Abstract
Cognitive decline is a major deficit that arises with age in humans. While some research on the underlying causes of these problems can be done in humans, harnessing the strengths of small model systems, particularly those with well-studied longevity mutants, such as the nematode C. elegans, will accelerate progress. Here we review the approaches being used to study cognitive decline in model organisms and show how simple model systems allow the rapid discovery of conserved molecular mechanisms, which will eventually enable the development of therapeutics to slow cognitive aging.
Keywords: aging, longevity, cognitive aging, model systems, cognitive decline
1. Introduction
Over the past century, the population of elderly individuals has greatly increased, leading to the emergence of age-related cognitive decline as a significant public health threat. Memory impairments are exhibited in many neurodegenerative diseases in which age is a risk factor, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and several forms of dementia. Additionally, cognitive decline is a prominent feature of normal aging, with decreased cognitive function beginning in mid-life and worsening with advanced age. In order to prevent these deficits, it is imperative to gain an understanding of how nervous system function is altered by the process of aging. Work in both vertebrate and invertebrate model systems has uncovered many features of neuronal aging and has determined how they are linked to age-related cognitive decline. More recent studies in model organisms have revealed that pathways that regulate lifespan may also play a role in the maintenance of cognition with age. Here we review features of neuronal and cognitive aging, and highlight how work in model systems has uncovered evolutionarily conserved pathways that regulate both longevity and age-related changes in learning and memory.
2. Model systems and assays of cognitive function
Studies of human cognitive function and aging are often difficult to carry out, and are typically limited to epidemiological studies, genome-wide association studies, or the identification of changes in regional activity or neuroanatomical structure with aging. Longitudinal studies can identify both genetic and environmental factors that are linked to cognitive aging, as well interventions that may slow cognitive aging, but this wealth of information can take decades to collect, and usually cannot determine causation. While these studies can provide information regarding correlates of cognitive aging, humans are an unsuitable system to directly test the role of specific genes and molecules by knockout or overexpression in cognitive aging. Furthermore, researchers cannot use humans to rapidly screen chemical libraries of compounds that may ameliorate age-related cognitive defects. Thus, the development of model systems has been invaluable in the discovery and analysis of the conserved mechanisms underlying learning, memory, and other neuronal abilities. Interestingly, analyses of both vertebrate and invertebrate systems have revealed that the pathways that regulate cognitive function are highly conserved [17].
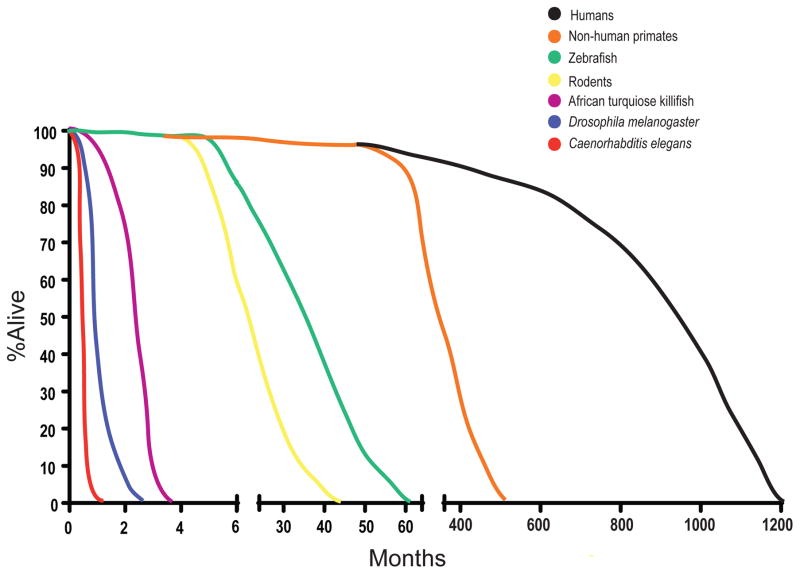
The ability to study aging in a simple, short-lived system is extremely useful. Aging studies in primates are generally impractical because they have decades-long lifespans and are not genetically tractable (Figure 1). Other mammalian systems that have genetic and molecular tools available, such as mice and rats, still live for years, making it difficult to rapidly perform aging studies (Figure 1). Even zebrafish, a relatively simpler non-mammalian vertebrate model, has a lifespan of 2–3 years (Figure 1). Recently, the African turquoise killifish has been identified as a promising model for aging. With a lifespan of only 4–6 months, it is the shortest-lived vertebrate that can be bred in captivity and will be useful for aging studies (Figure 1).
Figure 1.
Model systems allow for rapid study of longevity. Because of the long life of humans (black line), model systems are necessary to study regulators of aging. Non-human primates (orange line) still live for decades and thus are impractical for many aging studies. Simpler vertebrate models, such as rodents (yellow line) and zebrafish (green line), are short-lived relative to humans and non-human primates, but take years to reach an “aged” state. Turquoise killifish live for about 3 months, which is significantly shorter than other vertebrate models. Invertebrate models such as Drosophila (blue line) and C. elegans (red line) live for 3 months or 3 weeks, respectively, making them ideal systems for the rapid identification of genes and molecules involved in aging and age-related cognitive decline.
Invertebrate models such as Drosophila and C. elegans are excellent genetic models to study the process of aging, due to their lifespans of three months and three weeks, respectively (Figure 1). This short lifespan enables the rapid identification of genes that affect longevity [58, 65, 88, 101, 105, 118, 144, 158, 186, 207]. Moreover, a vast number of genetic techniques and tools are available that allow precise spatial and temporal control of genetic perturbations [38, 129]. Other invertebrate species, such as ants and bees, show promise for future studies in aging and cognition, but in the present review, the focus will be on more commonly used, genetically tractable organisms [4, 8, 36, 37, 66]. Though these features make invertebrate models attractive in the study of aging, there are some obvious caveats when working in such a simple system. They lack the analogous neuroanatomy that can be found in vertebrate models, and cannot fully recapitulate features of complex human diseases associated with neuronal dysfunction, such as AD. However, invertebrates remain useful for the rapid identification of genes that may be of interest for studies in higher organisms, and even more critically, can be used to determine the underlying molecular and genetic mechanisms before embarking on experiments in slower, more laborious systems. In the following section, we will review assays of cognitive and neuronal function across model systems, with a focus on evolutionarily conserved molecules and mechanisms.
2.1 Assays of cognitive and neuronal function in vertebrates
An obvious advantage of mammalian systems is that the nervous systems of rodents and non-human primates are well mapped and more closely resemble that of humans than do invertebrate systems, and age-related morphological changes in these have been determined [26, 138, 182]. Furthermore, there are many behavioral tasks developed to assess the function of these brain regions [9, 26, 221, 231]. This includes the circuits that control the cognitive processes that are most vulnerable to aging, the hippocampus and the prefrontal cortex (PFC) [138]. Moreover, these circuits and their activities that correlate to cognitive function can be easily measured by electrophysiological techniques, so the effects of age or interventions on these neuronal ensembles can be determined, even in behaving animals [69, 70, 96, 131]. Additionally, these circuits can be manipulated with precise spatiotemporal control using optogenetic techniques or Designer Receptors Exclusively Activated by Designer Drugs (DREADDS) [179, 229]. Furthermore, mammals can more closely model neurodegenerative diseases such as AD, PD, and other dementias due to similarities in brain structure with humans [115, 151]. However, a caveat to studying age-related neuronal dysfunction is that the subjects must be aged for more than a year even in rodents, while invertebrate models only require weeks to months to be considered “old”. Genetic manipulations are also laborious in mammals, although the use of techniques such as CRISPR may eventually help circumvent these difficulties.
Zebrafish have been traditionally been utilized in the study of developmental biology, but recent studies have demonstrated that they can also display associative learning. These learning tasks include association of food or images of conspecifics (other zebrafish) in a spatial location on a maze or in a shuttle-box [160, 189, 235]. The association of images with conspecifics with a location on a maze requires NMDA-type glutamate receptors, as treatment with MK-801, a non-competitive NMDA receptor antagonist, impairs this behavior [190]. Killifish also display associative learning on a modified version of the shuttle-box task, which pairs side of a tank with a visual cue (red light) and aversive stimulus (shock). Learning is measured by the success rate of escaping the side of the tank associated with the shock following presentation of the visual cue [219]. It still remains to be determined how well memory can be modeled in fish, though recent studies suggest that the shuttle-box task produces a short-term memory in zebrafish [50, 93]. The circuits and molecules that regulate associative learning in fish also remain largely unknown.
2.2 Assays of cognitive and neuronal function in invertebrates
Drosophila exhibit several types of learning, but olfactory learning is the form that has been most valuable in identifying required molecules and circuits, and has been applied to the study of aging, as well [74]. Briefly, flies learn to associate an odor (the conditioned stimulus) with a positive reinforcement (food reward), or negative reinforcement (mild electrical shock) [40, 216]. These training paradigms result in mechanistically and quantitatively different forms of performance, as measured by the memory expression at defined times post-conditioning. A single conditioning trial generates a memory that decays over the course of 24 hours [14, 108, 159, 208, 215]. Massed conditioning, which consists of multiple cycles with no rest between cycles, produces a robust initial memory that decays over a few days. By contrast, the memory formed after spaced conditioning lasts for 4–7 days and requires both protein synthesis and CREB activity at the time of conditioning, which are features of long-term associative memory in higher organisms [162, 188, 215, 233, 234]. The circuits that are responsible for olfactory memory formation are fairly well understood, and the Mushroom body (MB), a symmetrical structure that is composed of ~2500 neurons in each hemisphere, is thought to be the primary center for olfactory memory storage [47, 108, 128]. The memory circuit is modulated by the biogenic amine neurotransmitters dopamine and octopamine [74]. Microarray analysis of Drosophila mutants with defects in short-term, medium-term, and anesthesia-resistant memory identified differentially-expressed genes with putative roles in membrane excitability, synaptic transmission, cytoskeletal regulation, cell adhesion, and cell signaling, suggesting that these functions are involved in Drosophila learning and memory [73].
The position and connectivity of all 302 of C. elegans’ neurons have been mapped, and these nematodes carry out many complex behaviors despite having a relatively simple nervous system [220, 225]. The 302 neurons include sensory neurons, interneurons, and motor neurons [84], which together function to integrate sensory stimuli and exhibit both associative and non-associative behavioral plasticity. The fact that C. elegans has a very short lifespan (~21 days), is genetically tractable, and 80% of its genes have human orthologs [111] makes it a particularly attractive model for cognitive aging, as the effects of age on behavior can be rapidly assessed.
Although it has long been an established model for aging [58, 88, 101, 105], synaptic function [116, 170, 225], and behavior [42, 84], C. elegans is a relatively new model organism in the study of age-related cognitive decline [5, 100, 195]. Forms of non-associative behavioral plasticity include adaptation to inherently attractive odors [33] and habituation in response to multiple taps [171]. Associative behaviors include the ability to associate feeding state with a pathogen [236], temperature [82], salt concentration [181], and odor [33, 100, 181, 199, 212]. C. elegans is also able to form both short-term and long-term memory, lasting up to 24 hours [3, 33, 68, 99, 100, 171, 196, 199, 222]. The associative behaviors that have been used in the study of age-related learning and memory are described in more detail below.
