatives as antifungal agents. J. Enz. Inhib. Med. Chem. 2008;23:470–475. doi: 10.1080/14756360701709094. [DOI] [PubMed] [Google Scholar]
- 23.Kibirev VK, Osadchuk TV, Kozachenko OP, Kholodovych V, Fedoryak D, Brovarets VS. Synthesis, biological evaluation and docking of novel bisamidinohydrazones as non-peptide inhibitors of furin. Ukr. Biochem. J. 2015;87:55–63. [PubMed] [Google Scholar]
- 24.Calas M, Ouattara M, Piquet G, Ziora Z, Bordat Y, Ancelin ML, Escale R, Vial H. Potent antimalarial activity of 2-aminopyridinium salts, amidines, and guanidines. J. Med. Chem. 2007;50:6307–6315. doi: 10.1021/jm0704752. [DOI] [PubMed] [Google Scholar]
- 25.Boykin DW, Kumar A, Spychala J, Zhou M, Lombardy RJ, Wilson WD, Dykstra CC, Jones SK, Hall JE, Tidwell RR, et al. Dicationic diarylfurans as anti-Pneumocystis carinii agents. J. Med. Chem. 1995;38:912–916. doi: 10.1021/jm00006a009. [DOI] [PubMed] [Google Scholar]
- 26.Gambari R, Nastruzzi C. DNA-binding activity and biological effects of aromatic polyamidines. Biochem. Pharmacol. 1994;47:599–610. doi: 10.1016/0006-2952(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 27.Bielawski K, Bielawska A, Wolczynski S. Aromatic extended bisamidines: synthesis, inhibition of topoisomerases, and anticancer cytotoxicity in vitro. Arch. Pharm. (Weinheim) 2001;334:235–240. doi: 10.1002/1521-4184(200107)334:7<235::aid-ardp235>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 28.Nakayama T, Taira S, Ikeda M, Ashizawa H, Oda M, Arakawa K, Fujii S. Synthesis and structure-activity study of protease inhibitors. V. Chemical modification of 6-amidino-2-naphthyl 4-guanidinobenzoate. Chem. Pharm. Bull. (Tokyo) 1993;41:117–125. doi: 10.1248/cpb.41.117. [DOI] [PubMed] [Google Scholar]
- 29.Cavallini G, Massarani E, Nardi D, Mauri L, Mantegazza P. Antibacterial agents. Some new guanylhydrazone derivatives. J. Med. Pharm. Chem. 1961;4:177–182. doi: 10.1021/jm50017a013. [DOI] [PubMed] [Google Scholar]
- 30.Belenky P, Camacho D, Collins JJ. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013;3:350–358. doi: 10.1016/j.celrep.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delattin N, Cammue BP, Thevissen K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med. Chem. 2014;6:77–90. doi: 10.4155/fmc.13.189. [DOI] [PubMed] [Google Scholar]
- 32.Ngo HX, Shrestha SK, Garneau-Tsodikova S. Identification of ebsulfur analogues with broad spectrum antifungal activity. ChemMedChem. 2016;11:1507–1516. doi: 10.1002/cmdc.201600236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Chang W, Zhang M, Li X, Jiao Y, Lou H. Diorcinol D exerts fungicidal action against Candida albicans through cytoplasm membrane destruction and ROS accumulation. PloS one. 2015;10:e0128693. doi: 10.1371/journal.pone.0128693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995;80:795–803. doi: 10.1016/0092-8674(95)90358-5. [DOI] [PubMed] [Google Scholar]
- 35.Fermini B, Fossa AA. The impact of drug-induced QT interval prolongation on drug discovery and development. Nat. Rev. Drug. Discov. 2003;2:439–447. doi: 10.1038/nrd1108. [DOI] [PubMed] [Google Scholar]
- 36.Guth BD, Rast G. Dealing with hERG liabilities early: diverse approaches to an important goal in drug development. Br. J. Pharmacol. 2010;159:22–24. doi: 10.1111/j.1476-5381.2009.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]