C. elegans can be conditioned to associate a training temperature with the presence or absence of food [82, 107, 135]. After training, worms move to the training temperature that had been associated with food, or avoid a temperature associated with starvation, a behavior is termed isothermal tracking (IT). This conditioning can last for several hours [68], and is dependent on diacylglycerol kinase, a known memory regulator in mice [187], though it still remains unclear whether it requires the other known processes involved in long-term memory (transcription, translation, and cAMP response element-binding protein (CREB) activity).
Both positive and negative associative plasticity assays have been developed using olfactory cues. One form of negative associative plasticity, referred to as benzaldehyde starvation associative plasticity, occurs when worms avoid an attractive concentration of benzaldehyde after experiencing starvation in the presence of an aversive concentration of the same odor (benzaldehyde) [148]. Pairing the neutral odorant butanone at low concentrations with food induces positive association, termed “butanone enhancement” [203]. Brief starvation before food-butanone conditioning induces a stronger learning response, inducing a a 60% increase in their chemotaxis towards butanone [100]. Massed training of food associated with butanone results in short-term associative memory (STAM), while spaced training induces long-term associative memory (LTAM) [100]. STAM, which worms exhibit following a single conditioning session, declines within two hours [100]. STAM is transcription-independent, and can be broken down into translation-independent (30 minutes post-training) and translation-dependent (1 hour post-training) stages, suggesting that massed training can be used to test both short-term and intermediate-term memory [196]. LTAM lasts 16–24 h after training, and is dependent on transcription, translation, and CREB activity [100], factors that are required for long-term memory in other organisms, such as flies, Aplysia, and mice [188]. A similar paradigm has been developed for negative associative olfactory learning and memory [199, 222], in which worms are starved in the presence of the attractive odorant diacetyl. This training paradigm also results in STAM or LTAM after 1 or 3 training cycles, respectively [199, 222].
Kauffman et al. (2010) determined that LTAM performance also correlates with levels of activated CREB protein [100]. At Day 1 of adulthood, mutants of the C. elegans insulin receptor homolog, daf-2, have significantly extended LTAM performance when compared to wild-type animals. Examination of CREB expression and levels of activated protein in daf-2 and wild-type worms revealed that higher levels of active CREB correlates with daf-2’s enhanced LTAM performance [100]. Similarly, overexpression of CREB solely in neurons also improves and extends wild-type’s LTAM [100]. The downstream targets of CREB following LTAM training were recently identified [113]; while the basal targets of CREB primarily regulate growth and metabolism, many of the mammalian homologs of the CREB targets induced during LTAM training are involved in memory formation and neuronal functions in other organisms, as well [113]. The large set of CREB/memory induced genes (>750) includes indirect downstream targets of CREB, and identifies new mechanisms and novel components that warrant future study in cognitive function in higher organisms. Although many of the tested CREB/LTAM targets are required for long-term memory [113], C. elegans’ decline in memory with age correlate with declining levels of CREB itself [100], suggesting that the major intervention to prevent memory loss could be maintenance of CREB levels and activity.
3. Features of the aging nervous system
It was initially believed that there was significant neuronal loss with advanced age in humans [22, 34]. However, after the development of stereological principles, which allow objective counting of the number of objects in a three-dimensional space independent of the size of the objects [223], it was determined that significant cell death in the neocortex and hippocampus were not characteristic of normal aging in rodents [133, 173, 175], non-human primates [62, 102, 134, 163], or humans [152, 224]. More recently, it was reported that there is significant neuronal loss in area 8a of the dorsolateral prefrontal cortex of non-human primates, while studies in rodents regarding neuronal loss in the dorsal and ventral prefrontal cortex have yielded conflicting results [191, 201, 232]. This highlights a difference between normal aging and neurodegenerative disorders, such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, which are characterized by a significant decline in cell number [224]. Instead, small, region-specific changes in dendritic branching and spine density are more commonly observed effects of aging on neuronal cell morphology.
Some of these age-dependent alterations at the dendritic level include increased branching and length of dendrites in projections both to and within the dentate gyrus itself in aged individuals, which is distinct from the changes observed in brains of individuals with senile dementia, where there is extensive dendritic loss [23, 24, 54, 55]. This change in branching is not exhibited in all areas associated with memory formation, as there is no difference with age in other subregions of the hippocampus, such as areas CA1 and CA3 and the subiculum [56, 79], although some synaptic loss is observed in individuals with mild cognitive impairment [147]. Studies of hippocampal dendritic morphology and length in rodent models have also reported no regression of dendritic length with age, but there is some evidence of an increase in dendritic length in small subsets of neurons [167, 217]. Interestingly, neurons of the prefrontal cortex (PFC) appear to be more vulnerable to age-related morphology changes than hippocampal neurons are. Reduced dendritic branching is observed in aged pyramidal neurons in superficial cortical layers and the anterior cingulate in the rat, and in the medial PFC in humans [43, 71, 123]. It remains to be determined whether fish models also display age-related changes in neuron morphology, although the similarities between vertebrates and invertebrates, discussed below, suggests that such morphological changes may be an evolutionarily conserved feature of aging.
Drosophila models have been used extensively to study neurodegenerative disorders [6, 46, 52, 213], but neurological changes that occur in the fly brain as a result of normal aging remain mostly unknown. However, age-related changes are observed in circadian rhythms, sleep patterns, and olfactory acuity, suggesting there are aging-specific changes in the neural circuits controlling these processes [72, 178]. Studies of morphological changes in the aging brain have primarily focused on the number of dopaminergic neurons with age, because of their role in locomotion, which declines with age [226]. No significant changes in the number of dopaminergic neurons have been observed in aging flies [226]. More recent studies have examined other age-related neuronal alterations in Drosophila. Normal aging results in a progressive accumulation of neural aggregates containing insoluble ubiquinated proteins, which may correlate with impairments in olfaction that result in abnormal courtship activity during the night [12, 176]. A recent study by Haddadi et al. (2014) more closely examined the neurons of the MBs in young and aged flies. More vacuolated areas were observed in the MBs of 50-day-old flies when compared to 5-day-old animals, indicating neurodegeneration in the area, although it remains to be determined whether there is any significant loss of neuronal number in the region [76]. Electron microscopy of MBs revealed a reduction in the number of synapses and decreased numbers of mitochondria [76], which also tended to be irregular in size and shape in aged flies [76]. These mitochondrial abnormalities were also accompanied by a significant reduction in the activity of catalase and superoxide dismutase, two antioxidant enzymes [76].
It was originally believed that C. elegans neurons did not display age-related morphological decline at either a cellular or subcellular level, because EM studies indicated that axons of motor neurons appeared to remain intact with age, in contrast to other tissues, such as skin and muscle [61, 83]. This did not correlate with a preservation of neuronal function with age, as many sensory behaviors and motility decline with age, and more recent studies have further characterized neuronal morphology with age [5, 195]. Although the soma are still intact, age-related changes are observed in neuronal processes and subcellular structures [153, 206, 214], and neuronal activity [30, 141]. This is similar to findings in mammals and Drosophila, which also do not display gross neuronal loss with age, but instead exhibit age-related morphological abnormalities; some abnormalities begin to appear as early as Day 8 of adulthood [153, 206, 214]. These structural alterations include ectopic neurite branching from the soma and processes, bubble-like lesions and GFP beading within the process, and blebbing that results in a “wavy” process [153, 206, 214]. The extent and type of morphological changes that occur vary somewhat between neurons. In addition to morphological abnormalities, neuronal mitochondria progressively fragment with age in C. elegans [94], and mitochondria are found within some of the beaded processes and bubble-like lesions [94, 214]. A decrease in gentle touch response and mobility correlates with excess ectopic neurites, suggesting that the age-related morphological changes either cause or are correlated with impaired neuronal function [206]. Using electron microscopy, Toth et al. (2012) found that synapses in the nerve ring and ventral ganglion exhibit vesicle depletion at Day 15 of adulthood, and the severity of depletion of synaptic vesicles correlates with the overall condition and mobility of the worm.
4. Declines in cognitive function with age
In addition to changes in neuronal morphology, synaptic function appears to decline with age in mammals. Reductions in synapse number in the dentate gyrus [64] may account for the reduced field excitatory postsynaptic potentials recorded in this region [9, 10]. This reduction in synapse number also correlates with spatial memory deficits in aged rats [63]. In other areas, such as Schaffer collateral – CA1 synapses, there is no difference in total synapse number, but instead there is a reduction in postsynaptic density area of rats with age-related learning impairment compared to learning-unimpaired animals [147]. Additionally, electron microscopy studies have reported substantial synapse loss (~32%) in the PFC of primates [48, 164]. Further investigation into the types of synapses that are most vulnerable to aging revealed that most of the synapses lost in the PFC with age are thin spines, which have a high degree of plasticity, are associated with learning new information, and may represent immature ‘silent synapses’ [21, 27, 48, 85, 238]. This loss of functional synapses results in changes in long–term potentiation (LTP) and long-term depression (LTD), such as deficits in both the induction and maintenance of LTP and increased susceptibility to LTD [26, 182].
Gene expression and protein synthesis, two processes that are required for the maintenance of LTP, also change in aged animals. Blalock, et al. (2003) performed microarray analysis of area CA1 of aged rats following behavioral testing to identify gene expression alterations that correlate with age-related cognitive decline. Downregulated genes included the Immediate early genes Arc and Narp and many genes associated with energy metabolism, biosynthesis, and activity-regulated synaptogenesis, while upregulated genes are associated with inflammation and intracellular Ca2+ release [18]. It is therefore unsurprising that age-related declines are observed in tasks that require information processing in the hippocampus and PFC, which show age-related changes in synaptic function. Deficits in retrieving contextual details of episodic memories are present in aged humans when compared with younger adults [130, 194]. Additionally, aged mice [7], rats [9, 59, 125], dogs [81], monkeys [112, 174], and humans [227] all display deficits in tasks designed to test spatial navigation, indicating that hippocampal function is impaired with age. There are also deficits in humans [53], rabbits [193, 209], rats [106], and mice [104] in trace eyeblink conditioning, an associative learning task that requires the hippocampus as well as other brain structures, such as the cerebellum. While the hippocampus is involved in spatial episodic memory, the PFC is required for working memory and episodic function. Rats [49], non-human primates [139, 140, 172], and humans [120] show working memory impairments with age. Executive function has also been shown to decline in aged humans and monkeys when compared to young adult controls [136, 177].
Age-related cognitive phenotypes have been less well characterized in fish, but also show declines. Zebrafish exhibit decreased associative learning with age, with defects in spatial learning evident at around 18 months [180, 235, 237]. Inhibitory avoidance is also reduced by 24 months of age [122], and there is a corresponding reduction in the expression of plasticity-associated genes in the zebrafish telencephalon, such as bdnf, cart4, and pcna [122]. Killifish also display reduced performance on an active avoidance task by 9 weeks of age [218, 219]. Interestingly, this decline can be prevented by interventions that prolong lifespan, such as reducing the temperature of the fish’s habitat or by resveratrol treatment [218, 219].
There have been relatively few studies investigating the effects of aging on olfactory associative memory in Drosophila, though various forms of memory have been shown to decline with age [205, 210, 211]. A slight defect in associative learning is observed after 10 days of age, although no further decline at later days was reported [205]. Intermediate-term memory, which is defined as the physiological changes that occur in neurons as a result of learning, shows a progressive decline with age [205], and is associated with decline in the ITM cellular memory trace [41, 210]. More recently, protein synthesis-dependent long-term memory, along with an age-dependent disruption in the long-term memory trace in the Mushroom Body, were also found to decline with age [211].
Neuronal aging phenotypes appear rapidly in C. elegans, long before the animals start to die [5, 195]. These include simpler behaviors such as motility and maximum velocity, which decrease by more than 50% by day 10 [77, 90], midway through life. Sensory behaviors, such as odor chemotaxis, decline several days before motility, suggesting that separate mechanisms of molecular decline regulate these behaviors [67, 100]. Non-associative learning (e.g., habituation to a tap stimulus) also changes with age. Worms at Day 9 of adulthood respond to a tap with smaller reversals, and recover from habituation more slowly than worms at age Day 1 or Day 4 [13].
Associative learning and memory is the most sensitive to the effects of aging [5, 100, 195]. Isothermal tracking, in which worms associate temperature with the presence of food, shows a modest but significant decline by day 6 of adulthood [143]. The fraction of worms that display isothermal tracking after training decreases by half at Day 12, and is undetectable by Day 15 [143].
In order to determine how aging affects C. elegans olfactory associative memory in relation to other phenotypes, Kauffman et al. (2010) examined motility, chemotaxis, massed positive olfactory learning, spaced positive olfactory learning, and 16-hr long-term positive olfactory memory for the first week of adulthood [100]. Movement and chemotaxis were maintained during this time; however, 16-hr LTAM decreased by day 2 of adulthood, and was undetectable by day 5. Massed learning begins to decline on day 3 and is absent by day 6, while spaced learning is undiminished at day 3, but declined by day 7 of adulthood. These declines in olfactory associative learning and memory performance precede age-related changes in chemotaxis [67], isothermal tracking [143], habituation [13], and motility [77, 90], suggesting that these associative olfactory forms of learning and memory are most sensitive to age-related changes. Furthermore, these declines in cognitive function occur at timepoints prior to observable age-related morphological defects in neurons and muscles [5, 83, 153, 195, 206, 214]. It remains to be determined if negative associative olfactory behaviors or other associative behaviors such as taste avoidance learning show similar age-related declines. Kauffman et al. (2010) also determined that levels of active CREB are significantly reduced by day 4, suggesting that this decrease underlies the loss of the ability to form long-term memory with age [100]. At this age, STAM levels are still maintained, indicating that different molecular pathways maintain this behavior with age [100]. Together, these results suggest that the maintenance of each form of learning and memory is regulated independently, or at least that they are differentially susceptible to age-related declines.
5. Intersection of longevity pathways and cognitive function with age
5.1 Insulin Signaling
The insulin/IGF-1 (IIS) pathway was first found to regulate lifespan in C. elegans [101]. In worms, the IIS pathway consists of a single insulin receptor homolog daf-2, which has >30% amino acid identity to human insulin and IGF-1 receptors [103]. The effects of daf-2 on lifespan require activity of the forkhead box O (FOXO) transcription factor, DAF-16 [101, 149]. In brief, signaling through DAF-2 activates phosphoinositide 3-kinase (PI3K), which leads to the Akt-1/2 and PDK-1-dependent phosphorylation of DAF-16 [155, 156] and its transcriptional inactivation by sequestration to the cytoplasm [119, 149]. In daf-2 mutants, IIS is low, which allows DAF-16 to enter the nucleus and enact a transcriptional program that promotes longevity and stress resistance, through the expression of DAF-16 targets, many of which have been identified and characterized [144]. Though there is only one known insulin receptor homolog in C. elegans, the genome encodes approximately 40 insulin-like peptides that can act as either DAF-2 agonists or antagonists [144, 165]. Drosophila, like C. elegans, has a single insulin-like receptor that, when mutated, extends life span in a manner that is dependent on its daf-16 homolog, dFOXO [207], and also expresses several different insulin-like peptides (dILPs) [98].
Reduction of insulin signaling by mutations in daf-2 promotes longevity, extending the lifespan to more than twice that of wild type [101]. IIS is evolutionarily conserved, and components of the IIS pathway regulate lifespan in Drosophila, mice, and humans [19, 32, 203, 207]. Mice haploinsufficient for the IGF-1 receptor are long-lived, as are mice that have reduced levels of insulin receptor substrate 2, and adipose tissue-specific insulin receptor knockout mice also exhibit increases in lifespan [19, 86, 204]. Moreover, genetic variations in IIS pathway components are linked to long life in humans [161]. Mutations in a human daf-16 homolog, FOXO3a, are linked to increased longevity [228]. In addition, IGF-1R mutations are highly represented in populations of centenarians [203].
In addition to having dramatic effects on lifespan, IIS regulates neuronal phenotypes with aging. Older daf-2 worms display fewer neuronal abnormalities with age, when compared to similarly aged wild-type worms, and this reduction in age-related morphological defects is dependent on daf-16 [153, 206, 214]. IIS also influences behavioral plasticity with age. Mutants of age-1, the catalytic subunit of PI3K downstream of daf-2, have increased isothermal tracking in young and old animals, and a 210% extension in “high IT” ability, which is defined as a period when more than 75% of the worms exhibit IT [142]. This extension is mediated directly by AGE-1 activity in the AIY interneurons, and is not merely a side effect of organism-wide lifespan extension, which involves other tissues [107, 142]. daf-2 worms also have increased IT with age [142], and the effects of both daf-2 and age-1 depend on daf-16 [142]. daf-2 worms display significantly extended short- (STAM) and long-term associative memory (LTAM) on the first day of adulthood [100], as well as extended maintenance of learning and short-term memory with age (Arey & Murphy, unpublished) as compared to wild-type animals.
To determine the effects of insulin signaling pathway on learning and memory with age, worms’ massed learning performance was tested daily for the first 5 days of adulthood, and LTAM was tested at Day 4 of adulthood [100]. daf-2 mutants maintained day 1 massed learning ability until Day 5, an age at which it is undetectable in wild-type worms [100]. In contrast, LTAM at Day 4 of adulthood was abrogated in both daf-2 and wild-type animals, indicating that maintenance of these two behaviors occurs through different mechanisms. The limiting factor for LTAM is CREB: daf-2 worms, like wild-type animals, exhibit decreases in CREB expression and activation with age that correlate with decreased LTAM activity [100].
Recent studies in Drosophila have found that knocking down the insulin receptor substrate chico in the fly brain results in defects in negative olfactory associative learning and protein synthesis-dependent memory, although the effect of reduced insulin receptor function on positive associative learning has yet to be determined [28, 145]. The role of IIS in age-dependent changes in learning and memory in Drosophila has not yet been elucidated.
In addition to the insulin pathway, two other insulin-like peptides (ILPs), insulin-like growth factor 1 & 2 (IGF1 and IGF2), regulate neuronal function in mammals. IGF1 and IGF2 have specific receptors (IGF1R and IGF2R, respectively), but can bind with varying affinities to IGF1R, IGF2R, and to the insulin receptor IR [51]. All three ILPs have been examined for their roles in learning and memory. In young rodents, microinjection or introcerebroventricular administration of insulin improves memory performance [78, 132, 137, 157, 197]. Following intranasal delivery of insulin, humans also show enhanced cognitive function [15]. Injection of IGF1 into the hippocampus or amygdala has no detectable effects on memory formation [197]. By contrast, systemic or hippocampal injection of IGF2 can enhance retention of hippocampal-dependent memories [2, 29, 198]. This enhancement is dependent on the actions of the IGF2R [29]. Interestingly, insulin does not bind IGF2R [95], so the enhancement of memory by these two ILPs must occur via distinct signaling mechanisms.
Despite insulin’s ability to alter cognitive performance, loss of neuronal insulin receptor function does not result in obvious dysfunction, and the effects of the loss of IGF1R and IGF2R function have yet to be determined. When the brain/neuron insulin receptor knockout (BIRKO) mice were examined for neurological and behavioral abnormalities, they displayed no alteration in neuronal proliferation or survival or memory at seven weeks of age [184], but it remains to be determined if reduced neuronal insulin signaling may have beneficial or deleterious effects in aged mammals. Interestingly, a recent study determined that introcerebroventricular injection of insulin does not enhance spatial memory in the water maze test in old rats [75], unlike young rats, suggesting that the link between aging and the effects of IIS on cognition has yet to be fully elucidated.
Diabetes mellitus, in which individuals have absolute or relative insulin deficiency, is associated with cognitive dysfunction and dementia (reviewed in [16]), further suggesting that insulin response is required for proper cognitive function with age. However, reduced cognitive function in diabetes mellitus may be due to insulin resistance, as recent studies have suggested that superior health in old age is associated with maintenance of insulin sensitivity [150]. Therefore, diabetes and lowered insulin signaling as seen in long-lived IIS mutants are likely two independent pathways that may oppositely affect cognitive maintenance.
5.2 Dietary Restriction
Dietary restriction, in which caloric intake is reduced to about 60–70% of normal levels, has been shown to extend lifespan in many model organisms, including yeast, worms, flies, rodents, and primates [121, 126, 127]. C. elegans can undergo caloric restriction by feeding them diluted bacteria or in axenic media, by intermittent fasting, or via a genetic mutations that cause altered pharyngeal pumping and defective feeding, such as eat-2 [87, 114, 168]. DR-mediated lifespan extension is at least in part independent of the insulin signaling pathway, as some dietary restriction treatments and eat-2 mutations can extend the lifespan of daf-2 mutants [39, 89]; instead, they depend on the PHA-4/FOXA1 transcription factor [154]. However, lifespan extension by intermittent fasting does require signaling through DAF-16 [87].
Although eat-2 worms have an extended lifespan, they are not protected from age-related ectopic neurite branching [206], indicating that morphological defects with age are regulated by specific longevity pathways, and that a longer lifespan is not protective of all phenotypes. eat-2 mutants have also been examined for positive olfactory associative memory performance, and were revealed to have normal STAM at Day 1. Young eat-2 mutants worms displayed impaired LTAM (about 60% of wild-type levels) but this could be improved by increasing the number of training cycles [100]. This reduced performance is dependent on caloric restriction itself, as feeding eat-2 animals smaller, easier to digest bacteria, Comamonas sp., restores LTAM to wild-type levels [100]. Kauffman et al. (2010) also examined eat-2 LTAM performance with age. Although eat-2 animals exhibit reduced LTAM at Day 1 of adulthood, their memory ability is maintained until at least Day 4 of adulthood, in contrast to daf-2 and wild-type animals, which have no detectable LTAM at the same age; CREB levels in eat-2 mutants compared to wild-type again correlated with the maintained LTAM performance [100]. The differences between eat-2 and daf-2 mutants in STAM and LTAM performance demonstrate that longevity pathways differ in their regulation of learning and memory in early adulthood, which can be traced to their differential regulation of CREB levels and activation [100].
Dietary restriction also affects learning and memory performance in Drosophila. Following aversive olfactory conditioning, dietary-restricted young flies (5 days old), have enhanced memory when assessed for performance 60 minutes after training [25]. However, this effect is not maintained with age, as learning and memory performance decline to similar levels in old dietary-restricted and rich diet-fed flies [25].
Dietary restriction attenuates age-related memory deficits in tests of mouse and rat hippocampal-dependent memory [91, 92, 124, 166, 200], and more recently was shown to preserve working memory in mice [110]. Improved spatial memory performance in aged dietary-restricted rats correlated with protection against age-related decreases in AMPA and NMDA-type glutamate receptors in the CA3 region of the hippocampus [1]. Deep sequencing carried out on the CA1 region of the hippocampus revealed that dietary restriction reduces age-dependent changes in gene expression [183]. Enriched pathways of these “protected” genes include calcium, long-term potentiation, and CREB signaling, though aged calorically-restricted gene sets still differed significantly from young ones. Suppression of age-related gene expression changes may provide some mechanistic insight into how dietary restriction prevents age-related cognitive decline in rodents.
Older people (mean age 60.5) subjected to 3 months of caloric restriction (30% reduction) were subsequently tested for verbal memory (recall of a word list after 30 minutes); the CR group had significantly improved memory performance when compared to controls or to those fed an unsaturated fatty acid-enriched diet [230]. This suggests that the benefits of caloric restriction may be evolutionarily conserved from invertebrates to humans.
5.3 APOE
Another gene that has been linked to lifespan, healthspan, and cognitive aging in humans is Apolipopotein E (APOE). APOE is a polymorphic gene, with 3 common alleles, ε2, ε3, and ε4. The initial study linking APOE to age-related phenotypes identified the ε4 allele as a risk factor for the development of AD[202] while the ε2 allele is protective [35]. The APOE incidence of the ε2 allele was found to be increased in centenarians [185], and APOE is associated with longevity in a number of human genetics studies [44, 45, 57, 60, 146]. This is unlike other longevity pathways discussed here, which were known to affect lifespan before their effects on cognitive function with age were examined. Interestingly, a recent study found that APOE genotype does not modify the rate of change in cognition with age, and may only affect age of AD onset [192], suggesting that normal age-related cognitive decline and cognitive dysfunction in neurodegenerative disease are two very distinct processes.
5.4 CREB and other molecules involved in cognitive aging downstream of longevity pathways
As discussed previously, Kauffman et al. (2010) uncovered a link between CREB levels and memory performance with age. daf-2 animals have higher levels of active CREB than wild-type animals on Day 1 of adulthood, correlating with their enhanced LTAM performance. Conversely, eat-2 animals have lower levels of active CREB and reduced LTAM on day 1 of adulthood. When CREB levels were examined with age, daf-2 and wild-type worms both displayed a reduction in active CREB by day 4, while eat-2 animals maintained day 1 levels of CREB activity, correlating with maintenance of LTAM activity in eat-2 worms on day 4. The increased level of active CREB in daf-2 animals is an example of tissue-specific regulation of phenotypes by IIS [97]; Kaletsky et al. (2016) found that CREB (crh-1) is specifically up-regulated in the neurons of daf-2 (reduced IIS) animals, while crh-1 is downregulated in other tissues [97]. (Worms with loss-of-function mutations in crh-1, the C. elegans homolog of CREB, are defective in long-term memory, but their lifespan and STAM are unaffected [113].) Kaletsky et al. (2016) also identified the full suite of adult neuronal IIS/FOXO targets. Several of these genes are required for the extension of STAM observed in daf-2 animals in early adulthood; these targets included ion channels, transcription factors, G-proteins, and vesicle fusion proteins, suggesting that IIS regulates a broad number of processes to extend memory [97]. Many of these genes are required for wild-type STAM performance in young adult worms [97], and it will be interesting to determine if these molecules also have a role in the enhanced STAM performance with age in daf-2 animals.
The differential effects of these longevity mutants on various types of memory performance can be attributed to different mechanisms. LTAM performance correlates with levels of active CREB, and maintenance of CREB expression and activity could be predictive of memory performance with age. Indeed, CREB and CREB binding protein levels decrease in the hippocampus with age in rodents [31, 80, 169], and correlate with impaired performance in contextual fear conditioning [109]. A recent study in humans also supports this hypothesis, as single nucleotide polymorphisms in the CREB-dependent histone deacetylation pathway, CREB-binding protein and RbAP48, are associated with episodic memory performance in healthy elderly subjects [11].
Recently, daf-2’s enhanced STAM memory performance with age was discovered to also be due to maintenance of synaptic integrity [117]. The anterograde kinesin motor UNC-104/KIF1A regulates synapse distribution, synaptic transmission, and motility with age in C. elegans [117]. Li et al. (2016) determined that UNC-104 levels are increased in daf-2 animals, that UNC-104 functions downstream of IIS, and that UNC-104 is required for the memory extension of daf-2 animals at Day 1 as well as their maintenance of STAM performance with age [117]. The loss of synaptic integrity is a conserved feature of cognitive aging. In aged mammals, deterioration in synapse number and structure is associated with impaired memory in both the hippocampus and cortex [20, 48, 138, 164]. Thus, molecules that preserve synaptic integrity are promising targets for the development of treatments for age-related cognitive decline.
6. Conclusions
In summary, age-related cognitive decline is a part of the normal aging process across phyla; in all invertebrate and vertebrate model organisms that have been examined for alterations in learning and memory with age, decreases in cognitive function have been observed (Figure 2) [26, 100, 182, 195, 205, 210, 211]. These changes in normal aging are not due to gross neuronal cell loss, but instead are the result of subtle changes in neuronal morphology, synaptic integrity, cellular connectivity, gene expression, and other factors that result in altered plasticity of circuits that regulate learning and memory (Figure 2) [26, 94, 138, 153, 182, 214]. There is a growing body of evidence that the major signaling pathways that regulate longevity are also important for the maintenance of cognitive function with age. Recent work in C. elegans has begun to elucidate the targets of these pathways that are necessary for the preservation of cognitive function with age; CREB function correlates with enhanced long-term memory performance in longevity mutants and the maintenance of long-term memory performance with age [100]. Maintaining CREB activity may be a way to preserve cognitive function with age in higher organisms, because CREB levels correlate with cognitive performance in mammals [31, 80, 109, 169], but the exact location and levels of this CREB maintenance will be important to avoid deleterious effects. Loss of short-term memory with age is less studied than long term memory, but several IIS targets have been identified that are required for normal STAM performance [97], and IIS mutants maintain STAM performance with age due to preserved pre-synaptic function [117]. Future work in model systems, both vertebrate and invertebrate, will be invaluable in the study of these targets in age-related cognitive decline. The identification and characterization of new molecules that regulate the loss of cognitive function with age will enable the development of treatments that ameliorate this growing health problem.
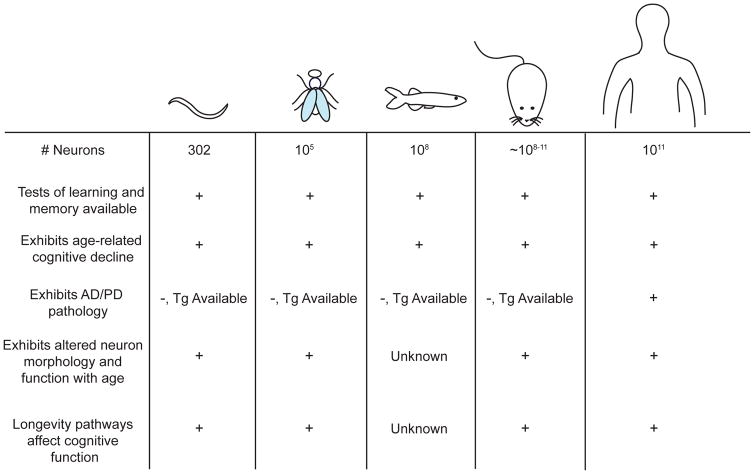
Figure 2.
Summary of neuronal aging phenotypes across model organisms. Despite having simpler nervous systems than humans, features of neuronal aging and age-related cognitive decline are evolutionarily conserved across model systems. Transgenic (Tg) models allow for the study of complex human neurological disease, even if the pathology does not normally occur in the model organism. Longevity pathways affect cognitive function in all organisms in which they have been examined.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams MM, Shi L, Linville MC, Forbes ME, Long AB, Bennett C, Newton IG, Carter CS, Sonntag WE, Riddle DR, Brunso-Bechtold JK. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp Neurol. 2008;211(1):141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberini CM, Ghirardi M, Huang YY, Nguyen PV, Kandel ER. A molecular switch for the consolidation of long-term memory: cAMP-inducible gene expression. Ann N Y Acad Sci. 1995;758:261–286. doi: 10.1111/j.1749-6632.1995.tb24833.x. [DOI] [PubMed] [Google Scholar]
- 3.Amano H, Maruyama IN. Aversive olfactory learning and associative long-term memory in Caenorhabditis elegans. Learn Mem. 2011;18(10):654–665. doi: 10.1101/lm.2224411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amdam GV. Social context, stress, and plasticity of aging. Aging Cell. 2011;10(1):18–27. doi: 10.1111/j.1474-9726.2010.00647.x. [DOI] [PubMed] [Google Scholar]
- 5.Ardiel EL, Rankin CH. An elegant mind: learning and memory in Caenorhabditis elegans. Learn Mem. 2010;17(4):191–201. doi: 10.1101/lm.960510. [DOI] [PubMed] [Google Scholar]
- 6.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science. 2002;295(5556):865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 7.Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci U S A. 1999;96(9):5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker N, Wolschin F, Amdam GV. Age-related learning deficits can be reversible in honeybees Apis mellifera. Exp Gerontol. 2012;47(10):764–772. doi: 10.1016/j.exger.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- 10.Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barral S, Reitz C, Small SA, Mayeux R. Genetic variants in a ‘cAMP element binding protein’ (CREB)-dependent histone acetylation pathway influence memory performance in cognitively healthy elderly individuals. Neurobiol Aging. 2014;35(12):2881.e2887–2881.e2810. doi: 10.1016/j.neurobiolaging.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett BJ, Isakson P, Lewerenz J, Sanchez H, Kotzebue RW, Cumming RC, Harris GL, Nezis IP, Schubert DR, Simonsen A, Finley KD. p62, Ref(2)P and ubiquitinated proteins are conserved markers of neuronal aging, aggregate formation and progressive autophagic defects. Autophagy. 2011;7(6):572–583. doi: 10.4161/auto.7.6.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck CD, Rankin CH. Effects of aging on habituation in the nematode Caenorhabditis elegans. Behav Processes. 1993;28(3):145–163. doi: 10.1016/0376-6357(93)90088-9. [DOI] [PubMed] [Google Scholar]
- 14.Beck CD, Schroeder B, Davis RL. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci. 2000;20(8):2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedict C, Hallschmid M, Schultes B, Born J, Kern W. Intranasal insulin to improve memory function in humans. Neuroendocrinology. 2007;86(2):136–142. doi: 10.1159/000106378. [DOI] [PubMed] [Google Scholar]
- 16.Biessels GJ, Reagan LP. Hippocampal insulin resistance and cognitive dysfunction. Nat Rev Neurosci. 2015;16(11):660–671. doi: 10.1038/nrn4019. [DOI] [PubMed] [Google Scholar]
- 17.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464(7288):529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23(9):3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blüher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 20.Bories C, Husson Z, Guitton MJ, De Koninck Y. Differential balance of prefrontal synaptic activity in successful versus unsuccessful cognitive aging. J Neurosci. 2013;33(4):1344–1356. doi: 10.1523/JNEUROSCI.3258-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr Opin Neurobiol. 2007;17(3):381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 22.BRODY H. Organization of the cerebral cortex. III. A study of aging in the human cerebral cortex. J Comp Neurol. 1955;102(2):511–516. doi: 10.1002/cne.901020206. [DOI] [PubMed] [Google Scholar]
- 23.Buell SJ, Coleman PD. Dendritic growth in the aged human brain and failure of growth in senile dementia. Science. 1979;206(4420):854–856. doi: 10.1126/science.493989. [DOI] [PubMed] [Google Scholar]
- 24.Buell SJ, Coleman PD. Quantitative evidence for selective dendritic growth in normal human aging but not in senile dementia. Brain Res. 1981;214(1):23–41. doi: 10.1016/0006-8993(81)90436-4. [DOI] [PubMed] [Google Scholar]
- 25.Burger JM, Buechel SD, Kawecki TJ. Dietary restriction affects lifespan but not cognitive aging in Drosophila melanogaster. Aging Cell. 2010;9(3):327–335. doi: 10.1111/j.1474-9726.2010.00560.x. [DOI] [PubMed] [Google Scholar]
- 26.Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7(1):30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- 27.Busetto G, Higley MJ, Sabatini BL. Developmental presence and disappearance of postsynaptically silent synapses on dendritic spines of rat layer 2/3 pyramidal neurons. J Physiol. 2008;586(6):1519–1527. doi: 10.1113/jphysiol.2007.149336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chambers DB, Androschuk A, Rosenfelt C, Langer S, Harding M, Bolduc FV. Insulin signaling is acutely required for long-term memory in Drosophila. Front Neural Circuits. 2015;9:8. doi: 10.3389/fncir.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen DY, Stern SA, Garcia-Osta A, Saunier-Rebori B, Pollonini G, Bambah-Mukku D, Blitzer RD, Alberini CM. A critical role for IGF-II in memory consolidation and enhancement. Nature. 2011;469(7331):491–497. doi: 10.1038/nature09667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chokshi TV, Bazopoulou D, Chronis N. An automated microfluidic platform for calcium imaging of chemosensory neurons in Caenorhabditis elegans. Lab Chip. 2010;10(20):2758–2763. doi: 10.1039/c004658b. [DOI] [PubMed] [Google Scholar]
- 31.Chung YH, Kim EJ, Shin CM, Joo KM, Kim MJ, Woo HW, Cha CI. Age-related changes in CREB binding protein immunoreactivity in the cerebral cortex and hippocampus of rats. Brain Res. 2002;956(2):312–318. doi: 10.1016/s0006-8993(02)03562-x. [DOI] [PubMed] [Google Scholar]
- 32.Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- 33.Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14(4):803–812. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 34.Coleman PD, Flood DG. Neuron numbers and dendritic extent in normal aging and Alzheimer’s disease. Neurobiol Aging. 1987;8(6):521–545. doi: 10.1016/0197-4580(87)90127-8. [DOI] [PubMed] [Google Scholar]
- 35.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Rimmler JB, Locke PA, Conneally PM, Schmader KE. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 36.Corona M, Hughes KA, Weaver DB, Robinson GE. Gene expression patterns associated with queen honey bee longevity. Mech Ageing Dev. 2005;126(11):1230–1238. doi: 10.1016/j.mad.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 37.Corona M, Velarde RA, Remolina S, Moran-Lauter A, Wang Y, Hughes KA, Robinson GE. Vitellogenin, juvenile hormone, insulin signaling, and queen honey bee longevity. Proc Natl Acad Sci U S A. 2007;104(17):7128–7133. doi: 10.1073/pnas.0701909104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corsi AK, Wightman B, Chalfie M. A Transparent Window into Biology: A Primer on Caenorhabditis elegans. Genetics. 2015;200(2):387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crawford D, Libina N, Kenyon C. Caenorhabditis elegans integrates food and reproductive signals in lifespan determination. Aging Cell. 2007;6(5):715–721. doi: 10.1111/j.1474-9726.2007.00327.x. [DOI] [PubMed] [Google Scholar]
- 40.Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- 41.Davis RL. Traces of Drosophila memory. Neuron. 2011;70(1):8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Bono M, Maricq AV. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. doi: 10.1146/annurev.neuro.27.070203.144259. [DOI] [PubMed] [Google Scholar]
- 43.de Brabander JM, Kramers RJ, Uylings HB. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10(4):1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- 44.Deelen J, Beekman M, Uh HW, Broer L, Ayers KL, Tan Q, Kamatani Y, Bennet AM, Tamm R, Trompet S, Guđbjartsson DF, Flachsbart F, Rose G, Viktorin A, Fischer K, Nygaard M, Cordell HJ, Crocco P, van den Akker EB, Böhringer S, Helmer Q, Nelson CP, Saunders GI, Alver M, Andersen-Ranberg K, Breen ME, van der Breggen R, Caliebe A, Capri M, Cevenini E, Collerton JC, Dato S, Davies K, Ford I, Gampe J, Garagnani P, de Geus EJ, Harrow J, van Heemst D, Heijmans BT, Heinsen FA, Hottenga JJ, Hofman A, Jeune B, Jonsson PV, Lathrop M, Lechner D, Martin-Ruiz C, Mcnerlan SE, Mihailov E, Montesanto A, Mooijaart SP, Murphy A, Nohr EA, Paternoster L, Postmus I, Rivadeneira F, Ross OA, Salvioli S, Sattar N, Schreiber S, Stefánsson H, Stott DJ, Tiemeier H, Uitterlinden AG, Westendorp RG, Willemsen G, Samani NJ, Galan P, Sørensen TI, Boomsma DI, Jukema JW, Rea IM, Passarino G, de Craen AJ, Christensen K, Nebel A, Stefánsson K, Metspalu A, Magnusson P, Blanché H, Christiansen L, Kirkwood TB, van Duijn CM, Franceschi C, Houwing-Duistermaat JJ, Slagboom PE. Genome-wide association meta-analysis of human longevity identifies a novel locus conferring survival beyond 90 years of age. Hum Mol Genet. 2014;23(16):4420–4432. doi: 10.1093/hmg/ddu139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Deelen J, Beekman M, Uh HW, Helmer Q, Kuningas M, Christiansen L, Kremer D, van der Breggen R, Suchiman HE, Lakenberg N, van den Akker EB, Passtoors WM, Tiemeier H, van Heemst D, de Craen AJ, Rivadeneira F, de Geus EJ, Perola M, van der Ouderaa FJ, Gunn DA, Boomsma DI, Uitterlinden AG, Christensen K, van Duijn CM, Heijmans BT, Houwing-Duistermaat JJ, Westendorp RG, Slagboom PE. Genome-wide association study identifies a single major locus contributing to survival into old age; the APOE locus revisited. Aging Cell. 2011;10(4):686–698. doi: 10.1111/j.1474-9726.2011.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng H, Dodson MW, Huang H, Guo M. The Parkinson’s disease genes pink1 and parkin promote mitochondrial fission and/or inhibit fusion in Drosophila. Proc Natl Acad Sci U S A. 2008;105(38):14503–14508. doi: 10.1073/pnas.0803998105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411(6836):476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- 48.Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30(22):7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunnett SB, Evenden JL, Iversen SD. Delay-dependent short-term memory deficits in aged rats. Psychopharmacology (Berl) 1988;96(2):174–180. doi: 10.1007/BF00177557. [DOI] [PubMed] [Google Scholar]
- 50.Fernandes Y, Talpos A, Gerlai R. Towards the characterization of short-term memory of zebrafish: effect of fixed versus random reward location. Prog Neuropsychopharmacol Biol Psychiatry. 2015;56:189–195. doi: 10.1016/j.pnpbp.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13(4):225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 52.Finelli A, Kelkar A, Song HJ, Yang H, Konsolaki M. A model for studying Alzheimer’s Abeta42-induced toxicity in Drosophila melanogaster. Mol Cell Neurosci. 2004;26(3):365–375. doi: 10.1016/j.mcn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Finkbiner RG, Woodruff-Pak DS. Classical eyeblink conditioning in adulthood: effects of age and interstimulus interval on acquisition in the trace paradigm. Psychol Aging. 1991;6(1):109–117. doi: 10.1037//0882-7974.6.1.109. [DOI] [PubMed] [Google Scholar]
- 54.Flood DG, Buell SJ, Defiore CH, Horwitz GJ, Coleman PD. Age-related dendritic growth in dentate gyrus of human brain is followed by regression in the ‘oldest old’. Brain Res. 1985;345(2):366–368. doi: 10.1016/0006-8993(85)91018-2. [DOI] [PubMed] [Google Scholar]
- 55.Flood DG, Buell SJ, Horwitz GJ, Coleman PD. Dendritic extent in human dentate gyrus granule cells in normal aging and senile dementia. Brain Res. 1987;402(2):205–216. doi: 10.1016/0006-8993(87)90027-8. [DOI] [PubMed] [Google Scholar]
- 56.Flood DG, Guarnaccia M, Coleman PD. Dendritic extent in human CA2-3 hippocampal pyramidal neurons in normal aging and senile dementia. Brain Res. 1987;409(1):88–96. doi: 10.1016/0006-8993(87)90744-x. [DOI] [PubMed] [Google Scholar]
- 57.Fortney K, Dobriban E, Garagnani P, Pirazzini C, Monti D, Mari D, Atzmon G, Barzilai N, Franceschi C, Owen AB, Kim SK. Genome-Wide Scan Informed by Age-Related Disease Identifies Loci for Exceptional Human Longevity. PLoS Genet. 2015;11(12):e1005728. doi: 10.1371/journal.pgen.1005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Friedman DB, Johnson TE. A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 1988;118(1):75–86. doi: 10.1093/genetics/118.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- 60.Garatachea N, Marín PJ, Santos-Lozano A, Sanchis-Gomar F, Emanuele E, Lucia A. The ApoE gene is related with exceptional longevity: a systematic review and meta-analysis. Rejuvenation Res. 2015;18(1):3–13. doi: 10.1089/rej.2014.1605. [DOI] [PubMed] [Google Scholar]
- 61.Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics. 2002;161(3):1101–1112. doi: 10.1093/genetics/161.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gazzaley AH, Thakker MM, Hof PR, Morrison JH. Preserved number of entorhinal cortex layer II neurons in aged macaque monkeys. Neurobiol Aging. 1997;18(5):549–553. doi: 10.1016/s0197-4580(97)00112-7. [DOI] [PubMed] [Google Scholar]
- 63.Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc Natl Acad Sci U S A. 1986;83(9):3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2(4):437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- 65.Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32(4):180–188. doi: 10.1016/j.tibs.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 66.Giraldo YM, Kamhi JF, Fourcassié V, Moreau M, Robson SK, Rusakov A, Wimberly L, Diloreto A, Kordek A, Traniello JF. Lifespan behavioural and neural resilience in a social insect. Proc Biol Sci. 2016;283(1822) doi: 10.1098/rspb.2015.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glenn CF, Chow DK, David L, Cooke CA, Gami MS, Iser WB, Hanselman KB, Goldberg IG, Wolkow CA. Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J Gerontol A Biol Sci Med Sci. 2004;59(12):1251–1260. doi: 10.1093/gerona/59.12.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomez M, De Castro E, Guarin E, Sasakura H, Kuhara A, Mori I, Bartfai T, Bargmann CI, Nef P. Ca2+ signaling via the neuronal calcium sensor-1 regulates associative learning and memory in C.elegans. Neuron. 2001;30(1):241–248. doi: 10.1016/s0896-6273(01)00276-8. [DOI] [PubMed] [Google Scholar]
- 69.Gothard KM, Skaggs WE, Moore KM, McNaughton BL. Binding of hippocampal CA1 neural activity to multiple reference frames in a landmark-based navigation task. J Neurosci. 1996;16(2):823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gray CM, Maldonado PE, Wilson M, McNaughton B. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J Neurosci Methods. 1995;63(1–2):43–54. doi: 10.1016/0165-0270(95)00085-2. [DOI] [PubMed] [Google Scholar]
- 71.Grill JD, Riddle DR. Age-related and laminar-specific dendritic changes in the medial frontal cortex of the rat. Brain Res. 2002;937(1–2):8–21. doi: 10.1016/s0006-8993(02)02457-5. [DOI] [PubMed] [Google Scholar]
- 72.Grotewiel MS, Martin I, Bhandari P, Cook-Wiens E. Functional senescence in Drosophila melanogaster. Ageing Res Rev. 2005;4(3):372–397. doi: 10.1016/j.arr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Guan Z, Buhl LK, Quinn WG, Littleton JT. Altered gene regulation and synaptic morphology in Drosophila learning and memory mutants. Learn Mem. 2011;18(4):191–206. doi: 10.1101/lm.2027111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 2014;21(10):519–526. doi: 10.1101/lm.034363.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haas CB, Kalinine E, Zimmer ER, Hansel G, Brochier AW, Oses JP, Portela LV, Muller AP. Brain Insulin Administration Triggers Distinct Cognitive and Neurotrophic Responses in Young and Aged Rats. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9494-6. [DOI] [PubMed] [Google Scholar]
- 76.Haddadi M, Jahromi SR, Sagar BK, Patil RK, Shivanandappa T, Ramesh SR. Brain aging, memory impairment and oxidative stress: a study in Drosophila melanogaster. Behav Brain Res. 2014;259:60–69. doi: 10.1016/j.bbr.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 77.Hahm JH, Kim S, DiLoreto R, Shi C, Lee SJ, Murphy CT, Nam HGC. elegans maximum velocity correlates with healthspan and is maintained in worms with an insulin receptor mutation. Nat Commun. 2015;6:8919. doi: 10.1038/ncomms9919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haj-ali V, Mohaddes G, Babri SH. Intracerebroventricular insulin improves spatial learning and memory in male Wistar rats. Behav Neurosci. 2009;123(6):1309–1314. doi: 10.1037/a0017722. [DOI] [PubMed] [Google Scholar]
- 79.Hanks SD, Flood DG. Region-specific stability of dendritic extent in normal human aging and regression in Alzheimer’s disease. I. CA1 of hippocampus. Brain Res. 1991;540(1–2):63–82. doi: 10.1016/0006-8993(91)90493-f. [DOI] [PubMed] [Google Scholar]
- 80.Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 81.Head E, Mehta R, Hartley J, Kameka M, Cummings BJ, Cotman CW, Ruehl WW, Milgram NW. Spatial learning and memory as a function of age in the dog. Behav Neurosci. 1995;109(5):851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- 82.Hedgecock EM, Russell RL. Normal and mutant thermotaxis in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1975;72(10):4061–4065. doi: 10.1073/pnas.72.10.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- 84.Hobert O. Behavioral plasticity in C. elegans: paradigms, circuits, genes. J Neurobiol. 2003;54(1):203–223. doi: 10.1002/neu.10168. [DOI] [PubMed] [Google Scholar]
- 85.Holtmaat A, Wilbrecht L, Knott GW, Welker E, Svoboda K. Experience-dependent and cell-type-specific spine growth in the neocortex. Nature. 2006;441(7096):979–983. doi: 10.1038/nature04783. [DOI] [PubMed] [Google Scholar]
- 86.Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–187. doi: 10.1038/nature01298. [DOI] [PubMed] [Google Scholar]
- 87.Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457(7230):726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- 88.Hosono R, Nishimoto S, Kuno S. Alterations of life span in the nematode Caenorhabditis elegans under monoxenic culture conditions. Exp Gerontol. 1989;24(3):251–264. doi: 10.1016/0531-5565(89)90016-8. [DOI] [PubMed] [Google Scholar]
- 89.Houthoofd K, Braeckman BP, Johnson TE, Vanfleteren JR. Life extension via dietary restriction is independent of the Ins/IGF-1 signalling pathway in Caenorhabditis elegans. Exp Gerontol. 2003;38(9):947–954. doi: 10.1016/s0531-5565(03)00161-x. [DOI] [PubMed] [Google Scholar]
- 90.Hsu AL, Feng Z, Hsieh MY, Xu XZ. Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol Aging. 2009;30(9):1498–1503. doi: 10.1016/j.neurobiolaging.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Idrobo F, Nandy K, Mostofsky DI, Blatt L, Nandy L. Dietary restriction: effects on radial maze learning and lipofuscin pigment deposition in the hippocampus and frontal cortex. Arch Gerontol Geriatr. 1987;6(4):355–362. doi: 10.1016/0167-4943(87)90014-8. [DOI] [PubMed] [Google Scholar]
- 92.Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42(1):78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- 93.Jia J, Fernandes Y, Gerlai R. Short-term memory in zebrafish (Danio rerio) Behav Brain Res. 2014;270:29–36. doi: 10.1016/j.bbr.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 94.Jiang HC, Hsu JM, Yen CP, Chao CC, Chen RH, Pan CL. Neural activity and CaMKII protect mitochondria from fragmentation in aging Caenorhabditis elegans neurons. Proc Natl Acad Sci U S A. 2015;112(28):8768–8773. doi: 10.1073/pnas.1501831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16(1):3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 96.Jung MW, McNaughton BL. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993;3(2):165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- 97.Kaletsky R, Lakhina V, Arey R, Williams A, Landis J, Ashraf J, Murphy CT. The C. elegans adult neuronal IIS/FOXO transcriptome reveals adult phenotype regulators. Nature. 2016;529(7584):92–96. doi: 10.1038/nature16483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kannan K, Fridell YW. Functional implications of Drosophila insulin-like peptides in metabolism, aging, and dietary restriction. Front Physiol. 2013;4:288. doi: 10.3389/fphys.2013.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kano T, Brockie PJ, Sassa T, Fujimoto H, Kawahara Y, Iino Y, Mellem JE, Madsen DM, Hosono R, Maricq AV. Memory in Caenorhabditis elegans is mediated by NMDA-type ionotropic glutamate receptors. Curr Biol. 2008;18(13):1010–1015. doi: 10.1016/j.cub.2008.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kauffman AL, Ashraf JM, Corces-Zimmerman MR, Landis JN, Murphy CT. Insulin signaling and dietary restriction differentially influence the decline of learning and memory with age. PLoS Biol. 2010;8(5):e1000372. doi: 10.1371/journal.pbio.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- 102.Keuker JI, Luiten PG, Fuchs E. Preservation of hippocampal neuron numbers in aged rhesus monkeys. Neurobiol Aging. 2003;24(1):157–165. doi: 10.1016/s0197-4580(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 103.Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277(5328):942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- 104.Kishimoto Y, Suzuki M, Kawahara S, Kirino Y. Age-dependent impairment of delay and trace eyeblink conditioning in mice. Neuroreport. 2001;12(15):3349–3352. doi: 10.1097/00001756-200110290-00040. [DOI] [PubMed] [Google Scholar]
- 105.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6(6):413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 106.Knuttinen MG, Gamelli AE, Weiss C, Power JM, Disterhoft JF. Age-related effects on eyeblink conditioning in the F344 x BN F1 hybrid rat. Neurobiol Aging. 2001;22(1):1–8. doi: 10.1016/s0197-4580(00)00194-9. [DOI] [PubMed] [Google Scholar]
- 107.Kodama E, Kuhara A, Mohri-Shiomi A, Kimura KD, Okumura M, Tomioka M, Iino Y, Mori I. Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 2006;20(21):2955–2960. doi: 10.1101/gad.1479906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krashes MJ, Waddell S. Rapid consolidation to a radish and protein synthesis-dependent long-term memory after single-session appetitive olfactory conditioning in Drosophila. J Neurosci. 2008;28(12):3103–3113. doi: 10.1523/JNEUROSCI.5333-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kudo K, Wati H, Qiao C, Arita J, Kanba S. Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Res. 2005;1054(1):30–37. doi: 10.1016/j.brainres.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 110.Kuhla A, Lange S, Holzmann C, Maass F, Petersen J, Vollmar B, Wree A. Lifelong caloric restriction increases working memory in mice. PLoS One. 2013;8(7):e68778. doi: 10.1371/journal.pone.0068778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lai CH, Chou CY, Ch’ang LY, Liu CS, Lin W. Identification of novel human genes evolutionarily conserved in Caenorhabditis elegans by comparative proteomics. Genome Res. 2000;10(5):703–713. doi: 10.1101/gr.10.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol Aging. 1995;16(6):947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- 113.Lakhina V, Arey RN, Kaletsky R, Kauffman A, Stein G, Keyes W, Xu D, Murphy CT. Genome-wide functional analysis of CREB/long-term memory-dependent transcription reveals distinct basal and memory gene expression programs. Neuron. 2015;85(2):330–345. doi: 10.1016/j.neuron.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lakowski B, Hekimi S. The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1998;95(22):13091–13096. doi: 10.1073/pnas.95.22.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee Y, Dawson VL, Dawson TM. Animal models of Parkinson’s disease: vertebrate genetics. Cold Spring Harb Perspect Med. 2012;2(10) doi: 10.1101/cshperspect.a009324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lewis JA, Wu CH, Levine JH, Berg H. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience. 1980;5(6):967–989. doi: 10.1016/0306-4522(80)90180-3. [DOI] [PubMed] [Google Scholar]
- 117.Li LB, Lei H, Arey RN, Li P, Liu J, Murphy CT, Xu XZ, Shen K. The Neuronal Kinesin UNC-104/KIF1A Is a Key Regulator of Synaptic Aging and Insulin Signaling-Regulated Memory. Curr Biol. 2016 doi: 10.1016/j.cub.2015.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315(5815):1133–1137. doi: 10.1126/science.1136610. [DOI] [PubMed] [Google Scholar]
- 119.Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- 120.Lyons-Warren A, Lillie R, Hershey T. Short- and long-term spatial delayed response performance across the lifespan. Dev Neuropsychol. 2004;26(3):661–678. doi: 10.1207/s15326942dn2603_1. [DOI] [PubMed] [Google Scholar]
- 121.Mair W, Dillin A. Aging and survival: the genetics of life span extension by dietary restriction. Annu Rev Biochem. 2008;77:727–754. doi: 10.1146/annurev.biochem.77.061206.171059. [DOI] [PubMed] [Google Scholar]
- 122.Manuel R, Gorissen M, Stokkermans M, Zethof J, Ebbesson LO, van de Vis H, Flik G, van den Bos R. The effects of environmental enrichment and age-related differences on inhibitory avoidance in zebrafish (Danio rerio Hamilton) Zebrafish. 2015;12(2):152–165. doi: 10.1089/zeb.2014.1045. [DOI] [PubMed] [Google Scholar]
- 123.Markham JA, Juraska JM. Aging and sex influence the anatomy of the rat anterior cingulate cortex. Neurobiol Aging. 2002;23(4):579–588. doi: 10.1016/s0197-4580(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 124.Markowska AL, Savonenko A. Retardation of cognitive aging by life-long diet restriction: implications for genetic variance. Neurobiol Aging. 2002;23(1):75–86. doi: 10.1016/s0197-4580(01)00249-4. [DOI] [PubMed] [Google Scholar]
- 125.Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, Pontecorvo MJ, Wenk GL, Olton DS. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol Aging. 1989;10(1):31–43. doi: 10.1016/s0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- 126.Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126(9):913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 127.McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size 1935. Nutrition. 1989;5(3):155–171. discussion 172. [PubMed] [Google Scholar]
- 128.McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293(5533):1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- 129.McGuire SE, Roman G, Davis RL. Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet. 2004;20(8):384–391. doi: 10.1016/j.tig.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 130.McIntyre JS, Craik FI. Age differences in memory for item and source information. Can J Psychol. 1987;41(2):175–192. doi: 10.1037/h0084154. [DOI] [PubMed] [Google Scholar]
- 131.McNaughton BL, O’Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods. 1983;8(4):391–397. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- 132.McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93(4):546–553. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J Comp Neurol. 2001;438(4):445–456. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- 134.Merrill DA, Roberts JA, Tuszynski MH. Conservation of neuron number and size in entorhinal cortex layers II, III, and V/VI of aged primates. J Comp Neurol. 2000;422(3):396–401. doi: 10.1002/1096-9861(20000703)422:3<396::aid-cne6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 135.Mohri A, Kodama E, Kimura KD, Koike M, Mizuno T, Mori I. Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics. 2005;169(3):1437–1450. doi: 10.1534/genetics.104.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol Aging. 2003;24(1):125–134. doi: 10.1016/s0197-4580(02)00054-4. [DOI] [PubMed] [Google Scholar]
- 137.Moosavi M, Naghdi N, Choopani S. Intra CA1 insulin microinjection improves memory consolidation and retrieval. Peptides. 2007;28(5):1029–1034. doi: 10.1016/j.peptides.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 138.Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13(4):240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moss MB, Killiany RJ, Lai ZC, Rosene DL, Herndon JG. Recognition memory span in rhesus monkeys of advanced age. Neurobiol Aging. 1997;18(1):13–19. doi: 10.1016/s0197-4580(96)00211-4. [DOI] [PubMed] [Google Scholar]
- 140.Moss MB, Rosene DL, Peters A. Effects of aging on visual recognition memory in the rhesus monkey. Neurobiol Aging. 1988;9(5–6):495–502. doi: 10.1016/s0197-4580(88)80103-9. [DOI] [PubMed] [Google Scholar]
- 141.Mulcahy B, Holden-Dye L, O’Connor V. Pharmacological assays reveal age-related changes in synaptic transmission at the Caenorhabditis elegans neuromuscular junction that are modified by reduced insulin signalling. J Exp Biol. 2013;216(Pt 3):492–501. doi: 10.1242/jeb.068734. [DOI] [PubMed] [Google Scholar]
- 142.Murakami H, Bessinger K, Hellmann J, Murakami S. Aging-dependent and -independent modulation of associative learning behavior by insulin/insulin-like growth factor-1 signal in Caenorhabditis elegans. J Neurosci. 2005;25(47):10894–10904. doi: 10.1523/JNEUROSCI.3600-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Murakami S, Murakami H. The effects of aging and oxidative stress on learning behavior in C. elegans. Neurobiol Aging. 2005;26(6):899–905. doi: 10.1016/j.neurobiolaging.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 144.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424(6946):277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 145.Naganos S, Horiuchi J, Saitoe M. Mutations in the Drosophila insulin receptor substrate, CHICO, impair olfactory associative learning. Neurosci Res. 2012;73(1):49–55. doi: 10.1016/j.neures.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 146.Nebel A, Kleindorp R, Caliebe A, Nothnagel M, Blanché H, Junge O, Wittig M, Ellinghaus D, Flachsbart F, Wichmann HE, Meitinger T, Nikolaus S, Franke A, Krawczak M, Lathrop M, Schreiber S. A genome-wide association study confirms APOE as the major gene influencing survival in long-lived individuals. Mech Ageing Dev. 2011;132(6–7):324–330. doi: 10.1016/j.mad.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 147.Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in size of perforated postsynaptic densities in hippocampal axospinous synapses and age-related spatial learning impairments. J Neurosci. 2004;24(35):7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nuttley WM, Atkinson-Leadbeater KP, Van Der Kooy D. Serotonin mediates food-odor associative learning in the nematode Caenorhabditiselegans. Proc Natl Acad Sci U S A. 2002;99(19):12449–12454. doi: 10.1073/pnas.192101699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ogg S, Paradis S, Gottlieb S, Patterson GI, Lee L, Tissenbaum HA, Ruvkun G. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389(6654):994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 150.Oh E, Miller RA, Thurmond DC. Syntaxin 4 Overexpression Ameliorates Effects of Aging and High-Fat Diet on Glucose Control and Extends Lifespan. Cell Metab. 2015;22(3):499–507. doi: 10.1016/j.cmet.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Onos KD, Sukoff Rizzo SJ, Howell GR, Sasner M. Toward more predictive genetic mouse models of Alzheimer’s disease. Brain Res Bull. 2015;122:1–11. doi: 10.1016/j.brainresbull.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pakkenberg B, Gundersen HJ. Neocortical neuron number in humans: effect of sex and age. J Comp Neurol. 1997;384(2):312–320. [PubMed] [Google Scholar]
- 153.Pan CL, Peng CY, Chen CH, McIntire S. Genetic analysis of age-dependent defects of the Caenorhabditis elegans touch receptor neurons. Proc Natl Acad Sci U S A. 2011;108(22):9274–9279. doi: 10.1073/pnas.1011711108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Panowski SH, Wolff S, Aguilaniu H, Durieux J, Dillin A. PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature. 2007;447(7144):550–555. doi: 10.1038/nature05837. [DOI] [PubMed] [Google Scholar]
- 155.Paradis S, Ailion M, Toker A, Thomas JH, Ruvkun G. A PDK1 homolog is necessary and sufficient to transduce AGE-1 PI3 kinase signals that regulate diapause in Caenorhabditis elegans. Genes Dev. 1999;13(11):1438–1452. doi: 10.1101/gad.13.11.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12(16):2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000;68(4):509–514. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 158.Partridge L, Barton NH. Evolution of aging: testing the theory using Drosophila. Genetica. 1993;91(1–3):89–98. doi: 10.1007/BF01435990. [DOI] [PubMed] [Google Scholar]
- 159.Pascual A, Préat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294(5544):1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- 160.Pather S, Gerlai R. Shuttle box learning in zebrafish (Danio rerio) Behav Brain Res. 2009;196(2):323–327. doi: 10.1016/j.bbr.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pawlikowska L, Hu D, Huntsman S, Sung A, Chu C, Chen J, Joyner AH, Schork NJ, Hsueh WC, Reiner AP, Psaty BM, Atzmon G, Barzilai N, Cummings SR, Browner WS, Kwok PY, Ziv E, Fractures SoO. Association of common genetic variation in the insulin/IGF1 signaling pathway with human longevity. Aging Cell. 2009;8(4):460–472. doi: 10.1111/j.1474-9726.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Perazzona B, Isabel G, Preat T, Davis RL. The role of cAMP response element-binding protein in Drosophila long-term memory. J Neurosci. 2004;24(40):8823–8828. doi: 10.1523/JNEUROSCI.4542-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex. 1994;4(6):621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- 164.Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152(4):970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, Liu LX, Doberstein SK, Ruvkun G. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15(6):672–686. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pitsikas N, Algeri S. Deterioration of spatial and nonspatial reference and working memory in aged rats: protective effect of life-long calorie restriction. Neurobiol Aging. 1992;13(3):369–373. doi: 10.1016/0197-4580(92)90110-j. [DOI] [PubMed] [Google Scholar]
- 167.Pyapali GK, Turner DA. Increased dendritic extent in hippocampal CA1 neurons from aged F344 rats. Neurobiol Aging. 1996;17(4):601–611. doi: 10.1016/0197-4580(96)00034-6. [DOI] [PubMed] [Google Scholar]
- 168.Raizen DM, Lee RY, Avery L. Interacting genes required for pharyngeal excitation by motor neuron MC in Caenorhabditis elegans. Genetics. 1995;141(4):1365–1382. doi: 10.1093/genetics/141.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40(4):835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- 170.Rand JB, Russell RL. Choline acetyltransferase-deficient mutants of the nematode Caenorhabditis elegans. Genetics. 1984;106(2):227–248. doi: 10.1093/genetics/106.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Rankin CH, Beck CD, Chiba CM. Caenorhabditis elegans: a new model system for the study of learning and memory. Behav Brain Res. 1990;37(1):89–92. doi: 10.1016/0166-4328(90)90074-o. [DOI] [PubMed] [Google Scholar]
- 172.Rapp PR, Amaral DG. Evidence for task-dependent memory dysfunction in the aged monkey. J Neurosci. 1989;9(10):3568–3576. doi: 10.1523/JNEUROSCI.09-10-03568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci U S A. 1996;93(18):9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8(8):1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- 175.Rasmussen T, Schliemann T, Sørensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol Aging. 1996;17(1):143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 176.Ratliff EP, Mauntz RE, Kotzebue RW, Gonzalez A, Achal M, Barekat A, Finley KA, Sparhawk JM, Robinson JE, Herr DR, Harris GL, Joiner WJ, Finley KD. Aging and Autophagic Function Influences the Progressive Decline of Adult Drosophila Behaviors. PLoS One. 2015;10(7):e0132768. doi: 10.1371/journal.pone.0132768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rhodes MG. Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychol Aging. 2004;19(3):482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- 178.Robertson M, Keene AC. Molecular mechanisms of age-related sleep loss in the fruit fly - a mini-review. Gerontology. 2013;59(4):334–339. doi: 10.1159/000348576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Roth BL. DREADDs for Neuroscientists. Neuron. 2016;89(4):683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ruhl T, Jonas A, Seidel NI, Prinz N, Albayram O, Bilkei-Gorzo A, von der Emde G. Oxidation and Cognitive Impairment in the Aging Zebrafish. Gerontology. 2015;62(1):47–57. doi: 10.1159/000433534. [DOI] [PubMed] [Google Scholar]
- 181.Saeki S, Yamamoto M, Iino Y. Plasticity of chemotaxis revealed by paired presentation of a chemoattractant and starvation in the nematode Caenorhabditis elegans. J Exp Biol. 2001;204(Pt 10):1757–1764. doi: 10.1242/jeb.204.10.1757. [DOI] [PubMed] [Google Scholar]
- 182.Samson RD, Barnes CA. Impact of aging brain circuits on cognition. Eur J Neurosci. 2013;37(12):1903–1915. doi: 10.1111/ejn.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schafer MJ, Dolgalev I, Alldred MJ, Heguy A, Ginsberg SD. Calorie Restriction Suppresses Age-Dependent Hippocampal Transcriptional Signatures. PLoS One. 2015;10(7):e0133923. doi: 10.1371/journal.pone.0133923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldiks N, Küstermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Brüning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc Natl Acad Sci U S A. 2004;101(9):3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schächter F, Faure-Delanef L, Guénot F, Rouger H, Froguel P, Lesueur-Ginot L, Cohen D. Genetic associations with human longevity at the APOE and ACE loci. Nat Genet. 1994;6(1):29–32. doi: 10.1038/ng0194-29. [DOI] [PubMed] [Google Scholar]
- 186.Shaw WM, Luo S, Landis J, Ashraf J, Murphy CT. The C. elegans TGF-beta Dauer pathway regulates longevity via insulin signaling. Curr Biol. 2007;17(19):1635–1645. doi: 10.1016/j.cub.2007.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shirai Y, Kouzuki T, Kakefuda K, Moriguchi S, Oyagi A, Horie K, Morita SY, Shimazawa M, Fukunaga K, Takeda J, Saito N, Hara H. Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKbeta in neurite spine formation, contributing to cognitive function. PLoS One. 2010;5(7):e11602. doi: 10.1371/journal.pone.0011602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- 189.Sison M, Gerlai R. Associative learning in zebrafish (Danio rerio) in the plus maze. Behav Brain Res. 2010;207(1):99–104. doi: 10.1016/j.bbr.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sison M, Gerlai R. Associative learning performance is impaired in zebrafish (Danio rerio) by the NMDA-R antagonist MK-801. Neurobiol Learn Mem. 2011;96(2):230–237. doi: 10.1016/j.nlm.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Smith DE, Rapp PR, McKay HM, Roberts JA, Tuszynski MH. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J Neurosci. 2004;24(18):4373–4381. doi: 10.1523/JNEUROSCI.4289-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Soldan A, Pettigrew C, Cai Q, Wang MC, Moghekar AR, O’Brien RJ, Selnes OA, Albert MS, Team BR. Hypothetical Preclinical Alzheimer Disease Groups and Longitudinal Cognitive Change. JAMA Neurol. 2016 doi: 10.1001/jamaneurol.2016.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Solomon PR, Groccia-Ellison ME. Classic conditioning in aged rabbits: delay, trace, and long-delay conditioning. Behav Neurosci. 1996;110(3):427–435. doi: 10.1037//0735-7044.110.3.427. [DOI] [PubMed] [Google Scholar]
- 194.Spencer WD, Raz N. Differential effects of aging on memory for content and context: a meta-analysis. Psychol Aging. 1995;10(4):527–539. doi: 10.1037//0882-7974.10.4.527. [DOI] [PubMed] [Google Scholar]
- 195.Stein GM, Murphy CT. The Intersection of Aging, Longevity Pathways, and Learning and Memory in C. elegans. Front Genet. 2012;3:259. doi: 10.3389/fgene.2012.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Stein GM, Murphy CTC. elegans positive olfactory associative memory is a molecularly conserved behavioral paradigm. Neurobiol Learn Mem. 2014;115:86–94. doi: 10.1016/j.nlm.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Stern SA, Chen DY, Alberini CM. The effect of insulin and insulin-like growth factors on hippocampus- and amygdala-dependent long-term memory formation. Learn Mem. 2014;21(10):556–563. doi: 10.1101/lm.029348.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Stern SA, Kohtz AS, Pollonini G, Alberini CM. Enhancement of memories by systemic administration of insulin-like growth factor II. Neuropsychopharmacology. 2014;39(9):2179–2190. doi: 10.1038/npp.2014.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Stetak A, Hörndli F, Maricq AV, van den Heuvel S, Hajnal A. Neuron-specific regulation of associative learning and memory by MAGI-1 in C. elegans. PLoS One. 2009;4(6):e6019. doi: 10.1371/journal.pone.0006019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stewart J, Mitchell J, Kalant N. The effects of life-long food restriction on spatial memory in young and aged Fischer 344 rats measured in the eight-arm radial and the Morris water mazes. Neurobiol Aging. 1989;10(6):669–675. doi: 10.1016/0197-4580(89)90003-1. [DOI] [PubMed] [Google Scholar]
- 201.Stranahan AM, Jiam NT, Spiegel AM, Gallagher M. Aging reduces total neuron number in the dorsal component of the rodent prefrontal cortex. J Comp Neurol. 2012;520(6):1318–1326. doi: 10.1002/cne.22790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Suh Y, Atzmon G, Cho MO, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105(9):3438–3442. doi: 10.1073/pnas.0705467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317(5836):369–372. doi: 10.1126/science.1142179. [DOI] [PubMed] [Google Scholar]
- 205.Tamura T, Chiang AS, Ito N, Liu HP, Horiuchi J, Tully T, Saitoe M. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron. 2003;40(5):1003–1011. doi: 10.1016/s0896-6273(03)00732-3. [DOI] [PubMed] [Google Scholar]
- 206.Tank EM, Rodgers KE, Kenyon C. Spontaneous age-related neurite branching in Caenorhabditis elegans. J Neurosci. 2011;31(25):9279–9288. doi: 10.1523/JNEUROSCI.6606-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292(5514):107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- 208.Tempel BL, Bonini N, Dawson DR, Quinn WG. Reward learning in normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1983;80(5):1482–1486. doi: 10.1073/pnas.80.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Thompson LT, Moyer JR, Disterhoft JF. Trace eyeblink conditioning in rabbits demonstrates heterogeneity of learning ability both between and within age groups. Neurobiol Aging. 1996;17(4):619–629. doi: 10.1016/0197-4580(96)00026-7. [DOI] [PubMed] [Google Scholar]
- 210.Tonoki A, Davis RL. Aging impairs intermediate-term behavioral memory by disrupting the dorsal paired medial neuron memory trace. Proc Natl Acad Sci U S A. 2012;109(16):6319–6324. doi: 10.1073/pnas.1118126109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Tonoki A, Davis RL. Aging impairs protein-synthesis-dependent long-term memory in Drosophila. J Neurosci. 2015;35(3):1173–1180. doi: 10.1523/JNEUROSCI.0978-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Torayama I, Ishihara T, Katsura I. Caenorhabditis elegans integrates the signals of butanone and food to enhance chemotaxis to butanone. J Neurosci. 2007;27(4):741–750. doi: 10.1523/JNEUROSCI.4312-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Torroja L, Chu H, Kotovsky I, White K. Neuronal overexpression of APPL, the Drosophila homologue of the amyloid precursor protein (APP), disrupts axonal transport. Curr Biol. 1999;9(9):489–492. doi: 10.1016/s0960-9822(99)80215-2. [DOI] [PubMed] [Google Scholar]
- 214.Toth ML, Melentijevic I, Shah L, Bhatia A, Lu K, Talwar A, Naji H, Ibanez-Ventoso C, Ghose P, Jevince A, Xue J, Herndon LA, Bhanot G, Rongo C, Hall DH, Driscoll M. Neurite sprouting and synapse deterioration in the aging Caenorhabditis elegans nervous system. J Neurosci. 2012;32(26):8778–8790. doi: 10.1523/JNEUROSCI.1494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79(1):35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 216.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157(2):263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 217.Turner DA, Deupree DL. Functional elongation of CA1 hippocampal neurons with aging in Fischer 344 rats. Neurobiol Aging. 1991;12(3):201–210. doi: 10.1016/0197-4580(91)90098-5. [DOI] [PubMed] [Google Scholar]
- 218.Valenzano DR, Terzibasi E, Cattaneo A, Domenici L, Cellerino A. Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell. 2006;5(3):275–278. doi: 10.1111/j.1474-9726.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- 219.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16(3):296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 220.Varshney LR, Chen BL, Paniagua E, Hall DH, Chklovskii DB. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput Biol. 2011;7(2):e1001066. doi: 10.1371/journal.pcbi.1001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Vukojevic V, Gschwind L, Vogler C, Demougin P, de Quervain DJ, Papassotiropoulos A, Stetak A. A role for α-adducin (ADD-1) in nematode and human memory. EMBO J. 2012;31(6):1453–1466. doi: 10.1038/emboj.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14(4):275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 224.West MJ, Coleman PD, Flood DG, Troncoso JC. Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer’s disease. Lancet. 1994;344(8925):769–772. doi: 10.1016/s0140-6736(94)92338-8. [DOI] [PubMed] [Google Scholar]
- 225.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314(1165):1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 226.White KE, Humphrey DM, Hirth F. The dopaminergic system in the aging brain of Drosophila. Front Neurosci. 2010;4:205. doi: 10.3389/fnins.2010.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wilkniss SM, Jones MG, Korol DL, Gold PE, Manning CA. Age-related differences in an ecologically based study of route learning. Psychol Aging. 1997;12(2):372–375. doi: 10.1037//0882-7974.12.2.372. [DOI] [PubMed] [Google Scholar]
- 228.Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci U S A. 2008;105(37):13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Williams SC, Deisseroth K. Optogenetics. Proc Natl Acad Sci U S A. 2013;110(41):16287. doi: 10.1073/pnas.1317033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci U S A. 2009;106(4):1255–1260. doi: 10.1073/pnas.0808587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yang Y, Lei C, Feng H, Sui JF. The neural circuitry and molecular mechanisms underlying delay and trace eyeblink conditioning in mice. Behav Brain Res. 2015;278:307–314. doi: 10.1016/j.bbr.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 232.Yates MA, Markham JA, Anderson SE, Morris JR, Juraska JM. Regional variability in age-related loss of neurons from the primary visual cortex and medial prefrontal cortex of male and female rats. Brain Res. 2008;1218:1–12. doi: 10.1016/j.brainres.2008.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79(1):49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 234.Yu D, Keene AC, Srivatsan A, Waddell S, Davis RL. Drosophila DPM neurons form a delayed and branch-specific memory trace after olfactory classical conditioning. Cell. 2005;123(5):945–957. doi: 10.1016/j.cell.2005.09.037. [DOI] [PubMed] [Google Scholar]
- 235.Yu L, Tucci V, Kishi S, Zhdanova IV. Cognitive aging in zebrafish. PLoS One. 2006;1:e14. doi: 10.1371/journal.pone.0000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Zhang Y, Lu H, Bargmann CI. Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature. 2005;438(7065):179–184. doi: 10.1038/nature04216. [DOI] [PubMed] [Google Scholar]
- 237.Zhdanova IV, Yu L, Lopez-Patino M, Shang E, Kishi S, Guelin E. Aging of the circadian system in zebrafish and the effects of melatonin on sleep and cognitive performance. Brain Res Bull. 2008;75(2–4):433–441. doi: 10.1016/j.brainresbull.2007.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61(2):247–258. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]